Abstract
Liver malignancies, either primary tumours (mainly hepatocellular carcinoma and cholangiocarcinoma) or secondary hepatic metastases, are a major cause of death, with an increasing incidence. Among them, hepatocellular carcinoma (HCC) presents with a dark prognosis because of underlying liver diseases and an often late diagnosis. A curative surgical treatment can therefore only be proposed in 20 to 30% of the patients. However, new treatment options for intermediate to advanced stages, such as internal radionuclide therapy, seem particularly attractive. Transarterial radioembolization (TARE), which consists in the use of intra-arterial injection of a radiolabelled embolising agent, has led to very promising results. TARE with 90Y-loaded microspheres is now becoming an established procedure to treat liver tumours, with two commercially available products (namely, SIR-Sphere® and TheraSphere®). However, this technology remains expensive and is thus not available everywhere. The aim of this review is to describe TARE alternative technologies currently developed and investigated in clinical trials, with special emphasis on HCC.
1. Introduction
Primary tumours (with hepatocellular carcinoma accounting for 75-80% of them) or secondary hepatic metastases are a major cause of death, with an increasing incidence [1, 2]. Hepatocellular carcinoma (HCC) is the fifth cancer in terms of incidence and the second leading cause of cancer death for men worldwide [3]. The vast majority of cases occurs in Southeast Asia [4], China alone representing half of the worldwide cases and deaths, and an increasing incidence in Europe. The presence of a cirrhosis, associated with various possible etiologies (such as hepatitis viral infections, alcohol, and haemochromatosis), is the primary risk factor. 73.4% of HCC can be attributed to hepatitis infections [5, 6], but the nature of the underlying disease mainly depends on geographical region or ethnic group. For instance, in Asia, 70-80% of all HCCs are attributable to hepatitis B virus (HBV) [4, 7], with the notable exception of Japan, where hepatitis C (HCV) is the main factor [4]. In the Western world, the main causes of HCC are alcoholism and nonalcoholic steatohepatitis (NASH), linked with a dramatic rise in obesity and diabetes occurrence, with also a strong increase in chronic hepatitis C [8, 9].
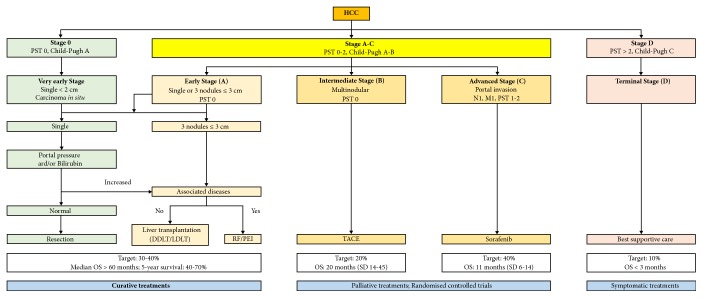
Despite the large range of treatment options developed over the years [10, 11], HCC prognosis remains dark, with a 5-year survival below 5%. The only really curative treatments are surgery (resection, transplantation) and ablative techniques (radiofrequency, cryotherapy, percutaneous alcohol injection). However, because of the underlying liver diseases (fibrosis, cirrhosis) and an often late diagnosis, a curative treatment can only be proposed in 20 to 30% of cases. Indeed, the presence of multiple foci, intraportal or extrahepatic metastases, and impaired hepatic functions are contraindications for liver transplantation or tumour resection. Additionally, even for such patients, recurrences are still likely to occur (up to 50% recurrence rate at 2 years for resection and, for transplantation, 10% within Milan criteria, but as high as 40% for patients outside Milan criteria) [12]. For nonoperable tumours, various palliative treatments may be proposed depending on the tumour staging. A large range of staging systems have been proposed [9, 13]. Most groups, especially in Europe, currently use the BCLC (Barcelona Clinic Liver Cancer), recommended by the EASL-EORTC (Figure 1). For intermediate stage HCCs, since external radiotherapy is of little use because of the high risk of hepatic toxicity, despite recent improvements in its efficacy and tolerance [14, 15], and systemic chemotherapy has not yet demonstrated effectiveness, with a low level of response without any improvement in survival [16], patients are generally proposed locoregional therapies [17]. For advanced HCCs, targeted treatment with sorafenib, and/or recently authorised regorafenib or other promising kinase inhibitors remains the only possibility [18, 19].
Figure 1.
BCLC staging system and therapeutic strategy according to EASL-EORTC guidelines. © European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. (Adapted from J Hepatol 2012; 56: 908-43.)
Due to both dual blood supply of the liver from the portal vein and the hepatic artery, and the sinusoidal cytoarchitecture of the liver parenchyma, the invasion of circulating tumour cells for establishing secondary hepatic metastatic foci is greatly favoured. 95% of hepatic malignancies are in fact secondary tumours [2]. So, for instance, liver metastases occur in approximately 50% of patients with colorectal carcinoma (CRC) [20], and in 44% of patients with neuroendocrine tumours (NET) [21]. Depending on volume and number of the metastases and histology of the original tumour, the median survival of patients with liver metastases ranges from 2 to 12 months [22]. To treat these intrahepatic metastases, surgery is considered the gold standard for CRC liver metastases [23] while its use remains controversial for metastases from non-colorectal primary tumours [21]. When surgery is not possible, because of the number of metastases, liver-directed therapies help in prolonging survival [17]. In the case of primary NET liver metastases, peptide-receptor radionuclide therapy (PRRT) with radiolabelled somatostatin analogues seems appealing [24–26].
Taking advantage of this dual blood supply and a rich vasculature, intra-arterially delivered treatments, such as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE), appear as really attractive treatment modalities. TACE, consisting in intra-arterial injection of an emulsion of Lipiodol and a chemotherapeutic drug, followed by the occlusion of the feeding artery, is currently considered as the standard of care for intermediate stage HCCs and some metastases [27], but its effectiveness remains a matter of debate. In this context, the development of a well-tolerated treatment modality appears particularly attractive. TARE, consisting in the intra-arterial delivery of a radioactive material to the tumour, limiting systemic irradiation and preserving the healthy liver to a maximum extent, appears to be a promising alternative to TACE [28–31]. Different materials (Lipiodol, glass, resin, or polymer microspheres) and radioisotopes have been used [32]. Only a few radionuclides have suitable characteristics for the treatment of tumours, among them are 32P, 90Y, 131I, 166Ho, 177Lu, and 186/188Re, all of which are β-emitters (Table 1). 90Y-loaded microspheres now have an established role and proved to be safe and effective in treating primary and secondary liver cancers, with favourable toxicity profile, with several tens of prospective or retrospective clinical studies completed or ongoing [15, 33–35]. There are, however, few Phase III studies, and published results are up to now disappointing [36, 37], but mostly because of suboptimal study designs or inadequate patient selection. Moreover, though this treatment modality has gained wide acceptance, it remains very expensive, especially for low-income countries, and is not available everywhere. Though not as common as TARE with 90Y-microspheres, TARE alternative methods are under clinical investigation, either in primary or in secondary liver cancers and will be presented here.
Table 1.
Radionuclides used for TARE.
| Radionuclide | t 1/2 (days) | E β (MeV) (%) | E γ (keV) (%) | Tissue penetration range (mm) | Production method |
|---|---|---|---|---|---|
| 32P | 14.3 | 1.71 (100) | / | 7.9 | Nuclear reactor |
|
| |||||
| 90Y | 2.7 | 2.284 (100) | / | 12 | 90Sr/90Y generator |
| Nuclear reactor for microspheres labelling | |||||
|
| |||||
| 131I | 8 | 0.81 (90) | 0.364 (81) | 2 | Nuclear reactor |
|
| |||||
| 166Ho | 1.1 | 1.84 (50.5) | 81 (6.4) | 8.7 | Nuclear reactor |
|
| |||||
| 177Lu | 6.7 | 0.497 (79) | 113 (6.4) | 2.2 | Nuclear ractor |
| 208 (11) | |||||
|
| |||||
| 186Re | 3.8 | 1.07 (72) | 137 (9) | 4.5 | Nuclear reactor |
|
| |||||
| 188Re | 0.7 | 2.118 (72) | 155 (15) | 11 | 188W/188Re generator |
| Nuclear reactor | |||||
Note: t1/2 (days), radioisotope half-life in days; Eβ (MeV) (%), maximum particle energy and respective decay abundance shown in parentheses; Eγ (KeV) (%), gamma ray energy useful for imaging and respective abundance in total energy emission shown in parentheses; tissue penetration range (mm), maximum tissue penetration shown in millimeters.
2. TARE with Radiolabelled Microspheres
The idea to utilise radiolabelled microspheres to treat tumours, liver being the first organ successfully treated, dates back to the 1960s [38]. First isotopes used were phosphorus-32 (Eβmax = 1.71 MeV; t1/2 = 14.3 d, max tissue penetration = 8 mm) and yttrium-90 (Eβmax = 2.28 MeV; t1/2 = 64.0 h, max tissue penetration = 12 mm), two pure β-emitters. It then fell out of use, before being revived in the 1980s until present day. There are currently two commercially available 90Y-labelled microspheres, based on different technologies [39]. One is based on glass microspheres (TheraSphere®, BTG International Ltd., London, United Kingdom); the other is made of ion-exchange resin (SIR-Sphere®, SIRTEX Medical Limited, North Sidney, New South Wales, Australia). Another TARE device, loaded with holmium-166 (Eβmax = 1.84 MeV; t1/2 = 26.8 h, max tissue penetration = 8.7 mm), has been recently made available (QuiremSpheres®, Quirem Medical BV, Utrecht, Netherlands) and is currently the subject of clinical trials in liver metastases. Besides these, numerous types of microspheres with various sizes and labelled with different isotopes have been developed and studied in humans [40].
2.1. Phosphorus-32 Microspheres
Phosphorus-32 was among the first isotopes used for therapeutic purposes, thanks to its suitable properties. In the middle of the 1960s, Caldarola et al. [41] used 32P-labelled resin microspheres for intra-arterial treatment of tumours. In 1979, Grady used 32P-CrPO4 colloids to treat intrahepatic metastases in a pilot study [38]. During the time of follow-up (2 years), 3 out of 4 patients were doing well, without significant side effects. However, with the rise of 90Y, 32P fell out of use [42], except in China where 44 patients with unresectable liver cancer were treated with moderate results with 32P-glass microspheres [43] and in Iran where 39 patients suffering from primary or secondary liver cancer were recently treated with intra-arterial injection of 32P-CrPO4 colloids [44]. This study was, however, based on imaging parameters and no result on the outcome of the patients is given.
2.2. Holmium-166 Microspheres
Compared with 90Y, 166Ho has the advantage of possessing a γ emission (81 keV) suitable for SPECT imaging. Moreover, holmium is highly paramagnetic, thus enabling MRI imaging and quantification [45, 46]. It has a 26.8 h half-life, resulting in a high dose-rate, and can be produced pure upon neutron bombardment, since natural holmium-165 has a 100% abundance and a large cross section [47]. 166Ho-loaded microspheres have been prepared using synthetic polymers [48], natural polymers [49], phosphate [50], resin [51], or glass [52].
2.2.1. Glass Microspheres
Glass is relatively resistant to radiation damage and nontoxic. Glass microspheres can easily be spheroidized in uniform sizes and can generally be produced with minimal radionuclidic impurities [53]. Holmium doped (Ho2O3) aluminium silicate glasses were “melted in an alumina crucible at 1600°C in a crucible type electric furnace. The liquid was held at this temperature for 2 hours and stirred each 30 min by using a silica rod to assure the liquid homogenization and gas release”. Brown et al. [54] prepared 166Ho-loaded glass particles with a small size of 2-5 μm for direct injection into mammary carcinoma tumours in mice, which led to an effective deposition of intense β-radiation. It could therefore be an effective modality for use in localised internal radionuclide therapy. Yet, no further studies were reported. In 2009, Costa et al. reported the preparation of 20-50 μm diameter glass particles (considered to be the ideal particle size to reach the arterioles of the liver), loaded with a specific activity up to 224 GBq/g of microsphere, suitable for therapeutic applications [52]. Their reported microspheres however contained more than 20% of 177Lu, because of the presence of lutetium oxide in the initial material.
2.2.2. Resin Microspheres
Compared with glass microspheres, ion-exchange resin-based microspheres present with the advantage of a lower density and commercial availability. Schubiger investigated different resins with 90Y and found that Bio-Rex 70 (Bio-Rad Inc, Hercules CA, USA) was the most suitable one [55]. Bio-Rex resin is made from acrylic polymer, with carboxylic groups used to bind the radiometal. Subramanian et al. recently investigated the feasibility of using Bio-Rex 70 with 166Ho [56]. 166Ho-labeled microspheres were obtained in high-yield (94.53% at pH 8.5), demonstrated high in vivo stability and showed very good retention in the liver (94.94 ± 1.51% at 72 h pi.). Besides Bio-Rex resins, Aminex resins (Bio-Rad Inc, Hercules CA, USA) were also explored. Aminex A-5, based on styrene divinylbenzene copolymer with sulphonic acid functional groups, was used [51]. Biodistribution in pigs demonstrated a reproducible fixation in the liver and dosimetry study, based on SPECT, allowed determining the required radiation absorbed dose to the liver to be 25 Gy to reach a therapeutic activity.
2.2.3. Polymer Microspheres
Polymer-based microspheres present with the advantage of near-plasma density, biodegradability, and biocompatibility [48]. However, this material is not able to withstand high thermal neutron fluxes [57, 58]. To circumvent this problem, microspheres can be loaded with holmium after neutron irradiation, like Suzuki et al. with chitosan microspheres [59] or Zielhuis et al. with alginate microspheres [49]. An alternative is to use additives and to select carefully the irradiation parameters (like avoiding any traces of water, < 1h irradiation in a high-flux reactor) [47, 60]. Another important parameter is the stability of the holmium-166 inside the polymer matrix. Mumper et al. showed that β-diketone chelates and particularly acetylacetonate (AcAc) formed strong complexes with holmium and that it moreover had a strong melting point amenable to withstand the heat inside the reactor without melting [61]. He thus developed poly-(L-lactic acid) (PLA or sometimes PLLA) microspheres for the prospective treatment of liver tumours, prepared by a simple solvent evaporation technique [62]. This method was soon used and furthered by a team from Utrecht [60]. Briefly, [165Ho]-AcAc complex and PLA are dissolved in chloroform, a volatile and water immiscible solvent, and subsequently added to polyvinyl alcohol aqueous solution. The resulting solution is emulsified by stirring, thus forming small organic droplets until the organic solvent evaporates. As chloroform evaporates, the droplets harden, forming microspheres, which can be collected through filtration. Microsphere size can be tuned through speed of stirring. The resulting microspheres, after drying, are then irradiated into a nuclear reactor to yield the desired 166Ho-loaded microspheres. Chloroform is a toxic solvent and should be avoided in patients. It has however been demonstrated that irradiation resulted in the complete removal of chloroform from the microspheres [63]. Holmium-loaded PLA-microspheres produced by this method (Figure 2) have been extensively characterised [47, 58, 64, 65] and are now produced under Good Manufacturing Practices [66]. Biodistribution studies demonstrated an effective tumour targeting in rats, with a 6.1 ± 2.9 tumour-to-liver ratio at one day after administration [67]. Animal studies have also established a low toxicity profile in rats followed up to 18 months, with only slight chronic inflammation, and no release of holmium load, as assessed with MRI [68]. They also demonstrated that correct administration of the microspheres was a critical step [69] and that it was feasible to quantify the dose through MR imaging [70, 71] and to predict dosimetry with the use of a scout dose of 166Ho-PLLA-microspheres [72].
Figure 2.

Scanning-electron microscope image of Holmium-PLA microspheres (from [73]).
A phase I trial for the treatment of unresectable, chemorefractory, liver metastases was consequently initiated [73]. 15 patients were treated with escalating aimed whole-liver absorbed doses of 20, 40, 60, and 80 Gy. At 6 weeks, treatment response was 1 partial response (PR), 7 stabilised disease (SD), and 7 disease progression (DP). At 12 weeks, treatment response was 1 PR, 1 SD, and 13 DP. The only PR was obtained for a patient treated with 20 Gy to the liver. Results from this trial showed “166Ho-radioembolization is feasible and safe for the treatment of patients with liver metastases” and enables image-guided treatment [74]. Maximum tolerated dose was determined to be 60 Gy. MRI and SPECT-based dosimetry were compared and found to be equivalent, with overall T/N ratios ranging from 0.9 to 2.7 with SPECT and 1.1 to 3.1 with MRI [75]. Use of a scout dose 166Ho-microspheres to assess lung shunting and predict dosimetry was demonstrated to be more accurate than 99mTc-MAA imaging [76] and proven to be safe, as possible extrahepatic deposition of the microspheres was estimated to have a low incidence of 1.3% [77]. Based on these promising results, a phase II study was launched, with a fixed aimed whole-liver dose of 60 Gy (or 3.8 GBq/kg of liver tissue, including the scout dose), in patients with liver metastases refractory to systemic therapy and ineligible for surgical resection (38 patients were included, one of whom was not evaluable). Besides 166Ho-scout dose to calculate dosimetry, a scout dose of 99mTc-MAA was administered to assess the lung shunts prior to treatment. In the recently published results [78], the target lesions showed complete response, partial response, or stabilised disease at 3 months for 27 patients (95% confidence interval [CI], 57% – 85%). The median overall survival was 14.5 months (95% CI, 8.6 – 22.8 months). The toxicity profile was acceptable. Compared to 90Y-microspheres TARE, the presence of γ-emission does increase radiation exposure to some extent, but only patients treated with more than 7 GBq of 166Ho and released within 6 h after treatment (instead of 24 h) would require contact restrictions. In all other cases, patients can be released without contact restrictions [79]. This study thus demonstrated clinical efficacy and further studies should be undergone. Another clinical study is currently ongoing, comparing a newly developed anti-reflux catheter to standard microcatheter for radioembolization of patients with liver-dominant colorectal metastases [80].
In parallel to the development of 166Ho-PLLA-microspheres for liver metastases, 166Ho-chitosan microspheres were investigated for HCC treatment [59]. Chitosan is a chitin derivative which can chelate metals and solidifies at alkaline pH. A phase IIb trial evaluated percutaneous injection of this device in 40 patients with small HCC [81], while another phase II trial investigated transarterial injection in 54 patients with a single large HCC [82]. Both studies reported good results, with survival rates at 1, 2, and 3 years of 87.2%, 71.8%, and 65.3%, respectively, in the first study and tumour necrosis achieved in 77.5% of patients with HCC < 3cm and 91.7% of patients with HCC < 2 cm. In the second study, where tumour size ranged from 3 to 13 cm, response rate was 78%, with 31 patients having a complete response for a 27-month median duration. Two treatment-related deaths were reported. Authors nonetheless found the toxicity to be acceptable, but patients should be carefully selected. When categorising patients according to their tumour size, it was found that patients with tumours of an intermediate size (3–5 cm) were the optimal candidates for this treatment. Phases III trials are therefore justified.
2.3. Rhenium-188 Microspheres
Rhenium-188 (Eβmax = 2.12 MeV; Eγ = 155 keV (15%); t1/2 = 16.9 h, max tissue penetration = 11 mm), available through a 188W/188Re generator, offers cost-effective convenient on-site availability, short half-life, energetic β-particle, and emission of γ-photons suitable for imaging [83, 84]. It is thus a promising isotope for radionuclide therapy, especially treatment of liver malignancies. Various particle materials have been considered for 188Re-microspheres preparation [85], but, so far, only albumin microspheres have been investigated in human.
2.3.1. Glass Microspheres
Chemically durable yttrium alumino–silicate (YAS) microspheres, incorporating more than 50 wt% of rhenium oxide (ReO2), have been prepared and neutron-irradiated in high-flux nuclear reactor [86]. These microspheres were investigated in hepatoma bearing rats, following intra-arterial injection, where they proved to be safe and effective in diminishing tumour growth [87]. However, low-cost and highly available natural rhenium consists of two neutron activatable radioisotopes (185Re and 187Re, both having large cross-sections for neutrons, yielding therapeutic amounts within a few h), thus producing a mixture of 186Re and 188Re, both β- and γ-emitting radionuclides, with different energies and different half-lives (cf. Table 1). The major disadvantage is that dosimetric calculations are complicated because of the two radioisotopes that must be considered. The alternative would be to use enriched 187Re, but at a much higher cost.
2.3.2. Resin Microspheres
There is only one example in the literature of resin-based microspheres loaded with 188Re [88]. Aminex A-27 resin (Bio-Rad Inc, Hercules CA, USA) was labelled by “adding [188Re]-perrhenate and SnCl2 to vacuum-dried resin particles. The mixture was boiled and centrifuged and microspheres were separated and resuspended in saline”. They were tested in hepatoma-bearing rats by direct intra-tumoural injection. “Survival over 60 days was significantly better in the treated versus control group (80% versus 27%)”. The same team compared this method with percutaneous ethanol injection in VX2 rabbit model [89]. Mean survival was 38.8 ± 6.2 days for the control group (rabbits treated with saline injection), 55.8 ± 11.8 days for the percutaneous ethanol injection group, and 68 ± 9.8 days for the rabbits treated with 188Re-microspheres. They thus concluded intra-tumoural injection of 188Re-microspheres could be an alternative to percutaneous ethanol injection and to intra-arterial injection.
2.3.3. Polymer Microspheres
Diverse natural and synthetic polymers have been tested to prepare 188Re-microspheres [85]. Poly(L-lactic acid) was mixed with jet-milled rhenium powder and the microspheres were prepared with the solvent evaporation similar to the method described above with holmium. 22 μm microspheres were neutron-activated for 3 h to give 188Re-PLA-microspheres [90]. Unfortunately, these biodegradable microspheres were damaged by the high neutron fluxes needed to achieve sufficiently high specific activity required for the treatment of liver tumours. This strategy was therefore abandoned. An alternative way to load PLA microspheres is to encapsulate a 188Re-labelled chelate inside the polymeric microspheres. Shukla et al. [91] encapsulated 188Re-DMSA, with a low 20-30% yield, while Yu et al. [92] derivatised PLA with a poly(histidine) tag to attach the [188Re(CO)3(H2O)3]+ complex directly onto the polymer chains. They thus reported a radiolabelling efficiency of 92%. Recently, encapsulation of 188Re-sulfur colloids in PLA microspheres by an oil in‐water (O/W) emulsion solvent extraction procedure was reported [93]. The 13-48 μm particles were labelled with over 99% efficiency and were injected intravenously into mice, showing high lung uptake. 188Re-colloids were also encapsulated in poly(L-lactide-co-glycolide) (PLGA) microparticles, along with doxorubicin, and used for transarterial chemoradioembolization in F344 rats [94]. 188Re/DOX@MS showed stronger tumour inhibition than 188Re@MS and DOX@MS alone.
Biodegradable starch microspheres have been proposed as an embolization agent to protect healthy liver tissue during TARE with 90Y-microspheres [95]. Verger et al. [96] proposed to chemically modify starch so that it could chelate 188Re and developed a cold-kit labelling method to prepare 188Re-starch-based microspheres with > 95% labelling yield. When injected in DENA-induced rats, the activity was essentially located in the tumorous parts of the liver. In the same way, human serum albumin (HSA) labelled with technetium-99m has been used for years for lung perfusion imaging, and 99mTc-labelled macroaggregates of albumin (MAA) are the current method of choice to assess lung shunts and predict 90Y-microspheres dosimetry [97]. In 2000, Wunderlich et al. proposed to label HSA with 188Re [98]. They found that HSA B20 (ROTOP Pharmaka GmbH, Dresden, Germany) gave uniform microparticles with a mean 25 μm diameter and were easily and stably labelled with generator-produced 188ReO4Na in the presence of tin chloride and HSA. They also demonstrated high in vivo stability, with a preferential lung uptake when injected intravenously in rats. A cold kit method was subsequently developed [99]. The proposed kit, consisting of three vials, enabled an almost quantitative labelling yield and minimum handling. The preparation was recently further optimised by Chen et al., using microwave heating, thus shortening the reaction time from 1 h to 3 min [100]. Tumour volume in the normal saline treated group was 1803.2 mm3 at 54 days after tumour inoculation, while tumour volumes in the 103.6 MBq and 240.5 MBq of 188Re-HSA microspheres treated groups were 381 and 267.4 mm3 (P = 0.001 and 0.004), respectively, demonstrating the high therapeutic efficacy of this modality.
188Re-HSA microspheres have been investigated in a feasibility study in 10 patients with HCC (3) or colorectal liver metastases (7) [101]. Patients were given0 13.6 ± 4.7 GBq of 188Re-microspheres selectively in the feeding artery of the tumour. The urinary excretion rate was low (8.9 ± 3.8%IA within 96 h). Response was assessed on CT: 2 patients had PR, 5 patients had SD, and 3 patients had DP. The treatment was well tolerated, with an acceptable rate of toxicity (30% of grades I and II, and 10% of grade III toxicity), which was reversible in most patients within 14 days after treatment. This treatment could thus represent a therapeutic option in these patients, but it is not possible to draw any firm conclusions, because of the small size and heterogeneity of the patient group. A larger patient cohort is necessary. A second study using 188Re-HSA microspheres was described by Nowicki et al. [102]. This study included 13 patients with progressive primary or secondary liver tumours. They received 3.8–12.4 GBq. The urinary excretion rate was low (6.5 ± 2.3%IA within 48 h). Mean overall survival was 7.1 months, and progression free survival was 5.1 months. Treatment adverse events were at an acceptable level, with 4 patients having grade 3 toxicity, mostly due to cancer progression. This study confirms the results of the first study and 188Re-HSA microspheres deserve to be further investigated.
3. TARE with radiolabelled Lipiodol
The most used vector for intrahepatic administration is Lipiodol. Lipiodol is an ethyl ester of iodinated fatty acids derived from poppy seeds with a proportion of iodine of approximately 38% by weight (i.e., 475 mg/mL). This iodised oil was discovered in 1901 by Guerbet and is the first organic iodinated contrast agent used for X-ray, in lymphography. Nakakuma et al. have shown that Lipiodol is selectively captured by hepatoma and by certain hepatic metastases, of colonic, neuroendocrine and mammary origin [103]. Lipiodol has therefore been used for the detection of HCC, then also for vectorising chemotherapeutic substances. Intratumour retention time has also been shown to be significantly greater than retention time in healthy liver, with retention in tumour cells up to several months [104]. Replacement of cold iodine with iodine-131, by an exchange reaction on Lipiodol [105], makes it possible to obtain radiolabelled Lipiodol, 131I-Lipiodol, which was successfully used for the treatment of nonoperable HCC [106, 107]. Still, Iodine-131 possesses a high energy gamma emission and long half-life (Eβmax = 0.81 MeV; Eγ = 364 keV (81%); t1/2 = 8.02 d) requiring hospitalisation in a radioprotected room for several days, thus limiting its potential. Other therapeutic nuclides, such as Yttrium-90 [108] and Rhenium-188 [109], have been proposed to advantageously replace it. Radiolabelling and clinical application of Lipiodol with Rhenium-188 have been extensively reviewed elsewhere [110].
3.1. Lipiodol Radiolabelling
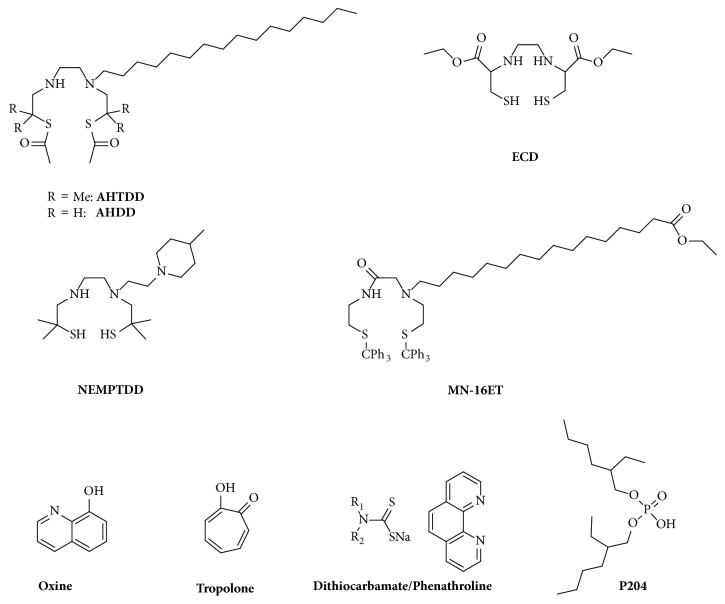
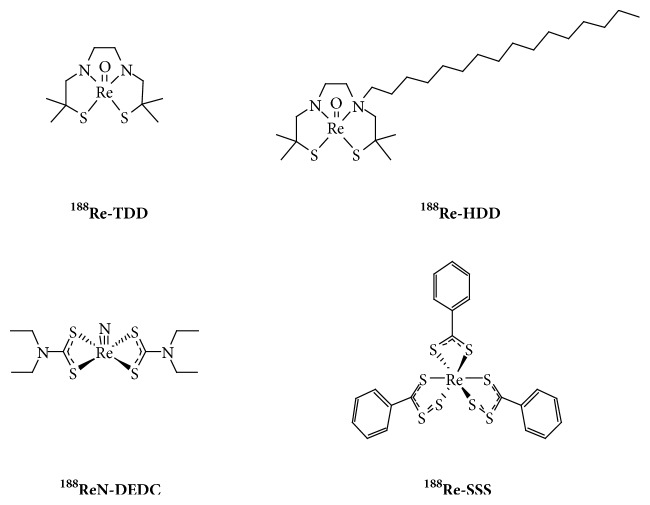
Initial trials to covalently label Lipiodol with 188Re and 90Y revealed unsuccessful [111–113]. Solubilisation of a lipophilic radiocomplex into Lipiodol was suggested as more suitable [114]. Several such 188Re-labelled complexes [115–127] were subsequently proposed (Figures 3 and 4).
Figure 3.
Ligands investigated with radiometals (188Re and 90Y/166Ho/177Lu) for Lipiodol labelling.
Figure 4.
188Re-chelates used to label Lipiodol and evaluated in human.
Lipiodol was also labelled with lipophilic chelates of yttrium-90 and radiolanthanides, although literature is scarce compared with rhenium-188. Oxine (8-hydroxyquinoline) forms a lipid-soluble complex with trivalent metals and has been used since the 1970s with indium-111 to label leukocytes [128]. Based on this, it has been proposed to label Lipiodol with yttrium-90 [129], holmium-166 [130], and lutetium-177 [131]. The procedure was however rather time-consuming and the resulting complex lacked sufficient stability, leading to undesirable bone uptake. Using the same analogy with 111In-labelled leukocytes, 90Y-tropolonate has been investigated to label Lipiodol [132]. The radiotracer showed increased stability compared to oxinate and high tumour uptake, but its stability was not satisfactory enough. Other compounds mentioned in the literature include dithiocarbamate/phenanthroline 90Y-complex [133] and di(2-ethylhexyl) orthophosphoric acid (P204), initially developed for solvent extraction of metals [134], with no biological data. Ligands used are summarised in Figure 3.
Since handling of high activities of beta-emitting therapeutic radionuclides can lead to a high radiation burden to the staff performing the preparations [135, 136], remote-controlled procedures were developed [137–139]. This led to a significant reduction of the received dose [140], in addition to a more GMP-compliant preparation.
3.2. Clinical Outcome
131I-labelled Lipiodol (Lipiocis®) dates back to the 1980s [141, 142]. Since then, many feasibility studies and a few Phase III trials have been carried out, essentially for the management of inoperable HCCs [107, 110, 143]. Three small randomised controlled trials were carried out for the palliative treatment of HCC with portal vein thrombosis (PVT) and without PVT, and adjuvant treatment after surgery, with respectively 129, 27, and 43 patients. Despite encouraging results, because of the suboptimal properties of 131I and a more than expected lung toxicity [144], Lipiocis® is not commercially available anymore in Europe. In-house labelled 131I-Lipiodol is however still under use in some countries, like for instance India [139]. To date, besides 131I-Lipiodol, only 188Re-labelled Lipiodol has been assessed in human. No 90Y- or radiolanthanide-labelled Lipiodol has been thus far investigated in clinics. Four 188Re-chelates have been evaluated to prepare clinical 188Re-labelled Lipiodol [110], i.e., 188Re-TDD [115], 188Re-HDD (now rebadged HTDD) [117], 188ReN-DEDC [125], and 188Re-SSS [126] (Figure 4). Except one nonconclusive case report with 188Re-TDD/Lipiodol [145], and two dose-escalation studies with 188ReN-DEDC/Lipiodol [125] and 188Re-SSS/Lipiodol [146], which both demonstrated promising preliminary outcomes, most clinical studies were carried out with 188Re-HDD/Lipiodol [147–158]. Despite high urinary excretion [150] and difficulties to obtain high activities because of low labelling yields [118], 188Re-HDD/Lipiodol demonstrated favourable responses and appeared to be well tolerated in dose escalation trials [148, 149], and in various feasibility studies in more advanced forms of the disease [151, 152], as well as in second-line therapy after relapse following a curative treatment [153, 154].
Following these encouraging results, an international IAEA-sponsored multicentric Phase II trial was launched [156–158]. 185 patients were included, receiving 1 to 4 treatment doses, with activities ranging from 0.78 to 13.45 GBq. The outcome of this study was 25% objective response (with 3% CR), 53% stabilised disease and 22% tumour progression, with a median follow-up of 455 days. One- and two-year survivals were, respectively, 46 and 23%. Tolerance was good. In view of these results, a larger randomised Phase III study appears to be required.
3.2.1. Other Therapeutic Applications
Lipiocis® had received marketing authorisation for the treatment of HCC with PVT. 131I-Lipiodol was however also investigated in other indications, such as in adjuvant and neoadjuvant settings, where it, respectively, led to an increase in overall and disease-free survivals [159] and to a decreased risk of recurrence before transplantation or resection [160, 161]. To date, only one preliminary study (with 5 patients) used 188Re-Lipiodol to stabilise patients awaiting liver transplantation, demonstrating its feasibility [162]. The advantage of 188Re-Lipiodol, compared to 131I-Lipiodol, is the shorter half-life of 188Re, making patients eligible for transplantation after 1 week, while they had to wait for 4 weeks when treated with 131I-Lipiodol. More patients are needed to be able to conclude on its potential use in this indication.
Besides HCC, liver can be home to other primary and secondary tumours, for which TACE with Lipiodol has shown its efficiency and tolerability [163, 164]. Limited studies using TARE with 131I-lipiodol have been published though. Contradictory results have been obtained in pilot studies with cholangiocarcinoma (CCA) [165, 166] and TARE is thus not recommended [167], while treatment of hepatic metastases could prove to be valuable [168, 169]. It would therefore be interesting to assess the potential interest of 188Re-Lipiodol in these indications, for which radiolabelled microspheres (with either 90Y, 166Ho, or 188Re) have proven their safety and efficacy.
4. Conclusions
Research on treatments for primary or secondary liver tumours has been particularly active, at the preclinical and clinical levels, following the successful introduction of 90Y-microspheres in the therapeutic armamentarium. Among the many methods to be investigated, 188Re-Lipiodol appears as a relatively economical, safe, and attractive alternative to 90Y-microspheres for the treatment of HCC. 166Ho-microspheres also look particularly appealing, thanks to the multimodal properties of holmium-166, and initial clinical findings, especially for liver metastases. Large randomised Phase III trials now need to take place, to confirm these encouraging Phase I/II results. In order to be able to define the place of TARE in treatment decision plans, comparisons with other existing treatment modalities, notably targeted therapies and newly developed immunotherapies, must be undertaken. It is most probable that future treatment strategies may rely on the combination with one or another of these therapies. Ultimately, choice of the TARE modality will be made based on local expertise and availability. Besides, many aspects of TARE still remain a challenge, such as optimisation of specific activity, determination of embolic distribution, microdosimetry, and personalised dosimetry (pre- and post-injection), as well as predictive response factors, requiring further clinical and preclinical studies.
Acknowledgments
This work was supported in part by Labex IRON [Grant no. ANR-11-LABX-0018].
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.London W. T., McGlynn K. A. Liver cancer. In: Schottenfeld D., Fraumeni J., editors. Cancer Epidemiology and Prevention. 3rd. New York, NY, USA: Oxford University Press; 2006. pp. 763–786. [Google Scholar]
- 2.Ahmed I., Lobo D. N. Malignant tumours of the liver. Surgery (Oxford) 2009;27(1):30–37. doi: 10.1016/j.mpsur.2008.12.005. [DOI] [Google Scholar]
- 3.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Zhu R. X., Seto W.-K., Lai C.-L., Yuen M.-F. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut and Liver. 2016;10(3):332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plummer M., de Martel C., Vignat J., Ferlay J., Bray F., Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. The Lancet Global Health. 2016;4(9):e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 6.de Martel C., Maucort-Boulch D., Plummer M., Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190–1200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharyya G. S., Govind Babu K., Malhotra H., Ranade A. A., Murshed S., Datta D. Hepatocellular carcinoma in India. Chinese Clinical Oncology. 2013;2(4) doi: 10.3978/j.issn.2304-3865.2013.09.05. [DOI] [PubMed] [Google Scholar]
- 8.Baffy G., Brunt E. M., Caldwell S. H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. Journal of Hepatology. 2012;56(6):1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Bertino G., Demma S., Ardiri A., et al. Hepatocellular carcinoma: Novel molecular targets in carcinogenesis for future therapies. BioMed Research International. 2014;2014 doi: 10.1155/2018/4560161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goutté N., Sogni P., Bendersky N., Barbare J. C., Falissard B., Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. Journal of Hepatology. 2017;66(3):537–544. doi: 10.1016/j.jhep.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M., Roayaie S., Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nature Clinical Practice Oncology. 2007;4(7):424–432. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M. B., Zhu A. X. Hepatocellular carcinoma: The need for progress. Journal of Clinical Oncology. 2005;23(13):2892–2899. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian S., Kelly R. K., Venook A. P. A review of hepatocellular carcinoma (HCC) staging systems. Chinese Clinical Oncology. 2013;2(4):33–44. doi: 10.3978/j.issn.2304-3865.2013.07.05. [DOI] [PubMed] [Google Scholar]
- 14.Keane F. K., Wo J. Y., Zhu A. X., Hong T. S. Liver-Directed Radiotherapy for Hepatocellular Carcinoma. Liver Cancer. 2016;5(3):198–209. doi: 10.1159/000367764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallicchio R., Nardelli A., Mainenti P., et al. Therapeutic strategies in HCC: Radiation modalities. BioMed Research International. 2016;2016 doi: 10.1155/2016/1295329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wörns M. A., Weinmann A., Schuchmann M., Galle P. R. Systemic therapies in hepatocellular carcinoma. Digestive Diseases. 2009;27(2):175–188. doi: 10.1159/000218351. [DOI] [PubMed] [Google Scholar]
- 17.Arciero C. A., Sigurdson E. R. Liver-directed therapies for patients with primary liver cancer and hepatic metastases. Current Treatment Options in Oncology. 2006;7(5):399–409. doi: 10.1007/s11864-006-0008-7. [DOI] [PubMed] [Google Scholar]
- 18.Medavaram S., Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Experimental Hematology and Oncology. 2018;7(77) doi: 10.1186/s40164-018-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raoul J., Kudo M., Finn R. S., Edeline J., Reig M., Galle P. R. Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treatment Reviews. 2018;68:16–24. doi: 10.1016/j.ctrv.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Ronot M., Clift A. K., Vilgrain V., Frilling A. Functional imaging in liver tumours. Journal of Hepatology. 2016;65(5):1017–1030. doi: 10.1016/j.jhep.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Page A. J., Weiss M. J., Pawlik T. M. Surgical management of noncolorectal cancer liver metastases. Cancer. 2014;120(20):3111–3121. doi: 10.1002/cncr.28743. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez R., Pappas S. G., Bentrem D. J. Clinical Features of Metastatic Hepatic Malignancies. Cancer Treatment and Research. 2016;168:185–202. doi: 10.1007/978-3-319-34244-3_9. [DOI] [PubMed] [Google Scholar]
- 23.Zampino M. G., Magni E., Ravenda P. S., et al. Treatments for colorectal liver metastases: A new focus on a familiar concept. Critical Review in Oncology/Hematology. 2016;108:154–163. doi: 10.1016/j.critrevonc.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Filice A., Fraternali A., Frasoldati A., et al. Radiolabeled somatostatin analogues therapy in advanced neuroendocrine tumors: A single centre experience. Journal of Oncology. 2012 doi: 10.1155/2012/320198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thang S. P., Lung M. S., Kong G., et al. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. European Journal of Nuclear Medicine and Molecular Imaging. 2018;45(2):262–277. doi: 10.1007/s00259-017-3821-2. [DOI] [PubMed] [Google Scholar]
- 26.Alexandraki K. I., Karapanagioti A., Karoumpalis I., Boutzios G., Kaltsas G. A. Advances and Current Concepts in the Medical Management of Gastroenteropancreatic Neuroendocrine Neoplasms. BioMed Research International. 2017;2017 doi: 10.1155/2017/9856140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giunchedi P., Maestri M., Gavini E., Dionigi P., Rassu G. Transarterial chemoembolization of hepatocellular carcinoma-agents and drugs: An overview. Part 2. Expert Opinion on Drug Delivery. 2013;10(6):799–810. doi: 10.1517/17425247.2013.796359. [DOI] [PubMed] [Google Scholar]
- 28.Burak K. W, Kneteman N. M. An Evidence-Based Multidisciplinary Approach to the Management of Hepatocellular Carcinoma (HCC): The Alberta HCC Algorithm. Canadian Journal of Gastroenterology & Hepatology. 2010;24:8. doi: 10.1155/2010/410574.410574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozkurt M. F., Salanci B. V., Uğur Ö. Intra-arterial radionuclide therapies for liver tumors. Seminars in Nuclear Medicine. 2016;46(4):324–339. doi: 10.1053/j.semnuclmed.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Hilgard P., Müller S., Hamami M., et al. Selective internal radiotherapy (radioembolization) and radiation therapy for HCC - Current status and perspectives. Zeitschrift für Gastroenterologie. 2009;47(1):37–54. doi: 10.1055/s-2008-1028002. [DOI] [PubMed] [Google Scholar]
- 31.Raoul J.-L., Boucher E., Rolland Y., Garin E. Treatment of hepatocellular carcinoma with intra-arterial injection of radionuclides. Nature Reviews Gastroenterology & Hepatology. 2010;7(1):41–49. doi: 10.1038/nrgastro.2009.202. [DOI] [PubMed] [Google Scholar]
- 32.Burrill J., Häfeli U., Liu D. M. Advances in radioembolization - embolics and isotopes. Journal of Nuclear Medicine Radiation Therapy. 2011;2(1):107–112. [Google Scholar]
- 33.Salem R., Thurston K. G. Radioembolization with yttrium-90 microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies - Part 3: Comprehensive literature review and future direction. Journal of Vascular and Interventional Radiology. 2006;17(10):1571–1593. doi: 10.1097/01.RVI.0000236744.34720.73. [DOI] [PubMed] [Google Scholar]
- 34.Khajornjiraphan N., Thu N. A., Chow P. K. Yttrium-90 microspheres: a review of its emerging clinical indications. Liver Cancer. 2015;4(1):6–15. doi: 10.1159/000343876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallini J. R., Gabr A., Salem R., Lewandowski R. J. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Advances in Therapy. 2016;33(5):699–714. doi: 10.1007/s12325-016-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sangha B. S., Nimeiri H., Hickey R., Salem R., Lewandowski R. J. Radioembolization as a Treatment Strategy for Metastatic Colorectal Cancer to the Liver: What Can We Learn from the SIRFLOX Trial? Current Treatment Options in Oncology. 2016;17(6) doi: 10.1007/s11864-016-0402-8. [DOI] [PubMed] [Google Scholar]
- 37.Vilgrain V., Pereira H., Assenat E., et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. The Lancet Oncology. 2017;18(12):1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 38.Grady E. D. Internal radiation therapy of hepatic cancer. Diseases of the Colon & Rectum. 1979;22(6):371–375. doi: 10.1007/bf02586901. [DOI] [PubMed] [Google Scholar]
- 39.Westcott M. A., Coldwell D. M., Liu D. M., Zikria J. F. The development, commercialization, and clinical context of yttrium-90 radiolabeled resin and glass microspheres. Advances in Radiation Oncology. 2016;1(4):351–364. doi: 10.1016/j.adro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nijsen J. F. W., van het Schip A. D., Hennink W. E., Rook D. W., van Rijk P. P., de Klerk J. M. H. Advances in nuclear oncology: Microspheres for internal radionuclide therapy in liver tumours. Current Medicinal Chemistry. 2002;9(1):73–82. doi: 10.2174/0929867023371454. [DOI] [PubMed] [Google Scholar]
- 41.Caldarola L., Rosa U., Badellino F., Sosi S., Cavalli A. Preparation of 32-p labelled resin microspheres for radiation treatment of tumours by intra-arterial injection. Minerva nucleare. 1964;55:169–174. [PubMed] [Google Scholar]
- 42.Vinjamuri S., Ray S. Phosphorus-32: the forgotten radiopharmaceutical? Nuclear Medicine Communications. 2008;29(2):95–97. doi: 10.1097/MNM.0b013e3282f1d4eb. [DOI] [PubMed] [Google Scholar]
- 43.Yan L., Li Z., Li L., et al. The relationship between effects and radiation doses of intra-arterial phosphorus-32 glass microspheres embolization therapy for patients with advanced liver cancer. Chinese journal of surgery. 2000;38(11):837–840. [PubMed] [Google Scholar]
- 44.Pirayesh E., Amoui M., Akhlaghpoor S., et al. Technical Considerations of Phosphorous-32 Bremsstrahlung SPECT Imaging after Radioembolization of Hepatic Tumors: A Clinical Assessment with a Review of Imaging Parameters. Radiology Research and Practice. 2014;2014:7. doi: 10.1155/2014/407158.407158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seevinck P. R., Van De Maat G. H., De Wit T. C., Vente M. A. D., Nijsen J. F. W., Bakker C. J. G. Magnetic resonance imaging-based radiation-absorbed dose estimation of166Ho microspheres in liver radioembolization. International Journal of Radiation Oncology Biology Physics. 2012;83(3):e437–e444. doi: 10.1016/j.ijrobp.2011.12.085. [DOI] [PubMed] [Google Scholar]
- 46.Van De Maat G. H., Seevinck P. R., Bos C., Bakker C. J. G. Quantification of holmium-166 loaded microspheres: Estimating high local concentrations using a conventional multiple gradient echo sequence with S 0-fitting. Journal of Magnetic Resonance Imaging. 2012;35(6):1453–1461. doi: 10.1002/jmri.23593. [DOI] [PubMed] [Google Scholar]
- 47.Vente M. A. D., Nijsen J. F. W., de Roos R., et al. Neutron activation of holmium poly(L-lactic acid) microspheres for hepatic arterial radioembolization: A validation study. Biomedical Microdevices. 2009;11(4):763–772. doi: 10.1007/s10544-009-9291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mumper R. J., Jay M. Biodegradable radiotherapeutic polyester microspheres: optimization and in-vitro/in-vivo evaluation. Journal of Controlled Release. 1992;18(3):193–203. doi: 10.1016/0168-3659(92)90165-N. [DOI] [Google Scholar]
- 49.Zeilhuis S. W., Seppenwoolde J. H., Bakker C. J. G., et al. Characterization of holmium loaded alginate microspheres for multimodality imaging and therapeutic applications. Journal of Biomedical Materials Research Part A. 2007;82(4):892–898. doi: 10.1002/jbm.a.31183. [DOI] [PubMed] [Google Scholar]
- 50.Arranja A., Hennink W., Denkova A., Hendrikx R., Nijsen J. Radioactive holmium phosphate microspheres for cancer treatment. International Journal of Pharmaceutics. 2018;548(1):73–81. doi: 10.1016/j.ijpharm.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 51.Turner J. H., Claringbold P. G., Klemp P. F. B., et al. 166Ho-microsphere liver radiotherapy: A preclinical SPECT dosimetry study in the pig. Nuclear Medicine Communications. 1994;15(7):545–553. doi: 10.1097/00006231-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Costa R. F., MB M., Nascimento N., Sene Martinelli F. F., Osso J. A. Production of microspheres labeled with holmium-166 for liver cancer therapy: the preliminary experience at IPEN-CNEN/SP. Proceedings of the International Nuclear Atlantic Conference - INAC; 2009. [Google Scholar]
- 53.Ehrhardt G. J., Day D. E. Therapeutic use of 90Y microspheres. International Journal of Radiation Applications and Instrumentation. Part B. Nuclear Medicine and Biology. 1987;14(3):233–242. doi: 10.1016/0883-2897(87)90047-X. [DOI] [PubMed] [Google Scholar]
- 54.Brown R. F., Lindesmith L. C., Day D. E. 166Holmium-containing glass for internal radiotherapy of tumors. International Journal of Radiation Applications and Instrumentation. Part B. Nuclear Medicine and Biology. 1991;18(7):783–790. doi: 10.1016/0883-2897(91)90018-G. [DOI] [PubMed] [Google Scholar]
- 55.Schubiger P. A., Beer H.-F., Geiger L., et al. 90Y-resin particles-Animal experiments on pigs with regard to the introduction of superselective embolization therapy. International Journal of Radiation Applications and Instrumentation. Part B. Nuclear Medicine and Biology. 1991;18(3):305–311. doi: 10.1016/0883-2897(91)90126-6. [DOI] [PubMed] [Google Scholar]
- 56.Subramanian S., Vimalnath K. V., Dash A. Preparation and preliminary in vivo evaluation of 166Ho-labeled microspheres for possible use in radioembolic therapy of liver cancer. Journal of Labelled Compounds and Radiopharmaceuticals. 2018;61(6):509–514. doi: 10.1002/jlcr.3616. [DOI] [PubMed] [Google Scholar]
- 57.Mumper R. J., Jay M. Poly(L-lactic Acid) Microspheres Containing Neutron-Activatable Holmium-165: A Study of the Physical Characteristics of Microspheres Before and After Irradiation in a Nuclear Reactor. Pharmaceutical Research: An Official Journal of the American Association of Pharmaceutical Scientists. 1992;9(1):149–154. doi: 10.1023/A:1018908600711. [DOI] [PubMed] [Google Scholar]
- 58.Zielhuis S. W., Nijsen J. F. W., Figueiredo R., et al. Surface characteristics of holmium-loaded poly(L-lactic acid) microspheres. Biomaterials. 2005;26(8):925–932. doi: 10.1016/j.biomaterials.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki Y. S., Momose Y., Higashi N., et al. Biodistribution and kinetics of holmium-166-chitosan complex (DW-166HC) in rats and mice. Journal of Nuclear Medicine. 1998;39(12):2161–2166. [PubMed] [Google Scholar]
- 60.Nijsen J. F. W., Zonnenberg B. A., Woittiez J. R. W., et al. Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: Effects of preparation and neutron activation techniques. European Journal of Nuclear Medicine and Molecular Imaging. 1999;26(7):699–704. doi: 10.1007/s002590050440. [DOI] [PubMed] [Google Scholar]
- 61.Mumper R. J., Jay M. Formation and stability of lanthanide complexes and their encapsulation into polymeric microspheres. The Journal of Physical Chemistry C. 1992;96(21):8626–8631. doi: 10.1021/j100200a076. [DOI] [Google Scholar]
- 62.Mumper R. J., Ryo U. Y., Jay M. Neutron-activated holmium-166-Poly(L-lactic acid) microspheres: A potential agent for the internal radiation therapy of hepatic tumors. Journal of Nuclear Medicine. 1991;32(11):2139–2143. [PubMed] [Google Scholar]
- 63.Zielhuis S. W., Nijsen J. F. W., Dorland L., Krijger G. C., van het Schip A. D., Hennink W. E. Removal of chloroform from biodegradable therapeutic microspheres by radiolysis. International Journal of Pharmaceutics. 2006;315(1-2):67–74. doi: 10.1016/j.ijpharm.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Bult W., Varkevisser R., Soulimani F., et al. Holmium nanoparticles: Preparation and in vitro characterization of a new device for radioablation of solid malignancies. Pharmaceutical Research. 2010;27(10):2205–2212. doi: 10.1007/s11095-010-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zielhuis S. W., Nijsen J. F. W., Krijger G. C., van het Schip A. D., Hennink W. E. Holmium-loaded poly(L-lactic acid) microspheres: In vitro degradation study. Biomacromolecules. 2006;7(7):2217–2223. doi: 10.1021/bm060230r. [DOI] [PubMed] [Google Scholar]
- 66.Zielhuis S. W., Nijsen J. F. W., De Roos R., et al. Production of GMP-grade radioactive holmium loaded poly(L-lactic acid) microspheres for clinical application. International Journal of Pharmaceutics. 2006;311(1-2):69–74. doi: 10.1016/j.ijpharm.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 67.Nijsen F., Rook D., Brandt C., et al. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: A biodistribution study. European Journal of Nuclear Medicine and Molecular Imaging. 2001;28(6):743–749. doi: 10.1007/s002590100518. [DOI] [PubMed] [Google Scholar]
- 68.Zielhuis S. W., Nijsen J. F. W., Seppenwoolde J.-H., et al. Long-term toxicity of holmium-loaded poly(l-lactic acid) microspheres in rats. Biomaterials. 2007;28(31):4591–4599. doi: 10.1016/j.biomaterials.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 69.Vente M. A. D., Nijsen J. F. W., De Wit T. C., et al. Clinical effects of transcatheter hepatic arterial embolization with holmium-166 poly(L-lactic acid) microspheres in healthy pigs. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35(7):1259–1271. doi: 10.1007/s00259-008-0747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nijsen J. F. W., Seppenwoolde J.-H., Havenith T., Bos C., Bakker C. J. G., Van Het Schip A. D. Liver Tumors: MR Imaging of Radioactive Holmium Microspheres - Phantom and Rabbit Study. Radiology. 2004;231(2):491–499. doi: 10.1148/radiol.2312030594. [DOI] [PubMed] [Google Scholar]
- 71.Seppenwoolde J.-H., Nijsen J. F. W., Bartels L. W., Zielhuis S. W., Van Het Schip A. D., Bakker C. J. G. Internal radiation therapy of liver tumors: Qualitative and quantitative magnetic resonance imaging of the biodistribution of holmium-loaded microspheres in animal models. Magnetic Resonance in Medicine. 2005;53(1):76–84. doi: 10.1002/mrm.20320. [DOI] [PubMed] [Google Scholar]
- 72.Vente M. A. D., De Wit T. C., Van Den Bosch M. A. A. J., et al. Holmium-166 poly(L-lactic acid) microsphere radioembolisation of the liver: Technical aspects studied in a large animal model. European Radiology. 2010;20(4):862–869. doi: 10.1007/s00330-009-1613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smits M. L. J., Nijsen J. F. W., van den Bosch M. A. A. J., et al. Holmium-166 radioembolization for the treatment of patients with liver metastases: design of the phase I HEPAR trial. Journal of Experimental & Clinical Cancer Research. 2010;29(70) doi: 10.1186/1756-9966-29-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smits M. L. J., Nijsen J. F. W., van den Bosch M. A. A. J., et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): A phase 1, dose-escalation study. The Lancet Oncology. 2012;13(10):1025–1034. doi: 10.1016/S1470-2045(12)70334-0. [DOI] [PubMed] [Google Scholar]
- 75.Smits M. L. J., Elschot M., Van Den Bosch M. A. A. J., et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho- microspheres for treatment of liver malignancies. Journal of Nuclear Medicine. 2013;54(12):2093–2100. doi: 10.2967/jnumed.113.119768. [DOI] [PubMed] [Google Scholar]
- 76.Elschot M., Nijsen J. F., Lam M. G., et al. 99mTc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with 166Ho-microspheres. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41(10):1965–1975. doi: 10.1007/s00259-014-2784-9. [DOI] [PubMed] [Google Scholar]
- 77.Prince J. F., van Rooij R., Bol G. H., de Jong H. W., van den Bosch M. A., Lam M. G. Safety of a Scout Dose Preceding Hepatic Radioembolization with 166Ho Microspheres. Journal of Nuclear Medicine. 2015;56(6):817–823. doi: 10.2967/jnumed.115.155564. [DOI] [PubMed] [Google Scholar]
- 78.Prince J. F., Van Den Bosch M. A. A. J., Nijsen J. F. W., et al. Efficacy of radioembolization with 166Ho-Microspheres in salvage patients with liver metastases: A phase 2 study. Journal of Nuclear Medicine. 2018;59(4):582–588. doi: 10.2967/jnumed.117.197194. [DOI] [PubMed] [Google Scholar]
- 79.Prince J. F., Smits M. L. J., Krijger G. C., et al. Radiation emission from patients treated with holmium-166 radioembolization. Journal of Vascular and Interventional Radiology. 2014;25(12):1956–1963.e1. doi: 10.1016/j.jvir.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 80.van den Hoven A. F., Prince J. F., Bruijnen R. C. G., et al. Surefire infusion system versus standard microcatheter use during holmium-166 radioembolization: Study protocol for a randomized controlled trial. Trials. 2016;17(1) doi: 10.1186/s13063-016-1643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J. K., Han K.-H., Lee J. T., et al. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (milican) for the treatment of small hepatocellular carcinoma. Clinical Cancer Research. 2006;12(2):543–548. doi: 10.1158/1078-0432.CCR-05-1730. [DOI] [PubMed] [Google Scholar]
- 82.Sohn J. H., Choi H. J., Lee J. T., et al. Phase II study of transarterial holmium-166-chitosan complex treatment in patients with a single, large hepatocellular carcinoma. Oncology. 2008;76(1):1–9. doi: 10.1159/000173735. [DOI] [PubMed] [Google Scholar]
- 83.Argyrou Maria, Valassi Alexia, Andreou Maria, Lyra Maria. Rhenium-188 Production in Hospitals, by W-188/Re-188 Generator, for Easy Use in Radionuclide Therapy. International Journal of Molecular Imaging. 2013;2013:7. doi: 10.1155/2013/290750.290750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knapp F. Continued Availability of the Tungsten-188/Rhenium-188 Generator to Enhance Therapeutic Utility of 188Re. International Journal of Nuclear Medicine Research. 2017;(Special - 2017) doi: 10.15379/2408-9788.2017.02. [DOI] [Google Scholar]
- 85.Wunderlich G., Pinkert J., Stintz M., Kotzerke J. Labeling and biodistribution of different particle materials for radioembolization therapy with 188Re. Applied Radiation and Isotopes. 2005;62(5):745–750. doi: 10.1016/j.apradiso.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Conzone S. D., Häfeli U. O., Day D. E., Ehrhardt G. J. Preparation and properties of radioactive rhenium glass microspheres intended for in vivo radioembolization therapy. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 1998;42(4):617–625. doi: 10.1002/(SICI)1097-4636(19981215)42:4<617::AID-JBM19>3.0.CO;2-4. doi: 10.1002/(SICI)1097-4636(19981215)42:4<617::AID-JBM19>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 87.Häfeli U. O., Casillas S., Dietz D. W., et al. Hepatic tumor radioembolization in a rat model using radioactive rhenium (186RE/188RE) glass microspheres. International Journal of Radiation Oncology Biology Physics. 1999;44(1):189–199. doi: 10.1016/S0360-3016(98)00554-9. [DOI] [PubMed] [Google Scholar]
- 88.Wang S.-J., Lin W.-Y., Chen M.-N., et al. Intratumoral injection of rhenium-188 microspheres into an animal model of hepatoma. Journal of Nuclear Medicine. 1998;39(10):1752–1757. [PubMed] [Google Scholar]
- 89.Lin Y.-C., Tsai S.-C., Hung G.-U., Lee J.-C., Huang Y.-S., Lin W.-Y. Direct injection of 188Re-microspheres in the treatment of hepatocellular carcinoma. Compared with traditional percutaneous ethanol injection: An animal study. Nuklearmedizin / Nuclear Medicine. 2005;44(3):76–80. doi: 10.1055/s-0038-1625689. [DOI] [PubMed] [Google Scholar]
- 90.Häfeli U. O., Roberts W. K., Pauer G. J., Kraeft S.-K., Macklis R. M. Stability of biodegradable radioactive rhenium (Re-186 and Re-188) microspheres after neutron-activation. Applied Radiation and Isotopes. 2001;54(6):869–879. doi: 10.1016/S0969-8043(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 91.Shukla J., Bandopadhyaya G. P., Varma I. K. 188Rhenium(V)-dimercaptosuccinic acid loaded poly(lactic-co-glycolic)acid microspheres for targeted radiotherapy: Production and effectivity. Die Pharmazie. 2005;60(8):583–587. [PubMed] [Google Scholar]
- 92.Yu J., Häfeli U. O., Xia J., et al. Radiolabelling of poly(histidine) derivatized biodegradable microspheres with the 188Re tricarbonyl complex [188Re(CO)3(H2O)3]+ Nuclear Medicine Communications. 2005;26(5):453–458. doi: 10.1097/00006231-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Jamre M., Shamsaei M., Erfani M., Sadjadi S., Ghannadi Maragheh M. Preparation and evaluation of. Journal of Labelled Compounds and Radiopharmaceuticals. 2018;61(8):586–594. doi: 10.1002/jlcr.3627. [DOI] [PubMed] [Google Scholar]
- 94.Chiang P., Peng C., Shih Y., et al. Biodegradable and Multifunctional Microspheres for Treatment of Hepatoma through Transarterial Embolization. ACS Biomaterials Science & Engineering. 2018;4(9):3425–3433. doi: 10.1021/acsbiomaterials.8b00635. [DOI] [PubMed] [Google Scholar]
- 95.Meyer C., Pieper C. C., Ezziddin S., Wilhelm K. E., Schild H. H., Ahmadzadehfar H. Feasibility of temporary protective embolization of normal liver tissue using degradable starch microspheres during radioembolization of liver tumours. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41(2):231–237. doi: 10.1007/s00259-013-2550-4. [DOI] [PubMed] [Google Scholar]
- 96.Verger E., Drion P., Meffre G., et al. 68Ga and 188Re starch-based microparticles as theranostic tool for the hepatocellular carcinoma: Radiolabeling and preliminary in vivo rat studies. PLoS ONE. 2016;11(10) doi: 10.1371/journal.pone.0164626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garin E., Boucher E., Rolland Y. 99mTc-MAA-based dosimetry for liver cancer treated using 90Y-loaded microspheres: Known proof of effectiveness. Journal of Nuclear Medicine. 2014;55(8):1391–1392. doi: 10.2967/jnumed.114.137422. [DOI] [PubMed] [Google Scholar]
- 98.Wunderlich G., Pinkert J., Andreeff M., et al. Preparation and biodistribution of rhenium-188 labeled albumin microspheres B 20: A promising new agent for radiotherapy. Applied Radiation and Isotopes. 2000;52(1):63–68. doi: 10.1016/S0969-8043(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 99.Wunderlich G., Drews A., Kotzerke J. A kit for labeling of [188Re] human serum albumin microspheres for therapeutic use in nuclear medicine. Applied Radiation and Isotopes. 2005;62(6):915–918. doi: 10.1016/j.apradiso.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Chen L.-C., Chang Y.-J., Chen S.-J., et al. Imaging, biodistribution and efficacy evaluation of 188Re-human serum albumin microspheres via intraarterial route in an orthotopic hepatoma model. International Journal of Radiation Biology. 2017;93(5):477–486. doi: 10.1080/09553002.2017.1276308. [DOI] [PubMed] [Google Scholar]
- 101.Liepe K., Brogsitter C., Leonhard J., et al. Feasibility of high activity rhenium-188-microsphere in hepatic radioembolization. Japanese Journal of Clinical Oncology. 2007;37(12):942–950. doi: 10.1093/jjco/hym137. [DOI] [PubMed] [Google Scholar]
- 102.Nowicki M. L., Ćwikła J. B., Sankowski A. J., et al. Initial study of radiological and clinical efficacy radioembolization using 188re-human serum albumin (HSA) microspheres in patients with progressive, unresectable primary or secondary liver cancers. Medical Science Monitor. 2014;20:1353–1362. doi: 10.12659/MSM.890480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakakuma K., Tashiro S., Hiraoka T., Ogata K., Ootsuka K. Hepatocellular carcinoma and metastatic cancer detected by iodized oil. Radiology. 1985;154(1):15–17. doi: 10.1148/radiology.154.1.2981111. [DOI] [PubMed] [Google Scholar]
- 104.Fairlie N. C., Adam A. N. Case report: Persistence of lipiodol for 13 months in metastatic deposits in the liver on computed tomography. Clinical Radiology. 1991;44(4):273–274. doi: 10.1016/S0009-9260(05)80196-5. [DOI] [PubMed] [Google Scholar]
- 105.Liebster J., Kočandrle V. Exchange labelling of ‘lipiodol’ ultra fluids with lodine-131 and its intra-lymphatic administration with a fistula on the thoracic duct. Nature. 1964;203(4946):777–778. doi: 10.1038/203777a0. [DOI] [PubMed] [Google Scholar]
- 106.Lintia-Gaultier A., Perret C., Ansquer C., Eugène T., Kraeber-Bodéré F., Frampas E. Intra-arterial injection of 131I-labeled Lipiodol for advanced hepatocellular carcinoma: a 7 years' experience. Nuclear Medicine Communications. 2013;34(7):674–681. doi: 10.1097/mnm.0b013e32836141a0. [DOI] [PubMed] [Google Scholar]
- 107.Raoul J.-L., Boucher E., Roland Y., Garin E. 131-iodine Lipiodol therapy in hepatocellular carcinoma. The Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2009;53(3):348–355. [PubMed] [Google Scholar]
- 108.Madsen M. T., Park C. H., Thakur M. L. Dosimetry of iodine-131 ethiodol in the treatment of hepatoma. Journal of Nuclear Medicine. 1988;29(6):1038–1044. [PubMed] [Google Scholar]
- 109.Lambert B., Bacher K., Defreyne L. Rhenium-188 based radiopharmaceuticals for treatment of liver tumours. The Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2009;53(3):305–310. [PubMed] [Google Scholar]
- 110.Lepareur N. Transarterial Radionuclide Therapy with 188Re-Labelled Lipiodol. International Journal of Nuclear Medicine Research. 2017;(Special - 2017) doi: 10.15379/2408-9788.2017.07. [DOI] [Google Scholar]
- 111.Wang S.-J., Lin W.-Y., Chen M.-N., et al. Radiolabelling of Lipiodol with generator-produced 188Re for hepatic tumor therapy. Applied Radiation and Isotopes. 1996;47(3):267–271. doi: 10.1016/0969-8043(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 112.Wang S.-J., Lin W.-Y., Chen M.-N., et al. Biodistribution of rhenium-188 Lipiodol infused via the hepatic artery of rats with hepatic tumours. European Journal of Nuclear Medicine and Molecular Imaging. 1996;23(1):13–17. doi: 10.1007/BF01736984. [DOI] [PubMed] [Google Scholar]
- 113.Wang S.-J., Lin W.-Y., Lui W.-Y., Chen M.-N., Tsai Z.-T., Ting G. Hepatic artery injection of yttrium-90-lipiodol: biodistribution in rats with hepatoma. Journal of Nuclear Medicine. 1996;37(2):332–335. [PubMed] [Google Scholar]
- 114.Jackson T. W., Kojima M., Lambrecht R. M. Rhenium Diamino Dithiol Complexes. III. Lipophilic Ligands for Endotherapeutic Radiopharmaceuticals. Australian Journal of Chemistry. 2000;53(12):p. 983. doi: 10.1071/CH96073. [DOI] [Google Scholar]
- 115.Jeong J. M., Kim Y. J., Lee Y. S. Lipiodol solution of a lipophilic agent, 188Re-TDD, for the treatment of liver cancer. Nuclear Medicine and Biology. 2001;28(2):197–204. doi: 10.1016/s0969-8051(00)00208-0. [DOI] [PubMed] [Google Scholar]
- 116.LEE Y., Jeong J. M., Kim Y. J., et al. Synthesis of 188Re-labelled long chain alkyl diaminedithiol for therapy of liver cancer. Nuclear Medicine Communications. 2002;23(3):237–242. doi: 10.1097/00006231-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 117.Paeng J. C., Jeong J. M., Yoon C. J., et al. Lipiodol solution of 188Re-HDD as a new therapeutic agent for transhepatic arterial embolization in liver cancer: Preclinical study in a rabbit liver cancer model. Journal of Nuclear Medicine. 2003;44(12):2033–2038. [PubMed] [Google Scholar]
- 118.Lee Y.-S., Min Jeong J., Joo Kim Y., et al. Development of acetylated HDD kit for preparation of 188Re-HDD/lipiodol. Applied Radiation and Isotopes. 2007;65(1):64–69. doi: 10.1016/j.apradiso.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 119.Banka V. K., Moon S.-H., Jeong J. M., et al. Development of 4-hexadecyl-4,7-diaza-1,10-decanedithiol (HDD) kit for the preparation of the liver cancer therapeutic agent Re-188-HDD/lipiodol. Nuclear Medicine and Biology. 2015;42(3):317–322. doi: 10.1016/j.nucmedbio.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 120.Luo T.-Y., Hsieh B.-T., Wang S.-J., et al. Preparation and biodistribution of rhenium-188 ECD/Lipiodol in rats following hepatic arterial injection. Nuclear Medicine and Biology. 2004;31(5):671–677. doi: 10.1016/j.nucmedbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Luo T.-Y., Shih Y.-H., Chen C.-Y., et al. Evaluating the potential of 188re-ecd/lipiodol as a therapeutic radiopharmaceutical by intratumoral injection for hepatoma treatment. Cancer Biotherapy and Radiopharmaceuticals. 2009;24(5):535–541. doi: 10.1089/cbr.2008.0603. [DOI] [PubMed] [Google Scholar]
- 122.Wang G.-Q., Zhang J., Luo S.-Z., et al. Synthesis and primary biological evaluation of 188ReN-NEMPTDD. Journal of Radioanalytical and Nuclear Chemistry. 2008;277(2):365–369. doi: 10.1007/s10967-007-7033-2. [DOI] [Google Scholar]
- 123.Tang I.-C., Luo T.-Y., Liu S.-W., et al. Synthesis and application of 188Re-MN-16ET/Lipiodol in a hepatocellular carcinoma animal model. Nuclear Medicine and Biology. 2011;38(7):1043–1052. doi: 10.1016/j.nucmedbio.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Huang P.-W., Tsai S.-C., Luo T.-Y., Kao C.-H., Lin W.-Y. Therapeutic efficacy of 188Re-MN-16ET lipiodol in an animal model of hepatocellular carcinoma. Annals of Nuclear Medicine. 2013;27(6):532–537. doi: 10.1007/s12149-013-0717-5. [DOI] [PubMed] [Google Scholar]
- 125.Boschi A., Uccelli L., Duatti A., et al. A kit formulation for the preparation of re-lipiodol: preclinical studies and preliminary therapeutic evaluation in patients with unresectable hepatocellular carcinoma. Nuclear Medicine Communications. 2004;25(7):691–699. doi: 10.1097/01.mnm.0000130241.22068.45. [DOI] [PubMed] [Google Scholar]
- 126.Lepareur N., Garin E., Noiret N., Herry J. Y. A kit formulation for the labelling of lipiodol with generator-produced 188Re. Journal of Labelled Compounds and Radiopharmaceuticals. 2004;47(12):857–867. doi: 10.1002/jlcr.863. [DOI] [Google Scholar]
- 127.Lepareur N., Ardisson V., Noiret N., Garin E. 188Re-SSS/Lipiodol: development of a potential treatment for HCC from bench to bedside. International Journal of Molecular Imaging. 2012;2012:9. doi: 10.1155/2012/278306.278306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thakur M. L., Segal A. W., Louis L., Welch M. J., Hopkins J., Peters T. J. Indium-111-labeled cellular blood components: mechanism of labeling and intracellular location in human neutrophils. Journal of Nuclear Medicine. 1977;18(10):1022–1026. [PubMed] [Google Scholar]
- 129.Yu J., Häfeli U. O., Sands M., Dong Y. 90Y-oxine-ethiodol, a potential radiopharmaceutical for the treatment of liver cancer. Applied Radiation and Isotopes. 2003;58(5):567–573. doi: 10.1016/S0969-8043(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 130.Das T., Chakraborty S., Sarma H. D., Venkatesh M., Banerjee S. Preparation of 166Ho-oxine-lipiodol and its preliminary bioevaluation for the potential application in therapy of liver cancer. Nuclear Medicine Communications. 2009;30(5):362–367. doi: 10.1097/MNM.0b013e328329981a. [DOI] [PubMed] [Google Scholar]
- 131.Subramanian S., Das T., Chakraborty S., et al. Preparation of 177Lu-labeled oxine in lipiodol as a possible agent for therapy of hepatocellular carcinoma: A Preliminary Animal Study. Cancer Biotherapy and Radiopharmaceuticals. 2010;25(5):539–543. doi: 10.1089/cbr.2010.0792. [DOI] [PubMed] [Google Scholar]
- 132.Lepareur N., Edeline J., Noiret N., Ardisson V., Garin E. Preparation and preliminary evaluation of 90Y-tropolonate-Lipiodol as a potential radiopharmaceutical for hepatocellular carcinoma therapy. European Journal of Nuclear Medicine and Molecular Imaging. 2016;43(Suppl. 1):p. S454. [Google Scholar]
- 133.Lopez A., Noiret N., Garin E., Lepareur N. Mixed-ligand complexes of yttrium-90 dialkyldithiocarbamates with 1,10-phenanthroline as a possible agent for therapy of hepatocellular carcinoma. Applied Radiation and Isotopes. 2014;94:241–246. doi: 10.1016/j.apradiso.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 134.Mu P. Y., Jiang X. L., Chen J., et al. Research on extracted 90Y with P204 in lipiodol for liver cancer. Journal of Radioanalytical and Nuclear Chemistry. 2007;272(3):669–671. doi: 10.1007/s10967-007-0644-9. [DOI] [Google Scholar]
- 135.Andreeff M., Wunderlich G., Behge K., Schönmuth T., Kotzerke J. β-radiation exposure with 188Re-labelled pharmaceuticals. Nuklearmedizin / Nuclear Medicine. 2005;44(3):94–98. doi: 10.1055/s-0038-1625712. [DOI] [PubMed] [Google Scholar]
- 136.Rimpler A., Barth I., Ferrari P., et al. Extremity exposure in nuclear medicine therapy with 90Y-labelled substances - Results of the ORAMED project. Radiation Measurements. 2011;46(11):1283–1286. doi: 10.1016/j.radmeas.2011.05.068. [DOI] [Google Scholar]
- 137.Uccelli L., Pasquali M., Boschi A., Giganti M., Duatti A. Automated preparation of Re-188 lipiodol for the treatment of hepatocellular carcinoma. Nuclear Medicine and Biology. 2011;38(2):207–213. doi: 10.1016/j.nucmedbio.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 138.Lepareur N., Ardisson V., Noiret N., et al. Automation of labelling of Lipiodol with high-activity generator-produced 188Re. Applied Radiation and Isotopes. 2011;69(2):426–430. doi: 10.1016/j.apradiso.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Mukherjee A., Subramanian S., Ambade R., Avhad B., Dash A., Korde A. Development of semiautomated module for preparation of 131I labeled lipiodol for liver cancer therapy. Cancer Biotherapy and Radiopharmaceuticals. 2017;32(1):33–37. doi: 10.1089/cbr.2016.2088. [DOI] [PubMed] [Google Scholar]
- 140.Lepareur N., Laffont S., Ardisson V., Noiret N., Garin E. Reduction of β-radiation exposure during preparation of 188Re-labelled Lipiodol for hepatocellular carcinoma treatment. Nuclear Medicine Communications. 2012;33(2):205–208. doi: 10.1097/MNM.0b013e32834e7580. [DOI] [PubMed] [Google Scholar]
- 141.Raoul J.-L., Bourguet P., Bretagne J.-F., et al. Hepatic artery injection of I-131-labeled lipiodol. Part I. Biodistribution study results in patients with hepatocellular carcinoma and liver metastases. Radiology. 1988;168(2):541–545. doi: 10.1148/radiology.168.2.2839866. [DOI] [PubMed] [Google Scholar]
- 142.Park C. H., Suh J. H., Yoo H. S., Lee J. T., Kim D. I. Evaluation of intrahepatic I-131 ethiodol on a patient with hepatocellular carcinoma. Therapeutic feasibility study. Clinical Nuclear Medicine. 1986;11(7):514–517. doi: 10.1097/00003072-198607000-00015. [DOI] [PubMed] [Google Scholar]
- 143.Ahmadzadehfar H., Sabet A., Wilhelm K., Biersack H. J., Risse J. Iodine-131-lipiodol therapy in hepatic tumours. Methods. 2011;55(3):246–252. doi: 10.1016/j.ymeth.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 144.Oger E., Lavenu A., Bellissant E., Garin E., Polard E. Meta-analysis of interstitial pneumonia in studies evaluating iodine-131-labeled lipiodol for hepatocellular carcinoma using exact likelihood approach. Pharmacoepidemiology and Drug Safety. 2011;20(9):956–963. doi: 10.1002/pds.2177. [DOI] [PubMed] [Google Scholar]
- 145.Sundram F. X., Yu S. W. K., Jeong J. M., et al. 188Rhenium-TDD-lipiodol in treatment of inoperable primary hepatocellular carcinoma - A case report. Annals, Academy of Medicine, Singapore. 2001;30(5):542–545. [PubMed] [Google Scholar]
- 146.Delaunay K., Garin E., Rolland Y., et al. Biodistribution and dosimetry assessments for hepatocellular carcinoma treated with 188Re-SSS Lipiodol: Preliminary results of the phase 1 trial Lip Re 1. Journal of Nuclear Medicine. 2018;59(supplement 1):p. 602. doi: 10.1007/s00259-019-04277-9. [DOI] [PubMed] [Google Scholar]
- 147.Keng G. H. W., Sundram F. X., Yu S. W. K., et al. Preliminary experience in radionuclide therapy of hepatocellular carcinoma using hepatic intra-arterial radio-conjugates. Annals, Academy of Medicine, Singapore. 2002;31(3):382–386. [PubMed] [Google Scholar]
- 148.Sundram F., Chau T. C. M., Onkhuudai P., Bernal P., Padhy A. K. Preliminary results of transarterial rhenium-188 HDD lipiodol in the treatment of inoperable primary hepatocellular carcinoma. European Journal of Nuclear Medicine and Molecular Imaging. 2004;31(2):250–257. doi: 10.1007/s00259-003-1363-2. [DOI] [PubMed] [Google Scholar]
- 149.Lambert B., Bacher K., Defreyne L., et al. 188Re-HDD/lipiodol therapy for hepatocellular carcinoma: An activity escalation study. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33(3):344–352. doi: 10.1007/s00259-005-1954-1. [DOI] [PubMed] [Google Scholar]
- 150.Lambert B., Bacher K., Defreyne L., et al. 188Re-HDD/lipiodol therapy for hepatocellular carcinoma: A phase I clinical trial. Journal of Nuclear Medicine. 2005;46(1):60–66. [PubMed] [Google Scholar]
- 151.Lambert B., Bacher K., De Keukeleire K., et al. 188Re-HDD/lipiodol for treatment of hepatocellular carcinoma: A feasibility study in patients with advanced cirrhosis. Journal of Nuclear Medicine. 2005;46(8):1326–1332. [PubMed] [Google Scholar]
- 152.Kumar A., Bal C., Srivastava D. N., et al. Transarterial radionuclide therapy with Re-188-HDD-lipiodol in case of unresectable hepatocellular carcinoma with extensive portal vein thrombosis. European Journal of Radiology Extra. 2005;56(2):55–59. doi: 10.1016/j.ejrex.2005.07.016. [DOI] [Google Scholar]
- 153.Kumar A., Srivastava D. N., Bal C. Management of postsurgical recurrence of hepatocellular carcinoma with rhenium 188-HDD labeled iodized oil. Journal of Vascular and Interventional Radiology. 2006;17(1):157–161. doi: 10.1097/01.RVI.0000195321.20579.F2. [DOI] [PubMed] [Google Scholar]
- 154.Kumar A., Bal C., Srivastava D. N., et al. Management of multiple intrahepatic recurrences after radiofrequency ablation of hepatocellular carcinoma with rhenium-188-HDD-lipiodol. European Journal of Gastroenterology & Hepatology. 2006;18(2):219–223. doi: 10.1097/00042737-200602000-00016. [DOI] [PubMed] [Google Scholar]
- 155.de Ruyck K., Lambert B., Bacher K., et al. Biologic dosimetry of 188Re-HDD/lipiodol versus 131I-lipiodol therapy in patients with hepatocellular carcinoma. Journal of Nuclear Medicine. 2004;45(4):612–618. [PubMed] [Google Scholar]
- 156.Bernal P., Raoul J.-L., Vidmar G., et al. Intra-arterial rhenium-188 lipiodol in the treatment of inoperable hepatocellular carcinoma: results of an IAEA-Sponsored Multination Study. International Journal of Radiation Oncology Biology Physics. 2007;69(5):1448–1455. doi: 10.1016/j.ijrobp.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 157.Bernal P., Raoul J.-L., Stare J., et al. International atomic energy agency-sponsored multination study of intra-arterial rhenium-188-labeled lipiodol in the treatment of inoperable hepatocellular carcinoma: results with special emphasis on prognostic value of dosimetric study. Seminars in Nuclear Medicine. 2008;38(2):S40–S45. doi: 10.1053/j.semnuclmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 158.Kumar A., Srivastava D. N., Chau T. T. M., et al. Inoperable hepatocellular carcinoma: Transarterial 188Re HDD-labeled iodized oil for treatment - Prospective multicenter clinical trial. Radiology. 2007;243(2):509–519. doi: 10.1148/radiol.2432051246. [DOI] [PubMed] [Google Scholar]
- 159.Furtado R., Crawford M., Sandroussi C. Systematic review and meta-analysis of adjuvant I131 lipiodol after excision of hepatocellular carcinoma. Annals of Surgical Oncology. 2014;21(8):2700–2707. doi: 10.1245/s10434-014-3511-2. [DOI] [PubMed] [Google Scholar]
- 160.Brans B., Dierckx R. A., De Winter F., et al. The anti-tumoral activity of neoadjuvant intra-arterial 131I-lipiodol treatment for hepatocellular carcinoma: A pilot study. Cancer Biotherapy and Radiopharmaceuticals. 2001;16(4):333–338. doi: 10.1089/108497801753131417. [DOI] [PubMed] [Google Scholar]
- 161.Raoul J.-L., Messner M., Boucher E., Bretagne J.-F., Campion J.-P., Boudjema K. Preoperative treatment of hepatocellular carcinoma with intra-arterial injection of 131I-labelled lipiodol. British Journal of Surgery. 2003;90(11):1379–1383. doi: 10.1002/bjs.4271. [DOI] [PubMed] [Google Scholar]
- 162.Lambert B., Praet M., Vanlangenhove P., et al. Radiolabeled lipiodol therapy for hepatocellular carcinoma in patients awaiting liver transplantation: Pathology of the explant livers and clinical outcome. Cancer Biotherapy and Radiopharmaceuticals. 2005;20(2):209–214. doi: 10.1089/cbr.2005.20.209. [DOI] [PubMed] [Google Scholar]
- 163.de Baere T., Arai Y., Lencioni R., et al. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. CardioVascular and Interventional Radiology. 2016;39(3):334–343. doi: 10.1007/s00270-015-1208-y. [DOI] [PubMed] [Google Scholar]
- 164.Herber S., Otto G., Schneider J., et al. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. CardioVascular and Interventional Radiology. 2007;30(6):1156–1165. doi: 10.1007/s00270-007-9032-7. [DOI] [PubMed] [Google Scholar]
- 165.Risse J. H., Grünwald F., Strunk H., Kleinschmidt R., Bender H., Biersack H. J. I-131-Lipiodol therapy in liver neoplasms. Hybridoma. 1999;18(1):83–85. doi: 10.1089/hyb.1999.18.83. [DOI] [PubMed] [Google Scholar]
- 166.Novell J. R., Green A. J., Hilson A. J. W., Dusheiko G., Dick R., Hobbs K. E. F. Selective Radionuclide Localisation in Primary Liver Tumours. HPB Surgery. 1994;7:16. doi: 10.1155/1994/93101.093101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Giammarile F., Bodei L., Chiesa C., et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38(7):1393–1406. doi: 10.1007/s00259-011-1812-2. [DOI] [PubMed] [Google Scholar]
- 168.Stansby G., Bhattacharya S., Hilson A. J. W., Lane D. M., Hobbs K. E. F. Localization of Lipiodol-radioiodine in hepatic metastases from renal cell carcinoma. British Journal of Radiology. 1994;67(800):822–824. doi: 10.1259/0007-1285-67-800-822. [DOI] [PubMed] [Google Scholar]
- 169.Al-Mufti R. A. M., Pedley R. B., Marshall D., et al. In vitro assessment of Lipiodol-targeted radiotherapy for liver and colorectal cancer cell lines. British Journal of Cancer. 1999;79(11-12):1665–1671. doi: 10.1038/sj.bjc.6690266. [DOI] [PMC free article] [PubMed] [Google Scholar]