Abstract
Background
Human hepatocellular carcinoma (HCC) is a common liver tumor and the main cause of cancer-related death. Tyrosine kinase inhibitors, such as imatinib and GNF5 which were developed to treat chronic myelogenous leukemia, regulate the progression of various cancers. The aim of this study was to confirm the anti-tumor activity of tyrosine kinase inhibitors through regulation of S-phase kinase-associated protein 2 (Skp2), an important oncogenic factor in various cancer cells, in human hepatocarcinoma SK-HEP1 cells.
Methods
Cell viability and colony formation assays were conducted to evaluate the effects of imatinib, GNF5 and GNF2 on the growth of SK-HEP1 cells. Using immunoblot analysis, we assessed change of the activation of caspases, PARP, Akt, mitogen-activated protein kinases, and Skp2/p27/p21 pathway by imatinib and GNF5 in SK-HEP1 cells. Using sh-Skp2 HCC cells, the role of Skp2 in the effects of imatinib and GNF5 was evaluated.
Results
Imatinib and GNF5 significantly inhibited the growth of SK-HEP1 cells. Treatment of imatinib and GNF5 decreased Skp2 expression and Akt phosphorylation, and increased the expression of p27, p21, and active-caspases in SK-HEP1 cells. In sh-Skp2 HCC cells, cell growth and the expression of Skp2 were inhibited by more than in the mock group treated with imatinib and GNF5.
Conclusions
These results suggest that the anti-growth activity of tyrosine kinase inhibitors may be associated with the regulation of p27/p21 and caspases through Skp2 blockage in HCC cells.
Keywords: Anti-growth activity, Hepatocellular carcinoma, Skp2, Imatinib, GNF5
INTRODUCTION
Human hepatocellular carcinoma (HCC) is the most common malignant tumor of primary liver worldwide, and approximately 1 million people die from this liver cancer. Particularly, HCC is the most frequent cause of death in patients with liver cirrhosis.1 The incidence of HCC is highest in Asia and Africa, where the infection rate of hepatitis B or C is high. In the treatment of HCC, local ablation surgery is more effective than chemotherapy, but is only suitable in case of early diagnosis.2 However, most HCC patients are diagnosed at an advanced stage. Thus, although systemic chemotherapy such as sorafenib is the only standard treatment for patients with advanced HCC, its efficacy is limited.3
S-phase kinase-associated protein 2 (Skp2) is an oncogenic protein that induce the degradation of tumor suppressor proteins, such as p27 and p21, which are expressed in many cancers.4 An in vivo study by Nakayama et al.5 showed that mice lacking Skp2 exhibited abnormal accumulation of cyclin E and p27. A previous study reported that Skp2 expression increased the proliferation of HCC cells, and decreased the levels of cell cycle regulators.6 These results suggest that Skp2 may plays an important role in the progression of various human cancers including HCC.
Imatinib, under the brand name Gleevec, was developed and used as an anticancer agent for chronic myelogenous leukemia (CML).7 This agent is a first-generation tyrosine kinase inhibitor for the breakpoint cluster region-Abelson murine leukemia (Bcr-Abl)-targeted therapy of CML, which is caused by high expression of the Bcr-Abl fusion protein.8 GNF2 and GNF5 also act as allosteric non-ATP competitive inhibitors against Bcr-Abl, and were reported to cooperate with imatinib to inhibit Bcr-Abl.9 Ramadori et al.10 reported the successful treatment of a patient with HCC using tyrosine kinase inhibitor such as imatinib. Particularly, Skp2 acts as a crucial mediator of Bcr-Abl because depletion of Skp2 weakens Bcr-Abl-induced CML.
In this study, we examined the effects of tyrosine kinase inhibitors on the growth and apoptosis of SK-HEP1 cells, derived from a patient with liver adenocarcinoma. Furthermore, the effect of Skp2 on tyrosine kinase inhibitor activity in HCC was examined using sh-Skp2 cells.
MATERIALS AND METHODS
1. Cell culture and reagents
The human hepatocarcinoma cell line SK-HEP1 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS and 1% penicillin/streptomycin. The cells were incubated at 37°C with 5% CO2 and 95% humidity. Imatinib, GNF2, and GNF5 (Fig. 1) were purchased from Cayman Chemical (Ann Arbor, MI, USA) and dissolved in dimethyl sulfoxide at 20 mM.
Figure 1.
Chemical structures of imatinib, GNF2 and GNF5.
2. Cell viability assay
Cell viability was measured by the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Rockville, MD, USA). Briefly, the cells were seeded into 96-well plates (2 × 103 cells/ well), and incubated after which 20 μM of compounds (imatinib, GNF2, and GNF5) were added. The cells were incubated for 0, 24, 48, or 72 hours at 37°C with 5% CO2 and then incubated for 2 hours with CCK-8 solution. Absorbance was determined at 450 nm using a microplate spectrophotometer (BMG LABTECH, Ortenberg, Germany).
3. Soft agar assay for colony formation
Cells (8 × 103 cells/well) were plated in triplicate on the top of a bottom layer of 0.5% agar in DMEM with 10% FBS and the indicated concentration of imatinib and GNF5. The cells were suspended in DMEM containing 0.3% agar, 10% FBS, and compounds. After four weeks, the colonies were counted under a light microscope.
4. Immunoblot analysis
Cells were lysed with radioimmuneprecipitation assay buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% TritonTM X-100, 0.5% sodium deoxycholate, and 0.1% SDS) and 1% protease inhibitor (GenDEPOT, Barker, TX, USA). Protein concentrations were measured by DCTM assay (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were subjected to SDS-PAGE, transferred to polyvinylidene fluoride membranes, and then incubated with suitable primary antibodies overnight at 4°C. These membranes were incubated with an appropriate secondary antibody for 1 hour at room temperature (20°C–25°C) after washing with tris-buffered saline containing Tween-20. The expression of specific proteins was detected using a Davinch-ChemiTM imaging system (Davinch-K, Seoul, Korea). Primary antibodies for Skp2, p21 and p27 and all secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against caspase-9, cleaved caspase-9, caspase-8, cleaved caspase-8, caspase-3, cleaved caspase-3, PI3K, p-Akt, Akt, p-ERK, ERK, p-JNK, JNK, and tubulin were purchased from Cell Signaling Technology (Danvers, MA, USA).
5. Lentiviral particle production and infection
SK-HEP1 cells were transfected for 24 hours with the pLKO.1-sh Mock or pLKO.1-sh SKP2 plasmid and a lentiviral envelope packaging plasmid (pRSV-Rev, pMDLg/pRRE) using the jetPEITM transfection reagent (Cosmogenetech, Seoul, Korea). Viral particles were collected 24 hours after initial transfection. Cells were incubated with viral particles for 48 hours in medium containing polybrene (Sigma-Aldrich, St. Louis, MO, USA). Cells were selected by treatment with puromycin (2 μg/mL) for 48 hours.
6. Statistical analysis
All experimental results are presented as the mean ± SD. Significant differences were determined by Student t-test. A value of P < 0.05 was considered to indicate statistical significance.
RESULTS
1. Effect of imatinib, GNF5 and GNF2 on the growth of SK-HEP1 cells
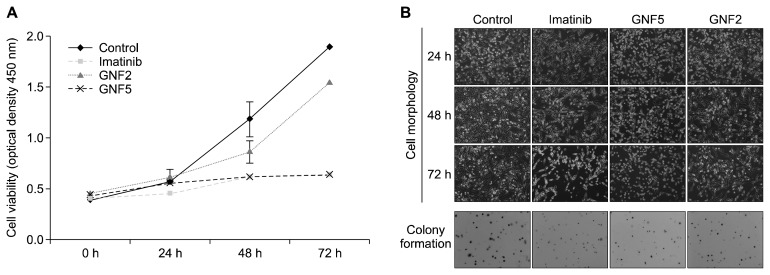
To investigate the growth of SK-HEP1 cells following treatment which imatinib, GNF5 and GNF2, we examined the change in the morphology, proliferation and colony formation of SK-HEP1 cells for 24, 48, and 72 hours. Figure 2A shows that all drugs at 20 μM time-dependently inhibited the proliferation of SK-HEP1 cells. Particularly, imatinib and GNF5 significantly decreased cell viability by more than 50% at 72 hours, which was confirmed by the cell morphology images (Fig. 2B). Additionlly, the colony formation assay showed that all compound-treated cells formed fewer colonies compared to the control group (Fig. 2B). Collectively, these results demonstrate that imatinib and GNF5 significantly inhibited the growth of SK-HEP1 cells, compared to the same concentration of GNF2. In subsequent experiments, therefore, imatinib and GNF5 were used.
Figure 2.
Effect of imatinib, GNF5 and GNF2 on the growth of SK-HEP1 cells. Cells were incubated with 20 μM of imatinib, GNF5 and GNF2 for 0, 24, 48, or 72 hours. (A) Cell viability was measured using the Cell Counting Kit-8 assay. (B) Cell morphology and colony formation by soft agar assay was observed under a light microscope (Cell morphology: ×100, Colony formation: ×50).
2. Effect of imatinib and GNF5 on apoptosis of SK-HEP1 cells
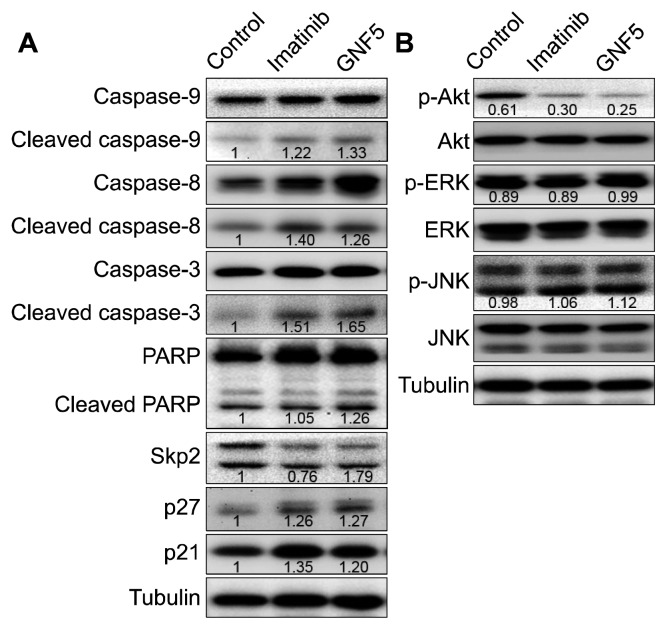
We investigated the apoptotic activity of imatinib and GNF5 by confirming the activation of caspases and PARP by Western blot analysis. As shown in Figure 3, both imatinib and GNF5 slightly increased the expression of the active cleaved forms of caspase-9, caspase-8, and caspase-3, but did not significantly affect activation of PARP. This indicates that imatinib and GNF5 induce the caspase family-dependent apoptosis of SK-HEP1 cells.
Figure 3.
Effect of imatinib and GNF5 on apoptosis, Skp2/p27/p21 pathway, and the phosphorylation of Akt and mitogen-activated protein kinases in SK-HEP1 cells. Cells were treated with/without im-atinib or GNF5. (A) The activation of caspases and PARP and Skp2/p27/p21 level was determined by immunoblot analysis and densitometry analysis (target protein/tubulin). (B) The expression of Akt, ERK and JNK proteins was determined by immunoblot analysis and densitometry analysis (phospho-form/total-form).
3. Effect of imatinib and GNF5 on Skp2/p27/p21 pathway in SK-HEP1 cells
Skp2 controls the levels of both p27 and p21, which plays important roles as cell cycle mediators in tumorigenesis.11 We evaluated the effect of imatinib and GNF5 on the Skp2/p27/p21 pathway in SK-HEP1 cells. By two compounds treatment, Skp2 expression was decreased and the expression of both p27 and p21 was increased (Fig. 3A). Our data suggest that imatinib and GNF5 enhances stabilization of both p27 and p21 proteins by downregulating of Skp2.
4. Effect of imatinib and GNF5 on phosphorylation of Akt and mitogen-activated protein kinases in SK-HEP1 cells
Activation of Akt and mitogen-activated protein kinases (MAPKs), which are known to modulate the survival, proliferation and cell cycle of various tumor cells, was measured in SK-HEP1 cells. As shown in Figure 3B, imatinib and GNF5 significantly inhibited the phosphorylation of Akt, and did not affect ERK and JNK. These data demonstrate that imatinib and GNF5 can be regulated by PI3K/Akt pathway rather than MAPK signaling pathways.
5. Effect of imatinib and GNF5 in SK-HEP1 sh-Skp2 cells
To explore the role of Skp2 protein on the above identified effects of imatinib and GNF5 in SK-HEP1 cells, we experimented using cells which were transfected with a control vector (mock) or Skp2 shRNA construct. Knockdown of Skp2 induced suppression of cell proliferation; the inhibition rate of imatinib and GNF5 was approximately 70%, which was higher than that in sh-mock cells (Fig. 4A). Similar results were observed in the colony formation assay (Fig. 4B).
Figure 4.
Effect of imatinib and GNF5 on SK-HEP1 sh-Skp2 cells. Cells with mock or Skp2 knockdown were incubated with/without imatinib or GNF5 for 0, 24, 48, or 72 hours. (A) Cell viability was measured using Cell Counting Kit-8 assay. (B) Colony formation by soft agar assay was observed under a light microscope (×50).
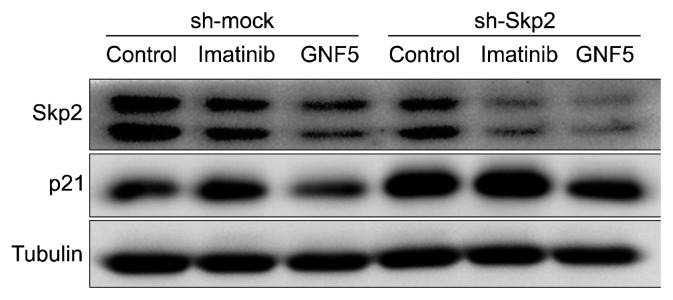
We also confirmed the effect of imatinib and GNF5 on the Skp2/p21 in SK-HEP1 sh-Skp2 cells. Compared in mock cells, Skp2 was also more inhibited by imatinib and GNF5 in sh-Skp2 cells, and expression of p21 was also increased as well (Fig. 5).
Figure 5.
Effect of imatinib and GNF5 on Skp2/p21 expression in SK-HEP1 sh-Skp2 cells. Cells with mock or Skp2 knockdown were treated with/without imatinib or GNF5. The expression of Skp2, p21, and tubulin proteins was determined by immunoblot analysis.
DISCUSSION
Uncontrolled kinase activity can directly or indirectly result in cancer.12 Particularly, Bcr-Abl causes malignant growth of cells as oncogenic tyrosine kinases, which is implicated in progression of carcinogenesis.13 Therefore, inhibition of oncogenic kinase activity is considered as an effective target for an anti-cancer therapy. Imatinib has potent pharmacological effects on CML by inhibiting Bcr-Abl tyrosine kinase activity.14 GNF2 and the analog GNF5, which were developed for CML patients with imatinib-resistant mutations, also suppress Bcr-Abl tyrosine kinase activity via a selective non-ATP competition.9 In this study, we confirmed the effect of imatinib, GNF2 and GNF5, belonging to the family of Bcr-Abl tyrosine kinase inhibitors, on HCC, a primary liver malignancy.
A previous study confirmed the cytotoxicity of imatinib in HCC HepG2 cells, and imatinib inhibited cell survival in a dose-dependent manner.15 Thus, we investigated the effect of GNF2 and GNF5 as well as imatinib on SK-HEP1 cells. Imatinib and GNF5 time-dependently inhibited the growth of the HCC cell line. However, treatment with GNF2 did not have a significant effect. Compared to the control group at 72 hours, imatinib and GNF5 inhibited cell viability by 66.1% and 66.0%, respectively. These growth-inhibitory effects were more pronounced in SK-HEP1 sh-Skp2 cells. Compared to the mock group, the sh-Skp2 cell survival rate was reduced by 44.9%, and was inhibited by over 70% by treatment with imatinib and GNF5. There results suggest that Skp2 directly affects the growth of liver cancer cells.
To determine whether the inhibition on SK-HEP1 cell growth by imatinib and GNF5 was caused by apoptosis or dysregulation of the cell cycle, we examined the activation of apoptotic caspases/PARP, and expression of cell cycle-regulatory proteins. Among the caspases that function as important regulators of apoptosis, caspase-3, a major effector in apoptosis progression, interacts with caspase-9 and caspase-8. In previous studies using HCC cells, the activation of caspase-9, caspase-8 and caspase-3 was investigated to evalutate caspase-dependent apoptosis.16,17 We found that imatinib and GNF5 induced caspase-mediated apoptosis, and slightly increased p27 and p21 protein level in SK-HEP1 cells. As an oncoprotein, Skp2 plays an important role in cancer progression by inducing the degradation of p27 and p21.6,18 Downregulation of Skp2 leads upregulation of p27 and p21. Our results showed that Skp2 reduction by imatinib and GNF5 treatment upregulated both p27 and p21 level in HCC cells. Ohkoshi et al.19 proposed p21 as a new therapeutic target for anti-HCC. Thus, we measured the change in p21 protein levels in sh-Skp2 HCC cells. Knockdown of Skp2 enhanced p21 expression, and inhibitory effect by imatinib and GNF5 was also increased. Taken together, the anti-growth activity of imatinib and GNF5 against HCC may be related to both apoptosis and cell cycle inhibition, and this effect can be enhanced by blocking Skp2.
To confirm the mechanism of anti-growth activity of imatinib and GNF5, the activation of Akt and MAPKs was measured in SK-HEP1 cells. Akt, MAPKs, and protein kinase C have been shown to be involved in Skp2 regulation in various cell types, including breast cancer, prostate cancer, and lung cancer.20–22 Andreu et al.23 reported that Bcr-Abl induces p27 degradation by increasing Skp2 expression through regulation of PI3K pathway. Because imatinib and GNF5 inhibited the phosphorylation of Akt, not MAPKs in SK-HEP1 cells, we will evaluate the PI3K/Akt-Skp2 pathway and detmonstrate the mechanism of Skp2 in HCC in the further study.
In conclusion, the anti-growth activity of imatinib and GNF5, which are tyrosine kinase inhibitors, was found to involve induction of caspase-dependent apoptosis, enhancement of p27 and p21, and downregulation of Akt phosphorylation in HCC cells. Blocking of Skp2 further enhanced the efficacy of imatinib and GNF5 on HCC. Collectively, tyrosine kinase inhibitors may be useful therapeutic candidates for HCC by regulating Skp2.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 2.Di Maio M, De Maio E, Perrone F, Pignata S, Daniele B. Hepatocellular carcinoma: systemic treatments. J Clin Gastroenterol 2002;35:S109–14. 10.1097/00004836-200211002-00007 [DOI] [PubMed] [Google Scholar]
- 3.Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: the present and the future. World J Hepatol 2017;9:907–20. 10.4254/wjh.v9.i21.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 2008;8:438–49. 10.1038/nrc2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J 2000;19:2069–81. 10.1093/emboj/19.9.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvisi DF, Ladu S, Pinna F, Frau M, Tomasi ML, Sini M, et al. SKP2 and CKS1 promote degradation of cell cycle regulators and are associated with hepatocellular carcinoma prognosis. Gastroenterology 2009;137:1816–26.e1–10. 10.1053/j.gastro.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 7.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 2005;105:2640–53. 10.1182/blood-2004-08-3097 [DOI] [PubMed] [Google Scholar]
- 8.Salesse S, Verfaillie CM. BCR/ABL: from molecular mechanisms of leukemia induction to treatment of chronic myelogenous leukemia. Oncogene 2002;21:8547–59. 10.1038/sj.onc.1206082 [DOI] [PubMed] [Google Scholar]
- 9.Adrián FJ, Ding Q, Sim T, Velentza A, Sloan C, Liu Y, et al. Allosteric inhibitors of Bcr-abl-dependent cell proliferation. Nat Chem Biol 2006;2:95–102. 10.1038/nchembio760 [DOI] [PubMed] [Google Scholar]
- 10.Ramadori G, Füzesi L, Grabbe E, Pieler T, Armbrust T. Successful treatment of hepatocellular carcinoma with the tyrosine kinase inhibitor imatinib in a patient with liver cirrhosis. Anticancer Drugs 2004;15:405–9. 10.1097/00001813-200404000-00014 [DOI] [PubMed] [Google Scholar]
- 11.Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med 2008;10:e19. 10.1017/S1462399408000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsatsanis C, Spandidos DA. The role of oncogenic kinases in human cancer (Review). Int J Mol Med 2000;5:583–90. [DOI] [PubMed] [Google Scholar]
- 13.Paul MK, Mukhopadhyay AK. Tyrosine kinase: role and significance in cancer. Int J Med Sci 2004;1:101–15. 10.7150/ijms.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan AQ, Sharma SV, Warmuth M. Allosteric inhibition of BCR-ABL. Cell Cycle 2010;9:3710–4. 10.4161/cc.9.18.13232 [DOI] [PubMed] [Google Scholar]
- 15.Keshavarz-Pakseresht B, Shandiz SA, Baghbani-Arani F. Imatinib induces up-regulation of NM23, a metastasis suppressor gene, in human Hepatocarcinoma (HepG2) cell line. Gastroenterol Hepatol Bed Bench 2017;10:29–33. [PMC free article] [PubMed] [Google Scholar]
- 16.Mitupatum T, Aree K, Kittisenachai S, Roytrakul S, Puthong S, Kangsadalampai S, et al. Hep88 mAb-mediated paraptosis-like apoptosis in HepG2 cells via downstream upregulation and activation of caspase-3, caspase-8 and caspase-9. Asian Pac J Cancer Prev 2015;16:1771–9. 10.7314/APJCP.2015.16.5.1771 [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Tagle R, Escobar CA, Romero V, Montorfano I, Armisén R, Borgna V, et al. Chalcone-induced apoptosis through caspase-dependent intrinsic pathways in human hepatocellular carcinoma cells. Int J Mol Sci 2016;17:260. 10.3390/ijms17020260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Z, Jiang X, Qiao H, Zhai B, Zhang L, Zhang Q, et al. STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal 2013;25:931–8. 10.1016/j.cellsig.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 19.Ohkoshi S, Yano M, Matsuda Y. Oncogenic role of p21 in hepatocarcinogenesis suggests a new treatment strategy. World J Gastroenterol 2015;21:12150–6. 10.3748/wjg.v21.i42.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auld CA, Caccia CD, Morrison RF. Hormonal induction of adipogenesis induces Skp2 expression through PI3K and MAPK pathways. J Cell Biochem 2007;100:204–16. 10.1002/jcb.21063 [DOI] [PubMed] [Google Scholar]
- 21.Bu W, Luo T. miR-1297 Promotes cell proliferation of non-small cell lung cancer cells: involving in PTEN/Akt/Skp2 signaling pathway. DNA Cell Biol 2017;36:976–82. 10.1089/dna.2017.3886 [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Wang H, Ma J, Xu J, Sheng C, Yang S, et al. The expression and prognosis of Emi1 and Skp2 in breast carcinoma: associated with PI3K/Akt pathway and cell proliferation. Med Oncol 2013;30:735. 10.1007/s12032-013-0735-0 [DOI] [PubMed] [Google Scholar]
- 23.Andreu EJ, Lledó E, Poch E, Ivorra C, Albero MP, Martínez-Climent JA, et al. BCR-ABL induces the expression of Skp2 through the PI3K pathway to promote p27Kip1 degradation and proliferation of chronic myelogenous leukemia cells. Cancer Res 2005;65:3264–72. 10.1158/0008-5472.CAN-04-1357 [DOI] [PubMed] [Google Scholar]