Abstract
Background:
It has been an established fact that diabetes mellitus (DM) is associated with lower levels of cognitive function and may be a risk factor for the development of mild cognitive impairment (MCI) and dementia. Most of these studies involved elderly diabetes patients and aging itself may contribute to cognitive impairment. Since a majority of the individuals with DM are between the ages of 40 and 59 years, it is crucial to determine the factors that contribute to cognitive impairment in these patients. So this study was done to correlate the various physical and metabolic parameters with MCI in young individuals with type 1 DM.
Materials and Methods:
In this cross-sectional study, 126 patients with type 1 DM underwent cognitive assessment by the Montreal Cognitive Assessment test and their cognitive levels were correlated with their HbA1c, lipid profile, and high-sensitivity C-reactive protein (hs-CRP).
Results:
The prevalence of MCI was 71.42%. MCI was significantly correlated with HbA1c, serum triglycerides, low-density lipoprotein, very low-density lipoprotein, and hs-CRP levels. The factors that were statistically insignificant were the duration of diabetes, body mass index, and high-density lipoprotein levels.
Conclusion:
Cognitive impairment is seen even in type 1 DM patients. It should be considered along with the other complications of DM.
Keywords: High-sensitivity C-reactive protein, mild cognitive impairment, montreal cognitive assessment, type 1 diabetes mellitus
INTRODUCTION
The prevalence of diabetes mellitus (DM) is rapidly increasing and it has become a major health problem in recent years in the world. It has been a known fact that diabetes is associated with lower levels of cognitive function and may be a risk factor for the development of mild cognitive impairment (MCI) and dementia.[1,2] It is reported that a diagnosis of diabetes increased the odds of cognitive decline 1.2-fold and future dementia 1.6-fold.[3] MCI, also known as incipient dementia or isolated memory impairment is a brain function syndrome involving the onset and evolution of cognitive impairments beyond those expected based on the age and education of the individual, but which are not significant enough to interfere with their daily activities.[4] It is often found to be a transitional stage between normal aging and dementia.
Although, the exact pathophysiology of cognitive impairment in diabetes is unclear, hyperglycemia, vascular disease, hypoglycemia, insulin resistance, amyloidosis, concomitant hypertension, and depression play significant roles.[4,5,6,7,8] Much research has been done on cognitive dysfunction in patients with type 1 DM (T1DM) and type 2 DM (T2DM), especially more in T2DM patients. Most of these studies involved elderly diabetes patients and aging itself may contribute to cognitive impairment. Since a majority of the individuals with DM are between the ages of 40 and 59 years,[9] it is crucial to determine whether the factors described above contribute to cognitive impairment in young individuals with DM as well. As for T1DM, which is less common and has an onset in childhood, information relating to cognitive function is relatively scanty.
This study was designed to examine the associations between diabetes control, dyslipidemia, chronic inflammation, and cognitive function of young T1DM patients.
MATERIALS AND METHODS
This cross-sectional study included young patients with T1DM attending medical outpatient department and those admitted to the hospital from January 2017 to December 2017. All the subjects included in the study were interviewed regarding age, gender, education level, duration, type of diabetes, history of smoking, history of alcohol abuse, sleep status (sleepless or not), history of hypertension, and dyslipidemia using a predesigned and pretested proforma. Medication history regarding the use of lipid-lowering medications, antidiabetes medications, antihypertensive medications, antiplatelet medications, or any drug causing cognitive impairment recorded through questionnaires and pill bottle review. Socioeconomic status was classified using the BG Prasad scale (updated for 2017).[10,11]
The patients between 15 to 59 years of age, who are either known or recently diagnosed to have T1DM (according to the ADA 2017 guidelines) and the patients willing to participate in the study were included.[12] The criteria for diagnosing the T1DM was fasting blood glucose ≥126 mg/dl or HbA1c ≥6.5% along with the positive test for the two autoantibodies of T1DM (GAD65, IAA, IA-2A, and ZnT-8).[12]
The exclusion criteria were age ≥60 years, T2DM patients, patients with established diagnosis of dementia due to any cause, patients who were seriously ill, patients who were on long-term corticosteroid therapy, patients on lipid-lowering medications, patients with thyroid disorder, patient with a history of cerebrovascular accidents, patients who were either known case of hypertension or recently diagnosed as hypertensive, patients with spine deformities, pregnant females/lactating females, patients on drugs like benzodiazepines, opiates, tricyclic antidepressants, corticosteroids, anticonvulsants, and so on that may cause cognitive impairment,[13,14] patients with chronic diseases like chronic liver disease, rheumatological disorders, chronic kidney disease, history of auditory disorders, and psychological disturbances which might interfere with the Montreal Cognitive Assessment (MoCA) test, history of alcohol or any drug abuse, and patients who were not co-operative.
After obtaining informed consent, the data were collected which included age, sex, duration, and relevant biochemical tests. For assessment of cognitive functions, MoCA (Hindi version 7.1) a rapid screening test for MCI was used.[15] It assesses different cognitive domains: attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation. The total possible score is 30 points; a score of 26 or above is considered normal.
Ethical clearance
The study was carried out in accordance with the declaration of Helsinki (2000) of the World Medical Association and approved by the institutional medical ethics committee. Informed consent was obtained from all participants.
Statistical analysis
The sample size was calculated by the formula Z2 × p × (1 − p)/d2, Z = standard normal variate (at 5% type 1 error it is 1.96), p = expected proportion, d = absolute error. The expected proportion was taken as 70% based on the previous study[16] and the absolute error was kept at 10%. The calculated sample size was 81.
The data obtained were tabulated on the Microsoft excel spreadsheet. Categorical data were expressed as rates, ratios, and percentages. Continuous data were expressed as the mean ± standard deviation (SD). Pearson's correlation coefficient (r) was used to assess the correlation between HbA1c, lipid profile, high-sensitive C-reactive protein (hs-CRP), and various domains of cognitive impairment. SPSS 17 trial version software was used for analysis.
RESULTS
A total of 383 patients with T1DM were screened and 126 patients were enrolled in the study after satisfying the inclusion and exclusion criteria. MCI was seen in 71.42% of patients.
Baseline characteristics
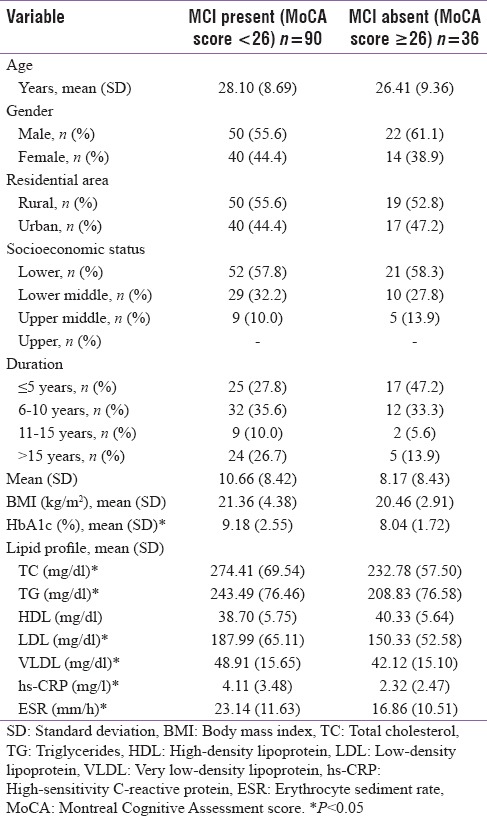
Most of the patients in this study were between 20 to 40 years of age (65.1%). The mean age group in patients with MCI was 28.10 years and without MCI was 26.41 years. Male patients (57.1%) were more in our study and they were represented more in both the groups. Gender did not have the significant impact on MCI. Patients from the rural area (54.8%) and low socioeconomic status (57.9%) were more in both the groups. The prevalence of MCI was also more in the patients from the rural area (72.5%) and low socioeconomic group (71.2%), but the observation was not significant.
Duration of T1DM and MCI
About 68.25% of patients had the disease of <10 years duration and the MCI was observed in 66.3% of patients in this group. Around 23% of patients had the disease of >15 years duration and 82.75% of the patients in this group had MCI. The mean duration of diabetes was also higher in patients with MCI and it was not significant.
Body mass index and MCI
About 19.8% of patients had body mass index (BMI) <18.5 kg/m2 and 68% of patients in this group had MCI. Only 11.1% had BMI ≥25 kg/m2 but the prevalence of MCI was 85.7% in this group. Although the mean BMI was higher in patients with MCI, it was not significant as 69% of patients had normal BMI [Table 1].
Table 1.
Baseline characteristics
Glycemic control and MCI
MCI with optimal glycemic control (HbA1c <7%) was observed in 11.1% of the patients and 61.3% had MCI without optimal glycemic control (HbA1c >7%). This observation was significant (P = 0.015). About 19% of the patients with HbA1c >7% had normal cognitive functions. HbA1c values correlated negatively with MoCA score (r = −0.387) [Table 2]. On correlating the components of MoCA test with HbA1c significant correlations were found with visuospatial/executive function (P < 0.001), delayed recall/memory (P < 0.001), and language (P < 0.05) [Figure 1].
Table 2.
Correlations coefficients of serum values of HbA1c, TG, LDL, VLDL, hs-CRP, MoCA score
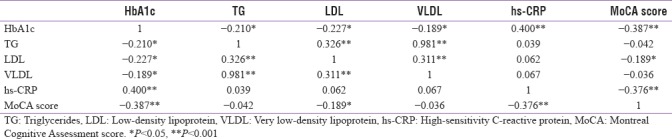
Figure 1.
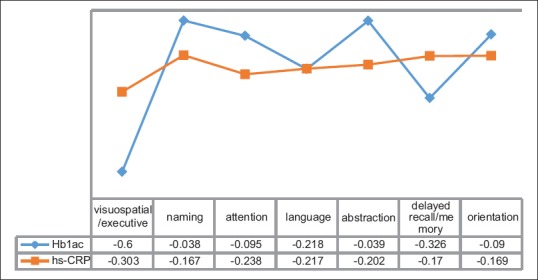
Correlation of different components of MoCA score with HbA1c and hs-CRP
Dyslipidemia and MCI
The prevalence of dyslipidemia [triglycerides (TG) >150 mg/dl] was 78.6% and the mean values were higher in patients with MCI. The observation was significant with total cholesterol (TC), TG, low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) levels. The significant correlations among the MoCA test were found with orientation (P < 0.001), delayed recall/memory (P < 0.001), naming (P < 0.05), and attention (P < 0.05) [Figure 2].
Figure 2.
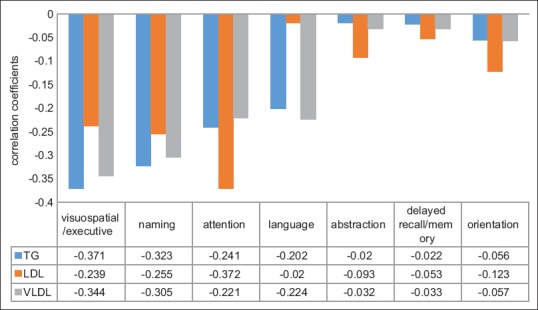
Correlation of different components of MoCA score with lipid profile
hs-CRP and MCI
In our study, 81.2% of patients with hs-CRP levels of >1 mg/l had MCI, while MCI was found in 43.3% of patients with hs-CRP levels of <1 mg/l. It was found that in patients with MCI the hs-CRP levels were higher and the observation was significant (P = 0.005). On correlating the hs-CRP values with components of MoCA test, significant correlations were found with visuospatial/executive functioning, attention, language, and abstraction (P < 0.05) [Figure 1].
We also found a significant correlation between MCI with erythrocyte sediment rate (ESR). Overall, delayed recall/memory was significantly affected, cognitive function affected in our study.
DISCUSSION
T1DM is also on the increase like T2DM, even though not in the same proportion, but still with a 3–5% increase/year. India accounts for most of the children with T1DM in South-East Asia.[9,17] Along with the focus on early identification of the microvascular and macrovascular complications in the T1DM, the screening for MCI should be considered because of the high prevalence in our study. The high prevalence of MCI may be due to the early onset of disease, more incidences of glycemic fluctuations and hypoglycemia due to insulin therapy, and the underestimated metabolic derangements in T1DM. The children with T1DM tend to perform less in cognitive and intellectual functions when compared to children with no diabetes.[18,19]
Age at the time of diagnosis, gender, residential area, and socioeconomic status had no significant effect on MCI in our study. It has also been observed that cognitive impairments appear in T1DM irrespective of their age. Studies have shown the appearance of MCI in TIDM patients who were diagnosed as early as 2 years of age, whereas some remain unaffected even at late stages of life.[20,21] A decline in verbal intelligence was seen in T1DM boys with worse glycemic control when compared to girls of similar ages.[22] The confounding factor of education was removed by employing MoCA test in our study. The residential area has shown not to influence the MCI in studies on both the T1DM and T2DM patients.[16,23,24] Socioeconomic status had no effect on MCI, may be due to the distribution of insulin at free of cost to T1DM patients in the community hospitals.
The mean duration of disease in patients with MCI was more when compared to patients without MCI, but the finding was statistically insignificant. The HbA1c values were higher in patients with MCI, which has a significant negative correlation with MoCA scores (r = −0.387). It suggests that rather than the duration of diabetes in T1DM, the degree of chronic hyperglycemia had more impact on cognitive functions.[20] Numerous underlying mechanisms of hyperglycemia that lead to MCI has been proven through multiple studies. Blood–brain barrier microvascular dysfunction may occur as a result of transient hyperglycemia.[25] Altered synthesis or reuptake of monoamine neurotransmitters as a result of altered precursor availability to the brain or changes in insulin availability to the brain are other possible explanations.[26,27] Complex effects on peptide neurotransmitters may be produced by uncontrolled diabetes.[28,29] Any one of these mechanisms alone may be insufficient, and several of these mechanisms could be additive.
The mean BMI was more in patients with MCI and it did not correlate with MCI in our study. Higher BMIs were associated with poorer cognitive performances in a linear way. Relationships between BMI and cognitive test scores have already been shown in a few previous studies such as the Framingham Study.[30]
In our study, we found that dyslipidemia contributed significantly to decrease in cognitive functions. The prevalence of dyslipidemia in T1DM was found to be >50% in large population-based studies.[31,32,33] Cognitive function has been reported to be significantly poorer in people with T2DM who have elevated levels of plasma TG[34,35] and in those with higher cholesterol levels,[34] but neither of these observations have been confirmed. TG, LDL, and VLDL levels had the weakly negative correlation with cognitive functions; whereas, HDL did not show the positive correlation with cognitive functions. The hyperglycemia may be a confounding factor for TG.
The mean hs-CRP and ESR were higher in patients with MCI and hs-CRP showed a negative correlation (r = −0.376) with MoCA scores. Inflammatory mediators may have a role in the accelerated development of cognitive impairment in individuals with DM either by a direct effect on the brain or through an influence on the development of vascular disease.[36] Two large cross-sectional studies (Edinburg study and GDMD study) has shown a significant association between chronic inflammation and MCI in T2DM.[36,37] This association has not been derived in T1DM, so further studies are required to confirm this association.
On comparing the components of MoCA test with the metabolic profile of the T1DM patients with MCI we found that delayed recall/memory, attention, visuospatial/executive function, and language were significantly affected. In a similar study done by Kumar et al., significant parameters affected were attention, followed by the delayed recall, naming, and abstraction.[16] The DCCT/EDIC study which was carried out in T1DM patients reported that motor speed and psychomotor efficiency were reduced more in patients with poor glycemic and metabolic control.[38,39]
There were few limitations in our study. It was conducted on a small sample size, which may not be a true representative of the whole population. Baseline cognitive functions were not available. All the participants were from the same health center and a selection bias could not be excluded.
CONCLUSION
Cognitive impairment is seen in early stages of T1DM. Poor glycemic control and associated metabolic derangements in T1DM are associated with cognitive impairment. The management of metabolic derangements from the early stage of diabetes may prevent the cognitive impairment and the risk of dementia. Individuals with T1DM should be screened for cognitive impairment along with other known macro- and microvascular complications of diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical approval
Approved by institutional ethical committee.
REFERENCES
- 1.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie K, Carriere I, Ritchie CW, Berr C, Artero S, Ancelin ML. Designing prevention programmes to reduce incidence of dementia: Prospective cohort study of modifiable risk factors. BMJ. 2010;341:1–9. doi: 10.1136/bmj.c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrabian S, Raycheva M, Gateva A, Todorova G, Angelova P, Traykova M, et al. Cognitive dysfunction profile and arterial stiffness in type 2 diabetes. J Neurol Sci. 2012;322:152–6. doi: 10.1016/j.jns.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: A cohort study. Ann Intern Med. 2014;161:785–93. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosway R, Strachan MW, Dougall A, Frier BM, Deary IJ. Cognitive function and information processing in type 2 diabetes. Diabet Med. 2001;18:803–10. doi: 10.1046/j.1464-5491.2001.00577.x. [DOI] [PubMed] [Google Scholar]
- 7.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–9. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 8.Biessels GJ, Staekenburg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurology. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 9.International Diabetes Federation. IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [PubMed] [Google Scholar]
- 10.Sharma R. Revision of Prasad's social classification and provision of an online tool for real time updating. South Asian J Cancer. 2013;2:157. doi: 10.4103/2278-330X.114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma R. Online interactive calculator for real-time update of the Prasad's social classification. [Last accessed on 2017 Jan 30]. Available from: http://www.prasadscaleupdate.weebly.com .
- 12.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl 1):S8–16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 13.Gray SL, Lai KV, Larson EB. Drug-induced cognition disorders in the elderly: Incidence, prevention, and management. Drug Saf. 1999;21:101–22. doi: 10.2165/00002018-199921020-00004. [DOI] [PubMed] [Google Scholar]
- 14.Moore AR, O’Keeffe TO. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15–28. doi: 10.2165/00002512-199915010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Nasreddine ZS. [Last accessed on 2016 Dec 15]. Available from: www.mocatest.org .
- 16.Kumar D, Singh VB, Meena B, Beniwal S, Singh K, Sidana S, et al. The spectrum of mild cognitive impairment in dyslipidemic nonelderly type 1 diabetics. Int J Res Med Sci. 2016;4:2246–51. [Google Scholar]
- 17.Das AK. Type 1 diabetes in India: Overall insights. Indian J Endocrinol Metab. 2015;19:S31–3. doi: 10.4103/2230-8210.155372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudieri PA, Chen R, Greer TF, Holmes CA. Cognitive function in children with type 1 diabetes: A meta-analysis. Diabetes Care. 2008;3:1892–7. doi: 10.2337/dc07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cato MA, Mauras N, Ambrosino J, Bondurant A, Conrad AL, Kollman C, et al. Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Soc. 2014;20:238–47. doi: 10.1017/S1355617713001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northam EA, Anderson PJ, Werther GA, Warne GL, Adler RG, Andrewes D. Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care. 1998;21:379–84. doi: 10.2337/diacare.21.3.379. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CM, Geckle MO. Circumscribed cognitive dysfunction in middle-aged adults with type 2 diabetes. Diabetes Care. 2000;23:1486–93. doi: 10.2337/diacare.23.10.1486. [DOI] [PubMed] [Google Scholar]
- 22.Schoenle EJ, Schoenle D, Molinari L, Largo RH. Impaired intellectual development in children with type 1 diabetes: Association with HbA1c, age at diagnosis and sex. Diabetologia. 2002;45:108–14. doi: 10.1007/s125-002-8250-6. [DOI] [PubMed] [Google Scholar]
- 23.Eze CO, Ezeokpo BC, Kalu UA, Onwuekwe IO. The prevalence of cognitive impairment amongst type 2 DM patients at Abakaliki South-East Nigeria. J Metab Syndr. 2015;4:171. [Google Scholar]
- 24.Kataria L, Pandya H, Shah S, Shah H, Gerg R. Prevalence and pattern of cognitive dysfunction in type 2 diabetes mellitus. Int J Med and Appl Sci. 2013;2:246–47. [Google Scholar]
- 25.Mooradian A. Central nervous system complications of DM: A perspective from the blood-brain barrier. Brain Res Brain Res Rev. 1997;23:210–8. doi: 10.1016/s0165-0173(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 26.Wurtman RJ. Effects of their nutrient precursors on the synthesis and release of serotonin, the catecholamines, and acetylcholine: Implications for behavioral disorders. Clin Neuropharmacol. 1988;11:S187–93. [PubMed] [Google Scholar]
- 27.Figlewicz DP, Brot MD, McCall AL, Szot P. Diabetes causes differential changes in CNS noradrenergic and dopaminergic neurons in the rat: A molecular study. Brain Res. 1996;736:54–60. doi: 10.1016/0006-8993(96)00727-5. [DOI] [PubMed] [Google Scholar]
- 28.Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44:147–51. doi: 10.2337/diab.44.2.147. [DOI] [PubMed] [Google Scholar]
- 29.Havel PJ, Hahn TM, Sindelar DK, Baskin DG, Dallman MF, Weigle DS, et al. Effects of streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expressions in rats. Diabetes. 2000;49:244–52. doi: 10.2337/diabetes.49.2.244. [DOI] [PubMed] [Google Scholar]
- 30.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: The Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–8. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 31.Idzior-Walus B, Mattock MB, Solnica B, Stevens L, Fuller JH. Factors associated with plasma lipids and lipoproteins in type 1 DM: The Eurodiab IDDM Complications Study. Diabet Med. 2001;18:786–96. doi: 10.1046/j.0742-3071.2001.00571.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim S-H, Jung I-A, Jeon YJ, Cho WK, Cho KS, Park SH, et al. Serum lipid profiles and glycemic control in adolescents and young adults with type 1 DM. Ann Pediatr Endocrinol Metab. 2014;19:191–6. doi: 10.6065/apem.2014.19.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawant, AM, Shetty D, Mankeshwar R, Ashavaid TF. Prevalence of dyslipidemia in young adult Indian population. J Assoc Physicians India. 2008;56:99–102. [PubMed] [Google Scholar]
- 34.Chen G, Cai L, Chen B, Liang J, Lin F, Li L, et al. Serum level of endogenous secretory receptor for advanced glycation end products and other factors in type 2 diabetic patients with mild cognitive impairment. Diabetes Care. 2011;34:2586–90. doi: 10.2337/dc11-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlmutter LC, Nathan DM, Goldfinger SH, Russo PA, Yates J, Larkin M. Triglyceride levels affect cognitive function in noninsulin-dependent diabetics. J Diabet Complications. 1988;2:2103. doi: 10.1016/s0891-6632(88)80011-4. [DOI] [PubMed] [Google Scholar]
- 36.Marioni RE, Strachan MWJ, Reynolds RM, Lowe GDO, Mitchell RJ, Fowkes FGR, et al. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes. Diabetes. 2010;59:710–3. doi: 10.2337/db09-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng T, Qin L, Chen B, Hu X, Zhang X, Liu Y et al. Association of plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: Results from the GDMD study in China. Diabetes Care. 2016;39:1594–601. doi: 10.2337/dc16-0316. [DOI] [PubMed] [Google Scholar]
- 38.The Diabetes Control and Complication Trial/Epidemiology of Diabetes Intervention and Complications (DCCT/EDIC) Study Research Group. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–52. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: An 18-year follow-up of diabetes control and complications trial (DCCT) cohort. Diabetologia. 2011;54:233–6. doi: 10.1007/s00125-010-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


