Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is a global health burden that affects 300 million people worldwide. Globally, COPD was reported as the fourth leading cause of death in 2004 and is projected to occupy the third position in 2030. The goal of the present project is to describe the prevalence and determine the causes and risk factors of COPD in five provinces of Iran.
Methods:
This study followed a stratified cluster sampling strategy with proportional allocation within strata. The target population is all noninstitutionalized inhabitants, aged 18 and over, who inhabit in different provinces in Iran in the year 2017. The stratification of the sample according to the 31 provinces of Iran is incorporated in the sampling process. The core questionnaire was developed from preexisting validated questionnaires. The single most important outcome measure obtained as part of this protocol was spirometry before and after the administration of 200 mg (two puffs) of salbutamol.
Results:
The most commonly reported respiratory symptoms were as follows: wheezing (N=217, 20.4%, 95% confidence interval [CI]: 18%–22.8%), sputum production (N=173, 16.5%, 95% CI: 14.3%–18.8%), and dyspnea (N=131, 12.3%, 95% CI: 10.3%–14.3%). The overall COPD prevalence defined by the postbronchodilator spirometric Global Initiative for Chronic Obstructive Lung Disease criteria was 4.9%, higher in men (6.4%) than in women (3.9%). The prevalence of COPD was strongly dependent on smoking status, age, and sex.
Conclusion:
COPD is considered a preventable disease, and avoidance of exposure to major risk factors can prevent the vast majority of cases. The present study findings add to the literature on the prevalence of COPD in Iran and will help policy-makers, specialists, and all stakeholders to strategize and evaluate medical services required for reducing the prevalence of respiratory diseases. The data from our present study will serve as baseline information for future national and regional studies of COPD.
KEY WORDS: Burden of Obstructive Lung Disease, chronic obstructive pulmonary disease, Iran, prevalence
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a global health burden that affects 300 million people worldwide resulting in >3 million deaths annually.[1,2] Globally, COPD was reported as the fourth leading cause of death (5.1%) in 2004 and is projected to occupy the third position (8.6%) in 2030.[3] The prevalence of COPD in adults has been reported differently. In PLATINO study, crude rates of Stage I or higher COPD were between 7.8% and 19.7% in samples from five Latin American countries.[4] This rate was reported between 0.2% in Japan and 37% in the USA.[5] COPD is characterized by chronic inflammation and nonfully reversible airflow obstruction, involving structural changes in the lung that can be demonstrated as a low ratio (<0.7) of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC).[6,7]
Tobacco is reported as the first risk factor for disease in high-income North America and Western Europe and second only to high blood pressure globally according to a recent systematic analysis.[8] It is proved that the strongest risk factors for airflow obstruction are smoking and exposure to environmental tobacco smoke,[9] but many areas of the world with high mortality rates from “COPD” still have low consumption of tobacco.[10,11,12] In our previous study, we found that the prevalence of airflow limitation was higher in individuals who had ever smoked, but among patients with COPD according to the spirometry tests, 12.6% were smokers and 18.3% ex-smokers.[13] In other words, nonfully reversible airflow obstruction also occurs in never smokers. Iran is a developing country where the smoking rate (11.9%) is lower than the observed in many developed countries.[14]
During the last decade, national estimates of COPD prevalence were mostly based on self-reported questionnaires. Several studies conducted in Iran,[15] the United States,[16] Spain,[17] Sweden,[18] Korea,[19] and South America[4] have demonstrated the underdiagnosis of COPD.
A clear example of this kind of underreport was observed in Japan, where the results of the 2004 population-based prevalence of COPD survey contrast with the estimates of the Japanese Ministry of Health (10.9% vs. 0.3%, respectively).[20]
The estimation of COPD prevalence indeed depends on the population characteristics and criteria used to diagnose COPD, with continuing debate over whether the fixed spirometric ratio of 0.7 or lower limit of normal should be the preferred diagnostic criteria.[21]
Carrying out research regarding different aspects of COPD is mentioned as health priorities by academic institutions and governments worldly wide,[22] and the burden of COPD in low- and middle-income countries has been rather high due to low COPD awareness, challenges with COPD diagnosis, and increased exposures to additional risk factors, especially combustion products of biomass fuels, dusty occupation, uncontrolled asthma, and sequela of tuberculosis.[23] The international, population-based Burden of Obstructive Lung Disease (BOLD) initiative was designed to develop robust models during the past two decades, which can be used to estimate the prevalence and current and future economic burden of COPD.[24] The goal of the present project is to describe the prevalence and determine the causes and risk factors of COPD in the population of Iran, and this report summarized primary results from five districts.
METHODS
The BOLD study protocol in Iran was published elsewhere.[25]
Population and sampling strategy
We used the same sampling protocol consistently throughout the project. The sampling frame in this study is the whole population of 31 provinces of Iran. It is considered that the present population in this area is nearly 78 million.
Sample size
Drawing upon our experience, a design effect of 1.5, prevalence rate of 50%, and a response rate of 90%, total sample size is calculated by 1152 in two different sexes for 40 years of age and above and 1152 for 18–39 years. In order to develop valid estimates of future burden of disease, researchers are encouraged to survey an additional cohort on the prevalence of smoking and other key risk factors.
Sampling plan
This study follows a stratified cluster sampling strategy with proportional allocation within strata. The target population is all noninstitutionalized inhabitants, aged 18–40 in one group and over 40 in another, who inhabit in different provinces in the year 2015.
The stratification of the sample according to the 31 provinces of Iran is incorporated in the sampling process. Proportional to the number of households in 31 provinces, the appropriate number of clusters is weighted according to each province. The decision about the number of clusters is based on total sample size, mean household members, and logistical facilities for subject enumeration, transport, and examination.
For each cluster, a team of three members (one male and one female aged <28 as interviewers dressed in white medical overall and a driver) approaches the index household, which is specified through the aforementioned random selection of clusters, and continues the enumeration in ten neighbor households in a systematic manner by proceeding round in a clockwise direction. In indexed household, if there is more than one person, interviewers are advised to use Kish method to choose the right participant(s).[26]
Examination protocol
The examination protocol includes a questionnaire covering respiratory symptoms, health status, activity limitation, and exposure to potential risk factors, such as tobacco smoke, occupational risk factors, and biomass exposure. They also perform pre- and postbronchodilator spirometry tests. Spirometry records provide the 1- and 6-s FEVs (FEV1 and FEV6) and the FVC.
Questionnaires
The core questionnaire was developed from preexisting validated questionnaires that had already been used in multinational studies.[1] The questionnaire obtains information about respiratory symptoms, exposure to potential risk factors, including smoking, occupation, respiratory diagnoses, comorbidities, health-care utilization, medication use, activity limitation, and health status.
Participants also are expected to complete an occupational questionnaire and (for current cigarette smokers) a “stages-of-change” questionnaire that assesses readiness to quit smoking. There is also a questionnaire to assess exposure to biomass fuels used in the home for either heating or cooking. All questionnaires were translated to Persian first and then back translated to English by a different translator. The questionnaires are administered by trained and certified staff; self-administration of questionnaires is not allowed.
Spirometry
The single most important outcome measure obtained as part of this protocol is spirometry before and after administration of 200 mg (two puffs) of salbutamol. To optimize quality control in this study, all teams are required to use the 2120 In2itive Vitalograph Spirometer, which was chosen because it provides an acceptable degree of accuracy, robustness, portability, and ease of storage. It can be used easily in the field and where there is no electric power available. The 2120 In2itive Vitalograph Spirometer has been approved by the National Research Institute of Tuberculosis and Lung Diseases as meeting predetermined performance criteria relating to reliability of measurement, suitability for field use, and ease of access to data.
Chronic obstructive pulmonary disease definitions
COPD definitions were as follows: (1) spirometry: postbronchodilator FEV1/FVC ratio <70%; (2) prior medical diagnosis: an affirmative response to: “have you ever had chronic bronchitis, emphysema, or COPD confirmed by a doctor?;” and (3) clinical definition: a positive criterion for the standard definition of chronic bronchitis. These definitions allowed comparison without the need for reference values and were a widely used standard that can be compared with other published studies.[7,27]
Statistical analysis
In calculating standard errors and the 95% confidence interval (CI) for categorical and continuous variables, the cluster sampling design is taken into account and adjusted for. In addition to descriptive analyses, odds ratios are calculated with multivariate logistic regression in order to control potential confounding variables and account for cluster design effects.
RESULTS
Participants
A total of 1065 individuals were interviewed and examined in five provinces. Four hundred and seventy-nine (45.1%) men and 583 (54.9%) women participated in the structured interviews. These provinces are located in different parts; north, south, east, and center of Iran. Table 1 shows the demographic characteristics.
Table 1.
Demographic characteristics
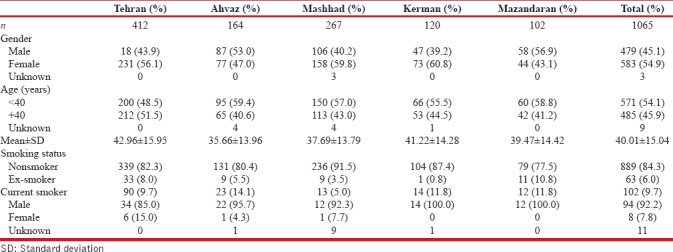
Smoking habits
The rate of current smoking was greater in men than women in all provinces. Of all participants, 102 (9.7%) were current smoker, and out of these participants, 94 (92.2%) were men and 8 (7.8%) women. Sixty-three (6.0%) among all were ex-smoker [Table 1].
Respiratory symptoms
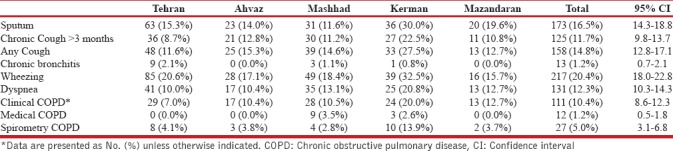
In this study's population, the most reported respiratory symptoms were as follows: 217 (20.4%) wheezing (95% CI: 18%–22.8%), 173 (16.5%) sputum production (95% CI: 14.3%–18.8%), and 131 (12.3%) dyspnea (95% CI: 10.3%–14.3%). As for clinical symptoms, self-reported chronic bronchitis, emphysema, or COPD, 111 (10.4%) (95% CI: 8.6%–12.3%) of the participants had these symptoms and diagnosis during last year.
The prevalence of prior medical diagnosis was nearly one-fourth of postbronchodilator spirometric COPD rate [Table 2].
Table 2.
COPD prevalence according clinical, prior diagnosis, and spirometry
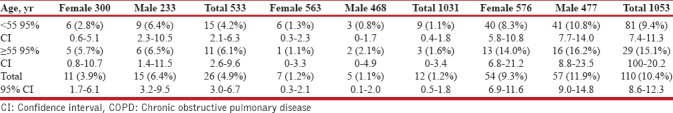
The overall COPD prevalence defined by the postbronchodilator spirometric functional criteria was 5.0%, higher in Kerman (13.9%), followed by Tehran, Ahvaz, Mazandaran, and Mashhad [Tables 2 and 3]. The prevalence was lower in participants younger than 55 years [15 (4.2%)] and was 50% higher [11 (6.1%)] in participants older than 55 years [Table 3]. Three (20%) of people with postbronchodilator spirometric for COPD criteria had >12% and 200 ml reversibility. The prevalence of prior medical diagnosis of asthma in the whole population was 73 (6.8%).
Table 3.
COPD prevalence according to age, gender, and copd definition criteria
Multivariate relationships
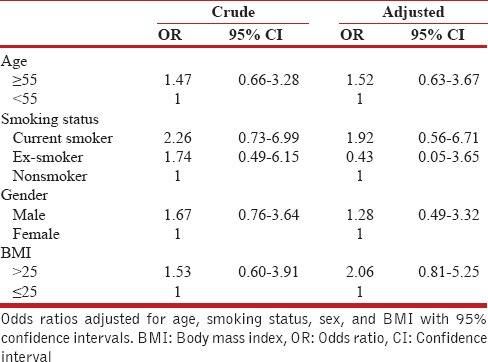
We performed univariate and multivariate logistic regression to assess the association of COPD and risk factors. When including age, gender, smoking habits, family history of obstructive airway disease, and socioeconomic group in the model, body mass index (BMI) and smoking revealed themselves as the two major risk factors. The prevalence of COPD was strongly dependent on BMI, current smoking, gender, and age [Table 4].
Table 4.
The odds ratio for having chronic obstructive pulmonary disease (according to spirometry results)
DISCUSSION
This study is the first systematic attempts to estimate the prevalence of COPD across different regions of Iran. In this study, we estimated the prevalence of COPD using questionnaires and spirometry-based data among people aged 18 years and older who were living in 31 different provinces, and for this report, we analyzed five centers of provinces including Tehran, Ahvaz, Mashhad, Kerman, and Mazandaran. The sample in this study was representative of Iran adult population, and the participation rates were exceptionally high for a comprehensive survey like this one.
In the current study, we estimated the total prevalence of COPD at 4.9% (95% CI: 3%–6.7%) according to the Global Initiative for Chronic Obstructive Lung Disease criteria. All residents were 40 years of age or over and had at least Stage I COPD, and this was more common in men than in women. The observations from this first report are similar to the results found in many other countries with an expected range of 4% to 10% using spirometry[4,28] though it had a wide range between 2.8% and 13.9% in different provinces.
Previous studies, like PLATINO multicenter study[4] which was conducted across five South American cities, reported crude prevalence of COPD ranging from 7.8% to 19.7% in five cities from Brazil, Chile, Mexico, Uruguay, and Venezuela. BOLD study in 2007 conducted in twelve sites from North America, Europe, Asia, and Africa reported that the prevalence of Stage II or higher COPD was 10.1% overall, 11.8% for men, and 8.5% for women.[27] In a study done in Peru, which was done in different provinces, overall prevalence of COPD in four different cities was 6.0% (95% CI: 5.1%–6.8%) but with marked variation across sites.[29] In the PREPOCOL study, the prevalence of COPD was 8.9% across five Colombian cities at different altitudes.[30] A study on the prevalence of COPD risk factors in 12 Asia-Pacific sites estimated about 57 million moderate-to-severe COPD cases in 2002, which is equivalent to a prevalence of 6.3% in the Asia-Pacific region.[28]
Moreover, in a national study by Varmaghani et al. pooled prevalence of chronic bronchitis was reported 5.57%.[31] According to a study done in Isfahan, a megacity of Iran, the prevalence of airflow limitation in the general population 40 years and older was 5.7%.[15]
A possible reason for the low prevalence of COPD in our first report could be the low prevalence of daily tobacco smoking which is mainly lower than every site in BOLD, PLATINO, and PREPOCOL studies.[4,27,30] Furthermore, the differences between COPD prevalence rates reported by different studies could be due to differences in data collection methods, sampling methods, time, and regions where the studies had been carried out.
In this study, we found that the prevalence of COPD was higher in current tobacco smokers similar to previous reports.[4,27,28] The odds for COPD were higher for current smokers. These figures suggest that the risk for developing COPD is approximately twice in current smokers compared that in nonsmokers. This amount of risk has been proposed from 15% to 50% in literature.[29,32] Our finding does prove the fact that smoking is, by far, one of the most important risk factors for COPD which rhymes with the WHO estimation that in many high-income countries, up to 73% of COPD deaths are related to tobacco smoking, and 90% of COPD deaths occur in low- and middle-income countries while 40% of these deaths are attributed to tobacco smoking.[33] These findings by no means imply that early detection of COPD could be helpful for early smoking cessation intervention. In our findings, a majority of patients with COPD were never smokers, and there was no significant relationship between passive smoking and COPD. The main reason could be that the participants in our study were from general population and were likely to be exposed to risk factors other than smoking that caused COPD.
In this study, the COPD prevalence in men is higher than that in women. This figure could be explained by the fact that Iranian women have not been as likely to smoke as men. In the study by van Durme et al. in the Netherlands, male sex and smoking status of current smokers were related to occurrence of COPD.[34] In another study, male sex, increasing age, and smoking history were strong risk factors for COPD, and these associations remained significant after adjustment for other variables.[35] This situation is similar to our previous study in Tehran[13] and posed the hypothesis that considering lower prevalence of tobacco smoking, other risk factors such as air pollution and fossil fuel pollution levels could have a greater effect in women compared with men.
Our study has some strength.First, this study similar to other BOLD studies across the world has a population-based sampling frame derived from five different geographical settings in north, south, center, and east across Iran. Second, we collected extensive demographic, clinical health status, and exposure to potential risk factors, such as tobacco smoke, while conducting standardized pulmonary function tests simultaneously. Third, the settings of this study provided a unique opportunity for us to examine all risk factors associated with COPD. Our study also has some limitations.First, at this point of our study, we were not powered enough to determine risk factors stratified by provinces. Second, as a cross-sectional population-based study, there are limitations in determining direction of causality for possible risk factors of COPD.
CONCLUSION
COPD is considered as a preventable disease, and avoidance of exposure to major risk factors can prevent the vast majority of cases of the disease. In particular, the wide gap between real and previously diagnosed COPD in this study provokes the necessity of raising awareness of this disease among general population and health professionals. The present study findings can upgrade the knowledge on the prevalence of COPD in Iran, and it can help governments, policy-makers, specialists, insurance companies, and all stakeholders to strategize and evaluate medical services required for reducing the prevalence of respiratory diseases. The data from our present study will be available as a baseline tool for future national and regional studies of COPD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management and prevention of Chronic Obstructive Pulmonary disease. Updated 2014. Global Initiative for Chronic Obstructive Lung Disease. 2014 [Google Scholar]
- 2.Cruz AA. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. World Health Organization. 2007 [Google Scholar]
- 3.World Health Organization. World Health Statistics. 2011. [Last accessed on 2011 Sep 04]. http://www. who. int/whosis/whostat/2011/en/index. html .
- 4.Menezes AM, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, Valdivia G, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): A prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 5.Gupta D, Agarwal R, Aggarwal AN, Maturu VN, Dhooria S, Prasad KT, et al. Guidelines for diagnosis and management of chronic obstructive pulmonary disease: Joint recommendations of Indian Chest Society and National College of Chest Physicians (India) Indian J Chest Dis Allied Sci. 2014;56:5–4. [PubMed] [Google Scholar]
- 6.Haahtela T, Tuomisto LE, Pietinalho A, Klaukka T, Erhola M, Kaila M, et al. A10 year asthma programme in Finland: Major change for the better. Thorax. 2006;61:663–70. doi: 10.1136/thx.2005.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 8.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. Acomparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper R, Burney P, Vollmer WM, McBurnie MA, Gislason T, Tan WC, et al. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project. Eur Respir J. 2012;39:1343–53. doi: 10.1183/09031936.00002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 11.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 12.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: Results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–63. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharifi H, Masjedi MR, Emami H, Ghanei M, Eslaminejad A, Radmand G, et al. Burden of obstructive lung disease study in Tehran: Prevalence and risk factors of chronic obstructive pulmonary disease. Lung India. 2015;32:572–7. doi: 10.4103/0970-2113.168129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharifi H, Sadr M, Emami H, Ghanei M, Eslaminejad A, Radmand G, et al. Prevalence of tobacco use and associated factors in Tehran: Burden of obstructive lung disease study. Lung India. 2017;34:225–31. doi: 10.4103/0970-2113.205323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amra B, Golshan M, Fietze I, Penzel T, Welte T. Correlation between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome in a general population in Iran. J Res Med Sci. 2011;16:885–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: Data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683–9. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 17.Peña VS, Miravitlles M, Gabriel R, Jiménez-Ruiz CA, Villasante C, Masa JF, et al. Geographic variations in prevalence and underdiagnosis of COPD: Results of the IBERPOC multicentre epidemiological study. Chest. 2000;118:981–9. doi: 10.1378/chest.118.4.981. [DOI] [PubMed] [Google Scholar]
- 18.Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: What is the true burden of disease? Chest. 2003;123:1684–92. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 19.Kim DS, Kim YS, Jung KS, Chang JH, Lim CM, Lee JH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: A population-based spirometry survey. Am J Respir Crit Care Med. 2005;172:842–7. doi: 10.1164/rccm.200502-259OC. [DOI] [PubMed] [Google Scholar]
- 20.Fukuchi Y, Nishimura M, Ichinose M, Adachi M, Nagai A, Kuriyama T, et al. COPD in Japan: The Nippon COPD Epidemiology study. Respirology. 2004;9:458–65. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 21.Vollmer WM, Gíslason T, Burney P, Enright PL, Gulsvik A, Kocabas A, et al. Comparison of spirometry criteria for the diagnosis of COPD: Results from the BOLD study. Eur Respir J. 2009;34:588–97. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gingter C, Wilm S, Abholz HH. Is COPD a rare disease? Prevalence and identification rates in smokers aged 40 years and over within general practice in Germany. Fam Pract. 2009;26:3–9. doi: 10.1093/fampra/cmn084. [DOI] [PubMed] [Google Scholar]
- 23.Mehrotra A, Oluwole AM, Gordon SB. The burden of COPD in Africa: A literature review and prospective survey of the availability of spirometry for COPD diagnosis in Africa. Trop Med Int Health. 2009;14:840–8. doi: 10.1111/j.1365-3156.2009.02308.x. [DOI] [PubMed] [Google Scholar]
- 24.Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, et al. The burden of obstructive lung disease initiative (BOLD): Rationale and design. COPD. 2005;2:277–83. [PubMed] [Google Scholar]
- 25.Sharifi H, Masjedi MR, Emami H, Ghanei M, Buist S. Burden of obstructive lung disease study in Tehran: Research design and lung spirometry protocol. Int J Prev Med. 2014;5:1439–45. [PMC free article] [PubMed] [Google Scholar]
- 26.Binson D, Catania JA. Random selection in a national telephone survey: A comparison of the Kish, next-birthday, and last-birthday methods. J Off Stat. 2000;16:53. [Google Scholar]
- 27.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): A population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 28.Regional COPD Working Group. COPD prevalence in 12 Asia-Pacific countries and regions: Projections based on the COPD prevalence estimation model. Respirology. 2003;8:192–8. doi: 10.1046/j.1440-1843.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 29.Jaganath D, Miranda JJ, Gilman RH, Wise RA, Diette GB, Miele CH, et al. Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res. 2015;16:40. doi: 10.1186/s12931-015-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caballero A, Torres-Duque CA, Jaramillo C, Bolívar F, Sanabria F, Osorio P, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study) Chest. 2008;133:343–9. doi: 10.1378/chest.07-1361. [DOI] [PubMed] [Google Scholar]
- 31.Varmaghani M, Farzadfar F, Sharifi F, Rashidian A, Moin M, Moradi-Lakeh M, et al. Prevalence of asthma, COPD, and chronic bronchitis in Iran: A systematic review and meta-analysis. Iran J Allergy Asthma Immunol. 2016;15:93–104. [PubMed] [Google Scholar]
- 32.World Health Organization. Burden of COPD. Geneva: WHO; 2014. [Last accessed on 2015 Nov 25]. Available from: http://www.who.int/respiratory/copd/burden/en/ [Google Scholar]
- 33.World Health Organization. Global burden of disease (GBD) 2002 estimates. In: World Health Report 2004. Geneva: WHO; 2004. [Last accessed on 2015 Nov 25]. Available from: http://www.who.int/healthinfo/global_burden_disease/estimates_regional_2002/en/ [Google Scholar]
- 34.van Durme YM, Verhamme KM, Stijnen T, van Rooij FJ, Van Pottelberge GR, Hofman A, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: The Rotterdam study. Chest. 2009;135:368–77. doi: 10.1378/chest.08-0684. [DOI] [PubMed] [Google Scholar]
- 35.Afonso AS, Verhamme KM, Sturkenboom MC, Brusselle GG. COPD in the general population: Prevalence, incidence and survival. Respir Med. 2011;105:1872–84. doi: 10.1016/j.rmed.2011.06.012. [DOI] [PubMed] [Google Scholar]


