Abstract
Effective management of biofilm-related oral infectious diseases is a global challenge. Oral biofilm presents increased resistance to antimicrobial agents and elevated virulence compared with planktonic bacteria. Antimicrobial agents, such as chlorhexidine, have proven effective in the disruption/inhibition of oral biofilm. However, the challenge of precisely and continuously eliminating the specific pathogens without disturbing the microbial ecology still exists, which is a major factor in determining the virulence of a multispecies microbial consortium and the consequent development of oral infectious diseases. Therefore, several novel approaches are being developed to inhibit biofilm virulence without necessarily inducing microbial dysbiosis of the oral cavity. Nanoparticles, such as pH-responsive enzyme-mimic nanoparticles, have been developed to specifically target the acidic niches within the oral biofilm where tooth demineralization readily occurs, in effect controlling dental caries. Quaternary ammonium salts (QAS) such as dimethylaminododecyl methacrylate (DMADDM), when incorporated into dental adhesives or resin composite, have also shown excellent and durable antimicrobial activity and thus could effectively inhibit the occurrence of secondary caries. In addition, custom-designed small molecules, natural products and their derivatives, as well as basic amino acids such as arginine, have demonstrated ecological effects by modulating the virulence of the oral biofilm without universally killing the commensal bacteria, indicating a promising approach to the management of oral infectious diseases such as dental caries and periodontal diseases. This article aims to introduce these novel approaches that have shown potential in the control of oral biofilm. These methods may be utilized in the near future to effectively promote the clinical management of oral infectious diseases and thus benefit oral health.
1. Introduction
Oral biofilm, a structured community which consists of a wide range of microbes embedded with self-organized matrix of extracellular polysaccharides (EPS), is clearly recognized as a virulence factor to many oral infectious diseases including dental caries, gingivitis, periodontitis, periapical periodontitis and peri-implantitis [1–5]. Controlling oral biofilm incurs large expenditures worldwide [6]. With recent boom of new technologies and increased knowledge of genetic pathways, physiological responses, and intracellular signal transduction pathways, our understanding of biofilms has progressed significantly since they were first formally defined in the mid-1980s [7, 8]. Classical biofilm lifecycle includes bacterial attachment, biofilm growth/maturation, and biofilm dispersal. Measures that can disrupt any stage of biofilm cycle are considered as potential approach to the control of biofilm. Due to the complexity of the oral cavity and the rapid clearance of saliva, topically applied antibacterial agents are not retained at the proper concentrations for a long enough duration [9]. Compared with bacteria in planktonic, mature biofilm tends to need higher concentrations of antimicrobial agents to be eradicated [1, 10, 11]. When mixed with antimicrobial drugs, planktonic cells expose all cells to the full dose [12] while matrix in biofilm may reduce some drug access, preventing penetration of drugs into its deep layer [13–17]. Recent studies have shown that the EPS matrix not only provides functions as scaffold for biofilm growth and maturation but also provides emergent properties of biofilms including surface adhesion, spatial and chemical heterogeneities, synergistic/competitive interactions, and increased tolerance to antimicrobial agents [14, 18]. By contrast, little is understood about the most economic and effective ways of controlling oral biofilm due to the enhanced resistance to antibiotics and other antimicrobial agents [7, 16].
There have been increased attempts to develop ideal antimicrobial agents for the emergence of antibiotic-resistant bacteria [19]. The ideal antibiofilm approach is to facilitate the dispersion of formed biofilms, eliminate pathogens, and inhibit the formation of new biofilms while avoiding the elimination of commensals which may cause microecology dysbiosis [20–23]. Different from conventional antibiotics such as chlorhexidine, some of the novel treatment strategies for biofilm infections aim at specifically targeting unique biofilm characteristics [1] to minimize or eliminate the drug resistance of oral biofilm.
This review will introduce some of the novel strategies for the disruption/inhibition of oral biofilm, including nanomaterials, quaternary ammonium salts, small molecules, arginine, and the natural products.
2. Nanomaterials
Nanomaterials have revolutionized the concept of what a material is and can be since its discovery in the 1980s. Since then, nanomaterials have been employed in many fields including medicine and are projected to have broad prospects for future development [24]. Many nanomaterials, such as silver, copper oxide, zinc oxide nanoparticles, titanium oxide, and graphene, can be used to control biofilm formation [25–27]. Quaternary ammonium polyethylenimine, chitosan, and silica nanoparticles have also been suggested effective in controlling biofilms [25, 28, 29]. Moreover, applying nanomaterials for drug delivery, either as a carrier with specific affinity to tooth surfaces or as a drug for its inherent antimicrobial properties, have garnered attention in recent years [11, 28, 30, 31].
Certain metal nanomaterials show their capacities in controlling the oral biofilm. Among them, silver nitrate and silver nanoparticles (AgNPs) are the most effective against oral pathogens [32]. The drawback of silver nitrate is to cause dentine discoloration [5, 33, 34] whereas a silver nanocoating directly on dentine can successfully prevent the biofilm formation on dentine surfaces and inhibit bacterial growth in the surrounding media, suggesting a promising approach to protecting from dental plaque and secondary caries when applied as a dentine coating [5]. AgNPs exhibit the antibiofilm potential against Enterococcus faecalis, which is identified as the main cause of secondary and persistent endodontic infections [35, 36]. The application mode of AgNPs affects its antibiofilm efficacy. Using 0.02% AgNPs gel as medicament can significantly disrupt the structural integrity of the E. faecalis biofilm [37]. However, using AgNPs solution as an irrigant shows less effective against E. faecalis than NaOCl, which is commonly used in the endodontic treatment [38]. In addition, AgNPs could be a promising vehicle for calcium hydroxide as a short-term intracanal medicament to eliminate E. faecalis from human dentin [39].
Chitosan is a nontoxic natural cationic polysaccharide with characteristics of adhesiveness, antimicrobial activity, biocompatibility, and biodegradability [40, 41]. Because of its poor solubility above pH 6.5, chitosan exhibits its antibacterial activity better in an acidic condition [42]. Previous studies showed that chitosan inhibited the growth and adherence of Streptococcus mutans and other streptococci [43–45]. The chitosan nanoparticles (CNPs), although smaller than chitosan, still have the antimicrobial activity [42]. Owing to a higher surface charge density, CNPs could interact with the negative charge surface of bacterial cells, causing bacterial cell death [46]. CNPs, especially those prepared from low molecular weights chitosans, exhibit high antimicrobial effect towards S. mutans biofilm [47, 48]. CNPs inhibit other streptococci (such as Streptococcus sobrinus, Streptococcus sanguinis and Streptococcus salivarius) at low concentrations ranging from 0.312 mg/mL to 0.625 mg/mL [48]. Moreover, CNPs show inhibitory effect against E. faecalis and its biofilm [40, 49]. The commonly used intracanal medicament calcium hydroxide can damage the bacterial DNA due to its alkaline pH [50]. However, E. faecalis are alkali resistant and thus cannot be killed by calcium hydroxide in the infected root canal [51]. Adding CNPs into calcium hydroxide, the mixed intracanal medicament shows increasing antibacterial activity against E. faecalis and inhibits bacterial recolonization on root canal dentin compared with calcium hydroxide alone [52]. Microorganisms on the dental implants are the major causative factor of implant failure and peri-implantitis. The Ag-conjugated CNPs can inhibit the growth and adherence of Porphyromonas gingivalis and S. mutans and reduce the biofilm formation on dental implants, thus representing a prospective coating material for titanium dental implants [53].
Mesoporous silica nanoparticles (MSNs) have been used as biocatalysts, biosensors, drug delivery system, as well as imaging modality for diagnosis and therapy. In comparison with conventional nanoparticles, MSNs have the unique characteristics of mesoporous structure, large surface area and pore volume, stable physicochemical property, and flexible surface modification [54–56]. It has been reported that silica nanoparticles could inhibit adherence of bacteria [57]. Moreover, MSNs have the advantage of loading drug molecules with high capacity, well dispersity, costing less, relatively high biocompatibility, and available for custom design [55, 56]. MSNs, whether spherical or wiry, while loaded with antimicrobial agents such as chlorhexidine (CHX), can attach on microbes and release CHX up to 48 hours [58]. The MSNs-encapsulated-CHX (CHX@MSN) has demonstrated potent antibacterial activity against S. mutans, S. sobrinus, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and E. faecalis, either in planktonic culture or in monospecies biofilms. It can also suppress multispecies biofilms of S. mutans, F. nucleatum, A. actinomycetemcomitans and P. gingivalis up to 72 h [59]. Addition of CHX@MSN to the dental filling materials such as glass ionomer cement (GIC) and resin composite can significantly inhibit the biofilm formation of S. mutans without compromise the mechanical properties of the filling materials [60, 61].
The “smart” drug delivery system is a delivery system of drugs by nanoparticles whose release is triggered by environmental stimuli such as pH, glucose or bacterial products [62]. Nanoparticles that are pH-responsive are stable at physiologic pH levels but degrade or disrupt at acidic pH levels to release the active drug [9]. Given that dental caries always occur at persistent low pH site around 4.5~5.5 on the teeth [63], where cariogenic organisms ferment sugar and create acidic niches, approaches to the control of cariogenic biofilms by targeting specific microenvironments have also been studied. This pH-responsive system makes full use of the acidic condition, with high affinity to hydroxyapatite, pellicle, and EPS surface, releasing drugs activated by low pH, where cariogenic bacteria prosper and actively develop biofilms. This delivery system can load up to ~22 wt% of farnesol, which is a hydrophobic antibacterial agent against planktonic S. mutans, but with limited activity against cariogenic biofilms. High affinity and capacity of this drug delivery system enhance the efficacy of farnesol, disrupt biofilms 4-fold more than free farnesol, and more importantly promote caries-reduction in rodent dental caries model [9].
Once inside the oral biofilm, bacteria are not easily disrupted, and it is therefore necessary to seek an approach that will disturb the matrix's integrity to eliminate the bacteria [64, 65]. Different from the nanoparticles with biological activity of antibacterial effects, catalytic nanoparticles (CAT-NP) can disrupt the matrix through its inherent enzyme mimic activity (e.g., peroxidase) when at acidic pH levels (greater catalytic efficiency at pH4.5-5.5, but minimal activity at neutral pH) [64]. Hence the catalytic nanoparticles are also termed nanozymes [66, 67]. Compared with other artificial enzymes that are based on organic molecules, CAT-NP possesses enhanced and versatile catalytic activities [68]. Hydrogen peroxide (H2O2) at proper concentration is commonly used as a disinfectant because it generates antimicrobial free radicals and degrades polysaccharides [65, 69]. CAT-NP can catalyze low concentrations (0.5–1%) of H2O2 in situ to simultaneously dissemble the biofilm EPS-matrix and kill embedded bacteria with high efficacy (>5-log reduction of cell viability; and >5000-fold more effective than H2O2 alone) [64]. Additionally, CAT-NP remains in the biofilm even after transient exposure [21]. CAT-NP is biocompatible because its catalytic activity is pH-dependent. At a physiological pH, free-radical production is minimized [64, 68]. No side effects to the oral mucosa tissue have been shown when CAT-NP is used in vivo with H2O2. In addition, CAT-NP can reduce apatite demineralization under acidic condition in vitro and thus attenuate the severity of carious lesions [64].
3. Quaternary Ammonium Salts (QAS)
Composite resin and adhesive system have been commonly used in clinical restoration for their esthetic effects [70–72]. However, nearly half of restorations fail within ten years [70, 72]. One primary reason is the development of secondary caries [72, 73], which is mainly caused by micro-leakage and dental plaque accumulation [74, 75]. So far, the mechanical properties and wear resistance of dental composites have been considerably improved while the antibacterial properties are still limited [75, 76]. Efforts have been made to inhibit secondary caries by adding antibacterial agents such as antibiotics and silver ions into the resin and adhesive systems [74, 77]. Polycations, such as quaternary ammonium salts (QAS), are of high molecular weight, non-volatile, chemically stable compared with conventional antibacterial agents [76], showing great potential to be added into the resin and adhesive system [78].
QAS, owing to their broad-spectrum of antimicrobial activity and low level of toxicity, were first used in mouthwash to control oral biofilm in the 1970s and later added into dental composite materials in the 1990s [79, 80]. The antibacterial mechanism of QAS is by binding their positive charge to the negatively charged bacterial cell membrane, causing lysis of cell membrane [76]. One type of QAS, quaternary ammonium dimethacrylate (QADM), has reactive groups on both ends of a dimethacrylate and can be incorporated in resin without compromising its mechanical properties [81].
12-methacryloyloxydodecyl-pyridinium bromide (MDPB), a QAS developed by Imazato et al., has shown strong antibacterial and antibiofilm effects against S. mutans, E. faecalis, F. nucleatum, and Prevotella nigrescens [82–84]. MDPB and methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB) can be incorporated into composites and inhibit the growth and adherence of oral pathogens [83–85]. Recent studies tend to mix QAS with other effective constituents to develop novel composites. A special QAS dimethacrylate monomer named ionic dimethacrylate monomers (IDMAs) can copolymerize with other methacrylate monomers (e.g., bisphenol A glycerolate dimethacrylate) and generate antibacterial polymers for dental composites and consequently reduce S. mutans colonization [86]. Composite with IDMA-1, when combined with calcium phosphate (CaP) particles and silver nanoparticles, demonstrates enhanced antibacterial activity with intact mechanical properties [87], indicating the potential of IDMAs in the development of novel antimicrobial composite resin.
Recently, new antibacterial monomers dimethylaminohexadecyl methacrylate (DMAHDM) and dimethylaminododecyl methacrylate (DMADDM) have been developed with enhanced antibacterial activity compared to QADM [88, 89]. The long chain polycations of these monomers bond to bacterial membrane is as a needle to a balloon [90]. DMADDM with a longer carbon chain length of 12 demonstrates stronger antimicrobial effect than DMAHM with a chain length of 6 [88]. It has been proven that the length of carbon chain can affect the antimicrobial efficacy [91, 92]. More importantly, DMADDM-containing adhesives showed an anticaries activity in a secondary caries animal model [93]. When DMADDM is incorporated with silver nanoparticles into adhesive system, it significantly reduces the metabolic activity of biofilm without affecting dentin bond strength [88]. Amorphous calcium phosphate (NACP) can neutralize acid attacks and release high levels of Ca and inorganic phosphate (Pi) ions, which promote tooth remineralization. Adding nanoparticles of DMADDM and NACP into composites and adhesive system results in a stronger antimicrobial potency, milder pulpal inflammation, and much more reparative dentin formation [94, 95]. Additionally, this NACP-and DMADDM-containing adhesive system possesses long-lasing antibacterial properties and strong bond strength [96]. Although the carbon chain length can affect antimicrobial efficacy, DMAHDM with a carbon chain length of 16 has a stronger antimicrobial effect than DMAODM with a chain length of 18 [91, 97, 98]. 10% DMAHDM of bonding agent can completely remove S. mutans biofilm in vitro [99]. Combination of DMAHDM with NACP into composites decreases biofilm metabolic activity, acid production, and the colony-forming units (CFU) of S. mutans [97].
Changing the functional group position of QAS may alter its anti-caries effects when incorporated into dental resin. Triethylaminododecyl acrylate (TEADDA), a new QAS with a different functional group position of DMADDM, when combined with adhesive resin, shows enhanced mechanical properties but reduced antibacterial effects compared with DMADDM [80].
However, frequent use of QAS may also lead to bacterial resistance [100]. CHX as the common disinfectant in mouthwash, has been proven to cause resistance in four oral bacteria species, i.e., Streptococcus gordonii, E. faecalis, F. nucleatum, and P. gingivalis [101]. MDPB induced no resistance in S. mutans and E. faecalis [83]. Another study tested the bacterial resistance of eight oral bacteria (S. mutans, S. sanguinis, S. gordonii, E. faecalis, A. actinomycetemcomitans, F. nucleatum, P. gingivalis, and Prevotella intermedia) after treatment with DMADDM and DMAHDM and found that DMADDM induced resistance in only one species (S. gordonii), while DMAHDM induced resistance in none of the tested species [101].
4. Small Molecules
A novel strategy to control oral biofilm is to disrupt its formation [102]. Small molecules are promising for controlling biofilm formation due to their good stability, activity at low concentrations, and low toxicity [103].
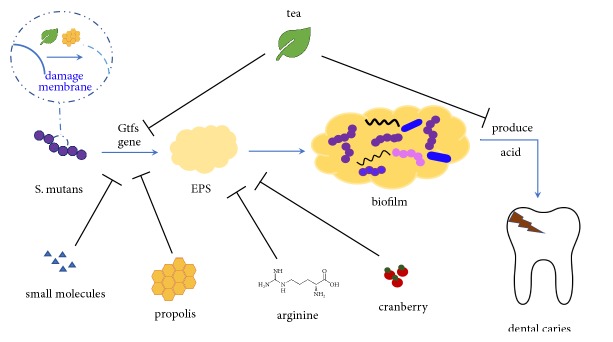
S. mutans is most widely regarded as the cariogenic bacterium in the oral biofilm. Though it may not be the most abundant, it can rapidly utilize dietary sucrose to synthesize EPS effectively, produce acid, and tolerate the acidic microenvironment in the cariogenic biofilm [104–106]. Three glucosyltransferases (GtfB, GtfC, and GtfD) of S. mutans have been identified, which synthesize adhesive EPS and contribute to the formation of cariogenic biofilms (Figure 1). Gtfs, specifically GtfB and GtfC secreted by S. mutans, can bind the pellicle formed on the tooth surface and produce glucans as specific binding sites for bacteria colonization [107, 108]. It has been proven that therapeutics aimed at interrupting the EPS synthesis by S. mutans are promising approaches to oral biofilm control [109], and glucosyltransferases may provide a good target for the inhibition of biofilm formation [110, 111]. GtfC is able to synthesizes both soluble and insoluble glucans [112, 113]. Lead compounds which target GtfC catalytic domain with high affinity and specificity have shown potential in controlling oral biofilm. Virtual screening from the small molecule library has identified two lead compounds, namely, #G43 and a quinoxaline derivative, 2-(4-methoxyphenyl)-N-(3-{[2-(4-methoxyphenyl) ethyl] imino}-1,4-dihydro-2- quinoxalinylidene) ethanamine. #G43 selectively inhibits the biofilm formation of S. mutans without disturbing the microecological balance between pathogens and commensal species (Figure 1) [110], while 2-(4-methoxyphenyl)-N-(3-{[2-(4-methoxyphenyl) ethyl] imino}-1,4-dihydro-2-quinoxalinylidene) ethanamine can inhibit the biofilm formation and promote the removal of mature biofilm of both S. mutans and S. sanguinis. More importantly, this small molecule shows good anticaries efficacy by significantly reducing the incidence and severity of smooth surface caries in vivo [111].
Figure 1.
S. mutans is well recognized as the main cariogenic bacterium in the oral biofilm. It can metabolize carbohydrates to synthesize EPS as the scaffold of biofilm, produce acid accumulating at the biofilm/enamel interface to demineralize tooth hard tissue, and ultimately cause visible dental decay. Antimicrobial agents inhibit the aforementioned cariogenic process of S. mutans and may have the translational potential in the control of dental caries.
A group of synthesized small molecules are inspired by some natural products such as garlic, ursine triterpenes, ginseng, etc. These small molecules have good antibacterial and antibiofilm activity and induce low drug resistance [20]. One group of small molecules inspired by marine natural products, are derivatives based on the 2-aminoimidazole (2-AI) or 2-aminobenzimidazole (2-ABI) subunit [113, 114]. Eight of them are proved to inhibit S. mutans in vitro at a concentration lower than 4μM without affecting the commensal bacteria. The most effective small molecule among them, 2A4, downregulates the expression of genes associated with biofilm and also inhibits the two key adhesins, antigen I/II and Gtfs, of S. mutans (Figure 1) [114]. 3F1, a compound in 2-aminoimidazole, can disperse mature S. mutans biofilm at 5μM while not affecting the biofilm of S. sanguinis and S. gordonii. Similarly to 2A4, 3F1 also targets key adhesins of S. mutans, and may represent a specific anticaries approach [115].
In addition, small molecules that target other oral pathogens have been developed. Candida albicans is a common commensal fugus in the oral cavity and can cause opportunistic fungal infection in susceptible populations [116]. Screening from a chemical library (NOVACore) has identified a unique series of diazaspiro-decane structural analogs that specifically targeted C. albicans. These compounds inhibit C. albicans filamentation and biofilm formation without affecting the growth of planktonic cells, making it a good candidate for the control of oral candidiasis [117].
5. Arginine
Frequent intake of dietary carbohydrates may lead to demineralization of tooth enamels by acidogenic bacteria in oral biofilm. However, periods of alkalization can promote remineralization to restore the integrity of the enamel [118]. Urea and arginine are two major substrates for alkali generation in oral biofilm. Some commensals are able to produce alkaline compounds to counter the acid stress imposed by acidogenic bacteria such as S. mutans and maintain a healthy oral biofilm [16, 119]. Oral commensals produce alkali mainly through the arginine and urea metabolism pathways [16]. A prevailing route of arginine metabolism is the arginine deiminase system (ADS), yielding ornithine, ammonia, CO2 and ATP [16, 118]. Oral streptococci, including S. sanguinis, S. gordonii, S. parasanguinis, S. intermedius, S. cristatus, and S. australis, certain Lactobacillus species, and a few spirochetes can express ADS [118]. Using arginine-containing toothpaste can significantly increase ADS activity in plaque of caries-active individuals, shifting the bacteria composition to one similar to that of caries-free individuals [120, 121]. Therefore, increasing the availability of exogenous arginine in the oral environment could be a novel approach to controlling biofilms.
Arginine as a natural dietary supplement has shown good activity against bacteria growth, virulence, coaggregation, and biofilm formation [122]. An arginine-rich polycationic protein, protamine, can inhibit oral pathogens such as A. naeslundii, A. odontolyticus, E. faecalis, Lactobacillus acidophilus, F. nucleatum, P. gingivalis, C. albicans, and A. actinomycetemcomitans [123]. Arginine suppresses the production and composition of extracellular membrane glucans, thereby inhibiting the adherence activity of S. mutans to the tooth surface [124]. L-arginine is able to reduce the biomass of polymicrobial dental biofilms, particularly inhibit the biofilm formation of S. mutans by reducing its water-insoluble EPS production (Figure 1) [119, 125]. A recent study showed that 1.5% L-arginine enriched S. gordonii while suppressing S. mutans. The arginine-treated biofilm exhibited significantly higher pH values at the biofilm-sHA interface [119]. In addition, L-arginine was shown to inhibit the coaggregation of P. gingivalis and Prevotella oris in vitro [126]. Addition of 7% arginine to the dental adhesives enhanced biofilm formation without compromising its mechanical properties [127]. More importantly, combinatory use of arginine with NaF could synergistically inhibit S. mutans but enrich S. sanguinis while suppressing the overgrowth of P. gingivalis in a multispecies biofilm, offering an ecological approach to the control oral biofilm [128].
6. Natural Products
Natural products, though its structure may be uncertain, exhibit biological activities that make them promising to be alternative or adjunctive therapies towards oral biofilm [129]. Polyphenols, which are defined as any substance that contains at least one aromatic ring with one or more hydroxyl groups and other substituents, have been identified as active compounds in many natural products such as tea, propolis, cranberry, Galla chinensis, grapes, coffee, and cacao polyphenols [130–132].
6.1. Tea
Tea (Camellia sinensis) has many health benefits including antioxidant, antimutagenic, antidiabetic, hypocholesterolemic, antibacterial, anti-inflammatory, and cancer-preventive properties [133–135]. Distinguished by the processing methods, there are three major types of tea: green tea (nonfermented), oolong tea (semifermented) and black tea (fermented) [136]. The anticaries effect of tea has been well suggested for decades. Using mouth wash containing green tea extract three times a day for a week could reduce salivary level of S. mutans and Lactobacilli [137]. The extracts of tea could inhibit the growth [138, 139], adherence [140], and acid production of the acidogenic oral streptococci [140, 141].
Although tea is rich in fluoride which is beneficial to caries prevention, its activity against oral biofilm formation is mainly attributed to polyphenols [133, 138, 139, 142, 143]. Tea catechins are polyphenols in green tea which have been recognized as the main antimicrobial components against oral pathogens [144, 145]. Tea polyphenols consist of (+)-catechin (C), (-)-epicatechin (EC), (+)-gallocatechin (GC), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECg), (-)-epigallocatechin gallate (EGCg), (-)-catechin gallate (Cg), and (-)-gallocatechin gallate (GCg) [144]. EGCg and ECg and ECg are the most abundant and active tea catechins [140, 144, 146], possibly due to the presence of galloyl groups [147]. The antimicrobial mechanism of tea catechins is the irreversible damage to the microbial cytoplasmic membrane (Figure 1) [144]. In addition, tea catechins also inhibit the activity of salivary amylase, leading to reduced cariogenicity of starch-containing foods [148, 149]. Xu et al. demonstrated that EGCg in green tea at sub-MIC levels suppressed multiple cariogenic virulence factors of S. mutans associated with carbohydrate metabolism and acid tolerance, thus promoting the control of dental caries (Figure 1) [150]. Lee et al. verified that EGCg in green tea was a powerful antimicrobial agent against planktonic E. faecalis and its biofilm, being able to suppress expression of genes related to its virulence and biofilm formation [151]. In addition, EGCg could also inhibit GtfB/C/D genes of S. mutans, and thus suppressed the biofilm formation of this cariogenic bacterium (Figure 1) [152].
Periodontitis is a multifactorial chronic infectious disease that affects periodontal tissue. Bacteria are the primary etiological factor of periodontal diseases. Periodontitis is closely associated with a special group of Gram-negative anaerobic bacteria (mainly P. gingivalis, Treponema denticola, and Tannerella forsythia) that interact with tooth supporting tissues and immune cells [153, 154]. An epidemiological study showed that frequent consumption of green tea was positively correlated with good periodontal health [155]. Consistently, in vitro studies showed EGCg and ECg in green tea could significantly inhibit the growth, adherence, and biofilm formation of P. gingivalis [156, 157], suppress the activity of collagenase [147] and MMPs [158], and enhance gingival keratinocyte integrity to protect from invasion of P. gingivalis [159]. Besides, EGCg can inhibit another periodontal pathogen, F. nucleatum, by decreasing the adherence and the biofilm formation of this bacterium [160]. Interestingly, EGCg can potentiate the effect of conventional antibiotics (metronidazole, tetracycline) which are used in periodontal therapy [161]. Moreover, tea catechins are able to reduce halitosis which is associated with volatile sulfur compounds (VSCs) produced mainly by oral anaerobes such as P. gingivalis and F. nucleatum [162]. Using mouth wash containing tea catechins for 4 weeks could reduce halitosis [163]. Yasuda et al. showed that EGCg could remove CH3SH, a major component of VSCs, through a chemical reaction in the presence of oxygen [164]. In addition, Xu et al. proved that EGCg inhibited CH3SH production by suppressing mgl gene expression of P. gingivalis [165].
In addition to its antibacterial activity, EGCg exhibits inhibitory effect on the growth and hyphal formation of C. albicans [166, 167], , and it can synergize with antimycotics against C. albicans biofilm [166, 168].
6.2. Propolis
Propolis is a hard, resinous, nontoxic natural product sourced from plants with a history of being used as a dietary supplement [169]. Ethanolic extracts of propolis (EEPs) have shown inhibitory effect against the growth and the adherence of S. mutans [170–173] and a comparable inhibitory effect to CHX when towards Lactobacilli, P. intermedia, P. gingivalis, A. israelii, and C. albicans [174]. Additionally, the anticaries effect of propolis was proven in desalivate rats [173, 175]. Among several compounds in propolis, flavonoid and cinnamic acid derivatives are considered to be the main bioactive constituent against bacteria [169]. Intriguingly, extracts from a novel type of propolis, which contain no traces of flavonoids and cinnamic acid derivatives, also show inhibition on Gtfs and growth and adherence of S. mutans due to its bioactive fraction containing a high abundance of fatty acids [176, 177].
Koo et al. identified two compounds, apigenin and trans-trans farnesol (tt-farnesol), which exhibited distinct biological activities against dental caries without impacting on bacterial viability [178, 179]. Apigenin, a 4β, 5, 7-trihydroxyflavone, is proven to effectively inhibit Gtfs, specifically GtfB and C (Figure 1). tt-farnesol is promising to be a novel adjunctive natural anticaries agent, as it is the most effective antibacterial compound in propolis. It is reported to reduce cell viability by disrupting membrane integrity and destabilizing the oral biofilm rather than affecting Gtfs activities (Figure 1) [178, 180]. Moreover, tt-farnesol can reduce the severity of smooth surface caries in rats [179]. The mechanism of tt-farnesol is attributed to the lipophilic moiety interaction with bacterial membrane, which is consistent with reduction of IPS accumulation in tt-farnesol treated S. mutans biofilms [179, 181]. One study examined the effect of topical applications (twice a day, 1 min exposure) of 1 mM apigenin, 5 mM tt-farnesol, and 13 mM fluoride (equivalent to 250 ppm F), alone or in combination, on the formation of S. mutans biofilms. The combination of the three shows the most effective impact on reducing the biofilm and acidogenicity of S. mutans [182]. Recently, Franca et al. designed a novel varnish with a propolis and chitosan base. This varnish adheres to the tooth surface, quickly forms film on the tooth surface, and continuously releases propolis for more than one week. The antimicrobial activity of the varnish against oral pathogens is similar to or even better than chlorhexidine varnish [183].
6.3. Cranberry
Cranberry is a highly nutritious fruit which is rich in a variety of bioactive compounds including flavonols, anthocyanins, tannins, flavan-3-ols and the phenolic acid derivatives [184]. Known for its high concentration in total polyphenols, the cranberry has been recognized as an excellent antioxidant and is beneficial to fighting bacterial infection [185]. It has well documented that cranberry is effective against oral infectious diseases, urinary tract disorders, cardiovascular diseases, and cancer [186, 187]. It has also shown antimicrobial activity against pathogens such as Helicobacter pylori, Salmonella, Staphylococcus aureus, and Escherichia coli [184]. Cranberries have demonstrated potential inhibitory effects against bacteria related to dental caries and periodontal diseases [188]. A cranberry-containing mouthwash could reduce the S. mutans counts after daily use for 6 weeks in a preliminary human trial [189]. Proanthocyanins (PACs) and flavonols are the most active components of the cranberry that can disrupt biofilm formation of S. mutans [190–192]. The highly purified A-type PAC (at 1.5 mg/ml) reduces biofilm formation of S. mutans and diminishes the acidogenicity of S. mutans even with lack of bactericidal effect [193]. Kim et al. also verified that topical application of PACs oligomers (100–300 μM) with myricetin (2 mM) of cranberry twice a day to cariogenic biofilm could break down its microarchitecture, reduce the amount of insoluble EPS (Figure 1), and increase the pH values at the biofilm-apatite interface [194]. Inhibition of biofilm formation is ascribed to PACs prevention of bacterial coaggregation, reduction of bacterial hydrophobicity, and alternation of cell surface molecules [195, 196]. Investigation of the degree-of-polymerization (DP) of PACs oligomer reveals that DP 4 and DP 8 to 13 are the most effective in disrupting bacterial adhesion to glucan-coated apatite surfaces [197]. PACs are more effective in reducing the development of carious lesions on the smooth surface than on the sulcal surface, but less effective than fluoride (at 225–250 ppm) in vivo [193]. Evidently, PACs of cranberry are promising novel alternatives or adjunctive anticaries chemotherapy [198].
PACs are also effective for the prevention and management of periodontitis. The A-type PAC reduces the biofilm formation, adherence and invasiveness to the human epithelial cells and proteinase activity P. gingivalis [199, 200]. Bodet et al. verified that the constituents of cranberry could inhibit metalloproteinases and elastase induced by lipopolysaccharide (LPS) in vitro [201]. La et al. proved that the A-type PAC of cranberry could inhibit MMP-1, -3, -7, -8, -9, and -13 production by LPS-stimulated macrophages [202] and decrease the secretion of chemoattractant such as IL-8 and CCL5 [199].
7. Conclusion and Future Prospects
Because of drug resistance, more attention is being drawn to identify alternative agents to biofilm control. The precision, effectiveness and efficiency of targeting oral biofilm are emphasized. Novel nanomaterials, which have the ability to load antimicrobial drugs or act as the drugs themselves, can precisely target the pathogen in response to specific environmental stimuli. QAS, which can be incorporated into the dental composite resin and adhesive system, exhibits excellent antibacterial activity to prevent secondary caries. Custom-designed small molecules that target key factors mediating bacterial adherence are also promising agents to disrupt oral biofilm. Arginine, as a base-generation substrate of oral bacteria, can function as an eco-modulator of oral biofilm and thus prevent dental caries. Furthermore, a group of natural products which contain polyphenols possess antimicrobial and antibiofilm activity with low drug tolerance towards oral biofilm.
However, it is noteworthy that current data available are mostly obtained from in vitro or animal studies using single species biofilm. The polymicrobial infection nature of dental caries and periodontitis would limit the clinical translation of the approaches developed based on single species biofilm. The complex environment in the oral cavity, particularly rapid clearance by saliva, will also affect the bioavailability and subsequently the effectiveness of the novel agents in vivo. More studies are needed to further evaluate the antimicrobial activities in humans to balance the bioactivity and biocompatibility of the novel agents as well.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81771099 and 81670978) and research grants from the Science and Technology Department of Sichuan Province (2016JY0006 and 2018SZ0121). Vivian Chen Received a Fulbright Research Grant to China.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Davies D. Understanding biofilm resistance to antibacterial agents. Nature Reviews Drug Discovery. 2003;2(2):114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 2.Ricucci D., Siqueira J. F., Jr. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. Journal of Endodontics. 2010;36(8):1277–1288. doi: 10.1016/j.joen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Noiri Y., Ehara A., Kawahara T., Takemura N., Ebisu S. Participation of bacterial biofilms in refractory and chronic periapical periodontitis. Journal of Endodontics. 2002;28(10):679–683. doi: 10.1097/00004770-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Liljemark W. F., Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Critical Reviews in Oral Biology Medicine. 2016;7(2):180–198. doi: 10.1177/10454411960070020601. [DOI] [PubMed] [Google Scholar]
- 5.Besinis A., De Peralta T., Handy R. D. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology. 2014;8(7):745–754. doi: 10.3109/17435390.2013.825343. [DOI] [PubMed] [Google Scholar]
- 6.Beikler T., Flemmig T. F. Oral biofilm-associated diseases: Trends and implications for quality of life, systemic health and expenditures. Periodontology 2000. 2011;55(1):87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- 7.Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S. A., Kjelleberg S. Biofilms: An emergent form of bacterial life. Nature Reviews Microbiology. 2016;14(9):563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 8.Liang Y., Yang L., Hong W., et al. Current understanding of multi‐species biofilms. International Journal of Oral Science. 2011;3(2):74–81. doi: 10.4248/IJOS11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horev B., Klein M. I., Hwang G., et al. PH-Activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 2015;9(3):2390–2404. doi: 10.1021/nn507170s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart P. S., Costerton J. W. Antibiotic resistance of bacteria in biofilms. The Lancet. 2001;358(9276):135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 11.Benoit D. S. W., Koo H. Targeted, triggered drug delivery to tumor and biofilm microenvironments. Nanomedicine. 2016;11(8):873–879. doi: 10.2217/nnm-2016-0014. [DOI] [PubMed] [Google Scholar]
- 12.Costerton J. W., Stewart P. S., Greenberg E. P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 13.Van Acker H., Van Dijck P., Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends in Microbiology. 2014;22(6):326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Lebeaux D., Ghigo J.-M., Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiology and Molecular Biology Reviews. 2014;78(3):510–543. doi: 10.1128/mmbr.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Kamesh A. C., Xiao Y., et al. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials. 2016;105:156–166. doi: 10.1016/j.biomaterials.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowen W. H., Burne R. A., Wu H., Koo H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends in Microbiology. 2018;26(3):229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao J., Klein M. I., Falsetta M. L., et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathogens. 2012;8(4) doi: 10.1371/journal.ppat.1002623.e1002623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo H., Xiao J., Klein M. I. Extracellular Polysaccharides Matrix — An Often Forgotten Virulence Factor in Oral Biofilm Research. International Journal of Oral Science. 2009;1(4):229–234. doi: 10.4248/IJOS.09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baltzer S. A., Brown M. H. Antimicrobial peptides—promising alternatives to conventional antibiotics. Journal of Molecular Microbiology and Biotechnology. 2011;20(4):228–235. doi: 10.1159/000331009. [DOI] [PubMed] [Google Scholar]
- 20.Rabin N., Zheng Y., Opoku-Temeng C., Du Y., Bonsu E., Sintim H. O. Agents that inhibit bacterial biofilm formation. Future Medicinal Chemistry. 2015;7(5):647–671. doi: 10.4155/fmc.15.7. [DOI] [PubMed] [Google Scholar]
- 21.Gao L., Koo H. Do catalytic nanoparticles offer an improved therapeutic strategy to combat dental biofilms? Nanomedicine. 2017;12(4):275–279. doi: 10.2217/nnm-2016-0400. [DOI] [PubMed] [Google Scholar]
- 22.Wu H., Moser C., Wang H.-Z., Høiby N., Song Z.-J. Strategies for combating bacterial biofilm infections. International Journal of Oral Science. 2015;7:1–7. doi: 10.1038/ijos.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L., Wen Y. The role of bacterial biofilm in persistent infections and control strategies. International Journal of Oral Science. 2011;3(2):66–73. doi: 10.4248/IJOS11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulla J., Sahu S., Hayes A. Nanotechnology: History and future. Human & Experimental Toxicology. 2015;34(12):1318–1321. doi: 10.1177/0960327115603588. [DOI] [PubMed] [Google Scholar]
- 25.Allaker R. P., Memarzadeh K. Nanoparticles and the control of oral infections. International Journal of Antimicrobial Agents. 2014;43(2):95–104. doi: 10.1016/j.ijantimicag.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Hemeg H. A. Nanomaterials for alternative antibacterial therapy. International Journal of Nanomedicine. 2017;12:8211–8225. doi: 10.2147/IJN.S132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rai M. K., Deshmukh S. D., Ingle A. P., Gade A. K. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. Journal of Applied Microbiology. 2012;112(5):841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- 28.Besinis A., De Peralta T., Tredwin C. J., Handy R. D. Review of nanomaterials in dentistry: interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano. 2015;9(3):2255–2289. doi: 10.1021/nn505015e. [DOI] [PubMed] [Google Scholar]
- 29.Shrestha A., Zhilong S., Gee N. K., Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. Journal of Endodontics. 2010;36(6):1030–1035. doi: 10.1016/j.joen.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Hannig M., Hannig C. Nanomaterials in preventive dentistry. Nature Nanotechnology. 2010;5(8):565–569. doi: 10.1038/nnano.2010.83. [DOI] [PubMed] [Google Scholar]
- 31.Cheng L., Zhang K., Weir M. D., Melo M. A. S., Zhou X., Xu H. H. K. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine. 2015;10(4):627–641. doi: 10.2217/nnm.14.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besinis A., de Peralta T., Handy R. D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology. 2014;8(1):1–16. doi: 10.3109/17435390.2012.742935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng J. J.-Y., Botelho M. G., Matinlinna J. P. Silver compounds used in dentistry for caries management: a review. Journal of Dentistry. 2012;40(7):531–541. doi: 10.1016/j.jdent.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A., Kumar V., Singh J., Hooda A., Dutta S. Drug-induced discoloration of teeth: An updated review. Clinical Pediatrics. 2012;51(2):181–185. doi: 10.1177/0009922811421000. [DOI] [PubMed] [Google Scholar]
- 35.Stuart C. H., Schwartz S. A., Beeson T. J., Owatz C. B. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. Journal of Endodontics. 2006;32(2):93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 36.Siqueira J. F., Jr., Rôças I. N. Exploiting molecular methods to explore endodontic infections: Part 2 - Redefining the endodontic microbiota. Journal of Endodontics. 2005;31(7):488–498. doi: 10.1097/01.don.0000157990.86638.49. [DOI] [PubMed] [Google Scholar]
- 37.Wu D., Fan W., Kishen A., Gutmann J. L., Fan B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. Journal of Endodontics. 2014;40(2):285–290. doi: 10.1016/j.joen.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues C. T., de Andrade F. B., de Vasconcelos L. R., et al. Antibacterial properties of silver nanoparticles as a root canal irrigant against. International Endodontic Journal. 2018;51(8):901–911. doi: 10.1111/iej.12904. [DOI] [PubMed] [Google Scholar]
- 39.Afkhami F., Pourhashemi S. J., Sadegh M., Salehi Y., Fard M. J. K. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. Journal of Dentistry. 2015;43(12):1573–1579. doi: 10.1016/j.jdent.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Ong T. H., Chitra E., Ramamurthy S., et al. Correction: Chitosan-propolis nanoparticle formulation demonstrates anti-bacterial activity against Enterococcus faecalis biofilms. PLoS ONE. 2017;12(4):p. e0174888. doi: 10.1371/journal.pone.0174888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong M., Chen X. G., Xing K., Park H. J. Antimicrobial properties of chitosan and mode of action: a state of the art review. International Journal of Food Microbiology. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Qi L., Xu Z., Jiang X., Hu C., Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research. 2004;339(16):2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Bae K., Jun E. J., Lee S. M., Paik D. I., Kim J. B. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clinical Oral Investigations. 2006;10(2):102–107. doi: 10.1007/s00784-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 44.Sano H., Shibasaki K.-I., Matsukubo T., Takaesu Y. Effect of molecular mass and degree of deacetylation of chitosan on adsorption of Streptococcus sobrinus 6715 to saliva treated hydroxyapatite. The Bulletin of Tokyo Dental College. 2002;43(2):75–82. doi: 10.2209/tdcpublication.43.75. [DOI] [PubMed] [Google Scholar]
- 45.Sano H., Shibasaki K.-I., Matsukubo T., Takaesu Y. Effect of chitosan rinsing on reduction of dental plaque formation. The Bulletin of Tokyo Dental College. 2003;44(1):9–16. doi: 10.2209/tdcpublication.44.9. [DOI] [PubMed] [Google Scholar]
- 46.Shi Z., Neoh K. G., Kang E. T., Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27(11):2440–2449. doi: 10.1016/j.biomaterials.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 47.de Paz L. E. C., Resin A., Howard K. A., Sutherland D. S., Wejse P. L. Antimicrobial effect of chitosan nanoparticles on Streptococcus mutans biofilms. Applied and Environmental Microbiology. 2011;77(11):3892–3895. doi: 10.1128/AEM.02941-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aliasghari A., Khorasgani M. R., Vaezifar S., Rahimi F., Younesi H., Khoroushi M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iranian Journal of Microbiology. 2016;8(2):93–100. [PMC free article] [PubMed] [Google Scholar]
- 49.Sireesha A., Jayasree R., Vidhya S., Mahalaxmi S., Sujatha V., Kumar T. S. S. Comparative evaluation of micron- and nano-sized intracanal medicaments on penetration and fracture resistance of root dentin – An in vitro study. International Journal of Biological Macromolecules. 2017;104:1866–1873. doi: 10.1016/j.ijbiomac.2017.05.126. [DOI] [PubMed] [Google Scholar]
- 50.Siqueira J. F., Jr., Lopes H. P. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. International Endodontic Journal. 1999;32(5):361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakajo K., Nakazawa F., Iwaku M., Hoshino E. Alkali-resistant bacteria in root canal systems. Oral microbiology and immunology. 2004;19(6):390–394. doi: 10.1111/j.1399-302x.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 52.del Carpio-Perochena A., Kishen A., Felitti R., et al. Antibacterial Properties of Chitosan Nanoparticles and Propolis Associated with Calcium Hydroxide against Single- and Multispecies Biofilms: An In Vitro and In Situ Study. Journal of Endodontics. 2017;43(8):1332–1336. doi: 10.1016/j.joen.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Divakar D. D., Jastaniyah N. T., Altamimi H. G., et al. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. International Journal of Biological Macromolecules. 2018;108:790–797. doi: 10.1016/j.ijbiomac.2017.10.166. [DOI] [PubMed] [Google Scholar]
- 54.Mccarthy C. A., Ahern R. J., Dontireddy R., Ryan K. B., Crean A. M. Mesoporous silica formulation strategies for drug dissolution enhancement: A review. Expert Opinion on Drug Delivery. 2016;13(1):93–108. doi: 10.1517/17425247.2016.1100165. [DOI] [PubMed] [Google Scholar]
- 55.Tang F., Li L., Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Advanced Materials. 2012;24(12):1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Zhao Q., Han N. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11(2):313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Cousins B. G., Allison H. E., Doherty P. J., et al. Effects of a nanoparticulate silica substrate on cell attachment of Candida albicans. Journal of Applied Microbiology. 2007;102(3):757–765. doi: 10.1111/j.1365-2672.2006.03124.x. [DOI] [PubMed] [Google Scholar]
- 58.Li X., Wong C. H., Ng T. W., et al. The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. International Journal of Nanomedicine. 2016;11:2471–2480. doi: 10.2147/IJN.S105681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seneviratne C. J., Leung K. C., Wong C., et al. Nanoparticle-Encapsulated Chlorhexidine against Oral Bacterial Biofilms. PLoS ONE. 2014;9(8):p. e103234. doi: 10.1371/journal.pone.0103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan H., Yang H., Li K., Yu J., Huang C. Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules. 2017;22(7):p. 1225. doi: 10.3390/molecules22071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J. F., Wu R., Fan Y., et al. Antibacterial dental composites with chlorhexidine and mesoporous silica. Journal of Dental Research. 2014;93(12):1283–1289. doi: 10.1177/0022034514555143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park K. Controlled drug delivery systems: past forward and future back. Journal of Controlled Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowen W. H. The Stephan Curve revisited. Odontology. 2013;101(1):2–8. doi: 10.1007/s10266-012-0092-z. [DOI] [PubMed] [Google Scholar]
- 64.Gao L., Liu Y., Kim D., et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272–284. doi: 10.1016/j.biomaterials.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao L., Giglio K. M., Nelson J. L., Sondermann H., Travis A. J. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale. 2014;6(5):2588–2593. doi: 10.1039/c3nr05422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei H., Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chemical Society Reviews. 2013;42(14):6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 67.Lin Y., Ren J., Qu X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Accounts of Chemical Research. 2014;47(4):1097–1105. doi: 10.1021/ar400250z. [DOI] [PubMed] [Google Scholar]
- 68.Cormode D. P., Gao L., Koo H. Emerging Biomedical Applications of Enzyme-Like Catalytic Nanomaterials. Trends in Biotechnology. 2018;36(1):15–29. doi: 10.1016/j.tibtech.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marshall M. V., Cancro L. P., Fischman S. L. Hydrogen peroxide: A review of its use in dentistry. Journal of Periodontology. 1995;66(9):786–796. doi: 10.1902/jop.1995.66.9.786. [DOI] [PubMed] [Google Scholar]
- 70.Ferracane J. L. Resin composite—state of the art. Dental Materials. 2011;27(1):29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 71.Pashley D. H., Tay F. R., Breschi L., et al. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27(1):1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demarco F. F., Corrêa M. B., Cenci M. S., Moraes R. R., Opdam N. J. M. Longevity of posterior composite restorations: not only a matter of materials. Dental Materials. 2012;28(1):87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Sarrett D. C. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dental Materials. 2005;21(1):9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Bürgers R., Eidt A., Frankenberger R., et al. The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Archives of Oral Biolog. 2009;54(6):595–601. doi: 10.1016/j.archoralbio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Beyth N., Domb A. J., Weiss E. I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35(3):201–206. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Beyth N., Yudovin-Farber I., Bahir R., Domb A. J., Weiss E. I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27(21):3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Jedrychowski J. R., Caputo A. A., Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. Journal of Oral Rehabilitation. 1983;10(5):373–381. doi: 10.1111/j.1365-2842.1983.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 78.Cheng L., Zhang K., Zhang N., et al. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. Journal of Dental Research. 2017;96(8):855–863. doi: 10.1177/0022034517709739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciancio S. G., Mather M. L., Bunnell H. L. Clinical evaluation of a quaternary ammonium containing mouthrinse. Journal of Periodontology. 1975;46(7):397–401. doi: 10.1902/jop.1975.46.7.397. [DOI] [PubMed] [Google Scholar]
- 80.Liang J. G., Li M. Y., Ren B., et al. The anti-caries effects of dental adhesive resin influenced by the position of functional groups in quaternary ammonium monomers. Dental Materials. 2017:S0109–S5641. doi: 10.1016/j.dental.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 81.Cheng L., Zhang K., Melo M. A. S., Weir M. D., Zhou X., Xu H. H. K. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91(6):598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Izutani N., Imazato S., Noiri Y., Ebisu S. Antibacterial effects of MDPB against anaerobes associated with endodontic infections. International Endodontic Journal. 2010;43(8):637–645. doi: 10.1111/j.1365-2591.2010.01716.x. [DOI] [PubMed] [Google Scholar]
- 83.Imazato S., Torii M., Tsuchitani Y., Mccabe J. F., Russell R. R. B. Incorporation of Bacterial Inhibitor into Resin Composite. Journal of Dental Research. 1994;73(8):1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 84.Kitagawa R., Kitagawa H., Izutani N., Hirose N., Hayashi M., Imazato S. Development of an antibacterial root canal filling system containing MDPB. Journal of Dental Research. 2014;93(12):1277–1282. doi: 10.1177/0022034514549808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li F., Chen J., Chai Z., et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37(4):289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Antonucci J. M., Zeiger D. N., Tang K., Lin-Gibson S., Fowler B. O., Lin N. J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28(2):219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng L., Weir M. D., Xu H. H. K., et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dental Materials. 2012;28(5):561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng L., Weir M. D., Zhang K., Arola D. D., Zhou X., Xu H. H. K. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41(4):345–355. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C., Cheng L., Weir M. D., et al. Primer containing dimethylaminododecyl methacrylate kills bacteria impregnated in human dentin blocks. International Journal of Oral Science. 2016;8(4):239–245. doi: 10.1038/ijos.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tiller J. C., Liao C.-J., Lewis K., Klibanov A. M. Designing surfaces that kill bacteria on contact. Proceedings of the National Acadamy of Sciences of the United States of America. 2001;98(11):5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li F., Weir M. D., Xu H. H. K. Effects of quaternary ammonium chain length on antibacterial bonding agents. Journal of Dental Research. 2013;92(10):932–938. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han Q., Li B., Zhou X., et al. Anti-Caries Effects of Dental Adhesives Containing Quaternary Ammonium Methacrylates with Different Chain Lengths. Materials . 2017;10(6):p. 643. doi: 10.3390/ma10060643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu T., Li B., Zhou X., et al. Evaluation of Novel Anticaries Adhesive in a Secondary Caries Animal Model. Caries Research. 2018;52(1-2):14–21. doi: 10.1159/000481832. [DOI] [PubMed] [Google Scholar]
- 94.Li F., Wang P., Weir M. D., Fouad A. F., Xu H. H. K. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomaterialia. 2014;10(6):2804–2813. doi: 10.1016/j.actbio.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou C., Weir M. D., Zhang K., Deng D., Cheng L., Xu H. H. K. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29(8):859–870. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang K., Cheng L., Wu E. J., Weir M. D., Bai Y., Xu H. H. K. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41(6):504–513. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang K., Cheng L., Weir M. D., Bai Y.-X., Xu H. H. K. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. International Journal of Oral Science. 2016;8:45–53. doi: 10.1038/ijos.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou H., Weir M. D., Antonucci J. M., Schumacher G. E., Zhou X.-D., Xu H. H. K. Evaluation of three-dimensional biofilms on antibacterial bonding agents containing novel quaternary ammonium methacrylates. International Journal of Oral Science. 2014;6(2):77–86. doi: 10.1038/ijos.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou H., Liu H., Weir M. D., Reynolds M. A., Zhang K., Xu H. H. K. Three-dimensional biofilm properties on dental bonding agent with varying quaternary ammonium charge densities. Journal of Dentistry. 2016;53:73–81. doi: 10.1016/j.jdent.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hegstad K., Langsrud S., Lunestad B. T., Scheie A. A., Sunde M., Yazdankhah S. P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microbial Drug Resistance. 2010;16(2):91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- 101.Wang S., Wang H., Ren B., et al. Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species? Dental Materials. 2017;33(10):1127–1138. doi: 10.1016/j.dental.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 102.Koo H. Strategies to Enhance the Biological Effects of Fluoride on Dental Biofilms. Advances in Dental Research. 2008;20(1):17–21. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]
- 103.Worthington R. J., Richards J. J., Melander C. Small molecule control of bacterial biofilms. Organic Biomolecular Chemistry. 2012;10(37):7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanzer J. M., Livingston J., Thompson A. M. The microbiology of primary dental caries in humans. Journal of Dental Education. 2001;65(10):1028–1037. [PubMed] [Google Scholar]
- 105.Tanner A. C. R., Mathney J. M. J., Kent R. L., Jr., et al. Cultivable anaerobic microbiota of severe early childhood caries. Journal of Clinical Microbiology. 2011;49(4):1464–1474. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiology and Molecular Biology Reviews. 1980;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vacca-Smith A. M., Bowen W. H. Binding properties of streptococcal glucosyltransferases for hydroxyapatite, saliva coated hydroxyapatite, and bacterial surfaces. Archives of Oral Biolog. 1998;43(2):103–110. doi: 10.1016/S0003-9969(97)00111-8. [DOI] [PubMed] [Google Scholar]
- 108.Schilling K. M., Bowen W. H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infection and Immunity. 1992;60(1):284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cegelski L., Marshall G. R., Eldridge G. R., Hultgren S. J. Erratum: The biology and future prospects of antivirulence therapies (Nature Reviews Microbiology (2008) vol. 6 (17-27) 10.1038/nrmicro1818) Nature Reviews Microbiology. 2009;7(11):p. 836. doi: 10.1038/nrmicro2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Q., Nijampatnam B., Hua Z., et al. Structure-Based Discovery of Small Molecule Inhibitors of Cariogenic Virulence. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-06168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ren Z., Cui T., Zeng J., et al. Molecule targeting glucosyltransferase inhibits Streptococcus mutans biofilm formation and virulence. Antimicrobial Agents and Chemotherapy. 2016;60(1):126–135. doi: 10.1128/AAC.00919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infection and Immunity. 1988;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Richards J. J., Melander C. Synthesis of a 2-aminoimidazole library for antibiofilm screening utilizing the sonogashira reaction. The Journal of Organic Chemistry. 2008;73(13):5191–5193. doi: 10.1021/jo800618q. [DOI] [PubMed] [Google Scholar]
- 114.Liu C., Worthington R. J., Melander C., Wu H. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrobial Agents and Chemotherapy. 2011;55(6):2679–2687. doi: 10.1128/AAC.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garcia S. S., Blackledge M. S., Michalek S., et al. Targeting of Streptococcus mutans Biofilms by a Novel Small Molecule Prevents Dental Caries and Preserves the Oral Microbiome. Journal of Dental Research. 2017;96(7):807–814. doi: 10.1177/0022034517698096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brown G. D., Denning D. W., Gow N. A. R., Levitz S. M., Netea M. G., White T. C. Hidden killers: human fungal infections. Science Translational Medicine. 2012;4(165) doi: 10.1126/scitranslmed.3004404.165rv13 [DOI] [PubMed] [Google Scholar]
- 117.Pierce C. G., Chaturvedi A. K., Lazzell A. L., et al. A novel small molecule inhibitor of Candida albicans biofilm formation, filamentation and virulence with low potential for the development of resistance. npj Biofilms and Microbiomes. 2015;1(1) doi: 10.1038/npjbiofilms.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burne R. A., Marquis R. E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiology Letters. 2000;193(1):1–6. doi: 10.1016/S0378-1097(00)00438-9. doi: 10.1016/S0378-1097(00)00438-9. [DOI] [PubMed] [Google Scholar]
- 119.He J., Hwang G., Liu Y., et al. L-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. Journal of Bacteriology. 2016;198(19):2651–2661. doi: 10.1128/JB.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nascimento M. M., Browngardt C., Xiaohui X., Klepac-Ceraj V., Paster B. J., Burne R. A. The effect of arginine on oral biofilm communities. Molecular Oral Microbiology. 2014;29(1):45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng X., He J., Wang L., et al. Ecological Effect of Arginine on Oral Microbiota. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-07042-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chakraborty B., Burne R. A., Liu S. Effects of Arginine on Streptococcus mutans Growth, Virulence Gene Expression, and Stress Tolerance. Applied and Environmental Microbiology. 2017;83(15) doi: 10.1128/AEM.00496-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim Y.-H., Kim S. M., Lee S. Y. Antimicrobial activity of protamine against oral microorganisms. Biocontrol Science. 2015;20(4):275–280. doi: 10.4265/bio.20.275. [DOI] [PubMed] [Google Scholar]
- 124.Sharma S., Lavender S., Woo J., et al. Nanoscale characterization of effect of L-arginine on Streptococcus mutans biofilm adhesion by atomic force microscopy. Microbiology. 2014;160(Pt_7):1466–1473. doi: 10.1099/mic.0.075267-0. [DOI] [PubMed] [Google Scholar]
- 125.Huang X., Zhang K., Deng M., et al. Effect of arginine on the growth and biofilm formation of oral bacteria. Archives of Oral Biolog. 2017;82:256–262. doi: 10.1016/j.archoralbio.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 126.Sato T., Nakazawa F. Coaggregation between Prevotella oris and Porphyromonas gingivalis. Journal of Microbiology, Immunology and Infection. 2014;47(3):182–186. doi: 10.1016/j.jmii.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 127.Geraldeli S., Soares E. F., Alvarez A. J., et al. A new arginine-based dental adhesive system: formulation, mechanical and anti-caries properties. Journal of Dentistry. 2017;63:72–80. doi: 10.1016/j.jdent.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng X., Cheng X., Wang L., et al. Combinatorial effects of arginine and fluoride on oral bacteria. Journal of Dental Research. 2015;94(2):344–353. doi: 10.1177/0022034514561259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jeon J.-G., Rosalen P. L., Falsetta M. L., Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Research. 2011;45(3):243–263. doi: 10.1159/000327250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoo S., Murata R. M., Duarte S. Antimicrobial traits of tea- and cranberry-derived polyphenols against streptococcus mutans. Caries Research. 2011;45(4):327–335. doi: 10.1159/000329181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng L., Li J., He L., Zhou X. Natural products and caries prevention. Caries Research. 2015;49:38–45. doi: 10.1159/000377734. [DOI] [PubMed] [Google Scholar]
- 132.Gazzani G., Daglia M., Papetti A. Food components with anticaries activity. Current Opinion in Biotechnology. 2012;23(2):153–159. doi: 10.1016/j.copbio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 133.Hamilton-Miller J. M. Antimicrobial properties of tea (Camellia sinensis L.) Antimicrobial Agents and Chemotherapy. 1995;39(11):2375–2377. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mitscher L. A., Jung M., Shankel D., Dou J.-H., Steele L., Pillai S. P. Chemoprotection: A review of the potential therapeutic antioxidant properties of green tea (Camellia sinensis) and certain of its constituents. Medicinal Research Reviews. 1997;17(4):327–365. doi: 10.1002/(SICI)1098-1128(199707)17:4<327::AID-MED2>3.0.CO;2-Y. doi: 10.1002/(SICI)1098-1128(199707)17:4<327::AID-MED2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 135.Wu C. D., Wei G.-X. Tea as a functional food for oral health. Nutrition Journal . 2002;18(5):443–444. doi: 10.1016/S0899-9007(02)00763-3. [DOI] [PubMed] [Google Scholar]
- 136.Sharma V., Rao L. J. M. A thought on the biological activities of black tea. Critical Reviews in Food Science and Nutrition. 2009;49(5):379–404. doi: 10.1080/10408390802068066. [DOI] [PubMed] [Google Scholar]
- 137.Ferrazzano G. F., Roberto L., Amato I., Cantile T., Sangianantoni G., Ingenito A. Antimicrobial properties of green tea extract against cariogenic microflora: an in vivo study. Journal of Medicinal Food. 2011;14(9):907–911. doi: 10.1089/jmf.2010.0196. [DOI] [PubMed] [Google Scholar]
- 138.Kawamura J., Takeo T. Antibacterial activity of tea catechin to Streptococcus mutans. Journal of the Japanese Society of Food Science and Technology. 1989;36(6):463–467. doi: 10.3136/nskkk1962.36.6_463. [DOI] [Google Scholar]
- 139.Sakanaka S., Kim M., Taniguchi M., Yamamoto T. Antibacterial Substances in Japanese Green Tea Extract Against Streptococcus Mutans, a Cariogenic Bacterium. Agricultural and Biological Chemistry. 1989;53(9):2307–2311. [Google Scholar]
- 140.Otake S., Makimura M., Kuroki T., Nishihara Y., Hirasawa M. Anticaries effects of poly phenolic compounds from japanese green tea. Caries Research. 1991;25(6):438–443. doi: 10.1159/000261407. [DOI] [PubMed] [Google Scholar]
- 141.Hirasawa M., Takada K., Otake S. Inhibition of acid production in dental plaque bacteria by green tea catechins. Caries Research. 2006;40(3):265–270. doi: 10.1159/000092236. [DOI] [PubMed] [Google Scholar]
- 142.Nakahara K., Kawabata S., Ono H., et al. Inhibitory effect of oolong tea polyphenols on glucosyltransferases of mutans Streptococci. Applied and Environmental Microbiology. 1993;59(4):968–973. doi: 10.1128/aem.59.4.968-973.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ooshima T., Minami T., Aono W., et al. Oolong tea polyphenols inhibit experimental dental caries in spf rats infected with mutans streptococci. Caries Research. 1993;27(2):124–129. doi: 10.1159/000261529. [DOI] [PubMed] [Google Scholar]
- 144.Taylor P. W., Hamilton-Miller J. M., Stapleton P. D. Antimicrobial properties of green tea catechins. Food Science Technology Bulletin: Functional Foods. 2005;2(7):71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamilton-Miller J. M. T. Anti-cariogenic properties of tea (Camellia sinensis) Journal of Medical Microbiology. 2001;50(4):299–302. doi: 10.1099/0022-1317-50-4-299. [DOI] [PubMed] [Google Scholar]
- 146.Hattori M., Kusumoto I. T., Namba T., Ishigami T., Hara Y. Effect of Tea Polyphenols on Glucan Synthesis by Glucosyltransferase from Streptococcus mutans. Chemical & Pharmaceutical Bulletin. 1990;38(3):717–720. doi: 10.1248/cpb.38.717. [DOI] [PubMed] [Google Scholar]
- 147.Makimura M., Hirasawa M., Kobayashi K., et al. Inhibitory effect of tea catechins on collagenase activity. Journal of Periodontology. 1993;64(7):630–636. doi: 10.1902/jop.1993.64.7.630. [DOI] [PubMed] [Google Scholar]
- 148.Kashket S., Paolino V. J. Inhibition of salivary amylase by water-soluble extracts of tea. Archives of Oral Biolog. 1988;33(11):845–846. doi: 10.1016/0003-9969(88)90110-0. [DOI] [PubMed] [Google Scholar]
- 149.Hara K., Ohara M., Hayashi I., et al. The green tea polyphenol (-)-epigallocatechin gallate precipitates salivary proteins including alpha-amylase: Biochemical implications for oral health. European Journal of Oral Sciences. 2012;120(2):132–139. doi: 10.1111/j.1600-0722.2012.00947.x. [DOI] [PubMed] [Google Scholar]
- 150.Xu X., Zhou X. D., Wu C. D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrobial Agents and Chemotherapy. 2011;55(3):1229–1236. doi: 10.1128/aac.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee P., Tan K. S. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Archives of Oral Biolog. 2015;60(3):393–399. doi: 10.1016/j.archoralbio.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 152.Xu X., Zhou X. D., Wu C. D. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Archives of Oral Biolog. 2012;57(6):678–683. doi: 10.1016/j.archoralbio.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 153.Pihlstrom B. L., Michalowicz B. S., Johnson N. W. Periodontal diseases. The Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 154.Sorsa T., Tjäderhane L., Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Diseases. 2004;10(6):311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 155.Kushiyama M., Shimazaki Y., Murakami M., Yamashita Y. Relationship between intake of green tea and periodontal disease. Journal of Periodontology. 2009;80(3):372–377. doi: 10.1902/jop.2009.080510. [DOI] [PubMed] [Google Scholar]
- 156.Asahi Y., Noiri Y., Miura J., et al. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. Journal of Applied Microbiology. 2014;116(5):1164–1171. doi: 10.1111/jam.12458. [DOI] [PubMed] [Google Scholar]
- 157.Sakanaka S., Aizawa M., Kim M., Yamamoto T. Inhibitory effects of green tea polyphenols on growth and cellular adherence of an oral bacterium, Porphyromonas gingivalis. Bioscience, Biotechnology, and Biochemistry. 1996;60(5):745–749. doi: 10.1271/bbb.60.745. [DOI] [PubMed] [Google Scholar]
- 158.Yun J.-H., Pang E.-K., Kim C.-S., et al. Inhibitory effects of green tea polyphenol (−)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. Journal of Periodontal Research. 2004;39(5):300–307. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 159.Lagha A. B., Groeger S., Meyle J., Grenier D. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathogens and Disease. 2018;76(4) doi: 10.1093/femspd/fty030. [DOI] [PubMed] [Google Scholar]
- 160.Ben Lagha A., Haas B., Grenier D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Scientific Reports. 2017;7(1) doi: 10.1038/srep44815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fournier-Larente J., Morin M.-P., Grenier D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Archives of Oral Biolog. 2016;65:35–43. doi: 10.1016/j.archoralbio.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 162.Lodhia P., Yaegaki K., Khakbaznejad A., et al. Effect of green tea on volatile sulfur compounds in mouth air. Journal of Nutritional Science and Vitaminology. 2008;54(1):89–94. doi: 10.3177/jnsv.54.89. [DOI] [PubMed] [Google Scholar]
- 163.Kaneko K., Shimano N., Suzuki Y., et al. Effects of tea catechins on oral odor and dental plaque. Oral Therapeutics and Pharmacology. 1993;12(3):189–197. [Google Scholar]
- 164.Yasuda H., Arakawa T. Deodorizing Mechanism of (−)-Epigallocatechin Gallate against Methyl Mercaptan. Bioscience, Biotechnology, and Biochemistry. 1995;59(7):1232–1236. doi: 10.1271/bbb.59.1232. [DOI] [Google Scholar]
- 165.Xu X., Zhou X. D., Wu C. D. Tea catechin EGCg suppresses the mgl gene associated with halitosis. Journal of Dental Research. 2010;89(11):1304–1308. doi: 10.1177/0022034510378682. [DOI] [PubMed] [Google Scholar]
- 166.Hirasawa M., Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. Journal of Antimicrobial Chemotherapy. 2004;53(2):225–229. doi: 10.1093/jac/dkh046. [DOI] [PubMed] [Google Scholar]
- 167.Chen M., Zhai L., Arendrup M. C. In vitro activity of 23 tea extractions and epigallocatechin gallate against Candida species. Medical Mycology. 2015;53(2):194–198. doi: 10.1093/mmy/myu073. [DOI] [PubMed] [Google Scholar]
- 168.Ning Y., Ling J., Wu C. D. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral Candida species. Archives of Oral Biolog. 2015;60(10):1565–1570. doi: 10.1016/j.archoralbio.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 169.Burdock G. A. Review of the biological properties and toxicity of bee propolis (propolis) Food and Chemical Toxicology. 1998;36(4):347–363. doi: 10.1016/S0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 170.Koo H., Gomes B. P. F. A., Rosalen P. L., Ambrosano G. M. B., Park Y. K., Cury J. A. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Archives of Oral Biolog. 2000;45(2):141–148. doi: 10.1016/S0003-9969(99)00117-X. [DOI] [PubMed] [Google Scholar]
- 171.Koo H., Vacca Smith A. M., Bowen W. H., Rosalen P. L., Cury J. A., Park Y. K. Effects of Apis mellifera Propolis on the Activities of Streptococcal Glucosyltransferases in Solution and Adsorbed onto Saliva-Coated Hydroxyapatite. Caries Research. 2000;34(5):418–426. doi: 10.1159/000016617. [DOI] [PubMed] [Google Scholar]
- 172.da Cunha M. G., Franchin M., Galvão L. C. D., et al. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complementary and Alternative Medicine. 2013;13, article 23 doi: 10.1186/1472-6882-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hayacibara M. F., Koo H., Rosalen P. L., et al. In vitro and in vivo effects of isolated fractions of Brazilian propolis on caries development. Journal of Ethnopharmacology. 2005;101(1–3):110–115. doi: 10.1016/j.jep.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 174.Akca A. E., Akca G., Topçu F. T., Macit E., Pikdöken L., Özgen I. Ş. The Comparative Evaluation of the Antimicrobial Effect of Propolis with Chlorhexidine against Oral Pathogens: An In Vitro Study. BioMed Research International. 2016;2016:8. doi: 10.1155/2016/3627463.3627463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koo H., Rosalen P. L., Cury J. A., Park Y. K., Ikegaki M., Sattler A. Effect of Apis mellifera propolis from two Brazilian regions on caries development in desalivated rats. Caries Research. 1999;33(5):393–400. doi: 10.1159/000016539. [DOI] [PubMed] [Google Scholar]
- 176.Duarte S., Koo H., Bowen W. H., et al. Effect of a novel type of propolis and its chemical fractions on glucosyltransferases and on growth and adherence of mutans streptococci. Biological & Pharmaceutical Bulletin. 2003;26(4):527–531. doi: 10.1248/bpb.26.527. [DOI] [PubMed] [Google Scholar]
- 177.Duarte S., Rosalen P. L., Hayacibara M. F., et al. The influence of a novel propolis on mutans streptococci biofilms and caries development in rats. Archives of Oral Biolog. 2006;51(1):15–22. doi: 10.1016/j.archoralbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 178.Koo H., Rosalen P. L., Cury J. A., Park Y. K., Bowen W. H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrobial Agents and Chemotherapy. 2002;46(5):1302–1309. doi: 10.1128/aac.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koo H., Hayacibara M. F., Schobel B. D., et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. Journal of Antimicrobial Chemotherapy. 2003;52(5):782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 180.Jeon J.-G., Pandit S., Xiao J., et al. Influences of transtrans farnesol, a membranetargeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixedspecies oral biofilms. International Journal of Oral Science. 2011;3(2):98–106. doi: 10.4248/IJOS11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koo H., Jeon J. G. Naturally Occurring Molecules as Alternative Therapeutic Agents against Cariogenic Biofilms. Advances in Dental Research. 2009;21(1):63–68. doi: 10.1177/0895937409335629. [DOI] [PubMed] [Google Scholar]
- 182.Koo H., Schobel B., Scott-Anne K., et al. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. Journal of Dental Research. 2005;84(11):1016–1020. doi: 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Franca J. R., de Luca M. P., Ribeiro T. G., et al. Propolis—based chitosan varnish: drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complementary and Alternative Medicine. 2014;14, article 478 doi: 10.1186/1472-6882-14-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Côté J., Caillet S., Doyon G., Sylvain J.-F., Lacroix M. Bioactive compounds in cranberries and their biological properties. Critical Reviews in Food Science and Nutrition. 2010;50(7):666–679. doi: 10.1080/10408390903044107. [DOI] [PubMed] [Google Scholar]
- 185.Vinson J. A., Bose P., Proch J., Al Kharrat H., Samman N. Cranberries and cranberry products: Powerful in vitro, ex vivo, and in vivo sources of antioxidants. Journal of Agricultural and Food Chemistry. 2008;56(14):5884–5891. doi: 10.1021/jf073309b. [DOI] [PubMed] [Google Scholar]
- 186.Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics - A Finnish perspective. Molecular Nutrition & Food Research. 2007;51(6):684–691. doi: 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- 187.Ruel G., Couillard C. Evidences of the cardioprotective potential of fruits: The case of cranberries. Molecular Nutrition & Food Research. 2007;51(6):692–701. doi: 10.1002/mnfr.200600286. [DOI] [PubMed] [Google Scholar]
- 188.Bodet C., Grenier D., Chandad F., Ofek I., Steinberg D., Weiss E. I. Potential oral health benefits of cranberry. Critical Reviews in Food Science and Nutrition. 2008;48(7):672–680. doi: 10.1080/10408390701636211. [DOI] [PubMed] [Google Scholar]
- 189.Weiss E. I., Kozlovsky A., Steinberg D., et al. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiology Letters. 2004;232(1):89–92. doi: 10.1016/S0378-1097(04)00035-7. [DOI] [PubMed] [Google Scholar]
- 190.Duarte S., Gregoire S., Singh A. P., et al. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiology Letters. 2006;257(1):50–56. doi: 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 191.Gregoire S., Singh A. P., Vorsa N., Koo H. Influence of cranberry phenolics on glucan synthesis by glucosyltransferases and Streptococcus mutans acidogenicity. Journal of Applied Microbiology. 2007;103(5):1960–1968. doi: 10.1111/j.1365-2672.2007.03441.x. [DOI] [PubMed] [Google Scholar]
- 192.Steinberg D., Feldman M., Ofek I., Weiss E. I. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. Journal of Antimicrobial Chemotherapy. 2004;54(1):86–89. doi: 10.1093/jac/dkh254. [DOI] [PubMed] [Google Scholar]
- 193.Koo H., Duarte S., Murata R. M., et al. Influence of cranberry proanthocyanidins on formation of biofilms by streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Research. 2010;44(2):116–126. doi: 10.1159/000296306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim D., Hwang G., Liu Y., et al. Cranberry Flavonoids Modulate Cariogenic Properties of Mixed-Species Biofilm through Exopolysaccharides-Matrix Disruption. PLoS ONE. 2015;10(12):p. e0145844. doi: 10.1371/journal.pone.0145844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yamanaka A., Kimizuka R., Kato T., Okuda K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral microbiology and immunology. 2004;19(3):150–154. doi: 10.1111/j.0902-0055.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 196.Feghali K., Feldman M., La V. D., Santos J., Grenier D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. Journal of Agricultural and Food Chemistry. 2012;60(23):5728–5735. doi: 10.1021/jf203304v. [DOI] [PubMed] [Google Scholar]
- 197.Feng G., Klein M. I., Gregoire S., Singh A. P., Vorsa N., Koo H. The specific degree-of-polymerization of A-type proanthocyanidin oligomers impacts Streptococcus mutans glucan-mediated adhesion and transcriptome responses within biofilms. Biofouling. 2013;29(6):629–640. doi: 10.1080/08927014.2013.794456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bonifait L., Grenier D. Cranberry polyphenols: Potential benefits for dental caries and periodontal disease. Journal of the Canadian Dental Association. 2010;76(1) [PubMed] [Google Scholar]
- 199.La V. D., Howell A. B., Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrobial Agents and Chemotherapy. 2010;54(5):1778–1784. doi: 10.1128/AAC.01432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Labrecque J., Bodet C., Chandad F., Grenier D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. Journal of Antimicrobial Chemotherapy. 2006;58(2):439–443. doi: 10.1093/jac/dkl220. [DOI] [PubMed] [Google Scholar]
- 201.Bodet C., Chandad F., Grenier D. Inhibition of host extracellular matrix destructive enzyme production and activity by a high-molecular-weight cranberry fraction. Journal of Periodontal Research. 2007;42(2):159–168. doi: 10.1111/j.1600-0765.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 202.La V. D., Howell A. B., Grenier D. Cranberry proanthocyanidins inhibit MMP production and activity. Journal of Dental Research. 2009;88(7):627–632. doi: 10.1177/0022034509339487. [DOI] [PubMed] [Google Scholar]