Abstract
DPP4 (dipeptidyl peptidase-4), a highly conserved transmembrane glycoprotein with an exo-peptidase activity, has been shown to contribute to glucose metabolism, immune regulation, signal transduction, and cell differentiation. Here, we show that DPP4 is involved in control of activin/nodal signaling in Xenopus early development. In support of this, gain of function of DPP4 augmented Smad2 phosphorylation as well as expression of target genes induced by activin or nodal signal. In addition, Dpp4 and Xnr1 showed synergistic effect on induction of ectopic dorsal body axis, when co-injected at suboptimal doses in early embryos. Conversely, saxagliptin, a DPP4 inhibitor repressed activin induction of Smad2 phosphorylation. Notably, overexpression of Dpp4 disrupted specification of dorsal body axis of embryo, leading to malformed phenotypes such as spina bifida and a shortened and dorsally bent axis. Together, these results suggest that DPP4 functions as a potentiator of activin/nodal signaling pathway.
Keywords: Activin/Nodal signaling, Dipeptidyl peptidase-4, DPP4, Embryo, Xenopus
INTRODUCTION
The transforming growth factor β (TGF-β) superfamily of secreted proteins has more than 30 members including TGF-βs, bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), and Activin and Nodal. These members are involved in regulation of early development and tissue homeostasis of metazoans (1–3). In particular, Activin/Nodal and BMPs are responsible for induction and subsequent patterning of mesoderm and endoderm germ layers, as well as for gastrulation in vertebrate early embryos (4–6). Signaling by TGF-β and related ligands uses two types of receptors, type I and type II transmembrane serine-threonine kinases. Ligand binding results in formation of heterotetrameric receptor complex in which type II receptor activates type I receptor via phosphorylation of its GS region. Subsequently, the activated type I receptor triggers phosphorylation of receptor-regulated Smads (R-Smads), such as Smad 1/5/8 in BMP pathway and Smad 2/3 in Activin, Nodal and TGF-β signaling pathways. Phosphorylated R-Smads interact with Co-Smad, Smad4 and then move into the nucleus to regulate transcription of a variety of target genes (1, 7).
DPP4 (dipeptidyl peptidase-4), also known as adenosine deaminase complexing protein 2 or CD26 (cluster of differentiation 26), is a highly conserved cell surface glycoprotein belonging to the serine protease family (8). It is ubiquitously expressed on a variety of tissues including immune, endothelial and epithelial cells (9). However, it also exists in soluble form in plasma and other body fluids (10). It consists of a short cytoplasmic domain, a transmembrane region and an extracellular domain with dipeptidyl peptidase activity, which selectively removes the N-terminal dipeptides such as X-Pro and X-Ala from polypeptide substrates (11). Activity of this transmembrane exo-peptidase exerts major influence on immune regulation, signal transduction, glucose metabolism, cell migration, and cell differentiation by controlling the activity of its substrates or by associating physically with other proteins (10). DPP4 acts indiscriminately on a broad range of substrates (12), including growth factors, chemokines, neuropeptides, and vasoactive peptides. In contrast to its crucial regulatory roles, DPP4 knockout mice show normal phenotypes, even with an ameliorated glucose metabolism and more physical activity (13). Either increase or decrease of DPP4 expression has been shown to underlie a variety of pathophysiological processes (9). Furthermore, it appears to work in the development of cancer and tumors (8, 14, 15).
In our initial attempt to identify the role of DPP4 in vertebrate early embryogenesis, it has been found that DPP4 is involved in control of TGF-β signaling. In this study, we present evidence that it specifically acts as a potentiator of activin/nodal signaling pathway.
RESULTS
DPP4 enhances downstream events induced by activin/nodal signal but not by BMP4 signal
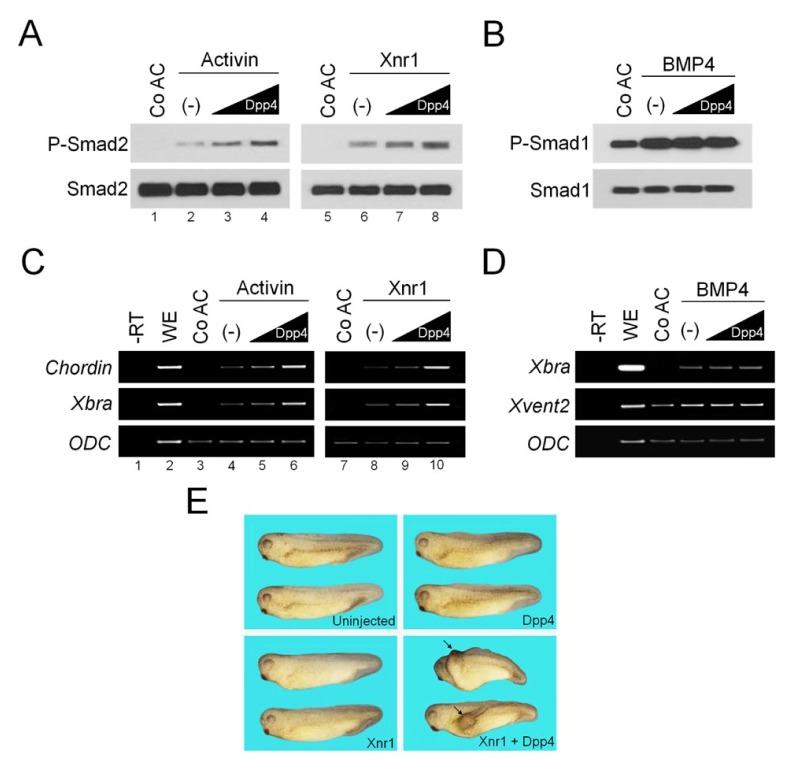
To unravel biological functions of DPP4 in Xenopus early development, we initially sought to investigate its activities in stem cell-like ectodermal tissue of early embryo. For this, we overexpressed Dpp4 mRNA in that tissue and analyzed its effects on downstream events induced in response to key cellular signalings, such as Activin/Nodal, BMP, FGF and Wnt signaling pathway. Overexpression of Dpp4 did not affect ERK phosphorylation induced by FGF signal and β-catenin stabilization by Wnt signal, as well as expression of target genes by both of them (data not shown). Of note, treatment with activin protein or injection of Xnr1 mRNA induced ectopically phosphorylation of Smad2, a downstream mediator of activin/nodal pathway, in ectodermal tissue (Fig. 1A, lanes 1, 2, 5 and 6), which could be further enhanced by expression of increasing doses of Dpp4 (Fig. 1A, lanes 3, 4, 7 and 8). In contrast, when co-injected, Dpp4 had no effect on phosphorylation of Smad1 induced in BMP4-stimulated explant tissues (Fig. 1B).
Fig. 1.
Dpp4 enhances activin/nodal signaling but not BMP4 signaling. (A–D) Four-cell stage embryos were injected in the animal pole region as indicated with increasing doses of Dpp4 (10, 1000 pg) alone or with Xnr1 (50 pg) or BMP4 (100 pg) mRNAs, and then animal cap explants were excised at stage 8.5 from injected or uninjected embryos, cultured to stage 10.5 for RT-PCR analysis (C and D) or 11 for Western blotting (A and B) in the presence or absence of activin protein (5 ng/ml) as shown. Smad1, Smad2 and ODC serve as loading controls. Co AC, uninjected control animal caps. (−), no injection of Dpp4. WE, stage 10.5 whole embryo. —RT, control in the absence of reverse transcriptase. (E) One blastomere of four-cell stage embryos was injected in the ventral marginal region with Dpp4 (50 pg) and/or Xnr1 (5 pg) mRNAs as indicated, and injected embryos were cultured until uninjected sibling embryos reached tadpole stages. Arrows denote the induced secondary dorsal axis. Embryos are shown in lateral views with anterior to the left.
Using RT-PCR analysis, we have also investigated whether the enhancing effects of DPP4 on activin/nodal signaling may be exerted at the transcriptional levels. As shown in Fig. 1C, ectopic expressions of mesodermal markers Chordin and Xbra were induced by stimulation with activin or Xnr1 in animal explants (lanes 3, 4, 7 and 8), and their expression levels were up-regulated gradually by co-injection of increasing amount of Dpp4 (lanes 5, 6, 9 and 10). In contrast, co-injection of Dpp4 did not influence increased levels of expression of target genes such as Xbra and Xvent2 induced by BMP4 in animal caps (Fig. 1D). These results are in line with the effects of DPP4 on levels of phosphorylated R-Smads in activin/nodal or BMP4-activated animal cap cells. Overall, we conclude that DPP4 acts as an enhancer of activin/nodal signaling, but not of BMP signaling.
DPP4 synergizes with nodal signal in inducing an ectopic dorsal axis of embryo
Injection of Xnr1 mRNA in the ventral region of embryos at the optimal levels can induce ectopically a secondary dorsal trunk with no head (16), whereas it causes no extra axis when injected at below optimal levels, i.e. suboptimal levels. Next, this secondary axis assay was used to test whether DPP4 could augment suboptimal levels of nodal signal, to levels sufficient to induce ectopic dorsal axis. As shown in Fig. 1E, single ventral injection of a range of concentration of Dpp4 could cause no secondary dorsal trunk. Likewise, Xnr1 did not induce any ectopic dorsal axis when injected at suboptimal levels. By contrast, the ectopic dorsal trunks were induced efficiently in embryos co-injected ventrally with Dpp4 and Xnr1 at the same doses. Thus, this synergism in inducing an ectopic dorsal axis supports that DPP4 may function to potentiate activin/nodal signaling pathway.
A DPP4 inhibitor suppresses Smad2 phosphorylation induced by activin signal
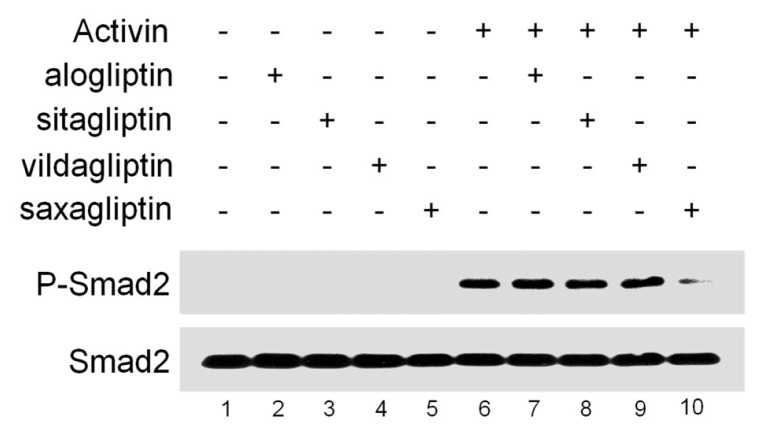
We next examined whether DPP4 activity is critical for activin/nodal signaling by using DPP4 inhibitors, including alogliptin, sitagliptin, vildagliptin and saxagliptin. Interestingly, stimulation with activin protein induced high levels of phosphorylated Smad2 in HEK293T cells (Fig. 2, lanes 1–6), which could be disturbed efficiently by treatment with saxagliptin but not with alogliptin, sitagliptin or vildagliptin (lanes 7–10). Since DPP4 inhibitors have unique drug-specific effects depending on the cell type (17), it is possible that only saxagliptin could interfere with DPP4 potentiation of activin signaling in HEK293T cells. Collectively, these data indicate that DPP4 activity is essential for activin/nodal signaling.
Fig. 2.
Saxagliptin, a DPP4 inhibitor represses Smad2 phosphorylation induced by activin. HEK293T cells were pre-treated for 1 hour with DPP4 inhibitors as indicated and subsequently incubated for 3 hours with or without activin (20 ng/ml), and harvested for Western blotting. Smad2 is a loading control.
Spatio-temporal expression pattern of Dpp4
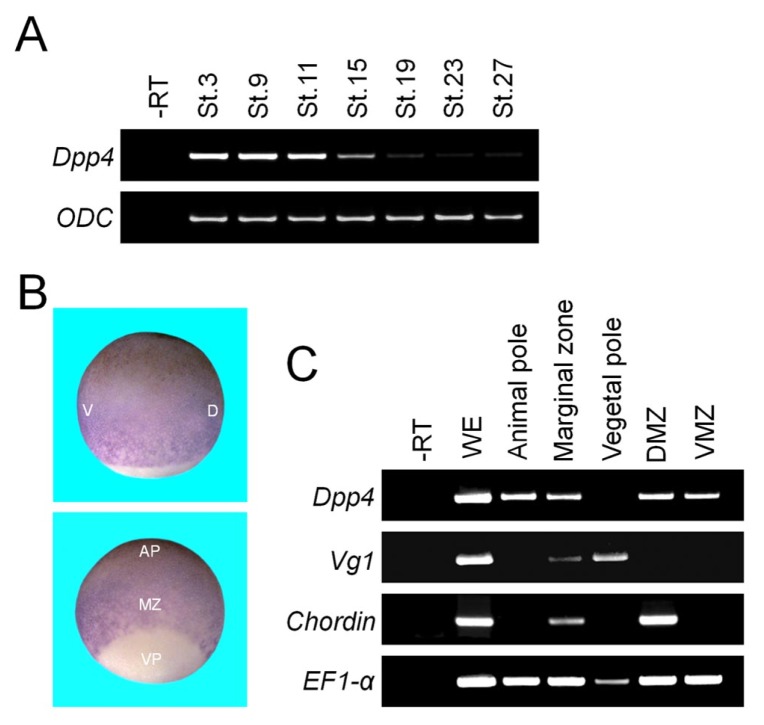
To investigate whether DPP4 enhancement of activin/nodal signaling could be observed at the organismal level during Xenopus early embryogenesis, we first examined the temporal expression pattern of Dpp4 by RT-PCR analysis, using cDNAs from whole embryos harvested at several embryonic stages. As shown in Fig. 3A, maternal mRNA messages of Dpp4 were abundant, but its zygotic transcription was barely detectable. In situ hybridization was also performed to determine the spatial expression pattern of Dpp4 in Xenopus early embryos. Of note, its expression was strong in the animal and marginal regions of early gastrulae and absent in the vegetal region (Fig. 3B). Consistently, this regional distribution of Dpp4 transcripts was also confirmed by RT-PCR experiment (Fig. 3C). Given that activin/nodal signal is responsible for mesoderm formation in the marginal zone at the blastula and gastrula stages (18), both the spatial and temporal expression patterns of Dpp4 transcripts suggest that it may function as a maternal factor to regulate formation of primitive germ layers.
Fig. 3.
Spatio-temporal expression pattern of Dpp4 in Xenopus early embryogenesis. (A) Temporal expression pattern of Dpp4 analyzed by RT-PCR. St., a developmental stage. ODC serves as a loading control. —RT, stage 27 control whole embryos in the absence of reverse transcriptase. (B and C) Whole mount in situ hybridization (B) and RT-PCR (C) showing spatial expression pattern of Dpp4 transcripts at stage 10.25. Embryos in (B) are shown in lateral (upper panel) or dorso-vegetal (lower panel) view with animal to the top. For (C), respective regional explants were dissected from stage 10.25 whole embryos. D, dorsal; V, ventral; VP, vegetal pole; MZ, marginal zone; AP, animal pole; DMZ, dorsal marginal zone; VMZ, ventral marginal zone. Chordin is a dorsal mesodermal marker, Vg1 is a member of TGF-β family expressed vegetally, and EF1-α is a loading control. —RT, stage 10.25 control whole embryos in the absence of reverse transcriptase.
Overexpression of Dpp4 disrupts formation of dorsal axial structures
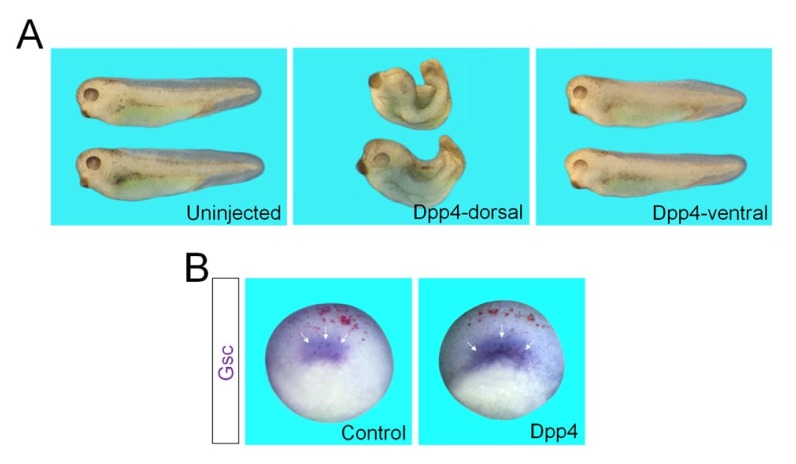
To determine whether DPP4 could affect germ layer specification, we next overexpressed Dpp4 mRNA into the animal, marginal or vegetal regions of Xenopus cleavage stage embryos, and observed resulting morphological phenotypes at the tadpole stages. In particular, when injected in the dorsal marginal region, Dpp4 caused a failure in gastrulation movements, which led to spina bifida and a reduced and dorsally kinked body axis (Fig. 4A). These malformed phenotypes are reminiscent of those of embryos exposed to increased levels of activin/nodal signals (19, 20), substantiating that DPP4 acts to up-regulate these signals. However, ventral injection of Dpp4 generated no morphological alterations (Fig. 4A). We also checked the effects of overexpression of Dpp4 on endogenous expression of mesodermal marker in early gastrulae. Notably, expression of a dorsal mesodermal marker Goosecoid was expanded in the LacZ staining-positive area of embryo overexpressing Dpp4 (Fig. 4B), compared with that in the control embryo injected with LacZ alone. Together, these results indicate that gain-of-function of DPP4 up-regulates expression of target genes of activin/nodal signal such as Goosecoid, thereby disrupting axial structures of Xenopus embryo.
Fig. 4.
Embryonic morphological phenotypes caused by overexpression of Dpp4. (A) Four-cell stage embryos were injected in the dorsal or ventral marginal regions with Dpp4 (500 pg) mRNA and cultured to tadpole stages. Embryos are shown in lateral views with anterior to the left. Uninjected, uninjected control embryos. (B) Four-cell stage embryos were injected in the dorsal marginal region with LacZ (50 pg) alone or with Dpp4 (1 ng) mRNA, cultured to stage 10.25, and fixed for LacZ staining (red) and subsequent in situ hybridization against Goosecoid (Gsc). Arrows indicate expression of Gsc in the dorsal lip. Embryos are shown in dorso-vegetal views with anterior to the top. Control, an embryo injected with LacZ alone.
DISCUSSION
It has been shown that DPP4 knockout mice are phenotypically normal, though it plays critical roles in immune regulation, cell migration, cell differentiation, glucose metabolism, and signal transduction (10). In this study, we have thus re-investigated the function of DPP4 at the organismal level using Xenopus early embryos with the result that it may act as a potentiator of activin/nodal signal to control germ layer specification. As shown by developmental RT-PCR analysis, strong expression of Dpp4 during the blastula and gastrula stages of embryos suggests its possible role as a maternal factor in early induction of germ layers. Given that in Xenopus, mesoderm is induced in the marginal zone of blastulae by signals from its vegetal region (21), spatial expression of Dpp4 in early gastrulae also indicates its probable function in mesoderm formation. In support of this, overexpression of Dpp4 caused morphological changes in embryos, including spina bifida, exo-gastrulation, and a shortened and kinked body axis, which recapitulate defective phenotypes of embryos experiencing up-regulated levels of mesoderm-inducing signal, nodal (22). Knockdown of Xenopus Lefty, a secreted antagonist of nodal signaling pathway, causes expansion of mesendoderm, thereby leading to exo-gastrulation (19). In addition, depletion of maternal Zic2 transcription factor, which impedes the expression of nodal-related genes during anteroposterior patterning, results in the same phenotypes as enhanced nodal signaling (20). Since gain-of-function of DPP4 causes the same embryonic defects as excess Nodal signal, it seems likely that DPP4 acts as a positive regulator to control nodal signaling. Consistently, overexpression of Dpp4 enhanced phosphorylation of Smad2 and transcription of target genes induced by activin or nodal signal. In contrast, DPP4 did not affect phosphorylation of Smad1 and expression of target genes induced by BMP4 signal. Furthermore, ventral injection of Dpp4 produced no morphological alteration, though BMP4 signal is highly active in the ventral region of embryo. Taking together, we conclude that DPP4 functions to potentiate only activin/nodal signal, but not BMP signal during formation of primitive germ layers.
DPP4 has been known to exist not only as a transmembrane glycoprotein but also in a secreted soluble form (10). This feature of DPP4 suggests that it may act either as a cell surface protein or as an extracellular matrix component to control activin/nodal signaling. Given the enhancing effects of DPP4 on Smad2 phosphorylation induced by activin or nodal ligand, it is possible that DPP4 facilitates access of the ligand to its cognate receptor and co-receptor, by interacting with the former on cell surface or outside the cell. Activin, nodal and BMP ligands share type II receptors for signaling, such as activin receptor type 2A (ACVR2A) and ACVR2B (2). Activin and nodal, but not BMP ligands, share type I receptors including ALK2, ALK4, and ALK7. As DPP4 augments activin as well as nodal signaling pathways but not BMP4 signaling, it may also promote assembly of the receptor complex, triggered by ligand binding, by associating with type I receptors shared by activin and nodal. In addition, it cannot be excluded that DPP4 may function as an exo-peptidase to modulate activity of substrates for regulation of activin/nodal signaling. Substrates may include activin/nodal ligands, their receptors, or extracellular enzymes responsible for ligand processing.
It is of note that among DPP4 inhibitors tested in this study, only saxagliptin disturbed activin induction of Smad2 phosphorylation but not alogliptin, sitagliptin and vildagliptin. While these inhibitors act commonly by inhibiting the catalytic activity of the DPP4 enzyme, they display unique drug-specific effects, depending upon the cell type (17). Unlike alogliptin and sitagliptin, saxagliptin and vildagliptin inhibit DPP4 by forming covalent enzyme-inhibitor complexes characterized by slow rates of binding and dissociation, which results in their relatively prolonged duration of activities (23). In addition, the half maximal inhibitory concentration (IC50) of saxagliptin is lower than that of vildagliptin, albeit in the low nanomolar range. These features of saxagliptin may contribute to its unique inhibition of activin signaling in HEK294T cells as shown in our data. However, more experiments are necessary to elucidate the precise mechanism underlying inhibition of activin signaling by saxagliptin.
In summary, we have found that DPP4 functions to enhance activin/nodal signaling for mesoderm formation in Xenopus early embryos. Future studies are warranted to identify activin/nodal signaling components with which DPP4 interacts, and to check the requirement of its exo-peptidase activity for this signaling, to gain deep insight into the molecular mechanism by which DPP4 potentiates activin/nodal signaling pathway.
MATERIALS AND METHODS
Embryo and in vitro fertilization
Xenopus laevis were obtained from NASCO & Korean Xenopus Resource Center for Research. Unfertilized eggs were in vitro fertilized using macerated testis, dejellied in 2% cysteine buffer (pH 8.0), and cultured in 0.33 × Modified Ringer (MR) until stage 8 and then transferred to 0.1 × MR with gentamycin. Developmental stages of embryos were determined according to Nieuwkoop and Faber’s normal table of development (24).
DNA constructs and RNA synthesis
The complete coding region of Xenopus laevis Dpp4 was amplified by PCR and inserted in the sites of pCS2+ vector (Dpp4-CS2). DNA constructs used in this study are as follows: Xnr1-CS2+ (21) and BMP4-CS2+ (25). Capped mRNAs were synthesized by in vitro transcription of NotI-linearized DNA constructs using mMESSAGE mMACHINE RNA synthesis kit (Ambion) and SP6 RNA polymerase.
Animal cap assay
Animal pole cells from Xenopus blastula stage embryos are pluripotent, and can differentiate into a variety of cell types depending on the signals they are exposed to. Thus, the stem cell-like animal cap explants are dissected at stage 8–8.5 from embryos animally injected with specific RNAs, which encode secreted signaling molecules or transcriptional regulators, cultured to desired stages in 1 × MR containing bovine serum albumin (BSA, 10 μg/ml) and gentamicin (50 μg/ml), and then subjected to further experiments.
RT-PCR (reverse transcriptase-polymerase chain reaction)
Total RNA was isolated from whole embryos and explant tissues using TRI Reagent (Sigma). For RT-PCR, the method of Wilson and Melton (26) was broadly followed except that DNA amplification was performed non-radioactively. Approximately 5 μg of DNase I-treated total RNA was reverse transcribed using a random hexamer and M-MLV reverse transcriptase (Promega). PCR reactions were performed in a standard 50 μl PCR reaction with Taq polymerase (TaKaRa). Primers were: Dpp4, (forward) 5′-GTGGGAATTGGGAAGTAACC-3′, (reverse) 5′-GTGGGCATCTGAATCTCATC-3′; Xbra, (forward) 5′-GCTGGAAGTAT GTGAATGGAG-3′, (reverse) 5′-TTAAGTGCTGTAATCTCTTCA-3′; Chordin, (forward) 5′-CCTCCAATCCAAGACTCCAGCAG-3′, (reverse), 5′-GGAGGAGGAGGAGCTTTGG GACAAG-3′; Vg1, (forward) 5′-ATGCCTATTGCTTCTATTTGC-3′, (reverse) 5′-GGTTTA CGATGGTTTCAC TCA-3′; ODC, (forward) 5′-GTCAATGATGGAGTGTATGGATC-3′, (reverse), 5′-TCCATTCCGCTCTCCTGAGCAC-3′; Xvent2, (forward) 5′-TGAGACTTGGG CACTGTCTG-3′, (reverse), 5′-CCTCTGTTGAATGGCTTGCT-3′; EF1-α, (forward) 5′-CCTGAACCACCCAGGCCAGATTGGTG-3′, (reverse) 5′-GAGGGTAGTCAGAGAAGC TCTCCACG-3′.
In situ hybridization
Whole-mount in situ hybridization was performed as described in (27). Anti-sense digoxigenin RNA probes were in vitro synthesized using template cDNA encoding Dpp4 and Gsc (28). For anti-sense Dpp4 probe, Dpp4-pGEM T vector was linearized with NcoI and transcribed with SP6 RNA polymerase. BM purple (Roche) was used as substrate for alkaline phosphatase.
Western blotting analysis
For Western blot analysis, animal cap explants or HEK293T cells were homogenized in Triton X-100 lysis buffer (1% Triton X-100, 50 mM NaCl, 1 mM EDTA, 50 mM Tris-Cl (pH 7.6), 10 mM NaF, 1 mM Na3VO4, 1mM PMSF, 20 μg/ml Aprotinin, 20 μg/ml Leupeptin). Equal amounts of protein were separated by 10 or 8% SDS–PAGE electrophoresis. Western blots were performed, according to standard protocol with P-Smad1 (Cell signaling, 1:1,000), Smad1 (Cell signaling, 1:1,000), P-Smad2 (cell signaling, 1:1,000) and Smad2 (Cell signaling, 1: 1000) primary antibodies.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016R1A2B4013355) and by the Ministry of Education (2017R1A6A3A01008239).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nat Rev Cancer. 2013;13:328–341. doi: 10.1038/nrc3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dale L, Jones CM. BMP signalling in early Xenopus development. Bioessays. 1999;21:751–760. doi: 10.1002/(SICI)1521-1878(199909)21:9<751::AID-BIES6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 6.Whitman M. Nodal signaling in early vertebrate embryos: themes and variations. Dev Cell. 2001;1:605–617. doi: 10.1016/S1534-5807(01)00076-4. [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 8.Pro B, Dang NH. CD26/dipeptidyl peptidase IV and its role in cancer. Histol Histopathol. 2004;19:1345–1351. doi: 10.14670/HH-19.1345. [DOI] [PubMed] [Google Scholar]
- 9.Glorie L, D’Haese PC, Verhulst A. Boning up on DPP4, DPP4 substrates, and DPP4-adipokine interactions: Logical reasoning and known facts about bone related effects of DPP4 inhibitors. Bone. 2016;92:37–49. doi: 10.1016/j.bone.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 11.Lone AM, Nolte WM, Tinoco AD, Saghatelian A. Peptidomics of the prolyl peptidases. AAPS J. 2010;12:483–491. doi: 10.1208/s12248-010-9208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X. Biochemical properties of recombinant prolyl dipeptidases DPP-IV and DPP8. Adv Exp Med Biol. 2006;575:27–32. doi: 10.1007/0-387-32824-6_3. [DOI] [PubMed] [Google Scholar]
- 13.Marguet D, Baggio L, Kobayashi T, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masur K, Schwartz F, Entschladen F, Niggemann B, Zaenker KS. DPPIV inhibitors extend GLP-2 mediated tumour promoting effects on intestinal cancer cells. Regul Pept. 2006;137:147–155. doi: 10.1016/j.regpep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Wesley UV, McGroarty M, Homoyouni A. Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway. Cancer Res. 2005;65:1325–1334. doi: 10.1158/0008-5472.CAN-04-1852. [DOI] [PubMed] [Google Scholar]
- 16.Niederlander C, Walsh JJ, Episkopou V, Jones CM. Arkadia enhances nodal-related signalling to induce mesendoderm. Nature. 2001;410:830–834. doi: 10.1038/35071103. [DOI] [PubMed] [Google Scholar]
- 17.Shi S, Kanasaki K, Koya D. Linagliptin but not Sitagliptin inhibited transforming growth factor-beta2-induced endothelial DPP-4 activity and the endothelial-mesenchymal transition. Biochem Biophys Res Commun. 2016;471:184–190. doi: 10.1016/j.bbrc.2016.01.154. [DOI] [PubMed] [Google Scholar]
- 18.Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branford WW, Yost HJ. Lefty-dependent inhibition of Nodal- and Wnt-responsive organizer gene expression is essential for normal gastrulation. Curr Biol. 2002;12:2136–2141. doi: 10.1016/S0960-9822(02)01360-X. [DOI] [PubMed] [Google Scholar]
- 20.Houston DW, Wylie C. Maternal Xenopus Zic2 negatively regulates Nodal-related gene expression during anteroposterior patterning. Development. 2005;132:4845–4855. doi: 10.1242/dev.02066. [DOI] [PubMed] [Google Scholar]
- 21.Osada SI, Wright CV. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- 22.Yun CH, Choi SC, Park E, et al. Negative regulation of Activin/Nodal signaling by SRF during Xenopus gastrulation. Development. 2007;134:769–777. doi: 10.1242/dev.02778. [DOI] [PubMed] [Google Scholar]
- 23.Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. 2012;51:501–514. doi: 10.1007/BF03261927. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Pub; New York: 1994. [Google Scholar]
- 25.Maeno M, Ong RC, Suzuki A, Ueno N, Kung HF. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci U S A. 1994;91:10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson PA, Melton DA. Mesodermal patterning by an inducer gradient depends on secondary cell-cell communication. Curr Biol. 1994;4:676–686. doi: 10.1016/S0960-9822(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 27.Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/S0091-679X(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 28.Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-A. [DOI] [PMC free article] [PubMed] [Google Scholar]