Abstract
Background:
Drug-resistant tuberculosis (DR-TB) is a global problem with only 52% reported cure rate. Extrapulmonary (EP) DR-TB poses a formidable diagnostic, therapeutic challenge. We aimed to study their clinical profile and treatment outcomes under the programmatic setting.
Materials and Methods:
This retrospective observational study included the database of consecutive EPDR-TB cases enrolled at the DR-TB center from 2012 to 2014. The demographic, clinical details, drug susceptibility tests (DSTs), follow-up, therapy, adverse events (AEs), and outcome were reviewed. Statistical analysis was done using percentages and mean.
Results:
Of total 1743 DR-TB patients, 76 (4.4%) EPDR-TB cases were included. These consisted of 53 (69.7%) adults and 23 (30.3%) children, with female preponderance. The mean age in adults and children was 27.96 (9.63) and 12.56 (3.83), respectively. EP sites involved were lymph nodes in 39 (51.3%), spine in 15 (19.7%), other bones in 6 (7.9%), pleural effusion in 9 (11.9%), central nervous system in 2 (2.6%), and disseminated EP disease in 5 (6.6%). Forty-one (53.9%) had multi-DR-TB (MDR-TB), 29 (38.2%) MDR-TB with fluoroquinolone resistance {preextensively DR-TB (Pre-XDR-TB (FQ)), 1 (1.3%) MDR-TB with aminoglycoside resistance (Pre-XDR-TB (AM)), and 5 (6.6%) extensively DR-TB (XDR-TB) on DST. Thirteen (17.11%) had comorbidities. None had HIV. Two (2.63%) had DM. Patients were treated as per the revised TB control program – programmatic management of DR-TB guidelines. Duration of intensive (IP) was 6.55 (1.22) months. Ten (13.2%) received shorter regimens, wherein therapy was stopped at 12–18 months due to severe adverse drug reactions and treatment response. Sixty-two (81.6%) completed treatment, 8 (10.5%) defaulted, 3 (4%) died, 2 (2.6%) failed, and 1 (1.3%) was transferred out. Two-third of patients reported AE.
Conclusion:
The prevalence of EP cases in DR-TB was 4.4%. Treatment completion rate was very high (81.6%). Shorter regimens were efficacious.
KEY WORDS: Extrapulmonary, shorter regimen, treatment outcome
INTRODUCTION
India is a high burden country for tuberculosis (TB).[1] It is also among one of the six countries that contribute to the major burden of drug-resistant TB (DR-TB) globally.[1] The World Health Organization (WHO) has reported a 52% cure rate which is much lower than that for drug-sensitive TB.[2] Rifampicin-resistant TB (RR-TB) is defined as the cases of TB resistant to at least rifampicin. RR-TB consists of the major DR-TB cases and both the terminologies are used interchangeably.[3] The WHO recommends reliable drug susceptibility tests (DSTs) should be performed only for rifampicin (R), isoniazid (H), aminoglycosides (AM), and fluoroquinolones (FQ).[3,4] Hence, DR-TB/RR-TB cases may further be subdivided as multi-DR-TB (MDR-TB), extensively DR-TB (XDR-TB), preextensively DR-TB (Pre-XDR-TB) on the basis of further DST. TB can involve any site and is classified as pulmonary and extrapulmonary TB (EP-TB). EP-TB is often a diagnostic and therapeutic dilemma. Similarly, EP DR-TB consists of a vast clinical spectrum and a formidable challenge with scarce literature. We embarked to study the clinical profile and treatment outcomes of EP DR-TB under the programmatic setting.
MATERIALS AND METHODS
This retrospective observational study was conducted with Institutional Ethics Committee permission. We included the database of consecutive EPDR-TB cases enrolled from 2012 to 2014 at the DR-TB center attached to a tertiary care center, Mumbai. Incomplete entries at enrollment were excluded from the study. All cases were culture positive. The smear status was not available in all patients. These culture isolates were processed with line probe assay or DST. The demographic, clinical details, DSTs, follow-up, therapy, adverse drug reactions (ADRs), and outcome were noted. The patients reported to the DR-TB center with DST reports to R, H, AM, and FQ done by the line probe assay/liquid culture method. DR-TB cases with resistance to R and H were defined as MDR-TB. MDR-TB cases with additional resistance to FQ/AM were defined as Pre-XDR-TB. Pre-XDR-TB with additional FQ resistance was defined as Pre-XDR-TB (FQ). Pre-XDR-TB with additional AM resistance was defined as Pre-XDR-TB (AM). MDR-TB cases with additional resistance to both FQ and AM were defined as XDR-TB.[5]
They were treated as per the May 2012 programmatic management of DR-TB (PMDT) guidelines[6] with Category 4 anti-TB therapy (ATT) with modification in cases of Pre-XDR-TB and XDR-TB. Resistance to FQ was managed with substitution with para-aminosalicylic acid. Resistance to kanamycin was managed with replacement with capreomycin. The DR-TB therapy regimen consisted of daily therapy with the omission of injectable on Sundays. The patients were followed up at the DR-TB center for adverse events (AEs), shift from intensive phase (IP) to continuation phase, and at the end of therapy. The outcome was defined as per Revised National TB Control Program (RNTCP) guidelines.[7] The therapy duration was 24 months. Postcompletion of therapy, this patient group enrolled from 2012 to 2014 had passive surveillance to document recurrence of DR-TB. If patients received shorter duration regimens (due to any reason), then they were actively followed up at 3 monthly interval till 2 years postcompletion of therapy to document any recurrence.
The statistical analysis of the qualitative data was done using percentages and mean. Unpaired t-test was applied for comparison to study significance.
RESULTS
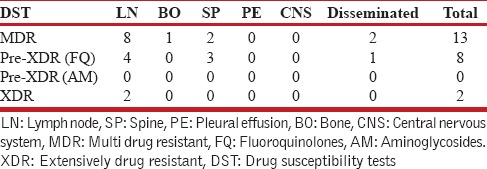
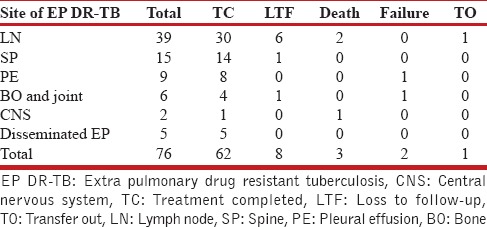
Total 1743 patients of DR-TB were registered at the DR-TB center in the study period. A total of 81 cases of EPDR-TB were registered. Of these, five cases had incomplete baseline entries hence were excluded from the study. The study thus included 76 patients of EPDR-TB. These consisted 4.4% of the total cases. Adults (18 years and above) accounted to 53 (69.7%) and children consisted of 23 (30.3%) of total patients. The adult patients consisted of 21 men and 32 women. The children were 7 boys and 16 girls. The female preponderance observed was statistically not significant (P = 0.61). The mean age was 27.96 (9.63) years in adults and 12.56 (3.83) years in children, respectively, at enrollment. The mean weight in adults and children was 50 (10.71) and 31.82 (12.94) kg, respectively, at enrollment. Body mass index could not be calculated as the older version of PMDT registers in use during the study period did not record height. Six patients had no previous exposure to ATT and were cases of primary DR-TB. Of these, 2 were adults and 4 were children. Various EP sites were involved in DR-TB. The site of affection was lymph nodes in 39 (51.3%), pleural effusion in 9 (11.9%), spine in 15 (19.7%), other bone and joint involvement in 6 (7.9%), central nervous system in 2 (2.6%), and disseminated disease in 5 (6.6%). The distribution in adult and pediatric groups with the site of EP DR-TB is given in Table 1. DST to second-line drugs was available in all patients either done by the second line probe assay or liquid culture method which reported the resistance/susceptibility to FQ and AM. The patient population consisted of 41 (53.9%) MDR-TB, 29 (38.2%) Pre-XDR-TB (FQ), 1 (1.3%) Pre-XDR-TB (AM), and 5 (6.6%) XDR-TB. The adult and pediatric distribution with respect to the DST is given in Table 2. The site-wise distribution of MDR-TB, Pre-XDR (FQ), Pre-XDR-TB (AM), and XDR-TB is given in Tables 3 and 4. Only 13 (17.1%) patients had comorbidities. The comorbidities included anemia in 4, DM in 2, hypothyroidism in 2, depression in 2, nonseminomatous germ cell tumor of testis in 1, renal failure in 1, elephantiasis and mental retardation in 1, hypertension in 1, and urolithiasis in 1. None of the patients had HIV.
Table 1.
Distribution as per site of extrapulmonary drug-resistant tuberculosis in adults and pediatric patients (The figures represent number)
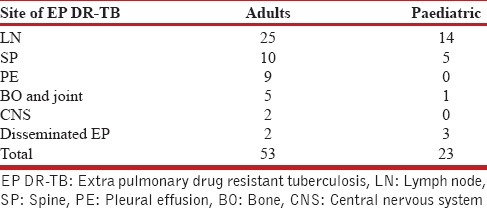
Table 2.
Drug susceptibility tests to second-line drugs fluoroquinolones and aminoglycosides (The figures represent number)
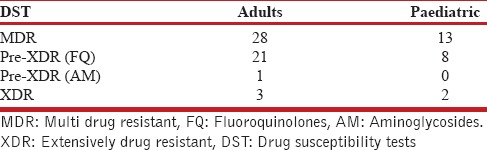
Table 3.
Site wise multidrug-resistant tuberculosis, preextensively drug-resistant tuberculosis, extensively drug-resistant tuberculosis in adults (The figures represent number)
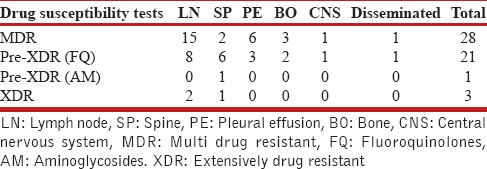
Table 4.
Site wise multidrug-resistant tuberculosis, preextensively drug resistant tuberculosis, extensively drug-resistant tuberculosis in children (The figures represent number)
The patients were treated with Category 4 therapy as per RNTCP PMDT 2012 guidelines with modification as per DST. The mean duration of IP was 6.55 (1.22) months. The response of therapy was assessed clinically in all and radiologically in 34 (44.7%). Most patients received therapy for 24 months. Optimal management of ADR and modification of therapy were attempted in all patients with severe ADR, for example, drug-induced psychosis. Of these, in 10 cases in spite of all these measures, the risks of continuation of therapy outweighed the benefits, and they showed a good clinicoradiological response. Hence, therapy was stopped in these 10. Thus, 10 (13.2%) received shorter regimens; wherein therapy was stopped at 12–18 months due to severe ADR and treatment response. The ten cases consisted of six cases of lymphadenopathy, two cases of pleural effusion, and 2 cases of disseminated TB. Of the two cases of disseminated TB, one patient had lymphadenopathy and spine involvement and second patient had lymphadenopathy and pleural effusion. On the basis of DST; the ten cases were divided as 9 MDR-TB and 1 Pre-XDR-TB (FQ). Those who had early interruption of treatment were given shorter regimens which consisted of similar regimens as given in other patients (as per DST); however, the therapy was interrupted before 24 months in view of severe AEs and clinicoradiological evidence of treatment response. These patients with shorter regimens were followed up till 2 years posttherapy completion for evidence of recurrence. None had a recurrence. Thus, all patients with shorter regimens completed treatment successfully. The treatment completed cases with conventional 24-month regimen and shorter regimen cases consisted of the total treatment completed cases. Sixty-two (81.6%) completed treatment, 8 (10.5%) were lost to follow-up, 3 (4%) died, 2 (2.6%) failed, and 1 (1.3%) was transferred out. Table 5 denotes the treatment outcome in adult and pediatric cases. Table 6 denotes the site-wise outcome. The mean duration of therapy in patients who completed therapy with conventional regimens was 22.22 (3.62) months. The mean duration of therapy in patients receiving shorter regimens was 15.4 (2.4) months. This difference of duration between the conventional regimen and shorter regimen was statistically significant (unpaired t-test; P < 0.0001).
Table 5.
Outcome in adult and children (The figures represent number)

Table 6.
Site-wise distribution of outcome of extrapulmonary drug-resistant tuberculosis (The figures represent number)
The DR-TB therapy is known to be associated with a spectrum of AE. Two-third of patients reported AE. Twenty eight (36.8%) patients reported nil AE. Twenty one (27.6%) had gastritis, 15 (19.7%) psychiatric ADR, 6 (7.9%) arthralgia, 3 (3.9%) ototoxicity, 3 (3.9%) hepatotoxicity, 3 (3.9%) hypothyroidism, and 1 (1.3%) skin rash.
DISCUSSION
EP TB has been exhaustively studied in literature with a varied range of clinical reports/case series of microbiologically confirmed EP DR-TB cases.[8,9] EP TB consists of 10%–15% of the total TB cases.[8,9,10] Their proportion among the DR-TB cases is variable. While laboratory data are available;[10,11] for the first time, we present data about the proportion of these cases at a DR-TB center in Mumbai. We report EP cases to consist of 4.4% of our DR-TB center patients. Although these figures are low as compared to the prevalence of the EP disease in drug-sensitive cases; the exact figures are unknown. Our results were in concordance with the Maharashtra data under PMDT (2011–2012 cohort) which had 3% of EP DR-TB cases.[12] More so, the prevalence is impacted by the availability and scale-up of the WHO-approved rapid diagnostics in the region. With the universal access to DST in Mumbai, EP DR-TB cases consist of a small, important, clinically challenging subgroup. The demographic data from our study give us an insight into the regional pattern of EP DR-TB in Mumbai. Our patients consisted of young adults and adolescents in majority. Our study had a female preponderance. These findings are consistent with previous reports from our region.[12,13,14,15] The mean weight of our patients in adult and children was higher than reported in various Indian studies by Sharma et al.[16] Although we report a higher 7.9% of primary DR-TB cases, this observation requires further validation within a wider patient population group. None of our patients had HIV infection correlating with a study by Sharma et al.[16]
Our study is one of the first kinds to present the distribution of DR-TB at the clinical end. While lymph node involvement is the most common in EP TB in general, we encountered a similar pattern in the EP DR-TB cases too. This result was consistent with a previous study from Maharashtra.[12] However, lymph node involvement was seen in one-third of their cases as against half in this study. The second common site of EP TB documented in literature is the pleura; however, it was the third common site in our study. We report spine DR-TB as the second common site. A study from Bangladesh reported lymph nodes followed by the pleura as the two most common site of DR-TB.[17] Data on the distribution of DR-TB as per specimen/samples are available from Indian supranational laboratory, NIRT and Delhi. NIRT detected DR-TB in 19% of EP TB specimens. Of them, 27% were fluids and aspirates, 23% pus, 19% lymph node biopsy, 10% CSF, and 6% urine samples.[10] While the New Delhi laboratory based study diagnosed DR-TB among 11% of its EP TB specimens. Pus and needle aspirates consisted of 23.7%, tissue 11.8%, body fluids 6.4%, urine 3.1%, and blood and bone marrow 2.2%.[11] We had no cases of DR-TB diagnosed on urine samples. Laboratory data on the DST in EP DR-TB are available.[10,11] However, ours is the first study to give the distribution of these cases as per DST at a DR-TB center. We saw a higher (around 40%) prevalence of Pre-XDR-TB (FQ) cases which is consistent with studies from Mumbai.[13,14,15] However, this percentage is lower than that reported in our Pulmonary DR-TB cases though the studies differ in methodology and time frame.[13,14]
We report “hope amidst despair” of challenging outcomes for DR-TB reported globally by documenting a treatment completion rate of around 82%. Thus, we identify EP TB as a factor associated with a good prognosis. Global TB report 2017 for India reports a treatment success rate of only 46%.[18] A study in pulmonary DR-TB cases from our center also showed a treatment success of only 48.4%.[14] While the Maharashtra data reported EP TB as a factor associated with favorable outcomes in 68% higher than their pulmonary cases and representing a similar trend as our study. Of their 87 EP DR-TB cases, unfavorable outcomes were reported in 28 (32%) with a loss to follow-up 15 (17%) and death in 5 (6%).[12] Shorter regimens have been approved in certain cases of pulmonary DR-TB by the WHO.[3,19] For the first time, we document efficacy of shorter regimens in EP cases. While the patients received these regimens unintentionally, this indirect observation is of significant importance paving its way for further research of shorter regimens in EP DR-TB.
Our study had limitations due to the retrospective study design. Unintentional referral and demographic bias were unavoidable as our study was confined to a single DR-TB center which drained one-third of the Mumbai population. A selection bias was inevitable due to the study design. Hence, we may not have got cases of HIV co-infection. Socioeconomic aspects and risk factors which could possibly affect outcome were not studied though these aspects were distributed uniformly in the study population. The follow-up was passive in most cases except those who received shorter regimens. Many patients required specialized radiological investigations and procedures such as aspiration, biopsy at the tertiary care center, and had more interaction with the health-care facility at various levels for monitoring in view of being EP cases. As we are a government-run hospital, these additional health-care costs were unaccounted for as provided free of cost. The patients were declared treatment completed on the basis of clinicoradiology. Microbiological conversion was documented only in six cases with residual lesions doubtful of active disease. There was a possible positive impact on the direct observation of therapy due to the patient-centered approach offered due to increased interactions at various levels of health-care system which could have definitely affected the outcomes. Similar, application of a patient-centered approach has been emphasized and documented in the STREAM Stage 1 report by WHO, thereby reiterating the basic principle of supervision and DOT being the crux of any TB therapy irrespective of drug resistance status and duration.[20]
CONCLUSION
EP DR-TB is a heterogeneous subgroup consisted of 4.4% among our DR-TB cases. The treatment completion rate was very high (81.6%). Thus, we identify a subgroup with a good prognosis. We document the efficacy of shorter regimens in EP TB.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.WHO. Tuberculosis Country Profile-India. [Last accessed on 2017 Dec 24]. Available from: https://www.extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=IN&LAN=EN&outtype=html .
- 2.WHO. Multi-Drug Resistant Tuberculosis (MDR-TB) 2016 Update. [Last accessed on 2017 Apr 07]. Available from: http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf?ua=1 .
- 3.WHO. WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update (WHO/HTM/TB/2016.04) Online Annexes 4, 5, 6. 2016. [Last accessed on 2017 Dec 24]. Available from: http://www.apps.who.int/iris/bitstream/10665/250125/5/9789241549639-webannexes-eng.pdf .
- 4.WHO. Policy Guidance on Drug-Susceptibility Testing (DST) of Second-Line Antituberculosis Drugs. 2008. [Last accessed on 2017 Dec 24]. Available from: http://www.apps.who.int/iris/bitstream/10665/70500/1/WHO_HTM_TB_2008.392_eng.pdf . [PubMed]
- 5.Desai U, Joshi JM. Unveiling the new definitions, diagnostic basis and therapeutic approaches. Astrocyte. 2017;4:27–33. [Google Scholar]
- 6.RNTCP. Guidelines on Programmatic Management of Drug Resistant Tuberculosis (PMDT) in India. 2012. May, [Last accessed on 2017 Aug 18]. Available from: http://www.tbcindia.nic.in/showfile.php?lid=3155 .
- 7.RNTCP. Technical and Operational Guidelines for Tuberculosis Control in India Case Findings and Diagnosis Strategy; Ch. 3. 2016. [Last accessed on 2017 Dec 25]. Available from: http://www.tbcindia.nic.in/showfile.php?lid=3216 .
- 8.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]
- 9.Joshi JM. Tuberculosis chemotherapy in the 21 century: Back to the basics. Lung India. 2011;28:193–200. doi: 10.4103/0970-2113.83977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dusthackeer A, Sekar G, Chidambaram S, Kumar V, Mehta P, Swaminathan S, et al. Drug resistance among extrapulmonary TB patients: Six years experience from a supranational reference laboratory. Indian J Med Res. 2015;142:568–74. doi: 10.4103/0971-5916.171284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raveendran R, Oberoi JK, Wattal C. Multidrug-resistant pulmonary & extrapulmonary tuberculosis: A 13 years retrospective hospital-based analysis. Indian J Med Res. 2015;142:575–82. doi: 10.4103/0971-5916.171285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suryawanshi SL, Shewade HD, Nagaraja SB, Nair SA, Parmar M. Unfavourable outcomes among patients with MDR-TB on the standard 24-month regimen in Maharashtra, India. Public Health Action. 2017;7:116–22. doi: 10.5588/pha.17.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adwani S, Desai U, Joshi JM. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai. JKIMSU. 2016;5:13–9. doi: 10.4103/lungindia.lungindia_182_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waghmare MA, Utpat K, Joshi JM. Treatment outcome of drug-resistant pulmonary tuberculosis under programmatic management of multi-drug resistant tuberculosis, at a tertiary care center in Mumbai. Med J DY Patil Univ. 2017;10:41–5. [Google Scholar]
- 15.Dalal A, Pawaskar A, Das M, Desai R, Prabhudesai P, Chhajed P, et al. Resistance patterns among multidrug-resistant tuberculosis patients in greater metropolitan Mumbai: Trends over time. PLoS One. 2015;10:e0116798. doi: 10.1371/journal.pone.0116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma SK, George N, Kadhiravan T, Saha PK, Mishra HK, Hanif M, et al. Prevalence of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A retrospective hospital-based study. Indian J Med Res. 2009;130:392–5. [PubMed] [Google Scholar]
- 17.Afroz H, Ali MA, Fakruddin M, Kamrunnahar, Datta S. Prevalence and treatment follow-up of drug-resistant extra-pulmonary tuberculosis in rural communities in Narshingdi, Bangladesh. Int J Adv Med. 2014;1:71–7. [Google Scholar]
- 18.WHO. Global Tuberculosis Report 2017. [Last accessed on 2017 Dec 25]. Available from: http://www.apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1 .
- 19.WHO. The Shorter MDR-TB Regimen. Update. 2016. [Last accessed on 2017 Dec 24]. Available from: http://www.who.int/tb/Short_MDR_regimen_factsheet.pdf?ua=1 .
- 20.WHO. Results from the STREAM Stage 1 Randomised Controlled Trial for Treatment of MDR-TB Using a Standardised Shorter Regimen. [Last accessed on 2017 Dec 11]. Available from: http://www.who.int/tb/features_archive/stream_stage1_MDRTB/en/


