Abstract
Diabetes is a metabolic disorder characterized by hyperglycemia and is associated with several comorbidities and complications. Genital infection is one such complication that is often associated with diabetes mellitus (DM). Even though abnormalities in immune system, high urine glucose, and bladder dysfunction are important contributors for the increased risk of genitourinary symptoms, yet the possible role of pharmacologically induced glucosuria cannot be completely overlooked in such patients. There are various classes of medications to control blood glucose levels. A new therapeutic option to manage hyperglycemia is to increase renal glucose excretion by inhibiting sodium-glucose cotransporter-2 (SGLT2) glucose transport proteins. SGLT2 inhibitors (SGLT2i) represent a novel class of oral antidiabetic drugs which are associated with drug-induced glucosuria. Currently, canagliflozin, dapagliflozin, and empagliflozin are the three SGLT2i approved for therapy in Type 2 DM (T2DM). Safety studies with these three SGLT2i have reported events of mild-moderate genital infections in patients on SGLT2i therapy. However, most of the reported infections responded to standard treatment. Apart from SGLT2i, factors including personal hygiene, menopause, and circumcision might have a possible role in reported events of genital infections among T2DM patients on SGLT2i therapy. The present review identifies the occurrence of genital infections in diabetic patients on SGLT2i therapy, factors affecting the incidence of genital infections, and management strategies in patients with T2DM on SGLT2i therapy.
Keywords: Balanitis, balanoposthitis, genital infection, glucosuria, SGLT2i, vulvovaginal candidiasis
INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disorder, characterized by hyperglycemia, resulting from an absolute or relative deficiency of insulin, its action or both. Complications of diabetes can be broadly classified into vascular and nonvascular. Infection is one of the important and frequently occurring nonvascular complications. Among many health challenges that a person with diabetes faces, infections have a profound impact in terms of morbidity and mortality. Infections precipitate metabolic derangements in diabetes, and conversely, the metabolic derangements facilitate infection. Patients with DM are at a two-fold higher risk of developing infections as compared with nondiabetic individuals.[1]
Patients with DM have increased risk of genital infections [Table 1]. Microbial flora causing genital infections are fungi, viruses, bacteria, and parasites. In diabetes, fungal genital infections are seen most frequently.
Table 1.
Genital infections commonly seen clinically
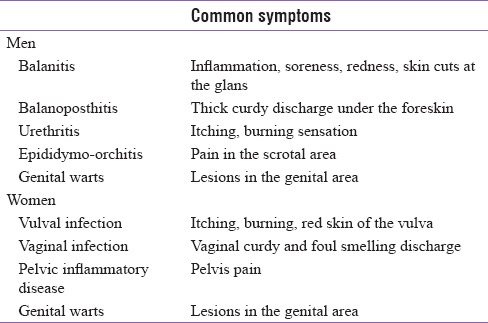
There is an overlap in the symptoms of genital and urinary infections. The present review aims to identify the occurrence of genital infections in diabetic patients and in patients on sodium-glucose cotransporter-2 inhibitors (SGLT2i) therapy, factors affecting the incidence of genital infections and discuss the various management strategies for genital infections in diabetic patients on SGLT2 inhibitor therapy.
PREVALENCE OF GENITAL INFECTIONS IN PATIENTS WITH DIABETES MELLITUS
The prevalence of genital infections in diabetes is similar in both the genders. Common mycotic genital infections found in diabetes patients are vulvovaginal infections in females and balanitis in males.[2] An Indian study identified vulvovaginal candidiasis (VVC) as the most common genital infection among women with diabetes.[3] Among the males, the incidence of Candida balanitis is reportedly higher, particularly in uncircumcised men with diabetes.[4] In a species-specific prevalence study of vaginal candidiasis among Indian women with diabetes, a vaginal swab of 36 cases was examined. The predominant Candida species isolated from study participants were Candida glabrata (39%), Candida albicans (26%), and Candida tropicalis (17%).[3] Globally, approximately 3% of uncircumcised men are affected with Candida balanoposthitis.[5] In uncircumcised males, the moist and warm space beneath the foreskin promotes the growth of the microorganisms.[4]
In the US Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes 2008 survey, it was found that women and men with Type 2 DM (T2DM) had 1.9 and 2.3 times higher chances of experiencing at least one episode of vaginitis and balanitis infection, respectively, compared with women and men without diabetes.[6] C. albicans is the most common pathogen causing balanitis in men, and C. glabrata is responsible for a maximum number of genital mycosis infections in women.[7,8]
WHY IS THERE INCREASED RISK OF GENITAL INFECTIONS IN PATIENTS WITH DIABETES?
Apart from hyperglycemia and glucosuria, decreased humoral and cellular immunity, more frequent need of medical interventions, are the factors which increase the susceptibility to infections in patients with diabetes.[1,9] Other predisposing factors contributing to genital infections include poor hygiene, topical corticosteroid use, pharmacologically induced glucosuria, pregnancy, estrogen, and oral contraceptive use.[7] The moist and warm environment of the genital area associated with hyperglycemia provides excellent growth conditions for various causative organisms, especially fungi responsible for genital infections.[8,10] Genital infections in diabetes are often not transmitted sexually and are of mycotic origin. However, the chances of sexually transmitted infections are also considerably high among the population with T2DM.[1,4]
In recent times, pharmacologically induced glucosuria is of vital clinical concern because it increases the chances of genital infections in diabetes. SGLT2i are one such class of novel oral antidiabetic therapeutic agents that have been under close scrutiny of clinicians and researchers for its anticipated side effects of increasing chances of genital infection in diabetes.[11] In females, a glucose-inducible protein, which is structurally and functionally similar to a complement receptor CD11b/CD18, a protein found on mammalian phagocytes, is produced by C. glabrata.[3] This protein enables the fungi to adhere strongly to the vaginal epithelium and multiply, thereby making C. glabrata as most common fungus causing vaginal candidiasis.[3]
GENITAL INFECTIONS AND SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITOR THERAPY
The SGLT2 inhibitors are responsible for pharmacologically induced urinary glucose concentration (renal glucosuria), thereby providing a favorable condition for pathogens to thrive which later results in genital infections [Table 4].[12] The mechanism of action of SGLT2 inhibitors is to prevent reabsorption of glucose by inhibiting SGLT2 protein present in proximal convoluted tubules of the kidney and facilitate its excretion in urine.[11] Currently, three SGLT2i, namely, canagliflozin, dapagliflozin, and empagliflozin, have been approved for pharmacological therapy of T2DM.
Table 4.
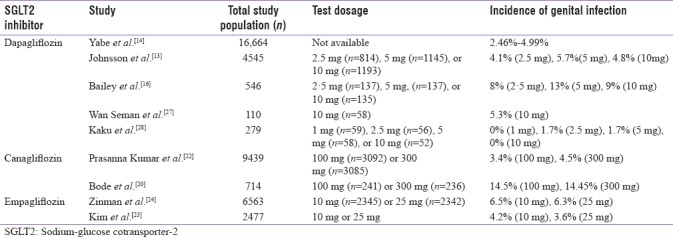
Prevalence of genital infection in Type 2 diabetes mellitus patients on sodium-glucose cotransporter-2 inhibitors therapy
Dapagliflozin
In a meta-analysis, safety data pooled from 12 placebo- controlled trials (n = 4545) of dapagliflozin demonstrated that when administered in doses of 2.5, 5, and 10 mg/day, the incidence of clinically diagnosed urogenital tract infection was 4.1%, 5.7%, and 4.8%, respectively [Table 1], compared to 0.9% in the placebo group.[13] Vulvovaginal mycotic infections, vaginal infections, and vulvovaginal pruritus were the most common infections seen in 8.5%–10.8% women receiving dapagliflozin versus 3.4% receiving placebo. Whereas among the males, the incidence of balanitis was low but more frequent in men who received dapagliflozin (1.0%–1.2%) compared with those receiving placebo (0.1%).[13] Furthermore, it was reported that most of the genital infections documented were mild to moderate in nature and responded to the standard antimicrobial treatment. Discontinuation of dapagliflozin was rare and no clear dose-response relationship between dapagliflozin and genital infections was observed. Similarly, another literature has reported that the incidence of genital infections and their progression can be significantly reduced by maintaining perineal hygiene and standard antifungal therapy and may rarely require discontinuation of SGLT2 inhibitor therapy.[14,15] A double-blind, parallel group, placebo-controlled study with 546 adults reported higher incidence of genital infections in the dapagliflozin group (2.5 mg, 11 patients [8%]; 5 mg, 18 [13%]; 10 mg, 12 [9%]) than in the placebo group (7 [5%]).[16]
Canagliflozin
Safety study with canagliflozin showed that canagliflozin when administered as 50 mg, 100 mg, 200 mg, 300 mg daily, or 300 mg twice daily for 12 weeks resulted in vaginal Candida colonization and symptomatic vulvovaginal adverse events in females.[17] However, the symptoms were resolved with standard antifungal therapy without interruption of SGLT2 inhibitor therapy.[17] Most of the safety studies with SGLT2 inhibitors showed that symptoms of genital infection usually appeared within the first 24–26 weeks of SGLT2 inhibitor treatment.[18,19] A single efficacy study in which patients received 100 and 300 mg of canagliflozin demonstrated early onset of genital infection symptoms in 14.5% and 14.4% patients, respectively.[20] However, pooled data from two recent randomized clinical trials with canagliflozin showed that the frequency of genital infections decreases over a period of time when patients were administered a standard treatment dose of 100–300 mg/day.[20,21] A high incidence of genital infections was reported within first 3 months of initiating therapy (females: canagliflozin 100 mg/day: 9.2%, canagliflozin 300 mg/day: 8.3%, control: 0.6%; males: canagliflozin 100 mg/day: 3.5%, canagliflozin 300 mg/day: 3.5%, control: 0.2%), which decreased gradually and was lowest after 21 months of therapy (females: canagliflozin 100 mg/day: 0.4%, canagliflozin 300 mg/day: 1.6%, control: 0.5%; males: canagliflozin 100 mg/day: 0.3%, canagliflozin 300 mg/day: 0.4%, control: 0.4%).[21] Data from the Indian subset of an efficacy and safety study of canagliflozin in T2DM patient (n = 1038) reported the incidence of genital infection only in canagliflozin group (1.2% [100 mg, n = 4] and 3.2% [300 mg, n = 11]) [Table 1], and no episode of genital infection was reported in placebo group.[22]
Empagliflozin
Safety data pooled from four randomized, placebo-controlled Phase III trials (n = 2477 patients) showed that patients on empagliflozin therapy had a higher incidence (empagliflozin 10 mg: 4.2%, empagliflozin 25 mg: 3.6%) of genital infections as compared with patients on placebo therapy (0.7%).[23] In addition, genital infections were more common in women (placebo: 1%, empagliflozin 10 mg: 6.3%, and empagliflozin 25 mg: 7.0%) than in men (placebo: 0.5%, empagliflozin 10 mg: 2.6%, and empagliflozin 25 mg: 1.1%). Most of the patients reporting these events had only one episode of genital infections, and only a few of them (empagliflozin 10 mg: 0.1%, empagliflozin 25 mg: 0.2%) discontinued empagliflozin therapy due to these events.[23] Another safety study with empagliflozin also reported a higher incidence of genital infection in empagliflozin group (6.4%, n = 4687) as compared with the placebo group (1.8%, n = 2333) [Table 1].[24]
GENITAL INFECTION IN SPECIAL POPULATIONS ON SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITOR THERAPY
Certain special populations including postmenopausal women and uncircumcised men with diabetes are anticipated to experience genital infections to a greater extent than other individuals. The prevalence of genital infections in postmenopausal diabetic women on SGLT2i therapy is still not clearly established due to scarcity of evidence.[25] However, it has been hypothesized that hormonal changes during and postmenopause are related to diminished immunity in the female reproductive tract, which increases the chances of having genital infections.[25] Similarly, circumcision in men is associated with maintenance of genital hygiene which is indirectly related to lower incidence of genital infection.[8] Pooled data from eight studies (n = 9439) with longer mean exposure (68 weeks of canagliflozin, 64 weeks of placebo) showed that the rate of male genital mycotic infection was more common in uncircumcised men (11%) than in circumcised men (3%).[26] A safety study which included 110 participants compared the safety of dapagliflozin with sulfonylurea during the fasting month of Ramadan and reported a lower risk of genital infections in the dapagliflozin group (5.3%) as compared with sulfonylurea group.[27] This possible reduction in the incidence of genital infection, particularly in males, could be related to higher rate of circumcision among the cases included in the Ramadan study.
Even though the literature is scarce, a few researchers claim that Japanese men with diabetes exhibit a considerably lower incidence of genital infections due to the maintenance of better hygiene. For instance, in an efficacy and safety study of dapagliflozin as a monotherapy in Japanese patients with T2DM (n = 279) who had inadequate glycemic control, only two patients (one case each in the dapagliflozin 2.5- and 5-mg groups) reported signs, symptoms, and other reports suggestive of genital infection.[28]
PREVENTION OF GENITAL INFECTION IN PATIENTS WITH DIABETES ON SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITOR THERAPY
Clinical evidence suggests that SGLT2 inhibitor induces glucosuria which leads to preferable conditions for pathogens to thrive and grow thereby resulting in genital infection. Proper counseling regarding pros and cons of SGLT2i therapy is essential for naïve patients for decreasing the risk of genitourinary infection [Figure 1].
Figure 1.
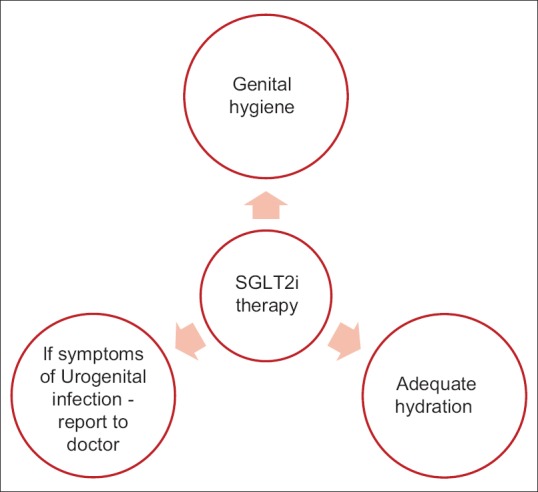
Overview of steps to prevent genital infections
Perineal hygiene
Patient education about perineal hygiene should be taken as a preemptive measure for all the diabetic patients irrespective of the antidiabetic therapy. This should include washing of genital organs followed by urination or defecation, routine hygienic wipes or sprays, women should be advised to wash from front-to-back, uncircumcised males should retract the prepuce before wash, use clean water for washing or mild soap if required, alcohol-based disinfectant should not be used for washing.[13]
TREATMENT OF GENITAL INFECTION IN PATIENTS ON SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITOR THERAPY
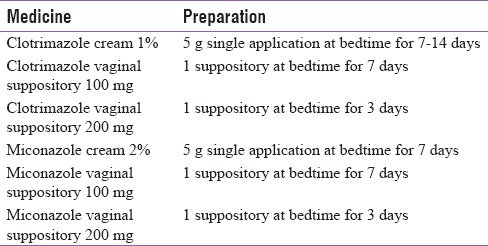
Although the chances of genital infections in patients with T2DM are considerably high, a better prognosis can be achieved with prompt treatment.[9,10] Safety and efficacy studies with SGLT2 inhibitors have shown that genital infections reported in the study population are often mild to moderate in severity and respond well to conventional therapy.[18,19] As per the Infectious Diseases Society of America, 2016, clinical practice guidelines for the management of candidiasis for the treatment of uncomplicated Candida vulvovaginitis, topical antifungal agents are recommended [Tables 2 and 3]. Whereas, for patients who have a recurrent infection, oral fluconazole 150 mg weekly for 6 months is recommended.[29] Recommendations by the Society of Obstetricians and Gynaecologists of Canada, 2015, recommended that symptomatic VVC should be treated with topical azoles. The topical antifungal agents comprise primarily of imidazoles such as miconazole and clotrimazole.[10] In addition, for effective management of recurrent VVC, initial therapy should be followed by weekly oral fluconazole for up to 6 months.[30,31]
Table 2.
Treatment for vulvovaginal candidiasis

Table 3.
Topical antifungal agents for vaginal candidiasis[31]
TREATMENT OF CANDIDA BALANITIS
Balanitis is usually associated with poor hygiene and hence frequent normal saline washes are recommended. Recommended regimen is clotrimazole cream 1% twice a day for 10 days. Miconazole cream 2% is also an alternative to clotrimazole cream. Relief is almost immediate, but treatment is to be continued for 10 days. Addition of topical steroids is not usually recommended in mild cases but where there is marked inflammation 1% hydrocortisone is used along with topical imidazoles. Topical therapy is usually sufficient in most of the cases of Candida balanitis.[32]
CONTINUATION OF SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITOR THERAPY AFTER AN EPISODE OF GENITAL INFECTION
The majority of the clinical trials with SGLT2 inhibitors have reported that discontinuation of therapy during an episode of genital infection is not related to better prognosis.[15,16,17,18] Genital infections in such patients are mild to moderate in nature which respond well to standard treatment, and do not require discontinuation of SGLT2 therapy.[15,16,17,18]
SUMMARY
Drug-induced glucosuria, decreased immune response, and altered microflora of the genital region are the main factors responsible for high incidence of genital infections in cases with diabetes. The anticipated association of SGLT2 inhibitors and increased risk of genital infection is a major cause of concern for diabetologists.
Genital infections following SGLT2 inhibitor therapy are mild to moderate in nature which respond well to the conventional therapy. Adequate counseling might help in preventing the risk of genital infections in patients with diabetes on SGLT2i treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
Hardik Vasnawala is employee of AstraZeneca Pharma India Ltd.
Acknowledgments
The authors acknowledge AstraZeneca Pharma India Ltd. and Turacoz, Healthcare Solutions for medical writing and editing support.
REFERENCES
- 1.Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, et al. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: Focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21(Suppl 2):S27–32. doi: 10.1016/j.cmi.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Gewirtzman A, Bobrick L, Conner K, Tyring SK. Epidemiology of sexually transmitted infections. In: Gross G, Trying SK, editors. Sexually Transmitted Infections and Sexually Transmitted Diseases. Springer-Verlag: Berlin Heidelberg; 2011. pp. 13–36. [Google Scholar]
- 3.Goswami R, Dadhwal V, Tejaswi S, Datta K, Paul A, Haricharan RN, et al. Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycaemic status. J Infect. 2000;41:162–6. doi: 10.1053/jinf.2000.0723. [DOI] [PubMed] [Google Scholar]
- 4.Nyirjesy P, Sobel JD. Genital mycotic infections in patients with diabetes. Postgrad Med. 2013;125:33–46. doi: 10.3810/pgm.2013.05.2650. [DOI] [PubMed] [Google Scholar]
- 5.Rajiah K, Veettil SK, Kumar S, Mathew EM. Study on various types of infections related to balanitis in circumcised or uncircumcised male and its causes, symptoms and management. Afr J Pharm Pharmacol. 2012;6:74–83. [Google Scholar]
- 6.Grandy S, Fox KM, Hardy E. Prevalence and recurrence of urinary tract and genital infections among adults with and without type 2 diabetes mellitus in the general population: A longitudinal cohort study. J Diabetes Res Clin Metab. 2013;2:5. [Google Scholar]
- 7.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–3. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 8.Shrivastav VK, Shukla D, Shrivastav A, Jana AM. Prevalence of vaginal candidiasis in diabetic women of Madhya Pradesh, India. Int J Curr Microbiol Appl Sci. 2015;4:834–46. [Google Scholar]
- 9.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: Impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103:373–81. doi: 10.1016/j.diabres.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–9. doi: 10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 12.Hirji I, Andersson SW, Guo Z, Hammar N, Gomez-Caminero A. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications. 2012;26:501–5. doi: 10.1016/j.jdiacomp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:479–84. doi: 10.1016/j.jdiacomp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Yabe D, Nishikino R, Kaneko M, Iwasaki M, Seino Y. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: Considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf. 2015;14:795–800. doi: 10.1517/14740338.2015.1034105. [DOI] [PubMed] [Google Scholar]
- 15.Kalra S, Baruah MP, Sahay R. Medication counselling with sodium glucose transporter 2 inhibitor therapy. Indian J Endocrinol Metab. 2014;18:597–9. doi: 10.4103/2230-8210.139206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–33. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 17.Nyirjesy P, Zhao Y, Ways K, Usiskin K. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin. 2012;28:1173–8. doi: 10.1185/03007995.2012.697053. [DOI] [PubMed] [Google Scholar]
- 18.Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: A randomized double-blind placebo-controlled 102-week trial. Diabet Med. 2015;32:531–41. doi: 10.1111/dme.12624. [DOI] [PubMed] [Google Scholar]
- 19.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S Dapagliflozin Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: Efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–36. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 20.Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 21.Arakaki RF. Sodium-glucose cotransporter-2 inhibitors and genital and urinary tract infections in type 2 diabetes. Postgrad Med. 2016;128:409–17. doi: 10.1080/00325481.2016.1167570. [DOI] [PubMed] [Google Scholar]
- 22.Prasanna Kumar KM, Mohan V, Sethi B, Gandhi P, Bantwal G, Xie J, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus from India. Indian J Endocrinol Metab. 2016;20:372–80. doi: 10.4103/2230-8210.179996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim G, Gerich J, Salsali A, Hach T, Hantel S, Woerle HJ. Empagliflozin (EMPA) increases genital infections but not Urinary Tract Infections (UTIs) in pooled data from four pivotal phase III trials. Diabetologie und Stoffwechsel. 2014;9:140. [Google Scholar]
- 24.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh M, Rodriguez-Garcia M, Wira CR. The immune system in menopause: Pros and cons of hormone therapy. J Steroid Biochem Mol Biol. 2014;142:171–5. doi: 10.1016/j.jsbmb.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyirjesy P, Sobel J, Fung A, Gassmann-Meyer C, Ways K, Usiskin K. Genital mycotic infections with canagliflozin (CANA) in subjects with type 2 diabetes mellitus (T2DM) Diabetes. 2013;62(Suppl 1):A276. [Google Scholar]
- 27.Wan Seman WJ, Kori N, Rajoo S, Othman H, Mohd Noor N, Wahab NA, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab. 2016;18:628–32. doi: 10.1111/dom.12649. [DOI] [PubMed] [Google Scholar]
- 28.Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: A phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2013;15:432–40. doi: 10.1111/dom.12047. [DOI] [PubMed] [Google Scholar]
- 29.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Schalkwyk J, Yudin MH Infectious Disease Committee. Vulvovaginitis: Screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can. 2015;37:266–74. doi: 10.1016/S1701-2163(15)30316-9. [DOI] [PubMed] [Google Scholar]
- 31.Thompson J. 2nd ed. Cambridge, MA, USA: Elsevier Publications; 2010. MPH Chapter - Infectious vaginitis. Infectious Disease Secrets. [Google Scholar]
- 32.Edwards SK. European Branch of the International Union against Sexually Transmitted Infection and the European Office of the World Health Organization. European guideline for the management of balanoposthitis. Int J STD AIDS. 2001;12(Suppl 3):68–72. doi: 10.1258/0956462011923976. [DOI] [PubMed] [Google Scholar]


