Abstract
Alpha-naphthyl isothiocyanate (ANIT)-induced liver damage is regarded as a useful model to study drug-induced cholestatic hepatitis. Ultra-performance liquid chromatography coupled with electrospray ionization quadrupole mass spectrometry (UPLC-ESI-QTOF MS)-based metabolomics revealed clues to the mechanism of ANIT-induced liver injury, which facilitates the elucidation of drug-induced liver toxicity. 1-Stearoyl-2-hydroxysn-glycero-3-phosphocholine (LPC 18:0) and 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC 18:1) were significantly increased in serum from ANIT-treated mice, and this increase resulted from altered expression of genes encoding the lipid metabolism enzymes Chka and Scd1. ANIT also increased NF-κB/IL-6/STAT3 signaling, and in vitro luciferase reporter gene assays revealed that LPC 18:0 and LPC 18:1 can activate NF-κB in a concentration-dependent manner. Activation of PPARα through feeding mice a Wy-14,643-containing diet (0.1%) reduced ANIT-induced liver injury, as indicated by lowered ALT and AST levels, and liver histology. In conclusion, the present study demonstrated a role for the lipid-regulated NF-κB/IL-6/STAT3 axis in ANIT-induced hepatotoxicity, and that PPARα may be a potential therapeutic target for the prevention of drug-induced cholestatic liver injury.
Keywords: NF-κB/IL-6/STAT3 axis, Metabolomics, Alpha-naphthyl isothiocyanate, Drug toxicity
Introduction
Alpha-naphthyl isothiocyanate (ANIT) is a hepatotoxicant used in rodents to model human intrahepatic cholestasis. ANIT-induced liver damage is regarded as a useful model to study drug-induced cholestasis, because some drugs (e.g., erythromycin estolate, chlorpromazine)-induced liver damage and cholestasis in humans can be mimicked by ANIT in mice and rats (Kossor et al. 1993). Determining the mechanism of ANIT-induced liver injury will be of great value for understanding the pathogenesis of drug-induced liver injury and the development of therapeutics. However, the mechanism of ANIT-induced cholestatic liver damage has not been entirely elucidated. Previous studies have indicated some potential mechanisms. ANIT-induced liver damage is mediated by an ANIT-GSH conjugate which can dissociate upon crossing the canalicular membrane to yield free GSH and ANIT in the bile (Dietrich et al. 2001). Furthermore, ANIT induces damage of the bile duct epithelium leading to cholestasis. Inflammatory factors were also found to play a key role in ANIT-induced hepatotoxicity (Faiola et al. 2010; Kodali et al. 2006).
Metabolomics is a technology to profile the small molecules that are altered by disease. The altered metabolites can lead to clues about mechanisms of toxicity and disease. For example, metabolomics was used to determine an important role for farnesoid X receptor (FXR) in the pathogenesis of nonalcoholic fatty liver disease (Jiang et al. 2015; Li et al. 2013). Metabolomics was also applied to elucidate the major contribution of trichloroacetic acid toward the adverse effects of trichloroethylene (Fang et al. 2013). Metabolomics analysis of bile acids components showed the preventative role of intestinal CYP3A4-catalyzed bile acid metabolism against lithocholic acid-induced hepatotoxicity (Cheng et al. 2014). The present study revealed the role of a lipid-regulated NF-κB/IL-6/STAT3 axis in ANIT-induced liver injury, leading to a new mechanism and potential target for the treatment of drug-induced cholestasis.
Materials and methods
Chemical reagents and animals
1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC 18:0) and 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC 18:1) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Bile acids TCA and Ta/β-MCA were obtained from Sigma-Aldrich (Steinheim, Germany).
All animal experiments were carried out in accordance with the Institute of Laboratory Animal Resources guidelines and approved by the National Cancer Institute Animal Care and Use Committee. Male 6- to 8-week-old Ppara-null and wild-type mice on the C57BL/6N background were used in the present study (Akiyama et al. 2001; Lee et al. 1995; Patterson et al. 2012). These measurements were repeated three times using different batches of mice.
Murine model of ANIT-induced liver toxicity and the administration of Wy-14,643
Oral gavage of a single dose of ANIT (75 mg/kg) was used to create the ANIT-induced cholestasis model. Mice were killed by CO2 asphyxiation 48 h after ANIT administration. Blood samples were collected in BD Microtainer™ serum separator tubes (Franklin Lakes, NJ) and serum obtained through centrifugation for 15 min at 8000×g. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in serum were determined using a commercial AST or ALT assay kit (Catachem, Bridgeport, CT) according to the manufacturer’s instructions. ELISA assays for IL-6 in mouse serum were performed according to the manufacturer’s instructions (BD Biosciences). Livers were collected and divided into two parts; one part was fixed in 10% PBS-buffered formalin for histological analysis, and the other was frozen in liquid nitrogen for mRNA and protein expression analysis. To investigate protection of the PPARα agonist Wy-14,643 toward ANIT-induced toxicity, groups of 6- to 8-week-old mice were fed a Wy-14,643-containing diet (0.1%) for 24 h before oral gavage of a single dose of ANIT (75 mg/kg) dissolved in corn oil. The mouse diet was purchased from Bioserve (Beltsville, MD). Wy-14,643 was provided by Dr. Janardan K. Reddy and obtained from Dr. Reddy’s Laboratories (Hyderabad India). The diet was uniformly mixed with Wy-14,643, and re-pelleted. The control animals were kept on a similar placebo diet. For Wy-14,643-containing food intake measurements, three mice were housed individually and daily food intake was determined by use of metabolic cages, and normalized against the initial body weight (g food/g body weight). These measurements were repeated three times using different batches of mice.
Liver histopathology analysis
Histopathology analysis was carried out as previously described (Patterson et al. 2012; Tanaka et al. 2012). Tissue blocks were embedded in paraffin and cut into 4-μm-thick sections. The sections were stained by the hematoxylin and eosin method.
UPLC-ESI-QTOFMS-based metabolomics analysis of serum
Serum samples were prepared through mixing 10 μL serum with 190 μL 66% aqueous acetonitrile containing 5 μM chlorpropamide. After 14,000×g centrifugation for 20 min, a 5 μL aliquot of the supernatants was injected into a Waters UPLC-ESI-QTOFMS system (Waters Corporation, Milford, MA) for analysis. Separation of serum components was performed using an Acquity UPLC BEH C18 column (1.7 μm, 2.1 × 50 mm, Waters Corp.). The mobile phase was comprised of 0.1% aqueous formic acid (A) and acetonitrile containing 0.1% formic acid (B). The running method was described in a previous publication (Fang et al. 2013).
Multivariate data analysis
MassLynx software (Waters Corp.) was used to acquire the chromatogram and mass spectrometric data in centroid format. After the generation of a multivariate data matrix, the data set was exported into SIMCA-P+12.0 (Umetrics, Kinnelon, NJ) for further analysis, as previously described (Fang et al. 2013; Li et al. 2012).
RNA analysis
For microarray analysis, dye-coupled complementary DNAs (cDNAs) were purified with a MiniElute PCR purification kit (Qiagen) and hybridized to an Agilent 44 K mouse 60-mer oligo microarray (Agilent Technologies, Santa Clara, CA). Genespring GX software (Agilent Technologies) was used to process and analyze the data. Compared with control samples, mRNAs with >twofold alterations were regarded as significantly changed. For quantitative polymerase chain reaction (qPCR) studies, TRIzol reagent (Invitrogen, Carlsbad, CA) was used to extract total liver RNA, and cDNA generated from 1 μg RNA with a SuperScript II™ Reverse Transcriptase kit and random oligonucleotides (Invitrogen, Grand Island, NY). Quantitative PCR (qPCR) was performed using SYBR green PCR master mix and ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Messenger RNA quantitation was performed using the comparative cycle threshold (CT) method, and results normalized to mouse 18S RNA.
Nuclear and cytoplasmic extract preparation and western blot analysis
Nuclear and cytoplasmic extraction was performed as described previously (Rothgiesser et al. 2010). For western blot detection, liver homogenates in protein lysis buffer were resolved by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Immunoblotting was performed through incubation of membranes with the antibodies against p65, total STAT3 and phospho-STAT3 (Cell Signaling Technologies, Danvers, MA) as previously described (Jiang et al. 2011).
Transfection and luciferase reporter assay
Transfections were performed using polyethylenimine (PEI, Polysciences, Inc.). For analysis of the effect of lipids LPC18:0 and LPC18:1 and taurocholic acid (TCA) on NF-κB activity, LO2 cells were transfected with the pGL3-NF-κB luciferase reporter plasmid and a control pRL-TK renilla luciferase reporter. Twenty four hour later, the cells were stimulated for 3 h with different compounds. Luciferase activity was measured with the Dual-luciferase Reporter Assay System (Promega).
Data analysis
The experimental data were presented as mean ± SEM. Statistical analysis was carried out using GraphPad Prism 5.0. Comparisons between two groups were performed using a two-tailed unpaired Student’s t test or Mann–Whitney U test. Multiple groups were compared using a two-way ANOVA.
Results
ANIT-induced liver damage through NF-κB/IL-6/STAT3 axis
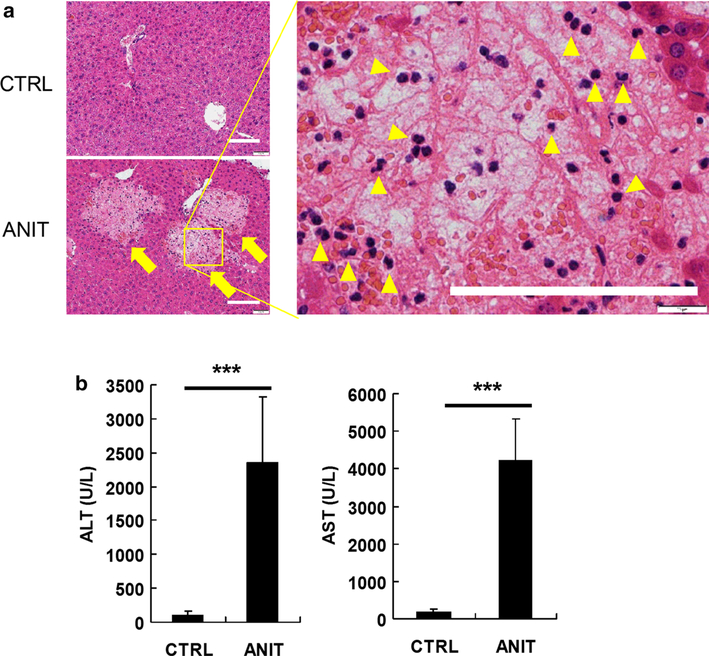
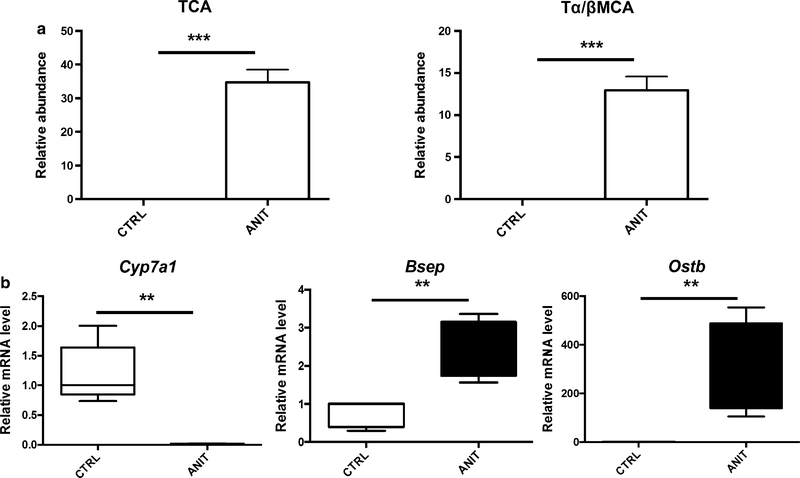
Two days after treatment with ANIT (75 mg/kg, p.o.), histological analysis of the liver revealed many foci of hepatocyte degeneration mainly in periportal areas (Fig. 1a, arrows). Degenerated hepatocytes were swollen with hydropic cytoplasm and unclear nucleus (Fig. 1a, right panel), indicating severe hepatocyte necrosis. Mononuclear/polymorphonuclear leukocytes were also observed in the necrotic areas (Fig. 1a, arrowheads in right panel). Consistent with the histological findings, ANIT treatment resulted in significantly increased serum ALT and AST activity (p < 0.001) (Fig. 1b). Through comparison with authentic bile acid standards (Supplemental Fig. 1A and B), TCA and Tα/βMCA were found to be significantly increased (p < 0.001) in ANIT-treated mice (Fig. 2a). Treatment with ANIT sharply increased expression of organic solute transporter b (Ostb) mRNA encoding a protein which functions to export bile acids from hepatocytes into blood, and inhibited expression of the bile acid-synthesizing enzyme Cyp7a1 mRNA. Expression of bile salt export pump (Bsep) mRNA was slightly increased.
Fig. 1.
a H&E staining of livers from control (CTRL) and ANIT-treated mice. Hepatocyte necrotic foci (arrows) and infiltration of leukocytes (arrowheads) were observed in ANIT-treated mouse livers. Bars represent 100 μm.
b Serum ALT and AST enzyme levels from control and ANIT-treated mice, 4–5 mice/group. The values are mean ± SEM. ***p < 0.001
Fig. 2.
a Comparison of the level of bile acids TCA and Tα/β-MCA in serum. b Expression of bile acids metabolism and transport-related genes Cyp7a1, Bsep, and Ostb. The values are mean ± SEM. **p < 0.01; ***p < 0.001
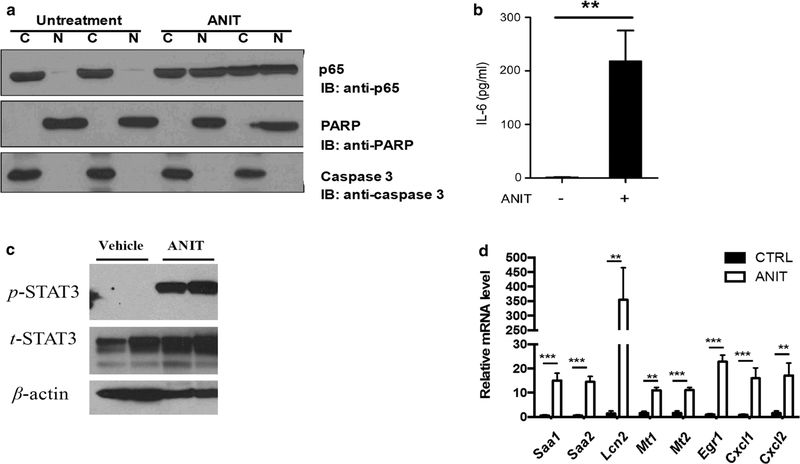
Since NF-κB plays a critical role in tissue injury and inflammation through the generation of inflammatory cytokines, the NF-κB signaling was measured. Western blot analysis showed that the expression of NF-κB (p65) significantly increased in hepatocyte nuclei of ANTI-treated mice, thus indicating that treatment with ANIT increased translocation of NF-κB (p65) into the nucleus (Fig. 3a). Compared with the control group, an increased of IL-6, which is dependent on NF-κB activation, was detected in serum of the ANIT-treated group (p < 0.01, Fig. 3b). As expected, an increased level of phospho-STAT3 was found in livers of mice treated with ANIT (Fig. 3c). Microarray analysis showed that compared with controls, 2372 genes were more than twofold differentially expressed in livers of ANIT-treated mice (p < 0.05) (Supplemental Fig. 2). The changed mRNAs included Saa1, Saa2, Lcn2, Mt1, Mt2, Egr1, Cxcl1 and Cxcl2, all of which are expressed from STAT3 target genes that were verified and quantified by qPCR (Fig. 3d).
Fig. 3.
a Western blotting analysis of transactivation of NF-κB. b IL-6 concentrations in serum from control and ANIT-treated mice. c Western blotting analysis of transactivation of phosphor-STAT3 and total STAT3; d QPCR of the expression of STAT3 downstream target gene mRNAs including Saa1, Saa2, Lcn1, Mt1, Mt2, Egr-1, Cxcl1, and Cxcl2. **p < 0.01; ***p < 0.001
ANIT-induced alteration of lipid metabolic profile
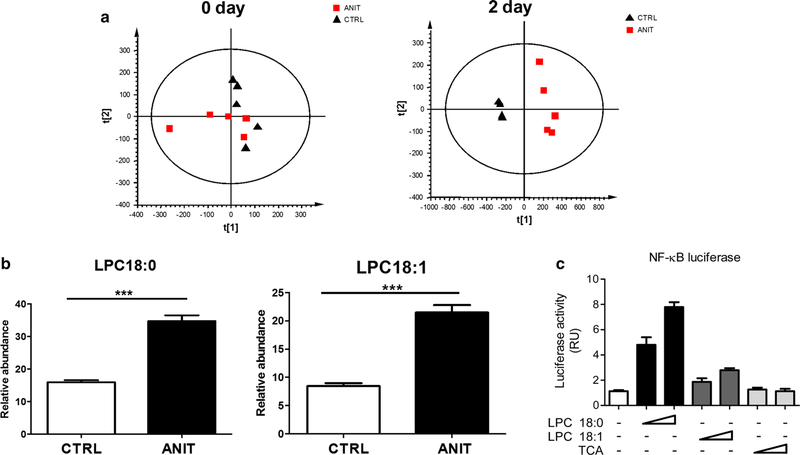
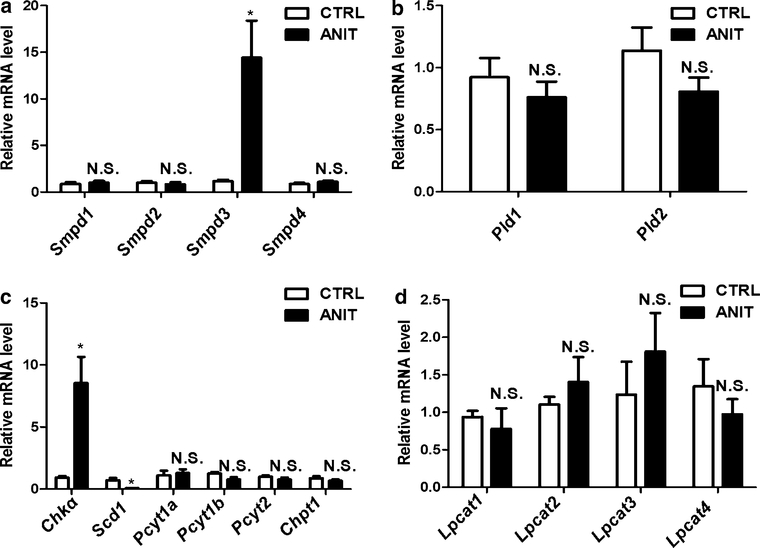
UPLC-ESI-QTOFMS analysis coupled with multiple discriminant analysis (MDA) was performed to profile the serum metabolome. Unsupervised principal components analysis (PCA) of UPLC-ESI-QTOFMS data from serum obtained from control and ANIT-treated groups showed that they were not separated at day 0. However, separation was observed between the ANIT group and control groups after a two day treatment with ANIT (Fig. 4a), with LPC18:0 and LPC18:1 (Supplemental Fig. 3A and B) significantly contributing to the group separation (Fig. 4b). LPC18:0 and LPC18:1 enhanced NF-κB activation in a concentration-dependent manner (Fig. 4c). To exclude the role of bile acids in NF-κB activation, the effect of TCA on NF-κB activation was also determined, and no activation was observed (Fig. 4c). Furthermore, the expression of lipid metabolism-relevant enzyme mRNAs was investigated; compared with the control group, the expression of Smpds3 and Chka mRNAs were increased (p < 0.05), and the expression of Scd1 mRNA decreased (Fig. 5). ANIT treatment did not alter the expression of other relevant enzymes related with the metabolism of lipids.
Fig. 4.
a PCA scores scatter plot of serum in control and ANIT-treated C57BL/6 wild-type mice at 0 and 2 days. Black triangle and red quadrangle represent control and ANIT-treated group, respectively. b Comparison of relevant abundance of LPC 18:0 and LPC 18:1 between control and ANIT-treated group. Chlorpropamide was used as the internal standard. ***p < 0.001. c Luciferase reporter assay for the activation of LPC 18:0, LPC 18:1 and TCA toward NF-κB
Fig. 5.
a Expression of mRNAs encoding lipid metabolism-relevant genes. The genes contained sphingomyelin phosphodiesterase (Smpd) 1–4 (a), phospholipase D (Pld) 1 and 2 (b), Chka, Scd1, Pcyt1a, Pcyt1b, Pcyt2, Chpt1 (c), and lysophosphatidylcholine transferase (Lpcat) 1–4 (d). *p < 0.05; NS not significant
Role of PPARα in protection against ANIT-induced liver toxicity
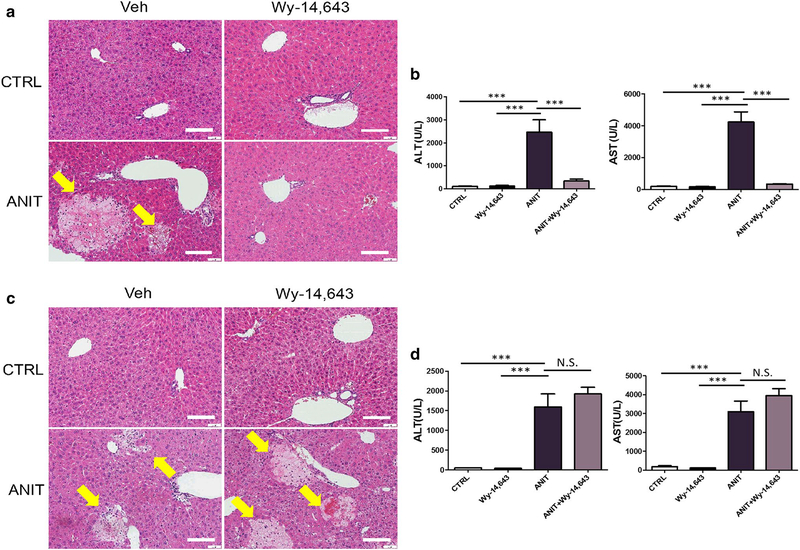
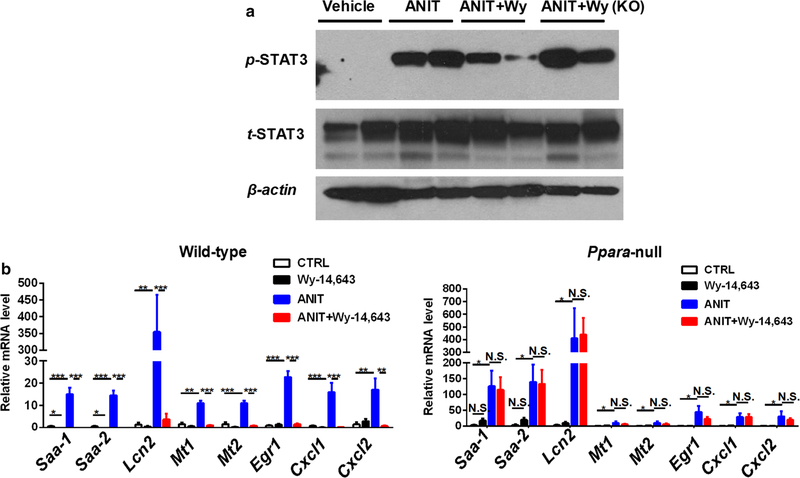
Due to the inhibitory effect of PPARα activation on NF-κB activation (Jeon et al. 2014; Shen et al. 2014), the role of PPARα in the treatment of ANIT-induced toxicity was investigated. Pretreatment with the PPARα agonist Wy-14,643 for 24 h before ANIT treatment resulted in the total protection toward ANIT-induced liver toxicity, as indicated by normal ALT and AST levels, and liver histology (Fig. 6a, b). In contrast, pretreatment with Wy-14,643 did not protect against ANIT-induced liver toxicity in Ppara-null mice, as indicated by hepatocyte necrosis and elevated ALT and AST levels in both ANIT-treated mice and ANIT + Wy-14,643-treated mice (Fig. 6e), and no significant difference for ALT and AST activity (Fig. 6c, d). Furthermore, Wy-14,643 treatment decreased activation of STAT3 and downstream gene expression in WT mice but not in Ppara-null mice (Fig. 7a, b).
Fig. 6.
a H&E staining of livers from control, Wy-14,643-treated, ANIT-treated and Wy-14,643/ANIT-treated wild-type mice. b Serum ALT and AST enzyme levels from control, Wy-14,643-treated, ANIT-treated and Wy-14,643/ANIT-treated wild-type mice. c H&E staining of livers from control, Wy-14,643-treated, ANIT-treated and Wy-14,643/ANIT-treated Ppara-null mice. d Serum ALT and AST enzyme levels from control, Wy-14,643-treated, ANIT-treated and Wy-14,643/ANIT-treated Ppara-null mice. In a and c, hepatocyte necrotic foci are indicated as arrows. Bars represent 100 μm. 4–6 mice in each group, and the values were given as mean ± SEM. ***p < 0.001; NS not significant
Fig. 7.
a Expression of STAT3 downstream genes control in wild-type mice treated with vehicle, Wy-14,643, ANIT, and Wy-14,643 + ANIT-treated Ppara-null mice. b Expression of STAT3 downstream genes control in Ppara-null mice treated with vehicle, Wy-14,643, ANIT, and Wy-14,643 + ANIT
Discussion
Pro-inflammatory cytokines have been implicated in the pathogenesis of acute tissue injury. IL-6, which acts as pro- and anti-inflammatory cytokine depending on the cellular context, is a critical mediator of injury and the acute-phase response (Rossi et al. 2015). IL-6 expression is regulated by a wide range of transcription factors, including NF-κB, CREB1, c-Fos, and c-Jun (Liu et al. 2013; Shi et al. 2015), depending on the cell type and ligand specificity. In the present study, activation and nuclear translocation of NF-κB was observed in livers from ANIT-treated mice. Serum analysis showed higher IL-6 in ANIT-treated mice than in the control group. Accompanied with NF-κB activation and IL-6 secretion, the level of phospho-STAT3 in liver from ANIT-treated mice was up-regulated, which induced STAT3 transcriptional activation of target genes. Expression of the STAT3 target gene mRNAs Saa, Lcn-2, Mt1, Mt2, Erg-1, Cxcl1 and Cxcl2 was up-regulated. SAA is a highly conserved, acute-phase protein mainly synthesized in the liver (Benditt and Eriksen 1971); the liver directs a significant proportion of its synthetic capability to producing SAA during acute inflammation, and SAA can activate inflammation through activating the inflammasome cascade, inducing the synthesis of several cytokines, and act as chemotactic for monocytes and polymorphonuclear leukocytes (Eklund et al. 2012). Lipocalin-2 (LCN-2), an important acute-phase protein, was demonstrated to exhibit higher expression after ANIT treatment, which is consistent with previous studies in which the level of LCN-2 was high during acute hepatic failure (Roth et al. 2013). Besides the increased expression of acute-phase protein, elevated expression of Mt1, Mt2, and Egr-1 mRNAs that were reported to exhibit high expression in cholestasis, were also detected. The over-expression of Cxcl1 and Cxcl2 mRNAs after ANIT treatment was also recently observed (Xu et al. 2004). The role of these genes in ANIT-induced liver damage was confirmed using knockout mice. For example, Cxcr2-null mice revealed a role for CXCL1 and CXCL2 in the pathogenesis of ANIT-induced liver toxicity (Xu et al. 2004). The current data reflected the role of NF-κB/IL-6/STAT3 in ANIT-induced liver injury.
Metabolomics was used to find lipid components, LPC18:0 and LPC18:1 that might stimulate the NF-κB/IL-6/STAT3 axis. LPCs were reported to exert some pro-inflammation functions. For example, LPC was demonstrated to induce mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells (Kume et al. 1992). LPC can also induce the production of IL-1β by human monocytes (Liu-Wu et al. 1998). The present study showed a potential function of LPCs in activating NF-κB.
The role of hepatic nuclear receptors in ANIT-induced liver injury has been the subject of a number of studies. The farnesoid X receptor (FXR) was demonstrated to play an important role in ANIT-induced cholestasis as revealed by the finding that FXR deficiency enhances susceptibility to ANIT-induced liver injury in mice (Cui et al. 2009). Treatment with the FXR agonist GW4064 also protects mice from cholestatic liver damage induced by ANIT treatment (Cui et al. 2009; Liu et al. 2003). Additionally, transgenic mice expressing FXR in intestine revealed that selective activation of intestinal FXR can protect the liver from ANIT-induced cholestasis through inducing intestinal fibroblast growth factor 15 (FGF15) expression which in turn reduces the bile acid pool in liver (Modica et al. 2012). Another bile acid sensor, pregnane X receptor, also plays little role in ANIT-induced liver damage (Cui et al. 2009). In addition, PPARα, that controls genes involved in glucose and lipid metabolism, also influences hepatocellular proliferation and the inflammatory response (Gervois and Mansouri 2012; Gonzalez and Shah 2008). Previous studies indicated an important role for PPARα in the suppression of chemically-induced liver damage. PPARα was demonstrated to play a key role in the protection of lipopolysaccharide-induced acute hepatic injury (Yoo et al. 2013) and inhibition of PPARα function can increase the susceptibility to dietary fat-induced nonalcoholic steatohepatitis (Abdelmegeed et al. 2011). Additionally, the activation of PPARα was reported to exhibit an inhibitory effect on NF-κB (Shen et al. 2014), and therefore PPARα might be a target for suppressing the NF-κB/IL-6/STAT3 axis involved in ANIT-induced liver damage. Indeed, activation of PPARα significantly protected ANIT-induced liver injury as revealed in the current study.
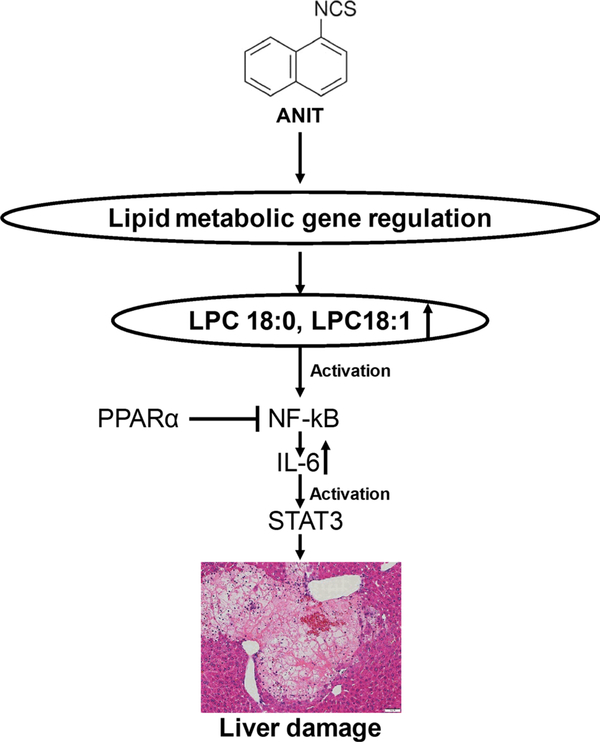
In conclusion, the role of lipid-regulated NF-κB/IL-6/STAT3 axis in ANIT-induced hepatotoxicity was demonstrated (Fig. 8) and this new mechanism was also successfully applied for developing new therapeutic strategies for the treatment of drug-induced cholestatic liver injury.
Fig. 8.
Summary of the role of lipid-regulated NF-κB/IL-6/STAT3 axis in ANIT-induced liver injury and the target role of PPARα. ANIT treatment altered the expression of lipid metabolism-relevant genes, which furtherly induced the elevation of LPC18:0 and LPC18:1. Elevated LPC18:0 and LPC18:1 activated NF-κB/IL-6/STAT3 axis, and finally resulted in the liver toxicity. The inhibition role of PPARα toward NF-κB activation prevented the ANIT-induced liver damage
Supplementary Material
Acknowledgements
This work was supported by the National Key Research and Development Program (2016YFC0903100, 2016YFC0903102) Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, the Tianjin Project of Thousand Youth Talents, Tianjin city-funded international projects to culture selected outstanding postdoctoral fellows, the Tianjin application foundation and advanced technology research plan (No. 15JCYBJC54700), China Postdoctoral Science Foundation (2016M590210), Tianjin Health Bureau Science Foundation Key Project (2014KR14), and project for Individualized diagnosis and treatment of colorectal cancer (No. LNCCC-B05-2015).
Footnotes
Conflict of interest The authors have declared that there are no conflicts of interest.
Compliance with ethical standards
Electronic supplementary material The online version of this article (doi:10.1007/s00204-016-1877-6) contains supplementary material, which is available to authorized users.
References
- Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ (2011) PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr 141(4):603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama TE, Nicol CJ, Fievet C et al. (2001) Peroxisome proliferator-activated receptor-alpha regulates lipid homeostasis, but is not associated with obesity: studies with congenic mouse lines. J Biol Chem 276(42):39088–39093 [DOI] [PubMed] [Google Scholar]
- Benditt EP, Eriksen N (1971) Chemical classes of amyloid substance. Am J Pathol 65(1):231–252 [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Fang ZZ, Kim JH et al. (2014) Intestinal CYP3A4 protects against lithocholic acid-induced hepatotoxicity in intestine-specific VDR-deficient mice. J Lipid Res 55(3):455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD (2009) Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci 110(1):47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP (2001) Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology 167(1):73–81 [DOI] [PubMed] [Google Scholar]
- Eklund KK, Niemi K, Kovanen PT (2012) Immune functions of serum amyloid A. Crit Rev Immunol 32(4):335–348 [DOI] [PubMed] [Google Scholar]
- Faiola B, Peterson RA, Kimbrough CL, Jordan HL, Cullen JM (2010) Acute ANIT toxicity in male IL-10 knockout and wild-type mice. Toxicol Pathol 38(5):745–755 [DOI] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Tanaka N et al. (2013) Metabolomics reveals trichloroacetate as a major contributor to trichloroethylene-induced metabolic alterations in mouse urine and serum. Arch Toxicol 87(11):1975–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois P, Mansouri RM (2012) PPARalpha as a therapeutic target in inflammation-associated diseases. Expert Opin Ther Targets 16(11):1113–1125 [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Shah YM (2008) PPARalpha: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology 246(1):2–8 [DOI] [PubMed] [Google Scholar]
- Jeon Y, Jung Y, Kim MC et al. (2014) Sargahydroquinoic acid inhibits TNFalpha-induced AP-1 and NF-kappaB signaling in HaCaT cells through PPARalpha activation. Biochem Biophys Res Commun 450(4):1553–1559 [DOI] [PubMed] [Google Scholar]
- Jiang C, Qu A, Matsubara T et al. (2011) Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60(10):2484–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Xie C, Li F et al. (2015) Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 125(1):386–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ (2006) ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol 291(2):G355–G363 [DOI] [PubMed] [Google Scholar]
- Kossor DC, Meunier PC, Handler JA, Sozio RS, Goldstein RS (1993) Temporal relationship of changes in hepatobiliary function and morphology in rats following alpha-naphthylisothiocyanate (ANIT) administration. Toxicol Appl Pharmacol 119(1):108–114 [DOI] [PubMed] [Google Scholar]
- Kume N, Cybulsky MI, Gimbrone MA Jr (1992) Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest 90(3):1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J et al. (1995) Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15(6):3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Patterson AD, Krausz KW, Tanaka N, Gonzalez FJ (2012) Metabolomics reveals an essential role for peroxisome proliferator-activated receptor alpha in bile acid homeostasis. J Lipid Res 53(8):1625–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Jiang C, Krausz KW et al. (2013) Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ et al. (2003) Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest 112(11):1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Hong YF, Huang CM, Chen CY, Huang TN, Hsueh YP (2013) TLR7 negatively regulates dendrite outgrowth through the Myd88-c-Fos-IL-6 pathway. J Neurosci 33(28):11479–11493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Wu Y, Hurt-Camejo E, Wiklund O (1998) Lysophosphatidylcholine induces the production of IL-1beta by human monocytes. Atherosclerosis 137(2):351–357 [DOI] [PubMed] [Google Scholar]
- Modica S, Petruzzelli M, Bellafante E et al. (2012) Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 142(2):355–365 [DOI] [PubMed] [Google Scholar]
- Patterson AD, Shah YM, Matsubara T, Krausz KW, Gonzalez FJ (2012) Peroxisome proliferator-activated receptor alpha-dependent induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 56:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JF, Lu ZY, Jourdan M, Klein B (2015) Interleukin-6 as a Therapeutic Target. Clin Cancer Res 21(6):1248–1257 [DOI] [PubMed] [Google Scholar]
- Roth GA, Nickl S, Lebherz-Eichinger D et al. (2013) Lipocalin-2 serum levels are increased in acute hepatic failure. Transplant Proc 45(1):241–244 [DOI] [PubMed] [Google Scholar]
- Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123(Pt 24):4251–4258 [DOI] [PubMed] [Google Scholar]
- Shen W, Gao Y, Lu B, Zhang Q, Hu Y, Chen Y (2014) Negatively regulating TLR4/NF-kappaB signaling via PPARalpha in endotoxin-induced uveitis. Biochim Biophys Acta 1842(7):1109–1120 [DOI] [PubMed] [Google Scholar]
- Shi X, Li D, Deng Q et al. (2015) NEFAs activate the oxidative stress-mediated NF-kappaB signaling pathway to induce inflammatory response in calf hepatocytes. J Steroid Biochem Mol Biol 145:103–112. doi: 10.1016/j.jsbmb.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ (2012) Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 56(1):118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lee G, Wang H, Vierling JM, Maher JJ (2004) Limited role for CXC chemokines in the pathogenesis of alpha-naphthylisothiocyanate-induced liver injury. Am J Physiol Gastrointest Liver Physiol 287(3):G734–G741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Abdelmegeed MA, Song BJ (2013) Activation of PPARalpha by Wy-14643 ameliorates systemic lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun 436(3):366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.