Abstract
Importance
Alcohol dependence (AD) and major depression (MD) are leading causes of disability that often co-occur. Genetic epidemiologic data have shown that AD and MD share a common possible genetic cause. The molecular nature of this shared genetic basis is poorly understood.
Objectives
To detect genetic risk variants for comorbid AD and MD and to determine whether polygenic risk alleles are shared with neuropsychiatric traits or subcortical brain volumes.
Design, Setting, and Participants
This genome-wide association study analyzed criterion counts of comorbid AD and MD in African American and European American data sets collected as part of the Yale-Penn study of the genetics of drug and alcohol dependence from February 14, 1999, to January 13, 2015. After excluding participants never exposed to alcohol or with missing information for any diagnostic criterion, genome-wide association studies were performed on 2 samples (the Yale-Penn 1 and Yale-Penn 2 samples) totaling 4653 African American participants and 3169 European American participants (analyzed separately). Tests were performed to determine whether polygenic risk scores derived from potentially related traits in European American participants could be used to estimate comorbid AD and MD.
Main Outcomes and Measures
Comorbid criterion counts (ranging from 0 to 14) for AD (7 criteria) and MD (9 criteria, scaled to 7) as defined by the DSM-IV.
Results
Of the 7822 participants (3342 women and 4480 men; mean [SD] age, 40.1 [10.7] years), the median comorbid criterion count was 6.2 (interquartile range, 2.3-10.9). Under the linear regression model, rs139438618 at the semaphorin 3A (SEMA3A [OMIM 603961]) locus was significantly associated with AD and MD comorbidity in African American participants in the Yale-Penn 1 sample (β = 0.89; 95% CI, 0.57-1.20; P = 2.76 × 10−8). In the independent Yale-Penn 2 sample, the association was also significant (β = 0.83; 95% CI, 0.39-1.28; P = 2.06 × 10−4). Meta-analysis of the 2 samples yielded a more robust association (β = 0.87; 95% CI, 0.61-1.12; P = 2.41 × 10−11). There was no significant association identified in European American participants. Analyses of polygenic risk scores showed that individuals with a higher risk of neuroticism (β = 1.01; 95% CI, 0.50-1.52) or depressive symptoms (β = 0.87; 95% CI, 0.32-1.42) and a lower level of subjective well-being (β = –0.94; 95% CI, –1.46 to –0.42) and educational attainment (β = –1.00, 95% CI, −1.57 to –0.44) had a higher level of AD and MD comorbidity, while larger intracranial (β = 1.07; 95% CI, 0.50 to 1.64) and smaller putamen volumes (β = –1.16; 95% CI, –1.86 to –0.46) were associated with higher risks of AD and MD comorbidity.
Conclusions and Relevance
SEMA3A variation is significantly and replicably associated with comorbid AD and MD in African American participants. Analyses of polygenic risk scores identified pleiotropy with neuropsychiatric traits and brain volumes. Further studies are warranted to understand the biological and genetic mechanisms of this comorbidity, which could facilitate development of medications and other treatments for comorbid AD and MD.
This genome-wide association study uses data from the Yale-Penn study to detect genetic risk variants for comorbid alcohol dependence and major depression and to determine whether polygenic risk alleles are shared with neuropsychiatric traits or subcortical brain volumes.
Key Points
Question
What specific genetic risk variants are associated with comorbid alcohol dependence and major depression?
Findings
A replicable genome-wide significant association at SEMA3A with comorbid alcohol dependence and major depression was detected in a sample of 4653 African American participants; there was no significant association in a sample of 3169 European American participants.
Meaning
This study enhances understanding of the genetic mechanisms shared between alcohol dependence and major depression and has implications both for development of medications and for other treatments.
Introduction
Alcohol dependence (AD) and major depression (MD) are among the world’s leading causes of disability1 and are frequently comorbid.2 Their co-occurrence is well documented in clinical and epidemiologic studies,3,4 and shared genetic risks for AD and MD have been identified.5,6,7,8,9,10,11 Thus, improved recognition and treatment for comorbid AD and MD could save lives and benefit society.12
Although the comorbidity of AD and MD is well established, the causal links between the 2 disorders have been debated. There is evidence both that AD increases the risk of MD13 and that MD leads to AD.14 Another possibility is that shared factors increase susceptibility to both disorders. Common genetic factors that predispose individuals to the co-occurrence of AD and MD have been sought in family, twin, and general population studies,15,16,17 with 1 study showing a sex-specific effect.16
Genome-wide association studies (GWASs) have reported genome-wide significant findings for AD18,19 and MD.20,21,22 However, thus far, no findings have been reported for comorbid AD and MD.23 In this study, we conducted a GWAS to detect novel genetic risks for comorbid criterion counts of AD and MD, which represented the overall severity of comorbid disorders. We also examined the genetic overlap between the comorbidity and other neuropsychological traits or subcortical brain volumes using a polygenic risk score (PRS) approach.24
Methods
Participants and Diagnostic Procedures
All participants were recruited for studies of the genetics of substance dependence conducted from February 14, 1999, to January 13, 2015, as previously described.19,25 The participants were interviewed using the Semi-structured Assessment for Drug Dependence and Alcoholism26 to derive DSM-IV27 diagnoses of lifetime AD and MD criteria. For AD, 7 DSM-IV criteria were assessed, and for MD, 9 DSM-IV criteria were assessed. The participants were grouped into Yale-Penn 1 and Yale-Penn 2 phases based on their epoch of recruitment and the genotyping platform used. Participants provided written informed consent and the study was approved by the institutional review board at each participating site (Yale Human Research Protection Program, University of Pennsylvania Institutional Review Board, University of Connecticut Human Subjects Protection Program, Medical University of South Carolina Institutional Review Board for Human Research, and the McLean Hospital Institutional Review Board). Certificates of confidentiality were obtained from the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Participants who were not exposed to alcohol (ie, who answered no for the question, “Have you ever had a drink of alcohol?”) or with any missing diagnostic criteria were excluded to reduce the misclassification of phenotypes. Of the total Yale-Penn sample of 7822 included in the present study, there were 3041 African American participants and 1618 European American participants (Yale-Penn 1) and, additionally, an identically ascertained 1612 African American participants and 1551 European American participants (Yale-Penn 2). We scaled the MD criteria uniformly into the same range as those of AD to weight them comparably for the GWAS. The comorbid criterion counts (ranging from 0 to 14) were then treated as the outcomes, representing the overall severity of comorbidity.
Genotyping, Quality Control, and Imputation
The Yale-Penn 1 sample was genotyped using the HumanOmni1-Quad array (Illumina) containing approximately 988 000 single-nucleotide polymorphisms (SNPs). The Yale-Penn 2 sample was genotyped using the HumanCore Exome array (Illumina) containing approximately 266 000 exomic SNPs and approximately 240 000 tagging SNPs for genome-wide imputation. Standard preimputation quality control included the removal of individuals and SNPs with call rates less than 98% and filtering out SNPs with a minor allele frequency less than 1%. To verify and correct the misclassification of self-reported race, we performed principal component (PC) analysis on SNPs common (pruning by linkage disequilibrium of r2 > 0.2) to each of the 2 individual genotyping arrays and the 1000 Genome phase 3 reference panels (African populations [AFR], European populations [EUR], East Asian populations [EAS], South Asian populations [SAS], and admixed American populations [AMR]) using EIGENSOFT.28,29 The first 10 PCs were used to cluster the participants, distinguish African American participants from European American participants, and remove outliers from the 2 groups, which were subsequently analyzed separately. We conducted a second PC analysis within groups, and the first 10 PCs were used to correct for population stratification. To correct for the pedigree relationships, a pairwise identity by descent was calculated using PLINK.30 Pairs of individuals who shared more than 25% of identity by descent were assigned to the same family, while pairs of individuals whose identity by descent proportions did not match the reported genetic relationship were assigned to 2 different families.
Additional single-nucleotide variants (SNVs) were imputed using Minimac3 implemented in Michigan Imputation Server (https://imputationserver.sph.umich.edu/index.html)31 and the 1000 Genomes phase 3 reference panel.32 The African American and European American samples were imputed separately. Single-nucleotide variants with a Hardy-Weinberg equilibrium P < 10−5 and a minor allele frequency less than 3% were excluded from downstream analysis. Single-nucleotide variants with an imputation accuracy of 0.8 or more in both the Yale-Penn 1 and Yale-Penn 2 samples were kept for the association analyses. In the Yale-Penn 1 sample, 7 773 845 SNVs in African American participants and 5 611 755 SNVs in European American participants were included in the association analyses; in the Yale-Penn 2 sample, 7 725 291 SNVs in African American participants and 5 595 246 SNVs in European American participants were analyzed.
Statistical Analysis
We performed association tests for the criterion counts (ranging from 0 to 14) for comorbid AD and MD. To account for family structure, a linear regression model embedded in the generalized estimation equation33 was applied in the R package GWAF.34 In the generalized estimation equation model, each family was treated as a cluster using the independence correlation matrix to estimate the robust variance. All SNVs, both genotyped and imputed, were tested using an additive model, adjusted by age, sex, and the first 10 PCs. Analyses were performed separately within each data set and ancestral group. The association results from 7 572 255 SNVs in African American participants were meta-analyzed across the 2 data sets using the inverse variance method implemented in the program METAL.35 In European American participants, 5 542 675 SNVs were meta-analyzed across data sets. In transpopulation meta-analysis, 5 086 170 SNVs were analyzed. A linkage disequilibrium score regression (LDSC) was used to distinguish confounding from polygenicity.36 Regional associations were plotted using LocusZoom.37
Polygenic Risk Scores
Polygenic risk scores constructed from GWAS summary statistics of the same or related traits in other data sets can be used to test the genetic relationship of those traits with the study trait, given the hypothesis that complex genetic traits are highly polygenic and the genetic risks are pleiotropic among different traits. As described previously,24 a PRS was calculated as the sum of the risk alleles with P values less than the threshold of significance, weighted by the effect sizes. The association between the constructed PRS and the phenotype was tested by a linear regression model in the generalized estimation equation, adjusting for age, sex, and the first 10 PCs. The Yale-Penn 1 and Yale-Penn 2 cohorts were analyzed separately and then meta-analyzed. Eight threshold P values (.00001, .0001, .001, .005, .01, .05, .10, and .50) were considered. Polygenic profiles of neuropsychological traits from the Social Science Genetic Association Consortium (https://www.thessgac.org/data), including depressive symptoms,21 educational attainment,38 neuroticism,21 and subjective well-being,21 were tested; polygenic profiles of neuropsychiatric diseases from the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/results-and-downloads), including binary anxiety disorders and quantitative anxiety factor scores,39 bipolar disorder,40 schizophrenia,41 Alzheimer diseases,42 and smoking behaviors,43 were tested; and polygenic profiles of human subcortical brain volumes from the ENIGMA (Enhancing Neuro Imaging Genetics through Meta-Analysis) Consortium44 (http://enigma.ini.usc.edu/research/gwasma-of-subcortical-structures/) were tested. Analyses of PRSs were performed only for the European American participants because all the public data were from European samples. The summary data were clumped by linkage disequilibrium with r2 < 0.2 in a 200-kb window. For comparison, the polygenic profiles of the above-mentioned traits were tested with AD (adjusting for MD) and MD (adjusting for AD). A correction for multiple testing was applied for all polygenic profiles with AD, MD, and the comorbidity at all threshold P values (504 tests in total) using the false discovery rate method.45
Results
In total, 7822 participants (3342 women and 4480 men; mean [SD] age, 40.1 [10.7] years) were included in the analysis. Among them, 6610 participants (84.5%) were diagnosed as having at least 1 criterion for AD or MD. The median comorbid criterion count was 6.2 (interquartile range, 2.3-10.9). A total of 3041 African American participants and 1618 European American participants were from the Yale-Penn 1 cohort, while 1612 African American participants and 1551 European American participants were from the Yale-Penn 2 cohort (Table). The distributions of comorbid criterion counts are shown in eFigure 1 in the Supplement.
Table. Demographic Characteristics of the Samples.
| Characteristic | Yale-Penn 1 Participants | Yale-Penn 2 Participants | Total (N = 7822) |
||
|---|---|---|---|---|---|
| African American (n = 3041) |
European American (n = 1618) |
African American (n = 1612) |
European American (n = 1551) |
||
| Female sex, No. (%) | 1402 (46.1) | 668 (41.3) | 664 (41.2) | 608 (39.2) | 3342 (42.7) |
| Age, mean (SD), y | |||||
| All participants | 41.1 (8.9) | 38.0 (10.8) | 41.0 (10.9) | 39.4 (13.0) | 40.1 (10.7) |
| Male participants | 41.9 (8.8) | 37.8 (11.0) | 41.9 (10.6) | 39.0 (12.7) | 40.4 (10.7) |
| Female participants | 40.2 (9.0) | 38.2 (10.5) | 39.7 (11.2) | 40.1 (13.4) | 39.6 (10.7) |
| Participants with at least 1 AD or MD criterion, No. (%) | 2541 (83.6) | 1484 (91.7) | 1260 (78.2) | 1325 (85.4) | 6610 (84.5) |
| Comorbid criterion count, median (IQR) | 5.9 (2.0-10.0) | 7.2 (4.2-11.7) | 5.9 (1.0-10.4) | 7.0 (3.0-11.3) | 6.2 (2.3-10.9) |
| Participants with at least 1 AD criterion, No. (%) | 2249 (74.0) | 1335 (82.5) | 1146 (71.1) | 1203 (77.6) | 5933 (75.9) |
| AD criterion count, median (IQR) | 3 (0-6) | 4 (1-6) | 3 (0-6) | 4 (1-6) | 3 (1-6) |
| Participants with at least 1 MD criterion, No. (%) | 1579 (51.9) | 1059 (65.5) | 817 (50.7) | 952 (61.4) | 4407 (56.3) |
| MD criterion count (scaled to 7), median (IQR) | 2.3 (0-6.2) | 4.7 (0-6.2) | 1.2 (0-6.2) | 3.9 (0-6.2) | 3.9 (0-6.2) |
Abbreviations: AD, alcohol dependence; IQR, interquartile range; MD, major depression.
Genome-Wide Significant Associations
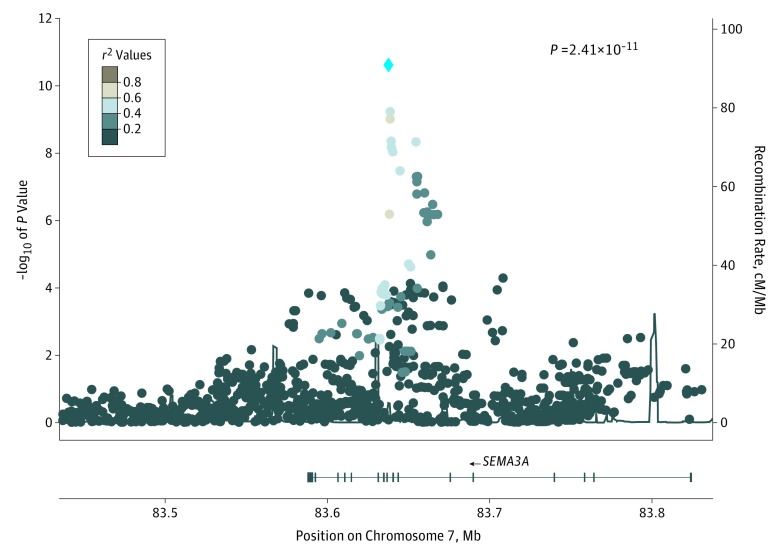
Genome-wide association studies were performed in each data set, followed by meta-analyses of African American participants and European American participants and transpopulation meta-analysis of all African American participants and European American participants (eFigure 2 and eTable 1 in the Supplement). A significant association was detected in the African American sample of the Yale-Penn 1 cohort (rs139438618, risk allele G, β = 0.89; 95% CI, 0.57-1.20; P = 2.76 × 10−8) and was replicated in the Yale-Penn 2 cohort (β = 0.83; 95% CI, 0.39-1.28; P = 2.06 × 10−4). By meta-analyzing all African American participants, the association was enhanced (β = 0.87; 95% CI, 0.61-1.12; P = 2.41 × 10−11) (Figure 1). A clear trend was observed in the criterion and minor allele frequency matrix showing that the higher the criterion count, the higher the frequency of the risk allele (eFigure 3 in the Supplement). This finding argues against the association being biased by criteria of a single disorder. The SNP rs139438618 is located in an intron of SEMA3A (OMIM 603961), which plays an important role in normal neuronal pattern development.
Figure 1. Regional Manhattan Plot of rs139438618 in African American Participants.
Association results from single-nucleotide polymorphisms (SNPs) in the 83.4- to 83.9-Mb region which encompass SEMA3A on chromosome 7. African American participants from the Yale-Penn 1 and Yale-Penn 2 cohorts were meta-analyzed. The blue diamond indicates lead SNP rs139438618. The SNPs are color coded according to the linkage disequilibrium (r2) in the 1000 Genomes African samples with the most significant SNP. cM/Mb indicates centimorgan per megabases.
Conditional Analyses
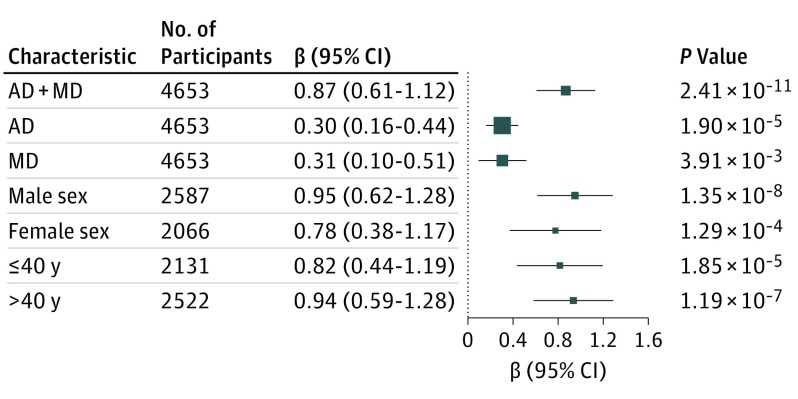
To demonstrate that the association of rs139438618 is contributed to by both disorders rather than being driven by only 1 of them, we tested the association with AD criterion counts (controlling for MD criterion counts) or MD criterion counts (controlling for AD criterion counts). Both of the associations were nominally significant (β = 0.30; 95% CI, 0.16-0.44; P = 1.90 × 10−5 for AD; β = 0.31; 95% CI, 0.10-0.51; P = 3.91 × 10−3 for MD), indicating an additive or synergistic association for comorbidity of AD and MD. To test whether the association was related to age or sex, we split the African American sample into older (>40 years of age) and younger groups (≤40 years of age, adjusting for sex and 10 PCs) and into male and female groups (adjusting for age and 10 PCs). Each of these approaches showed a similar association between rs139438618 and comorbid AD and MD, indicating that the associations were present in all of the different subgroups (Figure 2) rather than being influenced by either age or sex.
Figure 2. Conditional Analysis of rs139438618 and Associations in Different Groups.
Association with alcohol dependence (AD) was adjusted for major depression (MD); association with MD was adjusted for AD.
Polygenic Risk Scores
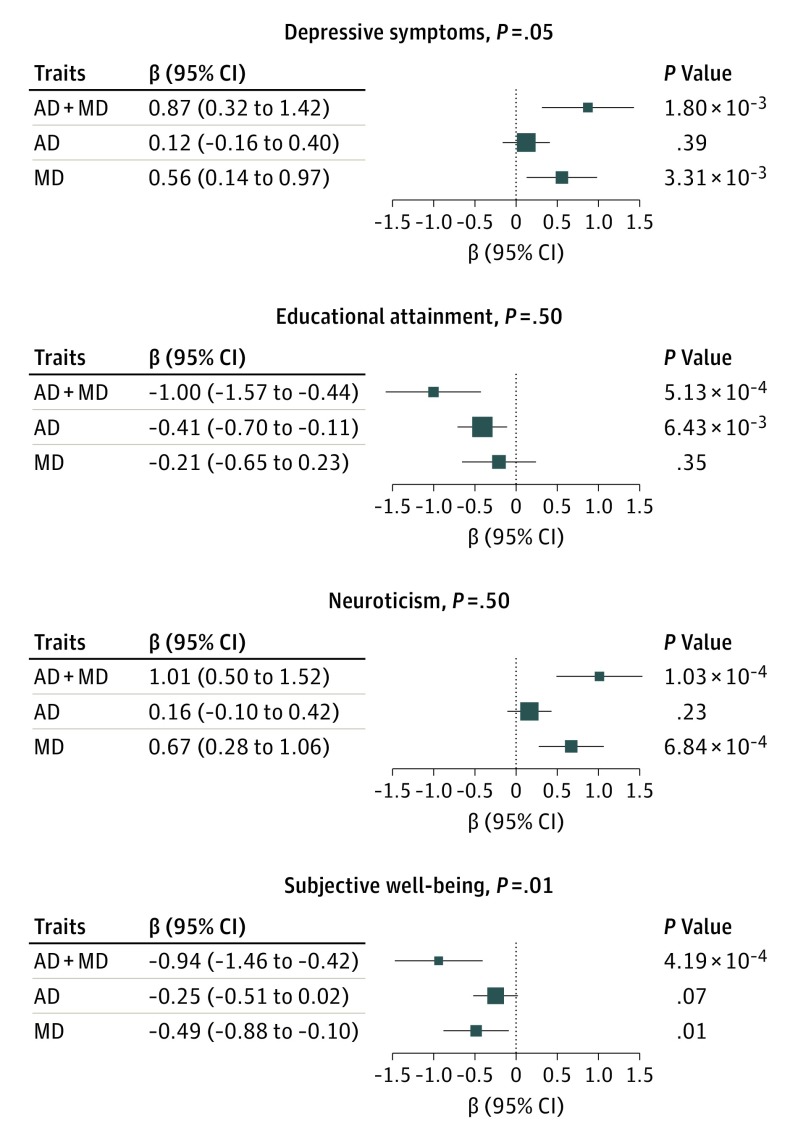
Polygenic risk scores of depressive symptoms (β = 0.87; 95% CI, 0.32-1.42; P = 1.80 × 10−3) and neuroticism (β = 1.01; 95% CI, 0.50-1.52; P = 1.03 × 10−4) were positively associated with the risk for comorbid AD and MD, although those associations were mainly driven by MD (although the association between depressive symptoms and MD did not survive multiple testing correction).46 This finding indicates that there are shared polygenic risks among neuroticism and MD; a significant positive genetic correlation between neuroticism and MD was reported in a previous study.47 The PRS of educational attainment (β = –1.00; 95% CI, –1.57 to –0.44; P = 5.13 × 10−4) and subjective well-being (β = –0.94; 95% CI, –1.46 to –0.42; P = 4.19 × 10−4) was negatively associated with risk for comorbidity, revealing shared genetic risks between neuropsychological traits and comorbid AD and MD. The association with educational attainment was driven mainly by AD, while the association with subjective well-being was contributed to by both disorders (Figure 3 and eTables 2-4 in the Supplement). However, no association was detected between the PRS of psychiatric traits and comorbid AD and MD. A PRS of smoking behavior (age at smoking initiation) was significantly associated with comorbid AD and MD (β = 0.95; 95% CI, 0.31-1.58; P = 3.34 × 10−3), which was explained mainly by AD alone (eFigure 4 in the Supplement). The PRS of intracranial volume was positively associated with the risk of comorbid AD and MD (β = 1.07; 95% CI, 0.50-1.64; P = 2.18 × 10−4) and was not driven by either single disorder. The PRS of putamen volume was negatively associated with comorbid AD and MD risk (β = –1.16; 95% CI, –1.86 to –0.46; P = 1.74 × 10−3) but was explained mainly by AD alone (eFigure 5 in the Supplement).
Figure 3. Associations Between Comorbid Alcohol Dependence (AD) and Major Depression (MD) and Polygenic Risk Scores for Neuropsychological Traits.
Association with AD was adjusted for MD; association with MD was adjusted for AD.
Discussion
Alcohol dependence and MD often co-occur owing, in part, to shared genetic risk factors. Prior GWAS analyses of AD have identified and/or confirmed risk variants, such as those that map to alcohol-metabolizing enzyme loci.19 In a GWAS of MD, variant discovery required either relatively homogenous samples with severe affection20 or very large samples.22 What is unusual about our results is that we identified at least 1 highly significant risk locus (genome-wide significant finding in the Yale-Penn 1 cohort and with a P ~ 2 × 10−4 in the Yale-Penn 2 cohort) that affects risk for the joint occurrence of these traits, but it was not identified previously in a GWAS of either trait separately. Thus, the phenotype definition that we used appears to have been a key factor in identifying this risk locus, which shows pleiotropic effects even on the single-gene level. This finding constitutes a specific example of pleiotropy that then results in comorbidity. Presumably, when large enough GWASs for AD and MD separately are completed, these same loci would be identified eventually. Large samples might be needed because, as seen in our results, the phenotype definition would in these cases (AD or MD taken individually) be incomplete.
We investigated both African American participants and European American participants and found genetic variants associated with comorbid AD and MD, which are the first such genetic findings obtained via a genome-wide design. In the GWAS analysis, we identified rs139438618, which maps to SEMA3A, as a genome-wide significant finding in African American participants in the Yale-Penn 1 cohort. The association was replicated in the Yale-Penn 2 cohort, and a remarkably strong association (especially considering the moderate sample size) was observed in the meta-analysis. No association was detected in this gene region in European American participants, indicating a population-specific genetic risk. Results of conditional analyses showed that the association was not driven by AD or MD alone. No association at this locus was detected in the previous GWAS of AD that used a subset of the same participant sample.19
SEMA3A belongs to the semaphorin family, which is a class of secreted and membrane proteins that are involved in axon guidance and neuronal connectivity. SEMA3A acts as either a chemorepulsive agent, inhibiting axonal outgrowth, or a chemoattractive agent, stimulating the growth of apical dendrites. A high level of expression of SEMA3A across various brain regions was observed in early fetal periods, with a decrease thereafter and a relatively low level maintained throughout adulthood (eFigure 6 in the Supplement; http://www.brainspan.org).48 Persistent expression of SEMA3A was observed in mature human and rat brains, including the olfactory system, the cerebral cortex, and the entorhinal-hippocampal system.49 Existing evidence indicates that SEMA3A is associated with many traits related to the central nervous system, including schizophrenia,50 Alzheimer disease,51 and iris patterns.52 However, the molecular mechanisms involved in AD and MD are largely unknown.
Changes in the expression of SEMA3A could change the neural circuits that predispose individuals to central nervous system traits.50,51,53 SEMA3A alleles have been shown to be associated with genetic disorders of neuronal migration, autism spectrum disorders, epilepsy, and several other disorders.53 The specific contribution of variation in SEMA3A to the possible causes of AD and MD remains to be determined. No expression quantitative trait loci effect was detected for the top SNP and variants sharing high linkage disequilibrium in the general health population by Genotype-Tissue Expression,54 but this outcome is consistent with the observed expression patterns and the hypothesis that the risk-associated variation alters expression changes in certain brain regions early in development that might trigger eventual vulnerability to comorbid AD and MD.
Analyses of PRSs tested for shared polygenic risk with several other central nervous system traits. As would be expected, there was an association between AD and MD comorbidity and depressive criteria (β = 0.87; 95% CI, 0.32-1.42; P = 1.80 × 10−3). A personality trait—neuroticism—was shown to be genetically correlated with psychiatric disorders, such as MD and anorexia nervosa.47 Consistent with this observation, we also observed shared genetic risks among individuals with neuroticism and individuals with comorbid AD and MD (β = 1.01; 95% CI, 0.50-1.52; P = 1.03 × 10−4), which was driven mainly by MD (β = 0.67; 95% CI, 0.28-1.06; P = 6.84 × 10−4).
There are complex links between genetic factors and social environment in depression, and negative correlations between MD and educational attainment55 and between MD and subjective well-being21 have been identified. However, the genetic correlation between comorbid AD and MD and these neuropsychological traits (educational attainment and subjective well-being) has not been studied previously, to our knowledge. We found that the PRSs of educational attainment (β = –1.00; 95% CI, –1.57 to –0.44; P = 5.13 × 10−4) and subjective well-being (β = –0.94; 95% CI, –1.46 to –0.42; P = 4.19 × 10−4) were, as expected, protective in relation to the risk of comorbid AD and MD.
We also found PRS associations with comorbid AD and MD. For AD alone, an association was observed for a PRS of anxiety factor scores (β = 0.58; 95% CI, 0.29-0.87; P = 7.00 × 10−5). A PRS for the age of onset of smoking was positively associated with comorbid AD and MD (β = 0.95; 95% CI, 0.31-1.58; P = 3.34 × 10−3), an association that was driven mainly by AD (β = 0.61; 95%, CI 0.29-0.93; P = 1.98 × 10−4), with a higher number of cigarettes per day associated with a greater risk of AD (β = 0.44; 95% CI, 0.14-0.74; P = 4.39 × 10−3). Opposite effects of smoking cessation were observed for AD and MD taken individually: current smoker status was strongly associated with a higher risk of AD (β = –0.96; 95% CI, –1.28 to –0.63; P = 7.56 × 10−9), while former smokers (defined as those who had quit smoking for >1 year43) showed a higher risk of MD (β = 0.88; 95% CI, 0.40-1.36; P = 3.26 × 10−4). Although smoking cessation could pose a risk for the development of MD,56 the genetic causal relationship between long-time smoking cessation and depression is still unknown. More research is needed to understand the genetic mechanisms and shared genetic risks among these and other psychiatric traits.
There was an association between the PRS for intracranial volume, such that a greater intracranial volume was associated with a greater risk of comorbid AD and MD (β = 1.07; 95% CI, 0.50-1.64; P = 2.18 × 10−4). Volumes of several specific subcortical regions have previously been shown to be associated with AD,57,58,59,60 with other regions associated with MD.61,62,63,64 We tested the volume of 7 different subcortical regions44 and found that a smaller putamen volume was associated with a greater risk of comorbid AD and MD (β = –1.16; 95% CI, –1.86 to –0.46; P = 1.22 × 10−3), an association that was explained by AD only (β = –0.78; 95% CI, –1.15 to –0.42; P = 2.77 × 10−5), consistent with a previous magnetic resonance imaging study.57 Furthermore, pallidum volume was negatively associated with risk for AD (β = –0.54; 95% CI, –0.84 to –0.24; P = 3.99 × 10−4). However, we did not observe any association between subcortical volume PRS and MD, in line with the results of the largest magnetic resonance imaging study to date, which reported that subcortical volumes might not differ between individuals with depression and healthy individuals.65
Several factors complicate prior research, such as small sample sizes in magnetic resonance imaging studies, trait heterogeneity, possible confounding by comorbid illnesses that frequently were not assessed, and the complex interactions between these traits and the underlying brain structure. Another limitation is that all of these PRS analyses were restricted to European American individuals owing to a lack of GWAS analyses for African American individuals. This restriction reflects a limitation in the published GWAS literature and the summary statistics that are available to the research community. Results from African American individuals could be different. There is a clear need to expand the diversity of populations in genetic studies.
Conclusions
Genetic variants in the SEMA3A gene were replicably associated with comorbid AD and MD in the African American individuals. Our results are specific to the African American participants. The trait distribution differs somewhat by population in our sample (Table). Our data do not allow for strong support of any particular hypothesis regarding the observed genetic differences by population. Differences in disease etiology by population cannot be excluded, but differences in environmental factors, epistasis, or random variation in our sample provide equally satisfactory possible explanations. The association signals detected in this study do not explain the underlying genetic architecture for susceptibility to comorbid AD and MD, although they do provide substantial insight into the problem and 1 novel mechanism. Analyses of PRSs provided evidence of shared polygenic risk variants between comorbid AD and MD and neuropsychological traits and subcortical brain volumes. Our findings thus support the conclusion that these comorbid traits may be to some extent, and may be considered for some purposes, a single diagnostic, or even genetic, entity: that is, among individuals with comorbid AD and MD, there are some in whom the risk for both illnesses is influenced by a single, or a few, variants. Further efforts to elucidate the molecular risk factors and the causal mechanisms for comorbid AD and MD will require larger samples to enable a focus on lower-frequency and rare variants that may have large effects.
eTable 1. Top Findings in Meta-Analyses (P value <1 × 10−4)
eTable 2. PRS Results for Comorbid AD and MD
eTable 3. PRS Results for AD, Adjusting for MD
eTable 4. PRS Results for MD, Adjusting for AD
eFigure 1. Distribution of Comorbid Criterion Counts
eFigure 2. Manhattan and QQ Plots of Meta-Analyses
eFigure 3. MAF of rs139438618 for Criteria
eFigure 4. Associations Between the Comorbidity and PRS of Psychiatric Traits
eFigure 5. Associations Between the Comorbidity and PRS of Subcortical Brain Volumes
eFigure 6. Spatio-Temporal Transcriptome of SEMA3A in Human Brain
References
- 1.Murray CJ, Vos T, Lozano R, et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2197-2223. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39(3):197-206. [DOI] [PubMed] [Google Scholar]
- 3.Merikangas KR, Gelernter CS. Comorbidity for alcoholism and depression. Psychiatr Clin North Am. 1990;13(4):613-632. [PubMed] [Google Scholar]
- 4.Petrakis IL, Gonzalez G, Rosenheck R, Krystal JH. Comorbidity of alcoholism and psychiatric disorders: an overview. Alcohol Res Health. 2002;26(2):81-89. [Google Scholar]
- 5.Wang JC, Hinrichs AL, Stock H, et al. . Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Mol Genet. 2005;14(16):2421-2434. [DOI] [PubMed] [Google Scholar]
- 7.Sjöholm LK, Kovanen L, Saarikoski ST, Schalling M, Lavebratt C, Partonen T. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. J Circadian Rhythms. 2010;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tambs K, Harris JR, Magnus P. Genetic and environmental contributions to the correlation between alcohol consumption and symptoms of anxiety and depression: results from a bivariate analysis of Norwegian twin data. Behav Genet. 1997;27(3):241-250. [DOI] [PubMed] [Google Scholar]
- 9.Nurnberger JI Jr, Foroud T, Flury L, et al. . Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. Am J Psychiatry. 2001;158(5):718-724. [DOI] [PubMed] [Google Scholar]
- 10.McEachin RC, Keller BJ, Saunders EF, McInnis MG. Modeling gene-by-environment interaction in comorbid depression with alcohol use disorders via an integrated bioinformatics approach. BioData Min. 2008;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen AM, Pietrzak RH, Kranzler HR, et al. . Polygenic scores for major depressive disorder and risk of alcohol dependence [published online August 16, 2017]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2017.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thase ME, Salloum IM, Cornelius JD. Comorbid alcoholism and depression: treatment issues. J Clin Psychiatry. 2001;62(suppl 20):32-41. [PubMed] [Google Scholar]
- 13.Fergusson DM, Boden JM, Horwood LJ. Tests of causal links between alcohol abuse or dependence and major depression. Arch Gen Psychiatry. 2009;66(3):260-266. [DOI] [PubMed] [Google Scholar]
- 14.Kuo PH, Gardner CO, Kendler KS, Prescott CA. The temporal relationship of the onsets of alcohol dependence and major depression: using a genetically informative study design. Psychol Med. 2006;36(8):1153-1162. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. Alcoholism and major depression in women: a twin study of the causes of comorbidity. Arch Gen Psychiatry. 1993;50(9):690-698. [DOI] [PubMed] [Google Scholar]
- 16.Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Arch Gen Psychiatry. 2000;57(8):803-811. [DOI] [PubMed] [Google Scholar]
- 17.Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20(2):173-189. [DOI] [PubMed] [Google Scholar]
- 18.Treutlein J, Cichon S, Ridinger M, et al. . Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66(7):773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelernter J, Kranzler HR, Sherva R, et al. . Genome-wide association study of alcohol dependence: significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19(1):41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CONVERGE Consortium Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523(7562):588-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okbay A, Baselmans BM, De Neve JE, et al. ; LifeLines Cohort Study . Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet. 2016;48(6):624-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyde CL, Nagle MW, Tian C, et al. . Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48(9):1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards AC, Aliev F, Bierut LJ, et al. . Genome-wide association study of comorbid depressive syndrome and alcohol dependence. Psychiatr Genet. 2012;22(1):31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell SM, Wray NR, Stone JL, et al. ; International Schizophrenia Consortium . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherva R, Wang Q, Kranzler H, et al. . Genome-wide association study of cannabis dependence severity, novel risk variants, and shared genetic risks. JAMA Psychiatry. 2016;73(5):472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierucci-Lagha A, Gelernter J, Feinn R, et al. . Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA). Drug Alcohol Depend. 2005;80(3):303-312. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 28.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2(12):e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904-909. [DOI] [PubMed] [Google Scholar]
- 30.Purcell S, Neale B, Todd-Brown K, et al. . PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das S, Forer L, Schönherr S, et al. . Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auton A, Brooks LD, Durbin RM, et al. ; 1000 Genomes Project Consortium . A global reference for human genetic variation. Nature. 2015;526(7571):68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121-130. [PubMed] [Google Scholar]
- 34.Chen MH, Yang Q. GWAF: an R package for genome-wide association analyses with family data. Bioinformatics. 2010;26(4):580-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pruim RJ, Welch RP, Sanna S, et al. . LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okbay A, Beauchamp JP, Fontana MA, et al. ; LifeLines Cohort Study . Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533(7604):539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otowa T, Hek K, Lee M, et al. . Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry. 2016;21(10):1485. [DOI] [PubMed] [Google Scholar]
- 40.Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4 [published correction appears in Nat Genet. 2012;44(9):1072]. Nat Genet. 2011;43(10):977-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. ; European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobacco and Genetics Consortium Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hibar DP, Stein JL, Renteria ME, et al. ; Alzheimer’s Disease Neuroimaging Initiative; CHARGE Consortium; EPIGEN; IMAGEN; SYS . Common genetic variants influence human subcortical brain structures. Nature. 2015;520(7546):224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289-300. [Google Scholar]
- 46.de Moor MH, van den Berg SM, Verweij KJ, et al. ; Genetics of Personality Consortium . Meta-analysis of genome-wide association studies for neuroticism, and the polygenic association with major depressive disorder. JAMA Psychiatry. 2015;72(7):642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo MT, Hinds DA, Tung JY, et al. . Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet. 2017;49(1):152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang HJ, Kawasawa YI, Cheng F, et al. . Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giger RJ, Pasterkamp RJ, Heijnen S, Holtmaat AJ, Verhaagen J. Anatomical distribution of the chemorepellent semaphorin III/collapsin-1 in the adult rat and human brain: predominant expression in structures of the olfactory-hippocampal pathway and the motor system. J Neurosci Res. 1998;52(1):27-42. [DOI] [PubMed] [Google Scholar]
- 50.Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8(2):148-155. [DOI] [PubMed] [Google Scholar]
- 51.Good PF, Alapat D, Hsu A, et al. . A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer’s disease. J Neurochem. 2004;91(3):716-736. [DOI] [PubMed] [Google Scholar]
- 52.Larsson M, Duffy DL, Zhu G, et al. . GWAS findings for human iris patterns: associations with variants in genes that influence normal neuronal pattern development. Am J Hum Genet. 2011;89(2):334-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Battum EY, Brignani S, Pasterkamp RJ. Axon guidance proteins in neurological disorders. Lancet Neurol. 2015;14(5):532-546. [DOI] [PubMed] [Google Scholar]
- 54.GTEx Consortium Human genomics: the Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peyrot WJ, Lee SH, Milaneschi Y, et al. ; Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium (Corporate Collaborator); Social Science Genetic Association Consortium Corporate Collaborator; Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium Corporate Collaborator; Social Science Genetic Association Consortium Corporate Collaborator . The association between lower educational attainment and depression owing to shared genetic effects? results in ~25,000 subjects. Mol Psychiatry. 2015;20(6):735-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsoh JY, Humfleet GL, Muñoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157(3):368-374. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry. 2005;57(7):768-776. [DOI] [PubMed] [Google Scholar]
- 58.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56(4):356-363. [DOI] [PubMed] [Google Scholar]
- 59.Wrase J, Makris N, Braus DF, et al. . Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165(9):1179-1184. [DOI] [PubMed] [Google Scholar]
- 60.Makris N, Oscar-Berman M, Jaffin SK, et al. . Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64(3):192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13(11):993-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hickie IB, Naismith SL, Ward PB, et al. . Serotonin transporter gene status predicts caudate nucleus but not amygdala or hippocampal volumes in older persons with major depression. J Affect Disord. 2007;98(1-2):137-142. [DOI] [PubMed] [Google Scholar]
- 63.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118. [DOI] [PubMed] [Google Scholar]
- 64.Husain MM, McDonald WM, Doraiswamy PM, et al. . A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40(2):95-99. [DOI] [PubMed] [Google Scholar]
- 65.Schmaal L, Veltman DJ, van Erp TG, et al. . Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21(6):806-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Top Findings in Meta-Analyses (P value <1 × 10−4)
eTable 2. PRS Results for Comorbid AD and MD
eTable 3. PRS Results for AD, Adjusting for MD
eTable 4. PRS Results for MD, Adjusting for AD
eFigure 1. Distribution of Comorbid Criterion Counts
eFigure 2. Manhattan and QQ Plots of Meta-Analyses
eFigure 3. MAF of rs139438618 for Criteria
eFigure 4. Associations Between the Comorbidity and PRS of Psychiatric Traits
eFigure 5. Associations Between the Comorbidity and PRS of Subcortical Brain Volumes
eFigure 6. Spatio-Temporal Transcriptome of SEMA3A in Human Brain