Abstract
Aim
The aim of this study was to investigate whether elderly people with impaired fasting glucose (IFG) or diabetes mellitus (DM) share the common risk factors for cognitive impairment as compared to normal blood glucose population.
Methods
This cross-sectional study assessed 10,039 community-dwelling participants aged ≥ 55 years in Beijing, China. According to the glycemic status, subjects were classified into three groups: normal fasting plasma glucose (NG, n=6399), impaired fasting glucose (IFG, n=873) and DM (n=2626). The Mini-Mental State Examination (MMSE) was applied to evaluate the cognitive function status of the study population. Potential demographic, clinical, and genetic risk factors for cognitive impairment were collected and compared across the three groups. Multivariate logistic regression model was performed to explore the risk factors associated with cognitive impairment.
Results
Education-modified MMSE scores in the participants with NG, IFG, and DM were 26.91±3.94, 26.67±4.00, and 26.58±4.11, respectively (P=0.0008). In the age- and sex-adjusted comparisons, the MMSE scores in subjects with DM and IFG were significantly lower than that in subjects with normal glucose (P=0.01 and P=0.02, respectively). The logistic regression analysis showed that risk factors only in the NG population were older age, female, apoEε4 carrier, normal or lower uric acid (UA) levels. Hypertension was an independent risk factor only in IFG group, and the history of stroke and depression were the risk factors associated with cognitive impairment only in the DM group.
Conclusion
Subjects with DM or IFG had a lower performance on the MMSE test compared with subjects who had normal blood glucose. The elderly with diabetes and IFG have some different risk factors for cognitive impairment as compared to those with normal blood glucose.
Keywords: cognition, pre-diabetes, dementia, cognitive decline, Mini-Mental State Examination
Introduction
Impairment of blood glucose level in old age is a very common phenomenon worldwide.1,2 At the same time, age-associated cognitive decline is another major health issue in old age.3 Moreover, with the population rapidly aging in China, these health issues could represent a major threat to the public health, in particular, given the increasing notion that altered blood glucose could lead to cognitive impairment in old age, which is also known to be early sign of dementia.4,5
Majority of the past studies have centered their focus on the impact of diabetes mellitus (DM) on cognitive decline in old age. However, a large portion of older adults in the community are known to have impaired fasting glucose (IFG) only (ie, with pre-diabetes).1 To our knowledge, studies investigating the association of IFG and cognitive impairment in the community-dwelling older Chinese population are sparse. Even among those few studies across the globe, results remain controversial.4,6,7 For instance, a study has shown pre-diabetic women to have impaired cognitive performance with greater risk of developing cognitive impairment.8 While another population-based cohort study has suggested that DM but not IFG to be associated with a higher decline of cognitive functions.9 The controversy is possibly due to methodological limitations including low sample size and the lack of diversity of selected participants. Furthermore, since older people with DM and IFG are particularly vulnerable to cognitive impairment, it is even more critical to identify those at high risk and the specific risk factors. Such findings could play an extremely important role to reduce the increasing burden of cognitive decline (including conditions such as dementia) in the Chinese community.
Therefore, we ought to investigate the association between elevated blood glucose level and cognitive impairment in a large representative community-dwelling Chinese older population. The objective of our study was to examine if the cognitive function of the elderly subjects with DM and IFG is worse than that of the elderly with normal blood glucose, and whether these population with elevated blood glucose share common risk factors as normal blood glucose population.
Materials and methods
Materials
The data for this article were obtained from the Beijing Longitudinal Study of Aging II study, which was initiated in 2009.10 A multistage cluster random sampling method was performed to select a representative community cohort. Long-term residents with age ≥55 years in each household were randomly selected from three urban districts and one rural county in Beijing.11 Total number of elderly people recruited was 10,039 from 11,214 community-living participants. Two follow-up studies were performed in 2010 and 2013. However, the follow-up study in 2013 included only participants living in urban areas. For this current analysis, 9,898 subjects with valid Mini-Mental State Examination (MMSE) and measures for serum glucose scores were included.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Xuanwu Hospital of the Capital Medical University. All patients provided written informed consent prior to undergoing any study-related procedures.
Methods
Data collection
A well-designed questionnaire and related clinical measurements and information were collected, which included demographic data (eg, age, gender, residence, marriage status, and education level), lifestyle factors (smoking, alcohol consumption, sleep, and exercise), diseases history (stroke, hypertension, DM, hypercholesterolemia, CVD, etc), medications, functional assessments, cognitive function, and blood samples. Fasting blood samples (12-hour overnight) were taken to test fasting blood glucose (FBG), total cholesterol (TCH), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), blood urea nitrogen, serum creatinine (CRE), and serum uric acid (sUA) for all subjects at baseline. All assays were conducted at the Laboratory of Analytical Biochemistry at Xuanwu Hospital, using automatic biochemical analyzer (BioTek Instrument, Inc., Beijing, China). In addition, genomic DNA was extracted from peripheral blood leukocytes via a modified phenol/chloroform extraction procedure. The apolipoprotein E (APOE) genotype was examined using one-stage PCR as described by Wenham et al.12 The MMSE and Geriatric Depression Scale-15 items (GDS-15) were performed on the day prior to blood testing. Depression was defined as GDS>5.13 Height, weight, and waist circumference were measured using a standard protocol for subjects during the physical examinations.14 Body mass index (BMI) was calculated as weight (kg) divided by squared height (m2).
Definitions
The criteria recommended by the Working Group on Obesity in China was incorporated to define obesity and abdominal obesity.15 Overweight and obesity were defined as BMI 25–28 and ≥28 kg/m2, respectively. Smoking status was referred to a current smoking habit of an individual. Hyperuricemia was defined as a serum uric acid (sUA) level >416 μmol/L in men and >356 μmol/L in women.16 The cutoff for high triglycerides was ≥2.26 mmol/L, for high TCH ≥6.22 mmol/L, for high LDL ≥4.14 mmol/L, and for low HDL <1.04 mmol/L. Subjects with cardiovascular diseases included participants with a history of coronary heart disease, atrial fibrillation, myocardial infarction, angina (self-report or cardiac medications use), or congestive heart failure. Stroke was defined as a history of previously diagnosed stroke by a hospital. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, use of antihypertensive medicines, or having already-diagnosed hypertension.
Cognitive assessment
A validated Chinese version of MMSE was performed to evaluate the cognitive function of the participants.17 The 30-item MMSE is a global test that evaluates orientation, attention, calculation, language, and recall. Cognitive impairment was defined as: MMSE ≤17 for illiterates; MMSE ≤20 for primary school graduates (≥6 years of education); and MMSE ≤24 for junior school graduates or above (≥9 years of education).18
Classification of groups
According to the 1999 World Health Organization diagnostic criteria,19 the subjects were classified into three groups:
Normal FBG (NG): Fasting glucose <6.1 mmol/L, n=6,399
Impaired fasting glucose (IFG): Fasting glucose ≥6.1 mmol/L <7.0 mmol/L in the absence of diabetes history, n=873
DM: Fasting glucose ≥7.00 mmol/L, or having pre-diagnosed type 2 diabetes, n=2,626.
Statistical analyses
Comparison of categorical variables among the three groups was performed via chi-squared tests, and Student’s t-test and ANOVA was used to compare the means of the groups for continuous variables. The participants were stratified by NG, IFG, and DM, and a multivariate logistic regression model was applied (multivariate analyses) to determine the association of potential risk factors with the presence of cognitive impairment in participants with NG, IFG, and DM. The logistic regression model was adjusted for age, gender, location, lifestyle, marital status, high UA, BMI ≥28 kg/m2, alcohol drinking, smoking, APOE ε4 carrier, hypertension, stroke, GDS >5, cardiovascular disease, high TCH, high TG, high LDL, and low HDL. The results are presented as OR and their 95% CI. Two-sided P-value was determined and P<0.05 was considered to be statistically significant. All statistical analyses for this study were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the study sample according to glycemic status
The characteristics of the three groups (NG, IFG, and DM) are shown in Table 1. The mean age of the participants was the highest among DM subjects (71.21±7.41 years), followed by NG participants (70.35±7.80 years), with the lowest among IFG subjects (69.87±7.86 years). Subjects with DM had the highest percentage of urban living, followed by NG group, and IFG group (82.42%, 77.62%, and 70.74%, respectively). There were no differences in gender, education level, apoEε4 carrier, and LDL cholesterol level between the three groups. Consistent with previous studies, BMI, TG, FBG, obesity, and SBP were higher in IFG and DM groups and so was the prevalence of hypertension and cardiovascular diseases (Table 1).
Table 1.
Characteristics of the study sample according to glycemic status
| Characteristics | NG | IFG | DM | P-value |
|---|---|---|---|---|
|
| ||||
| Total subjects, n | 6,399 | 873 | 2,626 | |
| Cognitive impairment, n (%) | 566 (8.85) | 82 (9.39) | 260 (9.9) | 0.28 |
| MMSE score, mean (SD) | 26.91±3.94 | 26.67±4.00 | 26.58±4.11 | 0.0008 |
| Sociodemographic | ||||
| Age, mean (SD), years | 70.35±7.80 | 69.87±7.86 | 71.21±7.41 | <0.0001 |
| Male, n (%) | 2,499 (38.98) | 357 (40.8) | 976 (37.05) | 0.09 |
| Urban, n (%) | 4,976 (77.62) | 619 (70.74) | 2,171 (82.42) | <0.0001 |
| Education level, n (%) | ||||
| Illiteracy | 680 (10.61) | 108 (12.34) | 303 (11.50) | 0.05 |
| Primary school graduates | 1,730 (26.98) | 235 (26.86) | 770 (29.23) | |
| Junior school graduates or above | 4,001 (62.41) | 532 (60.8) | 1,561 (59.26) | |
| Lifestyle, n (%) | ||||
| Married | 5,225 (81.50) | 726 (82.97) | 2082 (79.04) | 0.007 |
| Exercise <0.5 hours | 1,413 (22.11) | 208 (23.85) | 617 (23.46) | 0.25 |
| Sleep <6 hours | 1,125 (17.60) | 123 (14.11) | 467 (17.76) | 0.03 |
| Alcohol use, n (%) | 769 (12.00) | 127 (14.51) | 221 (8.39) | <0.0001 |
| Smoking, current, n (%) | 884 (13.79) | 117 (13.37) | 287 (10.90) | 0.0009 |
| Genetic | ||||
| apoEε4 carrier, n (%) | 472 (8.98) | 78 (10.44) | 215 (10.18) | 0.17 |
| Clinical | ||||
| BMI, mean (SD), kg/m2 | 24.68±3.37 | 25.55±3.49 | 25.25±3.35 | <0.0001 |
| Total cholesterol (mmol/L) | 5.36±1.00 | 5.48±1.00 | 5.36±1.11 | 0.003 |
| Triglycerides (mmol/L) | 1.49±0.80 | 1.70±1.00 | 1.73±1.12 | <0.0001 |
| HDL cholesterol (mmol/L) | 1.40±0.35 | 1.32±0.33 | 1.31±0.35 | <0.0001 |
| LDL cholesterol (mmol/L) | 3.30±2.01 | 3.36±0.84 | 3.28±0.89 | 0.49 |
| Fasting blood glucose (mmol/L) | 5.20±0.52 | 6.46±0.25 | 8.08±2.66 | <0.0001 |
| Systolic blood pressure (mmHg) | 127.52±11.90 | 130.02±12.72 | 129.51±12.58 | <0.0001 |
| Diastolic blood pressure (mmHg) | 78.10±7.91 | 79.198±.02 | 77.66±8.10 | <0.0001 |
| Uric acid (mmol/L) | 310.11±82.75 | 323.89±89.22 | 309.81±84.77 | <0.0001 |
| Obesity (BMI ≥28 kg/m2), n (%) | 973 (15.24) | 187 (21.49) | 507 (19.34) | <0.0001 |
| High waist circumference, n (%) | 4,564 (71.60) | 684 (78.71) | 2060 (78.51) | <0.0001 |
| Stroke | 870 (13.61) | 112 (12.84) | 449 (17.07) | <0.0001 |
| Hypertension | 3,649 (57.11) | 574 (65.90) | 1,836 (69.84) | <0.0001 |
| Cardiovascular disease* | 1,235 (19.32) | 186 (21.33) | 669 (25.44) | <0.0001 |
| GDS >5, n (%) | 607 (10.37) | 67 (8.59) | 286 (11.70) | 0.03 |
Notes:
Myocardial infarction, coronary heart disease, atrial fibrillation, congestive heart failure, or angina. The “P” significance in the table refers to “trend of groups.”
Abbreviations: apoEε4, apolipoprotein Eε4; DM, diabetes mellitus; BMI, body mass index; GDS, Geriatric Depression Scale; IFG, impaired fasting glucose; MMSE, Mini-Mental State Examination; NG, normal fasting plasma glucose.
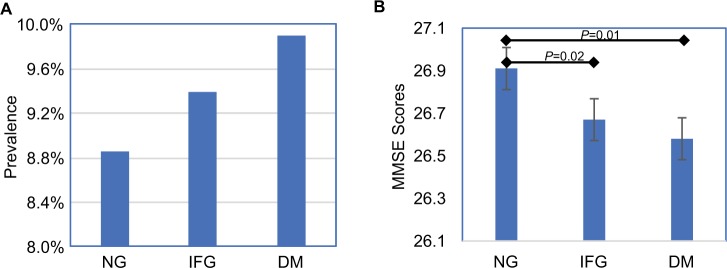
Among the 9,898 subjects with valid MMSE scores, 915 (9.14%) were classified as cognitively impaired. The prevalence of cognitive impairment in the participants with NG, IFG, and DM group was 8.85%, 9.39%, and 9.90%, respectively (Figure 1A).
Figure 1.
(A) Prevalence of cognitive impairment. (B) MMSE score based on glycemic status. Analyses are adjusted for age and sex.
Abbreviations: DM, diabetes mellitus; IFG, impaired fasting glucose; MMSE, Mini-Mental State Examination; NG, normal fasting plasma glucose.
Although there was an augmentation trend of cognitive impairment prevalence in those with IFG or DM, it was not statistically significant (P=0.28). However, the mean MMSE scores were significantly different among the three groups (P=0.0008) (Table 1). The MMSE score in DM and IFG group were significantly lower than that in NG group (P=0.01, P=0.02, respectively) and adjusted for age and gender (Figure 1B).
The percentage of stroke was the highest among DM subjects, followed by NG participants, with the lowest among IFG subjects (17.07%, 13.61%, and 12.84%, respectively) and so was the percentage of depression (11.70%, 10.37%, and 8.59%, respectively). The highest percentage of hypertension occurred in diabetic subjects, followed by IFG subjects, and then NG group (69.84%, 65.90%, and 57.11%, respectively) (Figure 2).
Figure 2.
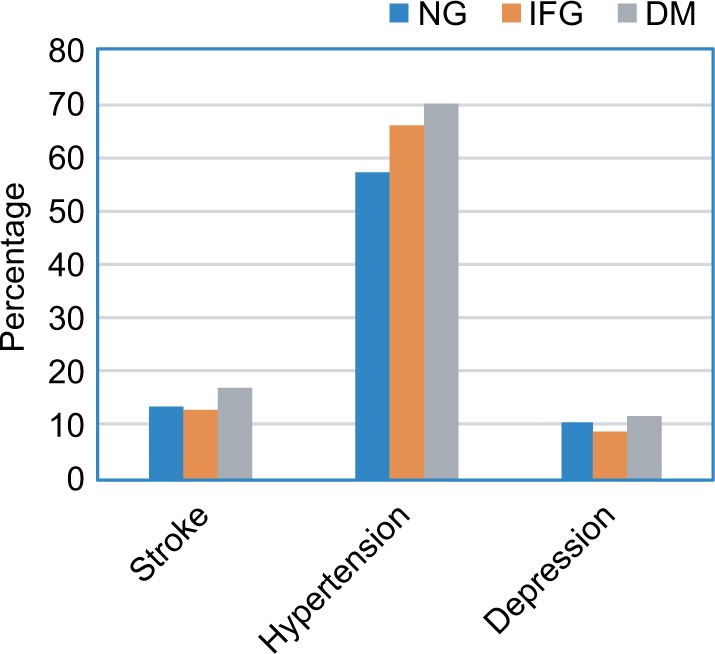
The percentage of stroke, hypertension, and depression in each of the three glycemic groups.
Abbreviations: DM, diabetes mellitus; IFG, impaired fasting glucose; MMSE, Mini-Mental State Examination; NG, normal fasting plasma glucose.
There was significant difference in the prevalence of cognitive impairment between the three groups for the participants with stroke and hypertension (P=0.038 and P=0.042, respectively). The highest prevalence of cognitive impairment occurred in the IFG group with hypertension or depression. The prevalence of cognitive impairment was the highest in DM group for participants with stroke (Figure 3).
Figure 3.
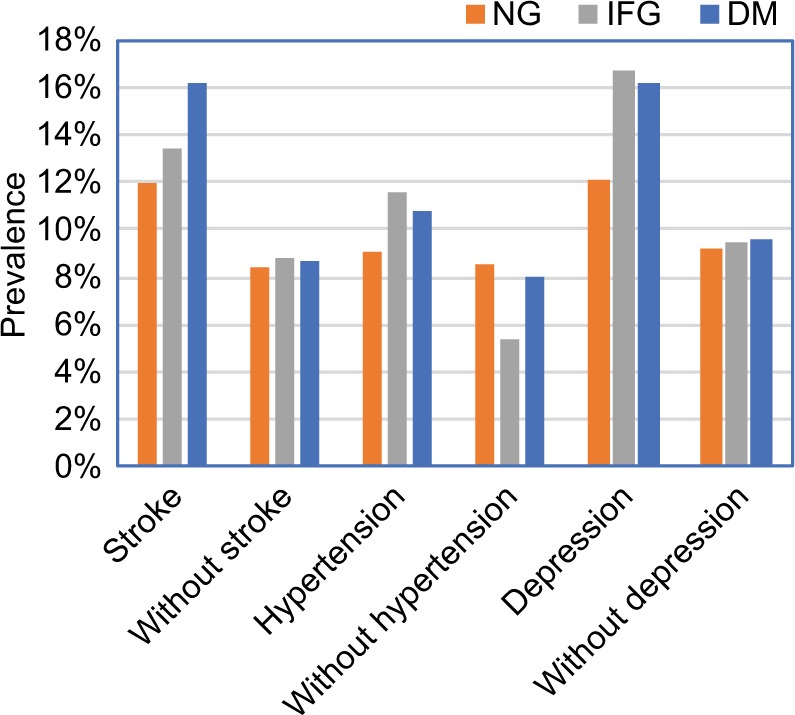
The prevalence of cognitive impairment for the participants with and without stroke, hypertension, and depression in each of the NG, IFG, and DM groups.
Abbreviations: DM, diabetes mellitus; IFG, impaired fasting glucose; MMSE, Mini-Mental State Examination; NG, normal fasting plasma glucose.
Characteristics based on cognitive function
Individuals with cognitive impairment were older in all three groups (Table 2). The percentages of living in urban, being married, and having sleep <6 hours per day were higher in subjects with cognitive impairment compared to subjects with normal cognition in each of the three groups. In the NG and DM groups, individuals with cognitive impairment showed significant increased prevalence of stroke and depression (GDS>5).
Table 2.
Characteristics based on cognitive function
| Characteristics | NG
|
IFG
|
DM
|
|||
|---|---|---|---|---|---|---|
| Cognitive
|
Normal n=5,833 | Cognitive
|
Normal n=791 | Cognitive
|
Normal n=2,366 | |
| Impairment n=566 | Impairment n=82 | Impairment n=260 | ||||
|
| ||||||
| MMSE score, mean (SD) | 19.04±4.71*** | 27.73±2.62 | 19.32±4.61*** | 27.50±0.10 | 19.29±4.39*** | 27.47±2.78 |
| Sociodemographic | ||||||
| Age, years (SD) | 72.81±8.27*** | 70.09±7.71 | 73.20±7.03*** | 69.52±7.85 | 73.51+7.14*** | 70.93+7.47 |
| Male, n (%) | 197 (34.81)* | 2,296 (39.36) | 29 (35.37) | 327 (41.34) | 89 (34.23) | 884 (37.36) |
| Urban, n (%) | 370 (65.37)*** | 4,600 (78.86) | 50 (60.98)** | 568 (71.81) | 196 (75.38)** | 1,971 (83.31) |
| Education level, n (%) | ||||||
| Illiteracy | 98 (17.31) | 582 (9.96) | 17 (20.73) | 91 (11.48) | 31 (11.92) | 267 (11.28) |
| Primary school graduates | 129 (22.79) | 1,601 (27.39) | 18 (21.95) | 217 (27.36) | 69 (26.54) | 698 (29.50) |
| Junior school graduates or above | 339 (59.89) | 3,662 (62.65) | 47 (57.32) | 485 (61.16) | 160 (61.54) | 1,401 (59.22) |
| Lifestyle, n (%) | ||||||
| Married | 410 (72.44)*** | 4,804 (82.36) | 58 (70.73)** | 667 (84.32) | 187 (71.92)** | 1,891 (79.92) |
| Exercise <0.5 hours | 168 (29.73)*** | 1,237 (21.27) | 26 (31.71) | 181 (22.97) | 90 (34.62)*** | 524 (22.18) |
| Sleep <6 hours | 151 (26.73)*** | 971 (16.70) | 20 (24.39)** | 103 (13.07) | 63 (24.23)** | 404 (17.10) |
| Alcohol use | 71 (12.54) | 697 (11.95) | 8 (9.76) | 119 (15.04) | 19 (7.31) | 201 (8.5) |
| Smoking, current | 86 (15.19) | 798 (13.68) | 5 (6.10)* | 112 (14.16) | 33 (12.69) | 252 (10.65) |
| Genetic | ||||||
| apoEε4 carrier | 70 (14.58)*** | 399 (8.37) | 11 (14.47) | 67 (10.00) | 26 (12.32) | 187 (9.87) |
| Clinical | ||||||
| BMI (kg/m2) | 24.26±3.78** | 24.73±3.33 | 25.63±3.56 | 25.54±3.49 | 25.24+3.43 | 25.26+3.34 |
| Total cholesterol (mmol/L) | 5.31±0.96 | 5.36±1.00 | 5.52±0.96 | 5.48±1.01 | 5.31±1.11 | 5.36±1.10 |
| Triglycerides (mmol/L) | 1.44±0.84 | 1.49±0.79 | 1.60±0.67 | 1.71±1.03 | 1.63±0.80 | 1.74±1.14 |
| HDL cholesterol (mmol/L) | 1.39±0.34 | 1.40±0.35 | 1.36±0.30 | 1.32±0.34 | 1.35±0.54 | 1.30±0.33 |
| LDL cholesterol (mmol/L) | 3.50±0.75** | 3.28±0.82 | 3.51±0.84 | 3.35±0.84 | 3.28±0.92 | 3.28±0.88 |
| Fasting blood glucose (mmol/L) | 5.25±0.47** | 5.19±0.52 | 6.43±0.24 | 6.46±0.25 | 8.31±3.02 | 8.05±2.61 |
| Systolic blood pressure (mmHg) | 129.5±14.29*** | 127.3±11.63 | 134.4±15.26** | 129.6±12.35 | 131.7±16.49 | 129.3±12.04 |
| Diastolic blood pressure (mmHg) | 78.88±9.27** | 78.02±7.76 | 80.18±9.75 | 79.09±7.83 | 79.05±9.79 | 77.50±7.88 |
| Uric acid (mmol/L) | 299.0±81.79*** | 311.3±82.76 | 311.3±81.17 | 325.2±90.07 | 306.6±85.37 | 310.1±84.69 |
| Obesity (BMI ≥28 kg/m2) | 86 (15.30) | 885 (15.23) | 23 (28.05) | 163 (20.74) | 52 (20.16) | 454 (19.28)) |
| Stroke | 104 (18.41)*** | 762 (13.10) | 15 (18.29) | 97 (12.31) | 72 (27.69)*** | 374 (15.83) |
| Hypertension | 333 (58.94) | 3,312 (56.99) | 66 (80.49)** | 507 (64.42) | 197 (75.77)* | 1,635 (69.25) |
| Cardiovascular disease* | 112 (19.82) | 1,121 (19.28) | 20 (24.39) | 165 (20.94) | 76 (29.23) | 592 (25.06) |
| GDS >5 | 73 (13.20)** | 531 (10.04) | 11 (14.10) | 55 (7.86) | 46 (18.25)*** | 239 (10.94) |
Notes: Values are presented as n (%) or mean ± SD.
P<0.05;
P<0.01;
P<0.001 vs cognitively normal group using χ2 for categorical variables and unpaired Student’s t-tests for continuous variables.
Abbreviations: BMI, body mass index; DM, diabetes mellitus; HDL, high-density lipoprotein; IFG, impaired fasting glucose; GDS, Geriatric Depression Scale; LDL, low-density lipoprotein; MMSE, Mini-Mental State Examination; NG, normal fasting plasma glucose.
The percentage of hypertension was higher in individuals with cognitive impairment in each of the IFG and DM group. In the NG group, participants with cognitive impairment had lower BMI and uric acid (UA), less common in male, more apoEε4 carrier and higher SBP, DBP, FBG and LDL cholesterol (Table 2).
The percentage of apoEε4 carrier was found to be higher in participants with cognitive impairment compared to participants with normal cognition only in the NG group (14.58% vs 8.37%, P<0.001). The prevalence of cognitive impairment for apoEε4 carriers or non-carriers was not different between the three groups (P=0.64, P=0.24, respectively) (Figure 4).
Figure 4.
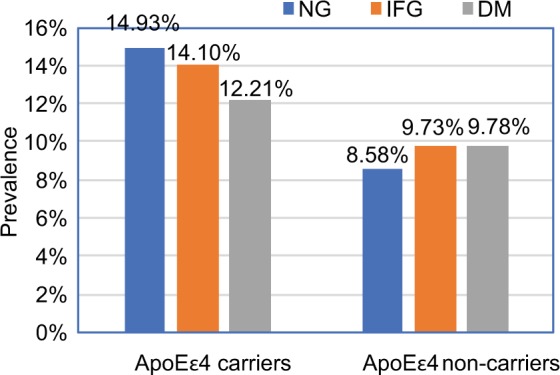
Prevalence of cognitive impairment based on apoEε4 carrier.
Abbreviations: DM, diabetes mellitus; IFG, impaired fasting glucose; NG, normal fasting plasma glucose.
Differences in cognitive severity
Cognitive severities measured by MMSE score in each of the NG, IFG, and DM groups are shown in Figure 5. According to the MMSE score, the participants were defined as having mild (MMSE score: 20–29), moderate (MMSE score: 10–19), or severe (MMSE score: 0–9) cognitive impairment. Most participants in all three groups were at a mild stage. The lowest number of participants had severe symptoms in all three groups. The severity of cognitive impairment was not different among participants with NG, IFG, and DM (P=0.84).
Figure 5.
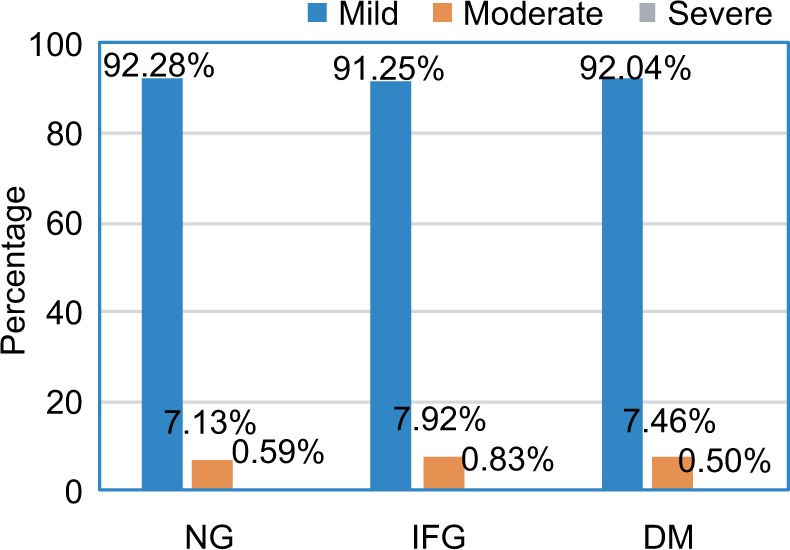
Severity of cognitive impairment among participants with NG, IFG, and DM.
Abbreviations: DM, diabetes mellitus; IFG, impaired fasting glucose; NG, normal fasting plasma glucose.
Risk factors associated with cognitive impairment
Logistic regression analysis for risk factors associated with cognitive impairment in each of the three groups are shown in Table 3. Comparing associated risk factors among the three groups, all of them shared some common risk factors such as rural residence, unmarried status, and daily exercise <0.5 hours. Higher serum UA level appeared to be protective (OR =0.71, 95% CI: 0.53–0.95, P=0.022), while apoEε4 carrier status was the independent risk factor (OR =1.70, 95% CI: 1.28–2.28 P<0.001) only for the NG group. Increased comorbidity with hypertension (OR =2.26, 95% CI: 1.21–4.22, P=0.01) was found for IFG group, while stroke (OR =1.64, 95% CI: 1.14–2.35, P=0.007) and depression status (OR =1.59, 95% CI 1.03–2.46, P=0.04) for DM (Table 3).
Table 3.
Logistic regression models for risk factors associated with cognitive impairment
| NG
|
IFG
|
DM
|
||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|
| ||||||
| Age group, years | ||||||
| 55–65 | Ref | Ref | Ref | |||
| 65–75 | 1.61 (1.23–2.10) | 0.02 | 2.06 (0.99–4.27) | 0.41 | 1.82 (1.16–2.83) | 0.57 |
| 75–85 | 2.67 (1.98–3.59) | 0.005 | 3.67 (1.58–8.50) | 0.17 | 2.45 (1.50–3.99) | 0.20 |
| ≥85 | 3.88 (2.18–6.91) | 0.001 | 5.43 (1.09–26.98) | 0.17 | 3.50 (1.23–9.97) | 0.13 |
| Male | 0.80 (0.65–0.99) | 0.042 | 0.77 (0.44–1.35) | 0.37 | 0.94 (0.67–1.30) | 0.69 |
| Urban | 0.32 (0.25–0.40) | <0.001 | 0.32 (0.17–0.59) | <0.001 | 0.34 (0.23–0.50) | <0.001 |
| Married | 0.67 (0.53–0.84) | 0.002 | 0.53 (0.29–0.99) | 0.04 | 0.66 (0.47–0.95) | 0.02 |
| Daily exercise <0.5 hours | 1.70 (1.36–2.13) | <0.001 | 1.50 (0.84–2.68) | 0.17 | 1.87 (1.36–2.59) | <0.001 |
| Sleep <6 hours | 1.61 (1.28–2.03) | <0.001 | 1.78 (0.95–3.32) | 0.07 | 1.30 (0.91–1.87) | 0.15 |
| APOE ε4 carrier | 1.70 (1.28–2.28) | <0.001 | 1.31 (0.61–2.82) | 0.48 | 1.27 (0.81–2.00) | 0.30 |
| High UA | 0.71 (0.53–0.95) | 0.022 | 0.63 (0.31–1.28) | 0.20 | 0.86 (0.58–1.28) | 0.46 |
| Hypertension | 0.99 (0.81–1.22) | 0.97 | 2.26 (1.21–4.22) | 0.01 | 1.27 (0.89–1.80) | 0.18 |
| Stroke | 1.19 (0.91–1.57) | 0.21 | 1.22 (0.61–2.44) | 0.57 | 1.64 (1.14–2.35) | 0.007 |
| GDS >5 | 1.19 (0.85–1.66) | 0.29 | 1.20 (0.49–2.96) | 0.70 | 1.59 (1.03–2.46) | 0.04 |
Notes: Analyses included the following covariates: age, gender, location, lifestyle, marital status, high UA, BMI ≥28 kg/m2, alcohol drinking, smoking, APOE ε4 carrier, hypertension, stroke, GDS >5, cardiovascular disease, high TCH, high TG, high LDL, and low HDL.
Abbreviations: DM, diabetes mellitus; GDS, Geriatric Depression Scale; IFG, impaired fasting glucose; NG, normal fasting plasma glucose; UA, uric acid.
Discussion
Diabetes and increased blood glucose level are the common comorbidities of aging, and both aging and diabetes can independently or interactively predispose individuals for cognitive dysfunction. It remains uncertain whether they share the same risk factors, eg, social status, lifestyle, genetics, and comorbidities. Our community-dwelling old population cohort study demonstrated that there was no significant difference in the prevalence of cognitive impairment among the elderly with normal NG, IFG, and DM (8.85%, 9.39%, and 9.90%, respectively), but in the age- and sex-adjusted comparisons, the MMSE scores in subjects with DM and IFG were significantly lower than that in subjects with normal glucose. However, while common risk factors of social status, age, and apoEε4 were associated with cognitive impairment in aging alone, hypertension and stroke were independent risk factors for IFG and DM elderly, respectively.
Most epidemiological studies have shown that diabetes is a risk factor for cognitive impairment. Few studies that investigated the association between “pre-diabetes” status and cognitive function have showed contradictory results, possibly due to different study population and design.20,21 In our study, some common risk factors such as age, female, and apoEε4 carrier were associated with cognitive dysfunction only in elderly individuals with normal blood glucose after adjustment. Previous studies have indicated that the presence of the apoEε4 may independently increase risk of Alzheimer’s disease and vascular dementia.22,43 We observed that there was a worse vascular risk factor profile, including overweight, stroke, and hypertension in IFG and DM subjects. The known relationship between these vascular risk factors and cognitive decline might interfere with our investigation of the association between apoEε4 carrier and cognitive impairment in subjects with IFG or DM, due to a larger impact of the vascular factors. However, most of the previous articles reported that there is no interaction between borderline diabetes and apoEε420 or between diabetes and apoEε4 carriers.23 This indicates that apoEε4 is far less enough to be recommended as an independent diagnosis predictor for cognitive deficit in the elderly with IFG or DM.
Hypertension was the risk factor for cognitive impairment only in IFG group in our study. Previous studies exploring the relationships between hypertension with cognitive impairment have reported inconsistent results.24,25 Longitudinal studies have suggested that high blood pressure in midlife is associated with late-life-related cognitive impairment.26 Some studies report that low blood pressure values in late-life is associated with an increased risk of dementia.27 These findings suggest the “U-phenomenon” to characterize the relationship between blood pressure and dementia.28 Besides as a leading risk factor for stroke, hypertension is thought to be associated with cognitive impairment through atherosclerosis, endothelial dysfunction, and white matter lesions of the brain.29 Slightly increased blood glucose as in IFG may potentiate hypertension-associated vascular damage leading to higher risk for cognitive deficit. However, reports have shown that the adequate cerebral perfusion that results from high blood pressure may be beneficial for cognitive function.30 The relationship between hypertension and cognitive impairment is complex, and further well-designed studies are needed to explore specific blood pressure management strategies for different population. In any event, our results suggest that management of blood pressure would be critical for people with IFG in order to prevent development of cognitive dysfunction.
Stroke is a well-known risk factor for dementia. The strong association of post-stroke dementia with multiple strokes indicated the central causal role of stroke itself as opposed to other vascular risk factors.31 In a systematic review of exploring the excess risk of incident dementia conferred by stroke, a history of stroke was found to confer approximately a doubling of the risk of dementia incidence in the elderly population. This association was not explained by common risk factors such as demographic or cardiovascular risk factors.32 In our study, stroke was the risk factor for cognitive impairment only in the participants with diabetes. In addition, our study showed that there were more atherosclerotic vascular risk factors in subjects with diabetes. Cerebrovascular disease is the most common mechanism of cognitive impairment or dementia of diabetes because diabetes is strongly related to vasculopathy and cerebrovascular disease.33
Previous studies showed inconsistent association between depressive symptoms and diabetes-associated cognitive decline.34,35 A national population-based cohort study of more than 2.4 million adults showed that depression and diabetes were independently associated with dementia, and the combined effect of both exposures was more than additive.36 Our findings showed that there was higher prevalence (11.7%) of depression in people with diabetes. The reason may be that depression and DM share common risk factors for cognitive impairment, such as insulin resistance, inflammation, and vascular disease.37 The results suggest that clinicians should pay more attention to manage stroke and depression when facing the patients with diabetes to prevent cognitive impairment.
Cardiovascular diseases include coronary artery disease (CAD), atrial fibrillation, and chronic heart failure. In most cases, CAD was found to be associated with global cognitive deficits in the older population.38,39 However, our study demonstrated that cardiovascular diseases were not independent risk factors for cognitive impairment in all participants after adjusted for cofounders though diabetes could be the cause of cardiovascular diseases. The discrepancies might be mainly associated with the selection bias of the study populations.
In addition, our study showed that the lack of exercise and sleep had direct influence on cognitive impairment. This has been proven in other observational studies in the general population.40,41 Thus, a healthy lifestyle with enough exercise and sleep might help to prevent cognitive impairment. Moreover, this study also demonstrated that high UA was the protective factor associated with cognitive impairment in NG group. The explanation could be that UA may reduce oxidative stress and play a protective role against cognitive impairment,42 which deserves future investigations.
Limitations and strengths
The strengths of this study are the use of the real-world data, as well as population-based design with a large population. Comprehensive risk factors such as social status, lifestyle, genetics, and comorbidities to cognitive decline were also included in this study. Furthermore, this is the first study to investigate the risk factors for cognitive impairment in the elderly with IFG. However, some limitations of this study are worth mentioning: An oral glucose tolerance test and glycosylated hemoglobin (HbA1c) levels were not performed in the present study. As it is in-practicable for such a large cohort, some participants with postprandial hyperglycemia might have been misclassified. A cross-sectional study can only demonstrate association, but not causation. Future studies in this subject could focus in the limitation of this study.
Conclusion
The current study demonstrated that although MMSE scores in subjects with DM and IFG were significantly lower than that in subjects with normal blood glucose level, they are independently associated with stroke or hypertension, respectively. Focusing on the management of blood pressure for people with IFG and of stroke with diabetes would be equally important for preventing elderly from cognitive deficit.
Acknowledgments
We acknowledge the support of all the grants for the project. The authors thank all the doctors, nurses, and participants in the communities who were involved in the study. This project was supported by grants from the Beijing Municipal Commission on Science and Technology (D07050701130000 and D07050701130701), Ministry of Health of China (201002011), Ministry of Science and Technology of China (2012AA02A514, 0S2012GR0150, 2012ZX 09303-005), and Beijing Municipal Administration of Hospitals (ZYLX201301). The funding sources had no involvement in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Xu Y, Wang L, He J, et al. 2010 China Noncommunicable Disease Surveillance Group Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 2.Tripathy JP. Burden and risk factors of diabetes and hyperglycemia in India: findings from the Global Burden of Disease Study 2016. Diabetes Metab Syndr Obes. 2018;11:381–387. doi: 10.2147/DMSO.S157376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014;10(1):1–9. doi: 10.1016/j.jalz.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Samaras K, Lutgers HL, Kochan NA, et al. The impact of glucose disorders on cognition and brain volumes in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 2014;36(2):977–993. doi: 10.1007/s11357-013-9613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012;69(9):1170–1175. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Euser SM, Sattar N, Witteman JC, et al. PROSPER and Rotterdam Study A prospective analysis of elevated fasting glucose levels and cognitive function in older people: results from PROSPER and the Rotterdam Study. Diabetes. 2010;59(7):1601–1607. doi: 10.2337/db09-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Manheim I, Sinnreich R, Doniger GM, Simon ES, Pinchas-Mizrachi R, Kark JD. Fasting plasma glucose in young adults free of diabetes is associated with cognitive function in midlife. Eur J Public Health. 2018;28(3):496–503. doi: 10.1093/eurpub/ckx194. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 9.Rouch I, Roche F, Dauphinot V, et al. Diabetes, impaired fasting glucose, and cognitive decline in a population of elderly community residents. Aging Clin Exp Res. 2012;24(4):377–383. doi: 10.1007/BF03325269. [DOI] [PubMed] [Google Scholar]
- 10.Xiu S, Zheng Z, Guan S, Zhang J, Ma J, Chan P. Serum uric acid and impaired cognitive function in community-dwelling elderly in Beijing. Neurosci Lett. 2017;637:182–187. doi: 10.1016/j.neulet.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Chhetri JK, Zheng Z, Xu X, Ma C, Chan P. The prevalence and incidence of frailty in Pre-diabetic and diabetic community-dwelling older population: results from Beijing longitudinal study of aging II (BLSA-II) BMC Geriatr. 2017;17(1):47. doi: 10.1186/s12877-017-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337(8750):1158–1159. doi: 10.1016/0140-6736(91)92823-k. [DOI] [PubMed] [Google Scholar]
- 13.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4(3):173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 14.Obesity: preventing and managing the global epidemic Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 15.Chen C, Lu FC, Department of Disease Control Ministry of Health, PR China The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 16.Hong JY, Lan TY, Tang GJ, Tang CH, Chen TJ, Lin HY. Gout and the risk of dementia: a nationwide population-based cohort study. Arthritis Res Ther. 2015;17:139. doi: 10.1186/s13075-015-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27(4):428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 18.Cui GH, Yao YH, Xu RF, et al. Cognitive impairment using education-based cutoff points for CMMSE scores in elderly Chinese people of agricultural and rural Shanghai China. Acta Neurol Scand. 2011;124(6):361–367. doi: 10.1111/j.1600-0404.2010.01484.x. [DOI] [PubMed] [Google Scholar]
- 19.Department of Noncommunicable Disease Surveillance . Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999. [Google Scholar]
- 20.Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56(1):211–216. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- 21.Yan-Ling Z, Chang-Quan H, Li Y, Bi-Rong D. Association of fasting serum insulin and fasting serum glucose levels with cognitive impairment in Chinese nonagenarians/centenarians. Age (Dordr) 2014;36(1):427–434. doi: 10.1007/s11357-013-9547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fei M, Yan Ping Z, Ru Juan M, Ning Ning L, Lin G. Risk factors for dementia with type 2 diabetes mellitus among elderly people in China. Age Ageing. 2013;42(3):398–400. doi: 10.1093/ageing/afs188. [DOI] [PubMed] [Google Scholar]
- 23.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63(7):1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Kinoshita LM, Noda A, et al. Effects of body mass index-related disorders on cognition: preliminary results. Diabetes Metab Syndr Obes. 2014;7:145–151. doi: 10.2147/DMSO.S60294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Relation of blood pressure to risk of incident Alzheimer’s disease and change in global cognitive function in older persons. Neuroepidemiology. 2006;26(1):30–36. doi: 10.1159/000089235. [DOI] [PubMed] [Google Scholar]
- 26.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the radiation effects research foundation adult health study. J Am Geriatr Soc. 2003;51(3):410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]
- 27.Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60(2):223–228. doi: 10.1001/archneur.60.2.223. [DOI] [PubMed] [Google Scholar]
- 28.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009;8(2):61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Duron E, Hanon O. Hypertension, cognitive decline and dementia. Arch Cardiovasc Dis. 2008;101(3):181–189. doi: 10.1016/s1875-2136(08)71801-1. [DOI] [PubMed] [Google Scholar]
- 30.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62(5):810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 32.Savva GM, Stephan BC, Alzheimer’s Society Vascular Dementia Systematic Review Group Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41(1):e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Song D, Leng SX. Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin Interv Aging. 2015;10:549–560. doi: 10.2147/CIA.S74042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darwish L, Beroncal E, Sison MV, Swardfager W. Depression in people with type 2 diabetes: current perspectives. Diabetes Metab Syndr Obes. 2018;11:333–343. doi: 10.2147/DMSO.S106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koekkoek PS, Rutten GE, Ruis C, et al. Mild depressive symptoms do not influence cognitive functioning in patients with type 2 diabetes. Psychoneuroendocrinology. 2013;38(3):376–386. doi: 10.1016/j.psyneuen.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Katon W, Pedersen HS, Ribe AR, et al. Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry. 2015;72(6):612–619. doi: 10.1001/jamapsychiatry.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail K, Winkley K, Stahl D, Chalder T, Edmonds M. A cohort study of people with diabetes and their first foot ulcer: the role of depression on mortality. Diabetes Care. 2007;30(6):1473–1479. doi: 10.2337/dc06-2313. [DOI] [PubMed] [Google Scholar]
- 38.Selnes OA, Grega MA, Bailey MM, et al. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88(2):445–454. doi: 10.1016/j.athoracsur.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh-Manoux A, Sabia S, Lajnef M, et al. History of coronary heart disease and cognitive performance in midlife: the Whitehall II study. Eur Heart J. 2008;29(17):2100–2107. doi: 10.1093/eurheartj/ehn298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo JC, Loh KK, Zheng H, Sim SK, Chee MW. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–1178. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004;3(4):219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 43.Rohn TT. Is apolipoprotein E4 an important risk factor for vascular dementia? Int J Clin Exp Pathol. 2014;7(7):3504–11. [PMC free article] [PubMed] [Google Scholar]