Abstract
Background:
Obesity is one of the most common yet neglected public health problems in both the developed and developing countries. Metabolic syndrome (MS) is a multiplex of risk factor for the development of type 2 diabetes (T2D) and cardiovascular disease (CVD) and it reflects the clustering of multiple risk factors resulting from obesity and insulin resistance. Despite its predominance in obese individuals, MS does occur in non-obese individuals. Many individuals characterised as normal weight as per their body mass index (BMI), have increased visceral adiposity thereby leading to an unfavourable inflammatory cytokine profile. There are limited studies from India with respect to inflammatory cytokines in obesity and MS in general and non-obese patients with MS in particular.
Materials and Methods:
An observational cross-sectional study was carried out in patients with MS with or without obesity. Anthropometric parameters such as height, weight and waist girth were measured and BMI was calculated. Serum levels of TNF-α, IL-6 and adiponectin were measured by using the enzyme-linked immunosorbent assay.
Results:
A significant proportion of individuals categorised as normal weight had an increased waist circumference which correlated with BMI, acanthosis nigricans (AN) and fatty liver. There was no statistically significant difference in the cytokine levels in obese and non-obese patients with MS; similarly among non-obese patients with MS, cytokine levels were comparable in patients with or without abdominal obesity. However, triglycerides inversely correlated with adiponectin levels and there was no significant correlation between the cytokines and other parameters of MS.
Conclusion:
There was no significant difference in various metabolic and inflammatory parameters between obese and non-obese patients with MS. Even in non-obese group, there were no differences in metabolic and inflammatory markers between individuals with or without abdominal obesity. This finding indicates that apart from adipose tissue, other factors are also responsible for the development of MS and its associated proinflammatory profile. There could be a significant contribution of genetic and epigenetic factors which needs to be further explored.
Keywords: Cytokines, inflammation, insulin resistance, metabolic syndrome, obesity
INTRODUCTION
Obesity is a serious metabolic disorder that predisposes an individual to multiple pathological conditions like diabetes, renal diseases, gastrointestinal disorders and cancer. According to the World Health Organization (WHO), obesity has more than doubled across nations since 1980. In 2014, >1.9 billion adults (18 years and older) were overweight, and of these over 600 million were obese.[1] Previously considered a problem only in high-income countries, obesity is now dramatically on the rise in low- and middle-income countries. India, a country with 1.2 billion people is currently experiencing rapid epidemiological transition. Undernutrition, due to poverty is being rapidly replaced by obesity associated with affluence.[2,3,4] As per the 2007 National Family Health Survey, 12.1% of men and 16% of women are overweight or obese.[5] With an increase in the prevalence of obesity, a parallel surge in obesity-related metabolic disorders like type 2 diabetes (T2D), cardiovascular disease (CVD), hypertension, dyslipidaemia and non-alcoholic fatty liver disease (NAFLD) are being observed.[6] Additionally, inflammation is presently considered a fundamental feature of obesity and T2D.[7] Individuals with normal body weight as per the body mass index (BMI) criteria but with a higher proportion of visceral fat have a proinflammatory cytokine profile similar to obese people.
Though metabolic syndrome (MS) is more common in obese individuals, it does occur in normal weight individuals as well. Studies of inflammatory markers in obese and non-obese people with MS have revealed varying results. The modest sample size of the study populations, and selective enrolment of women and older men are some of the reasons behind these diverse findings.[8,9] However, despite its apparent clinical significance, not many studies have assessed the relationship between inflammatory cytokines and various components of MS.[10,11,12,13] We therefore undertook the present study to analyse the inflammatory markers in obese and non-obese patients with MS and to assess the correlation between inflammatory cytokines and components of MS.
MATERIALS AND METHODS
Subject population
Present work is an observational cross-sectional study carried out at the Department of Endocrinology, in a tertiary care centre in north India between October 2014 and June 2016. The study was approved by the Institutional Ethical Committee (IEC) and an informed consent was obtained from all participants. A total of 76 individuals were included in the study, out of which 37 were obese (Group A) and 39 non-obese (Group B). Each recruited individual was interviewed and examined as per a predefined protocol. Only adult subjects (18 years or older) with MS with or without obesity were included in the study. Patients with chronic kidney disease, overt CVD, chronic liver disease, malignancy, acute or chronic inflammatory or infectious disease and autoimmune disorders were not included in the study. Furthermore, diabetic patients on insulin also were excluded from the study.
Anthropometry
Height and weight were measured as per the standard recommendations; BMI was calculated as weight in kg divided by height in m2, waist circumference (WC) was measured with the help of non-stretchable measurement tape at the level just above the iliac crest at the end of expiration and hip circumference was measured at the widest point.[14] An individual was considered obese if his/her BMI was >25 kg/m2.[15] For the diagnosis of MS, the National Cholesterol Education Program (NCEP-ATP III) criteria were used, namely three or more of the following: abdominal obesity (WC ≥102 cm in men and ≥88 cm in women), hypertriglyceridaemia (≥150 mg/dl), low high-density lipoprotein (HDL <40 mg/dl in men and <50 mg/dl in women), hypertension (BP >130/85 mm Hg or on treatment) and fasting glucose (>110 mg/dl).[16] Normal weight individuals with MS were further subdivided into two groups based on the presence of abdominal obesity (WC cut-off 90 cm for men and 80 cm for women).[17]
Biochemical and cytokine evaluation
After an overnight fast, a venous blood sample was drawn for investigations like haemogram, kidney and liver function, fasting blood glucose and lipid profile. Serum was separated within 1 h, and preserved at −80°C for the estimation of cytokines [tumour necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and adiponectin]. Plasma IL-6, TNF-α and adiponectin concentrations were measured with a high-sensitivity enzyme-linked immunosorbent assay (ELISA) for human TNF-α, IL-6 and adiponectin. ELISA for TNF-α was performed by the Diaclone TNF-α ELISA kit (Diaclone SAS, Besancon Cedex, France); the overall coefficient of variation was 3.3%. ELISA for IL-6 was performed by the Diaclone IL-6 ELISA kit (Diaclone SAS, Besancon Cedex, France); the overall coefficient of variation was 4.2%. ELISA for adiponectin was performed by the BioVender adiponectin kit (BioVender, Karasek, Czech Republic); the coefficient of variation was 3.9% for intra-assay and 6.3% for inter-assay.
Statistical analysis
The statistical software SPSS Version 20 (IBM SPSS Statistics for Windows, Version 20 Armonk, NY: IBM Corp) was used to analyse the data. The normality of the distribution of each variable was tested. Data were expressed as mean ± SD for normally distributed variables. Logarithmic transformations were applied to TNF-α and IL-6. The Pearson correlation coefficient was used to quantify the univariate associations between variables. The unpaired Students t-test was used to compare mean values between different groups. All the results have been described on 5% level of significance, i.e., P value <0.05 considered as significant.
RESULTS
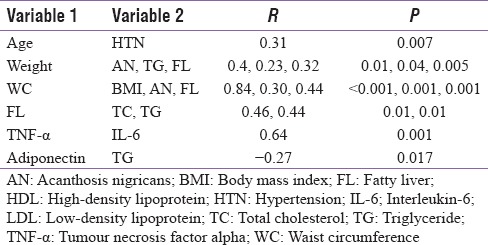
Seventy six patients (44 men and 32 women) with MS as per the NCEP-ATP III criteria were included in this study. These patients were further subdivided into obese and non-obese based on the BMI. Those having BMI of ≤25 kg/m2 were considered as non-obese, while those with BMI >25 kg/m2 were considered as obese. The lipid parameters and liver enzymes did not differ significantly in obese and non-obese patients, though fatty liver was commoner in former. There were no significant differences in the levels of TNF-α, IL-6 and adiponectin between obese and non-obese patients with MS [Table 1]. Results of cytokine analysis also revealed that levels of TNF-α, IL-6 and adiponectin were not different among non-obese patients with or without abdominal obesity [Table 2]. No significant correlation was found between the cytokine levels and different parameters of MS [Table 3].
Table 1.
Anthropometric, clinical and biochemical details of the subjects
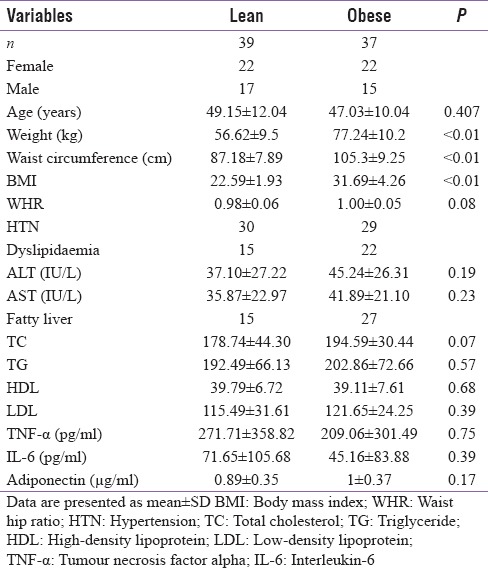
Table 2.
Metabolic and inflammatory parameters in patients with and without abdominal obesity in normal weight group
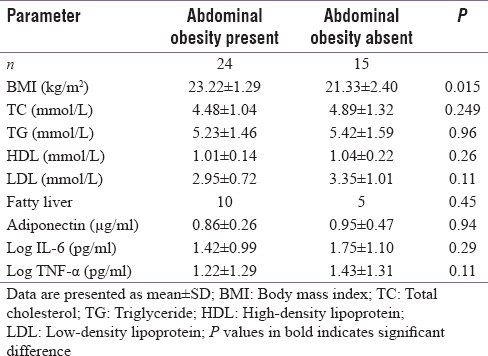
Table 3.
Correlation between different variables and cytokines of both groups (lean vs obese) of patients
DISCUSSION
MS represents clustering of multiple CVD risk factors within an individual. NCEP-ATP III criteria are commonly used for the diagnosis of MS because of their ease of use in clinical settings.[18] Though obesity is an important constituent of MS with clustering of cardiovascular risk factors, such risk factors are also seen in some normal weight individuals often referred to as ‘metabolically obese normal weight individuals’ (MONW).[19]
Chronic low-grade inflammation invariably accompanies MS and is associated with adverse proinflammatory cytokine profile both in serum and adipose tissue.[20] In the current study, the obese MS patients were more often female and were younger than the non-obese MS patients. This could be a referral bias that young people with obesity seek medical attention earlier. Moreover, in developing countries, young people are gaining weight because of changes in lifestyle and food habits.[21] The female preponderance in the current study could be a true reflection of increased prevalence of MS in women in India. A recent study from India demonstrated that 29% of women had MS as against 26% of men.[22] Data from the National Health and Nutrition Examination Survey (NHANES) also revealed a progressive rise in abdominal obesity in both men and women between 1988 and 1999 with overall prevalence of abdominal obesity progressively increasing in women from 46% in 1988 to 58% in 1999; despite normal body weight, abdominal obesity was present in 80% females and 47% males in the non-obese group.[23] A study from US revealed that among non-obese (BMI <25) individuals, 19.6% of women had increased WC, while <2% men had increased WC.[19]
In recent studies, WC has been documented as an important clinical risk parameter predicting the presence of obesity and fatty liver.[24,25] The present study also demonstrates a strong correlation between WC and BMI (r = 0.84, P < 0.001). Also we found a strong correlation between WC and fatty liver (r = 0.30, P < 0.001). Though fatty liver was prevalent in 72% of patients in obese group compared to 38% in non-obese group, in the latter group it was more often seen in those with abdominal obesity. Previous studies have shown that increase in WC can reliably predict the risk of NAFLD in obese adolescents.[26]
Acanthosis nigricans (AN), a reliable marker of insulin resistance (IR) and an independent risk factor for the development of diabetes mellitus, is commonly associated with MS.[27,28] Our results revealed that AN was more common in obese patients than normal weight individuals and it correlated with weight, BMI and WC.
Adverse cardiovascular risk factors like high, low-density lipoprotein (LDL) and low HDL were seen in both obese and non-obese patients with MS. Though half of our patients with MS were non-obese as per the BMI criteria, abdominal obesity was present in majority of these patients. There was no significant difference in lipid parameters like TC, TG, HDL and LDL between obese and non-obese group. Abdominal obesity has been found to have significant association with adverse lipid profile.[29] A recent study demonstrated that, in MONW people, dyslipidaemia was seen in 30%.[19] The adiposity associated with IR leads to an increased lipolysis causing increased free fatty acid delivery to liver resulting in fatty liver disease.[30] Our results also showed that elevated serum TG or free fatty acids strongly correlated with the presence of fatty liver.
TNF-α; a proinflammatory cytokine is elevated in various pathophysiological conditions in adipose tissues, if given exogenously, TNF-α causes IR.[31,32,33,34,35] Our study demonstrated a high prevalence of elevated TNF-α in patients with MS, though there was no statistically significant difference between obese and non-obese group There was no correlation between TNF-α and other components of MS, however, a strong correlation between TNF-α and another inflammatory marker IL-6 was seen (r = 0.654, P < 0.001).
In recent past, Phillips and Perry reported significantly higher levels of TNF-α in both obese and lean people with MS as compared with metabolically healthy individuals,[36] similar findings were reported in people with diabetes in whom the levels of TNF-α progressively increased with increase in IR.[37] In addition to obese women, inflammatory cytokine like TNF-α has been found to be increased in normal weight women with body fat in excess of 30% (a condition defined as normal weight obese) compared with normal weight controls.[38] However, Rajkovic et al., demonstrated high level of TNF-α in obese, overweight and lean people with diabetes, moreover, significantly higher levels were seen in obese/overweight patients compared with lean patients.[39]
Numerous studies reported increased levels of IL-6 in patients with MS and T2D. IL-6 is one of the earliest cytokines to be associated with IR, CVD and CV mortality, and it also plays an important role in hepatic IR.[40,41] The recently reported finding that visceral fat secretes more IL-6 than the subcutaneous fat may partly underlie with high (risk of) IR from abdominal obesity.[42] Our study showed IL-6 levels to be significantly higher in both obese and non-obese patients with MS. There was a significant correlation between TNF-α and IL-6 level (r = 0.654, P < 0.001). Levels of IL-6 were not significantly associated with any other component of MS.
Goyal et al. also found high levels of IL-6 in both lean and obese individuals with T2D compared with controls.[43] In agreement with our finding Phillips and Perry also found significantly higher IL-6 levels in metabolically unhealthy individuals irrespective of body weight.[36] Another study comprising a small cohort of elderly people, found higher IL-6 levels in obese patients with T2D compared with controls. Possibly a small sample size and advanced age of the population could have affected the results.[39] In a study of British population, level of IL-6 strongly correlated with body weight and WC.[8] Difference in ethnicity could be responsible for the variation from our results.
An anti-inflammatory cytokine adiponectin is an adipocyte-derived hormone which reduces IR in muscle and fat and improves insulin sensitivity when administered exogenously.[44,45] Adiponectin in the present study was significantly lower in patients with MS irrespective of weight and correlated negatively with clinical parameters like waist hip ratio and biochemical parameters like serum TG levels. Phillips and Perry also showed significantly decreased adiponectin levels in patients with MS irrespective of body weight as compared with controls.[36] Another study also revealed a significant decline in adiponectin in patients with T2D compared with controls and the difference decreased in patients with IR without T2D compared with controls.[46] In agreement with our study, no correlation was found between parameters of fatness and adiponectin level.[46] An inverse correlation was also found between adiponectin, TC and TG by Tamang et al.[47] Another study revealed a significant negative correlation between adiponectin and weight, BMI, fat mass, adverse lipid levels like TC and TG and a positive correlation with serum HDL levels.[9] The study consisted of a small sample size of patients with more pronounced obesity.
CONCLUSION
A significant proportion of individuals categorised as normal weight were found to have an increased WC which significantly correlated with BMI, AN and fatty liver. Despite differences in BMI and WC, there was no significant difference in various metabolic and inflammatory parameters between obese and non-obese patients with MS. Even in non-obese group, no difference in metabolic and inflammatory markers was observed between individuals with or without abdominal obesity. This finding indicates that apart from adipose tissue, other factors could also be responsible for the development of MS and its associated proinflammatory profile. There could be a significant contribution of genetic and epigenetic factors which needs to be further explored.
Financial support and sponsorship
The study was supported by financial support from Dean SKIMS as an academic grant.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are thankful to the Department of Immunology and Molecular Medicine for expert technical services.
REFERENCES
- 1.World Health Organization. Fact sheet: Obesity and overweight. [Last accessed on 2016 Dec 24]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Mohan V, Deepa R. Obesity & abdominal obesity in Asian Indians. Indian J Med Res. 2006;123:593–6. [PubMed] [Google Scholar]
- 3.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 4.Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47) Eur J Clin Nutr. 2009;63:259–67. doi: 10.1038/sj.ejcn.1602920. [DOI] [PubMed] [Google Scholar]
- 5.Kalra S, Unnikrishnan AG. Obesity in India: The weight of the nation. J Med Nutr Nutraceut. 2012;1:37–41. [Google Scholar]
- 6.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 7.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Whincup PH, Rumley A, Lowe GD. Inter-relationships of interleukin-6, cardiovascular risk factors and the metabolic syndrome among older men. J Thromb Haemost. 2007;5:1637–43. doi: 10.1111/j.1538-7836.2007.02643.x. [DOI] [PubMed] [Google Scholar]
- 9.Geloneze B, Pereira JA, Pareja JC, Lima MM, Lazarin MA, Souza IC, et al. Overcoming metabolic syndrome in severe obesity: Adiponectin as a marker of insulin sensitivity and HDL-cholesterol improvements after gastric bypass. Arq Bras Endocrinol Metabol. 2009;53:293–300. doi: 10.1590/s0004-27302009000200022. [DOI] [PubMed] [Google Scholar]
- 10.Pischon T, Hu FB, Rexrode KM, Girman CJ, Manson JE, Rimm EB. Inflammation, the metabolic syndrome, and risk of coronary heart disease in women and men. Atherosclerosis. 2008;197:392–9. doi: 10.1016/j.atherosclerosis.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KM, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, et al. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2007;75:235–40. doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs M, van Greevenbroek MM, van der Kallen CJ, Ferreira I, Blaak EE, Feskens EJ, et al. Low-grade inflammation can partly explain the association between the metabolic syndrome and either coronary artery disease or severity of peripheral arterial disease: The CODAM study. Eur J Clin Invest. 2009;39:437–44. doi: 10.1111/j.1365-2362.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- 13.Ingelsson E, Hulthe J, Lind L. Inflammatory markers in relation to insulin resistance and the metabolic syndrome. Eur J Clin Invest. 2008;38:502–9. doi: 10.1111/j.1365-2362.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 14.WHO (2011) Waist circumference and waist-Hip ratio: Report of a WHO expert consultation, Geneva. Technical report, World Health Organization. 2008 Dec;:8–11. [Google Scholar]
- 15.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. For consensus group, consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 16.Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.The IDF consensus worldwide definition of the metabolic syndrome. Part 1. Worldwide definition for use in clinical practice. [Last accessed on 2016 Dec 24]. Available from: http://www.idf.org .
- 18.Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: New definition of the metabolically obese, normal-weight individual. Diabetes Care. 2004;27:2222–8. doi: 10.2337/diacare.27.9.2222. [DOI] [PubMed] [Google Scholar]
- 20.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and T2D. Diabetes Res Clin Pract. 2014;105:141–50. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigh SH, Jain S. Prevalence of metabolic syndrome and gender differences. Bioinformation. 2012;8:613–6. doi: 10.6026/97320630008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pradhan AD. Sex differences in the metabolic syndrome: Implications for cardiovascular health in women. Clin Chem. 2014;60:44–52. doi: 10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]
- 24.Gierach M, Gierach J, Ewertowska M, Arndt A, Junik R. Correlation between body mass index and waist circumference in patients with metabolic syndrome. ISRN Endocrinol 2014. 2014:514589. doi: 10.1155/2014/514589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motamed N, Sohrabi M, Ajdarkosh H, Hemmasi G, Maadi M, Sayeedian FS, et al. Fatty liver index vs. waist circumference for predicting non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:3023–30. doi: 10.3748/wjg.v22.i10.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemente AP, Netto BD, de Carvalho-Ferreira JP, da Silveira Campos RM, de Piano Ganen A, Tock L, et al. Waist circumference as a marker for screening nonalcoholic fatty liver disease in obese adolescents. Rev Paul Pediatr. 2016;34:47–55. doi: 10.1016/j.rppede.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirschler V, Aranda C, Oneto A, Gonzalez C, Jadzinsky M. Is acanthosisnigricans a marker of insulin resistance in obese children? Diabetes Care. 2002;25:2353. doi: 10.2337/diacare.25.12.2353. [DOI] [PubMed] [Google Scholar]
- 28.Ayaz T, BaydurŞahin S, Şahin OZ. Relation of Acanthosisnigricans to metabolic syndrome in overweight and obese women. Metab Syndr Relat Disord. 2014;12:320–3. doi: 10.1089/met.2013.0145. [DOI] [PubMed] [Google Scholar]
- 29.Paccaud F, Schlüter-Fasmeyer V, Wietlisbach V, Bovet P. Dyslipidemia and abdominal obesity: An assessment in three general populations. J Clin Epidemiol. 2000;53:393–400. doi: 10.1016/s0895-4356(99)00184-5. [DOI] [PubMed] [Google Scholar]
- 30.Loria P, Carulli L, Bertolotti M, Lonardo A. Endocrine and liver interaction: The role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol. 2009;6:236–47. doi: 10.1038/nrgastro.2009.33. [DOI] [PubMed] [Google Scholar]
- 31.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111–16. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krogh-Madsen R, Plomgaard P, Møller K, Mittendorfer B, Pedersen BK. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab. 2006;291:E108–14. doi: 10.1152/ajpendo.00471.2005. [DOI] [PubMed] [Google Scholar]
- 33.Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, et al. Targeted disruption of the tumor necrosis factor-alpha gene: Metabolic consequences in obese and nonobese mice. Diabetes. 1997;46:1526–31. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- 34.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–9. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610–9. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 37.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–40. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 38.De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: Early inflammation? Am J Clin Nutr. 2007;85:40–5. doi: 10.1093/ajcn/85.1.40. [DOI] [PubMed] [Google Scholar]
- 39.Rajkovic N, Zamaklar M, Lalic K, Jotic A, Lukic L, Milicic T, et al. Relationship between obesity, adipocytokines and inflammatory markers in type 2 diabetes: Relevance for cardiovascular risk prevention. Int J Environ Res Public Health. 2014;1:4049–65. doi: 10.3390/ijerph110404049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller S, Martin S, Koenig W, Hanifi-Moghaddam P, Rathmann W, Haastert B, et al. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-α or its receptors. Diabetologia. 2002;45:805–12. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- 41.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: Association of acute phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 42.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 43.Goyal R, Faizy AF, Siddiqui SS, Singhai M. Evaluation of TNF-α and IL-6 levels in obese and non-obese diabetics: Pre- and postinsulin effects. N Am J Med Sci. 2012;4:180–4. doi: 10.4103/1947-2714.94944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erikson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 46.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–59. [PubMed] [Google Scholar]
- 47.Tamang HK, Timilsina U, Singh KP, Shrestha S, Pandey B, Basnet S, et al. Assessment of adiponectin level in obese and lean Nepalese population and its possible correlation with lipid profile: A cross-sectional study. Indian J Endocrinol Metab. 2013;17:S349–54. doi: 10.4103/2230-8210.119618. [DOI] [PMC free article] [PubMed] [Google Scholar]


