Abstract
Dystonia is a neurological disorder characterized by involuntary, repetitive movements. Although the precise mechanisms of dystonia development remain unknown, the diversity of its clinical phenotypes is thought to be associated with multifactorial pathophysiology, which is linked not only to alterations of brain organization, but also environmental stressors and gene mutations. This chapter will present an overview of the pathophysiology of isolated dystonia through the lens of applications of major neuroimaging methodologies, with links to genetics and environmental factors that play a prominent role in symptom manifestation.
Dystonia is a neurological movement disorder, which is characterized by sustained or intermittent muscle contractions, causing abnormal, often repetitive movements, postures, or both (Albanese et al., 2013). Isolated dystonia (formerly known as primary), where dystonic symptoms are the only disease manifestation, is a rare disorder with a prevalence of about 3–30 per 100,000 in the general population (Asgeirsson, Jakobsson, Hjaltason, Jonsdottir, & Sveinbjornsdottir, 2006; de Carvalho Aguiar & Ozelius, 2002; Nutt, Muenter, Melton, Aronson, & Kurland, 1988). It typically develops spontaneously and progresses into a chronic debilitating condition, oftentimes severely impacting not only the freedom of movements but also various other aspects of patient’s life, leading to continuous stress, social embarrassment, and even loss of employment. Dystonia comprises a large number of clinical syndromes, with adult-onset focal dystonia being the most common phenotype, followed by segmental dystonia, and much rare early (childhood)-onset generalized dystonia. Some forms of dystonia selectively affect higher-order motor control, resulting in impairments of unique patterns of highly learned behaviors, such as writing, speaking, or playing a musical instrument.
The exact pathophysiological mechanisms of isolated dystonia remain unknown. Based on the current state of knowledge, the diversity of clinical phenotypes of dystonia is thought to be associated with multifactorial pathophysiology, which is linked not only to alterations of brain organization, but also environmental stressors and gene mutations (Fig. 1). This chapter will present an overview of the pathophysiology of isolated dystonia through the lens of applications of major neuroimaging methodologies, with links to genetics and environmental factors that play a prominent role in symptom manifestation.
Fig. 1.
Schematic representation of multifactorial pathophysiology of isolated dystonia with major factors including abnormal brain structural, functional and neurochemical organization, underlying gene mutations, and environmental triggers, a combination of which leads to the manifestation of dystonic symptoms.
1. MULTI-MODAL NEUROIMAGING APPLICATION IN DYSTONIA
Rapid advances in brain imaging have had a tremendous impact on the evolution of our understanding of dystonia development. Narrowly considered for decades as a basal ganglia disorder due to the predilection for striatal lesions to trigger secondary (or combined) dystonias (Marsden, Obeso, Zarranz, & Lang, 1985; Vitek, 2002), neuroimaging studies addressing different aspects of brain organization have been transformative in changing our views about the complex pathophysiological mechanisms underlying this disorder and opening new perspectives for its objective diagnosis and therapeutic interventions. The early neuroimaging studies were heavily rooted in the use of positron emission tomography that captured brain glucose metabolism and cerebral blood flow alterations in dystonia patients compared to healthy subjects. Further advances in functional brain imaging using functional magnetic resonance imaging (MRI) and magnetoencephalography as well as structural imaging using diffusion weighted and high-resolution MR sequences provided deeper insights into brain regional alterations at both structural and functional levels. Finally, relatively recent applications of mathematical modeling of larger-scale brain networks revealed global neural abnormalities that were not recognized in initial studies, reinforcing the notion that isolated dystonia is a network disorder. In parallel, neuroreceptor mapping studies with positron emission tomography and magnetic resonance spectroscopy helped define the aberrations of dopaminergic and GABAergic neurotransmission across different forms of dystonia as one of the potential underlying causes of altered network function.
Recent studies have also attempted to link and understand the impact of gene mutations on the development of brain abnormalities in dystonia. Starting with the first discovery of DYT1 (TOR1A) gene for early-onset generalized dystonia in 1997 (Ozelius et al., 1997), several studies have been designed to elucidate distinct brain disorganization in manifesting and non-manifesting mutation carriers. Further on, rapid advances in next-generation whole-exome sequencing led to identification of additional dystonia genes, including DYT6 (THAP 1), DYT24 (ANO3), DYT25 (GNAL), DYT4 (TUBB4), and DYT23 (CIZ1), with the last two still pending an independent confirmation (Klein, 2014). These mutations are known to predominantly cause segmental dystonias that affect adjacent body regions. Again, neuroimaging studies revealed important associations between some of these genetic factors and neural aberrancies.
Arguably, the least explored aspect in the pathophysiology of dystonia is the role of environmental variables, such as extended sun exposure for blepharospasm, neck trauma and surgery in cervical dystonia, viral infections in laryngeal dystonia, and overuse practice in focal hand dystonia and musician’s dystonia. The current effort is directed to elucidating the possible environmental stressors that may influence the development of brain alterations and by this trigger the manifestation of dystonic symptoms.
2. STRUCTURAL NEUROIMAGING OF DYSTONIA
2.1. Gray Matter Structural Organization
The absence of gross structural abnormalities on conventional MRI is one of the clinical hallmarks of dystonia. In fact, it is often a criterion confirming the differential diagnosis of dystonia. On the other hand, detailed evaluation of high-resolution T1-weighted and diffusion-weighted MR sequences in numerous neuroimaging studies led to identification of microstructural cortical and subcortical alterations across all forms of isolated dystonia.
One of the first studies dates back to late 1990s, which applied MRI-based stereological volumetry to reveal a 10% increase of putaminal volume in patients with blepharospasm and focal hand dystonia (Black, Ongur, & Perlmutter, 1998). With the focused investigation on the striatum, this finding was contemporary and supportive of prevailing knowledge at the time that dystonia is a basal ganglia disorder due to intrinsic structural changes. However, further development of methodologies, such as voxel-based morphometry and cortical thickness analyses, allowed for evaluation of volumetric changes within and/or between patient and control groups based on statistical parametric mapping. Analytic pipelines for both methodologies use a refined and fully automated approach of segmentation of high-resolution T1-weighted brain images into gray matter, white matter, and cerebrospinal fluid. Voxel-based morphometry measures the quantity of tissue within a voxel and is dependent on local cortical thickness and/or cortical surface area. In contrast, cortical thickness measurements are based on determining the inner and outer cortical boundaries or surfaces and have a high sensitivity in detecting cortical changes, especially those prone to gene mutations. Both methodologies are complimentary, with voxel-based morphometry providing information about the volumetric organization of the entire brain and cortical thickness measurements being more limited to the cortical ribbon.
The use of these analytic methods made it apparent that putaminal volumetric changes are indeed a common feature of gray matter alterations across different forms of dystonia, including both focal and generalized phenotypes (Draganski et al., 2009; Draganski, Thun-Hohenstein, Bogdahn, Winkler, & May 2003; Etgen, Muhlau, Gaser, & Sander, 2006; Granert, Peller, Jabusch, Altenmuller, & Siebner, 2011; Obermann et al., 2007; Pantano et al., 2011; Simonyan & Ludlow, 2012) (Fig. 2A). In addition, these studies revealed that gray matter changes in focal dystonias further extend to the other divisions of the basal ganglia, most commonly including caudate nucleus and globus pallidus, as well as thalamus and cerebellum (Delmaire et al., 2005; Draganski et al., 2003; Egger et al., 2007; Filip et al., 2017; Obermann et al., 2007; Pantano et al., 2011; Simonyan & Ludlow, 2012; Waugh et al., 2016; Zeuner et al., 2015). More importantly, structural alterations were shown to involve some of the key cortical regions, responsible for sensorimotor processing, integration and motor execution, such as frontoparietal, supplementary motor and primary sensorimotor areas (Delmaire et al., 2005; Draganski et al., 2003; Egger et al., 2007; Garraux et al., 2004; Granert, Peller, Jabusch, et al., 2011; Horovitz, Gallea, Najee-Ullah, & Hallett, 2013; Martino et al., 2011; Pantano et al., 2011; Ramdhani et al., 2014; Simonyan & Ludlow, 2012). Among these, primary sensorimotor changes tended to localize to dystonia-affected body regions within the sensorimotor homunculus. For example, gray matter volumetric increases in patients with focal hand dystonia were observed in the hand area of sensori-motor cortex (Delmaire et al., 2005; Egger et al., 2007; Garraux et al., 2004), whereas in patients with laryngeal dystonia these were found within the larynx representation (Bianchi et al., 2017; Kostic et al., 2016; Simonyan & Ludlow, 2012), further showing relevance of cortical aberrations to the pathophysiology of distinct forms of dystonia.
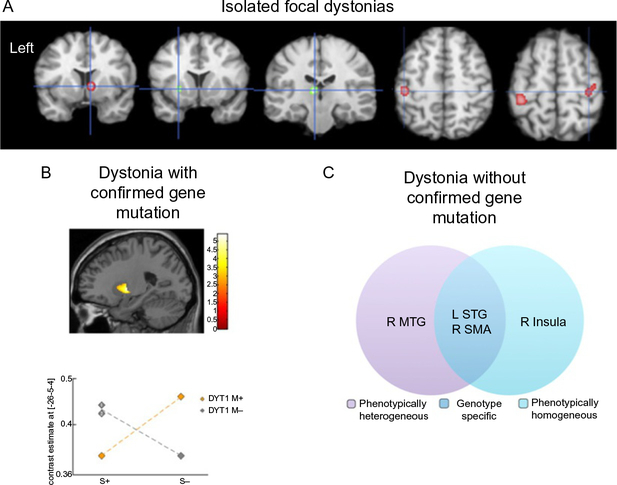
Fig. 2.
Microstructural gray matter abnormalities in isolated dystonia. (A) Anatomic likelihood estimation meta-analysis of common gray matter volumetric abnormalities across focal dystonias. (B) Gray matter volumetric increases in bilateral putamen in non-manifesting DYT1 mutation carriers and non-DYT1 dystonia patients compared to manifesting DYT1 mutation carriers and healthy subjects. The plot shows crossover interaction between genotype and phenotype factors. DYT M+, mutation-positive dystonia; DYT M−, mutation negative dystonia. (C) Cortical thickness changes across different phenotypes and putative genotypes (without confirmed gene mutation but based on a family history of dystonia) in patients with laryngeal dystonia. MTG, middle temporal gyrus; STG, superior temporal gyrus; SMA, supplementary motor area; R, right; L, left. Panel (A): Adapted from Zheng, Z., Pan, P., Wang, W., & Shang, H. (2012). Neural network of primary focal dystonia by an anatomic likelihood estimation meta-analysis of gray matter abnormalities. Journal of the Neurological Sciences, 316(1–2), 51–55. https://doi.org/10.1016/j.jns.2012.01.032. Panel (B): Adapted from Draganski, B., Schneider, S. A., Fiorio, M., Kloppel, S., Gambarin, M., Tinazzi, M., et al. (2009). Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. NeuroImage, 47(4), 1141–1147. doi:10.1016/j.neuro-image.2009.03.057. Panel (C): Adapted from Bianchi, S., Battistella, G., Huddlestone, H., Scharf, R., Fleysher, L., Rumbach, A. F., et al. (2017). Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Movement Disorders, 32(4), 560–568. https://doi.org/10.1002/mds.26920.
In contrast to this body of literature in focal dystonias, examinations of gray matter organization in isolated dystonias with underlying gene mutations (hereditary dystonias) are scarce. There are only a few reports showing genotype-phenotype interactions at the level of structural alterations. One study revealed that non-manifesting DYT1 mutation carriers and non-DYT1 dystonia patients have significantly increased bilateral putaminal volume compared to manifesting DYT1 mutation carriers and healthy subjects (Draganski et al., 2009) (Fig. 2B). Another study reported a volumetric increase of globus pallidus in patients with generalized dystonia, although the DYT1 status was not known in this cohort (Egger et al., 2007). As gene discovery for isolated focal dystonias remains stagnant and extremely challenging due, in part, to rare availability of large families, low penetrance, and variable expressivity, the design of neuroimaging studies has also been limited by somewhat arbitrary assignments of experimental cohorts based on a confirmed family history of dystonia only. Based on such approach, one recent study in patients with sporadic and familial forms of laryngeal dystonia identified phenotype-specific gray matter abnormalities in primary and associative areas of motor control that were distinct from genotype-specific changes within the cortical regions controlling sensory processing (Bianchi et al., 2017) (Fig. 2C).
Along with the progress in mapping and characterizing gray matter organization in dystonia, questions pertaining to the exact cellular mechanisms and causes of gray matter alterations as revealed by voxel-based morphometry and cortical thickness measurements remain poorly understood. It is possible that gray matter volumetric increases in some forms of dystonia, especially in musician’s dystonia and other task-specific dystonias, may be due to abnormal motor training and overuse that generally lead to volumetric increases in related brain regions in healthy populations (Ceccarelli et al., 2009; Draganski et al., 2004; Granert, Peller, Gaser, et al., 2011; Quallo et al., 2009). Another possible mechanisms may involve the formation of abnormal new connections by dendritic spine growth and axonal remodeling, and/or the strengthening of existing synaptic connections (Chklovskii, 2004; Chklovskii, Mel, & Svoboda, 2004; Holtmaat, Wilbrecht, Knott, Welker, & Svoboda, 2006; Sur & Rubenstein, 2005). Future ultra-high-resolution imaging of gray matter, ideally coupled with postmortem neuropathology, may be able to reveal which of these causative mechanisms are responsible for observed neuroimaging changes across dystonias.
2.2. White Matter Structural Organization
Most of the knowledge about white matter organization in isolated dystonia comes from the use of diffusion tensor imaging, which is a form of diffusion-weighted imaging that examines axonal organization in brain tissue. It is based on the principal of random displacement of water molecules and differential quantification of their diffusivity in parallel and perpendicular directions along the axonal course. Diffusion imaging measures include anisotropy indices (e.g., fractional anisotropy) to quantify the directionality of water diffusivity, reflecting axonal integrity and tissue coherence. Other diffusivity indices (e.g., mean diffusivity, trace of diffusion tensor) examine the magnitude of water movement independent of the direction and provide information about the organization of the extra-cellular space and the intracellular water content. Similar to voxel-based morphometry and cortical thickness measurements, diffusivity measures are sensitive to microstructural white matter abnormalities not evident on conventional MRI.
In addition to the assessment of regional white matter organization, diffusion imaging methods allow for in vivo reconstruction of the white matter tracts using deterministic and probabilistic tractography. This is considered as a valid method for examination of white matter connectivity due to its capability to model fiber direction(s) per voxel (single with deterministic tractography and multiple with probabilistic tractography), providing realistic estimates of the wiring strength of gross white matter projections. However, tractography is still limited for mapping the connectivity between brain regions with high fiber complexity and uncertainty about the fiber directions, especially in the presence of dense local cortico-cortical connections. The likelihood of tractography is excessively higher in estimation of the tracts from the regions of interest to immediately surrounding areas than those in more distant brain regions, leading to the distance bias. Similarly, the tractography algorithm is prone to higher propagation of tracts to larger targets and even to generating false-positive tracts. Moreover, tractography by-passes monosynaptic connections and does not allow inferring directionality of the connection, which makes it difficult to assess the structural influence of one brain region upon another. While a powerful analytic method for in vivo examination of structural connectivity in humans, the results of diffusion tractography warrant a careful comparison to those obtained with neuroanatomical tract tracing methods in non-human primates, most importantly to avoid identification of spurious “novel” connectivity and its impairments in diseased populations.
Studies assessing regional white matter organization in isolated dystonia have been mostly focused on the use of various diffusivity measures between and within different groups of patients and healthy subjects. The general unifying finding across these studies is identification of reduced axonal integrity and increased water diffusivity, involving both cortical and sub-cortical structures along the cortico-striato-pallido-thalamic and cerebellothalamo-cortical pathways (Blood et al., 2012; Bonilha et al., 2007; Colosimo et al., 2005; Delmaire et al., 2009; Fabbrini et al., 2008; Horovitz, Ford, Najee-Ullah, Ostuni, & Hallett, 2012; Prell et al., 2013; Simonyan et al., 2008) (Fig. 3A). Specificity of these alterations is relevant to the distinct forms of dystonia, such as reduced white matter integrity was reported in the posterior limb of internal capsule, including the corticospinal tract, in focal hand dystonia (Delmaire et al., 2009) and in the genu of internal capsule, involving corticobulbar tract, in laryngeal dystonia (Simonyan et al., 2008). The latter finding has been substantiated by neuropathological evidence of decreased axonal density and myelin content, potentially underlying the change in water diffusivity (Simonyan et al., 2008) (Fig. 3B).
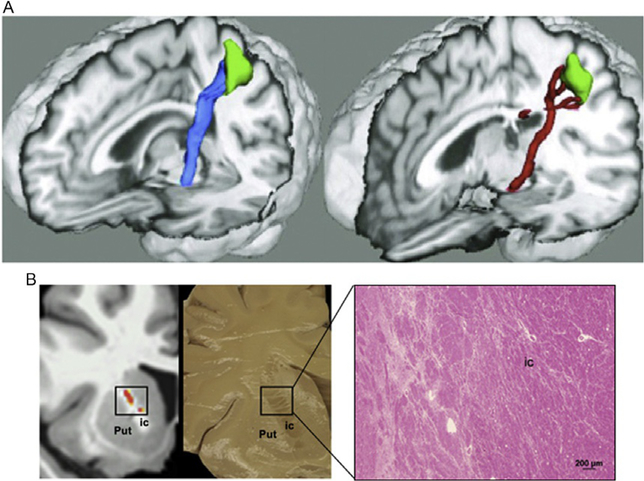
Fig. 3.
(A) Decreased gray matter volume in the facial portion of the precentral gyrus and reduced volume of the corticobulbar tract in healthy subjects (left) and patients with blepharospasm (right). (B) Specificity of white matter changes depending on the phenotype of dystonia: reduced white matter integrity along the corticobulbar tract in laryngeal dystonia, with subsequent postmortem neuropathology confirming axonal degeneration and demyelination within the corticobulbar tract in laryngeal dystonia. Put, putamen; ic, internal capsule. Panel (A): Adapted from Horovitz, S. G., Ford, A., Najee-Ullah, M. A., Ostuni, J. L., & Hallett, M. (2012). Anatomical correlates of blepharospasm. Translational Neurodegeneration, 1(1), 12. https://doi.org/10.1186/2047–9158-1–12. Panel (B): Adapted from Simonyan, K., Tovar-Moll, F., Ostuni, J., Hallett, M., Kalasinsky, V. F., Lewin-Smith, M. R., et al. (2008). Focal white matter changes in spasmodic dysphonia: A combined diffusion tensor imaging and neuropathological study. Brain, 131(Pt. 2), 447–459. https://doi.org/10.1093/brain/awm303.
Among the hereditary forms of dystonia, both manifesting and non-manifesting DYT1 carriers showed reduced white matter integrity in subgyral white matter of sensorimotor cortex, albeit with greater abnormalities in manifesting than non-manifesting carriers (Carbon, Kingsley, et al., 2004; Carbon, Kingsley, Tang, Bressman, & Eidelberg, 2008). A similar finding was observed in the study of manifesting DYT6 carriers (Cheng et al., 2012). In addition, DYT1 carriers showed abnormal white matter organization of dorsal pontine tegmentum adjacent to the left superior cerebellar peduncle (Carbon et al., 2008). Diffusion tractography, examining the cerebellar involvement, identified reduced integrity in the cerebellar lobule VI adjacent to dentate nucleus in DYT1 and DYT6 manifesting carriers as well as reduced thalamo-cortical connectivity in non-manifesting dystonia gene carriers (Argyelan et al., 2009). On the other hand, substantial phenotypic variability of hereditary dystonia appears to be linked to reduced integrity of somatotopic projections related to asymptomatic body regions (Vo, Sako, Niethammer, et al., 2015). Similarly, non-manifesting carriers showed additionally reduced connectivity within the distal thalamo-cortical segment of the cerebello-thalamo-cortical tract, pointing to the protective neural mechanisms underlying the manifestation of hereditary dystonia. Corroborating these findings, an ex vivo study in the heterozygous DYT1 knock-in mouse model showed reduced connectivity of the cerebello-thalamic, thalamo-cortical and thalamo-striatal pathways in mutants compared to wild type (Ulug et al., 2011). Another study reported deficits of free water diffusivity and widespread increases in functional connectivity of the stratum with somatosensory cortex, thalamus, cerebellum and brainstem in the conditional knock-out mice of the DYT1 protein torsinA (DeSimone et al., 2017). As the cerebello-thalamo-cortical pathway facilitates intracortical inhibition via projections to interneurons in the sensorimotor cortex (Molinari, Filippini, & Leggio, 2002), these findings may suggest the presence of the intermediary pattern in non-manifesting carriers that may act as a buffer against the aberrant outflow from the proximal part of this pathway (Niethammer, Carbon, Argyelan, & Eidelberg, 2011).
Taken together, the use of volumetric and diffusion imaging helped establish the presence of microstructural alterations of gray and white matter in dystonia that are not restricted to the putamen but rather extend to surrounding subcortical and further cortical and cerebellar regions, predominantly within the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuitries. Identification of structural alterations specific to the disorder genotype and phenotype has been important for providing a critical step toward future delineation of imaging markers and the potential targets for novel therapeutic interventions in this disorder.
3. FUNCTIONAL NEUROIMAGING OF DYSTONIA
3.1. Positron Emission Tomography
Early studies in dystonia relied heavily on the use of positron emission tomography to capture the cerebral blood flow and glucose metabolism in patient within-group and between-group comparisons with healthy subjects. Positron emission tomography is based on radioactivity emitted after a radiolabeled tracer is injected intravenously. The nucleus of the radioisotope emits a positron, which collides with an electron in the tissue and in the process converts mass to energy in the form of two photons. The tomograph uses scintillation crystals placed around the subject’s head to detect these photons. The crystals absorb the photons, producing light that is converted into an electrical signal.
One of the most commonly used tracers to quantify brain activity via glucose metabolism in dystonia is [18F]-fluorodeoxyglucose ([18F]FDG), which is taken up by brain regions depending on metabolic needs. As regional metabolism is dependent on synaptic activity, changes in tracer uptake correlate with changes in regional neuronal activity. Using [18F]FDG, several studies reported abnormal metabolism in basal ganglia, thalamus, cerebellum, and sensorimotor cortex in patients with focal dystonia (Esmaeli-Gutstein, Nahmias, Thompson, Kazdan, & Harvey, 1999; Hutchinson et al., 2000; Kerrison et al., 2003; Magyar-Lehmann et al., 1997; Suzuki et al., 2007). During induced sleep in patients with blepharospasm, hypometabolic activity was additionally found in the frontal eye field (Brodmann area 8), which is involved in planning of complex movements and is associated with supra-nuclear control of the eyelid opening (Hutchinson et al., 2000). Evaluation of the effectiveness of botulinum toxin treatment in blepharospasm showed an association with normalization of metabolic activity in the cerebellum and pons (Suzuki et al., 2007).
Studies in hereditary dystonias also identified distinct patterns of regional metabolic activity. Specifically, DYT1 carriers showed metabolic increases in the striatum and cerebellum; DYT6 carriers had metabolic reductions in the putamen, thalamus, cerebellar cortex, and upper brainstem; and DYT11 mutation carriers had both metabolic increases in the deep cerebellar nuclei and vermis and metabolic decreases in the medial prefrontal cortex and pre-supplementary motor area (Carbon & Eidelberg, 2009; Carbon, Su, et al., 2004). The findings in DYT1 patients were substantiated in the Tor1a heterozygous knock-out mouse, which showed metabolic abnormalities in the striatum and cerebellum as well as sensorimotor cortex and subthalamic nucleus (Vo, Sako, Dewey, Eidelberg, & Ulug, 2015).
Another commonly used radiotracer in dystonia research is [15O] H2O, which allowed the assessment of the regional cerebral blood flow during production of both symptomatic and asymptomatic tasks while in the scanner (Ali et al., 2006; Ceballos-Baumann et al., 1995; Ibanez, Sadato, Karp, Deiber, & Hallett, 1999; Lerner et al., 2004; Playford, Passingham, Marsden, & Brooks, 1998). Similar to glucose metabolism, the regional cerebral blood flow appeared to be abnormal in the basal ganglia, thalamus, cerebellum, and sensorimotor cortex in focal dystonia. In addition, wider spread abnormalities were identified involving prefrontal, posterior parietal and temporal regions necessary for motor planning and sensorimotor processing. Examination of the regional covariance pattern during writing and at rest in patients with writer’s cramp further revealed reduced correlations between putamen and premotor cortex and between bilateral premotor cortex (Ibanez et al., 1999), suggesting that not only regional brain activity but also functional connectivity may be abnormal in dystonia. Deficient activity and connectivity of the preparatory cortical regions and basal ganglia pointed to a loss of inhibition during the generation of motor commands, likely arising from the striatal region.
3.2. Functional MRI During Task Production and Resting State
The development of functional MRI methodology in early 1990s (Kwong et al., 1992; Ogawa, Lee, Nayak, & Glynn, 1990) has completely changed the landscape of neuroimaging studies examining brain activity and functional connectivity in dystonia. Functional MRI is based on a blood oxygen-level dependent contrast, which captures the hemodynamic response by relying on differential magnetic susceptibility of oxyhemoglobin and deoxyhemoglobin due to changes during neuronal activity. Functional MRI has been used during various tasks, both eliciting and not eliciting dystonic movements. Similar to positron emission tomography and structural imaging studies, this line of research again confirmed the presence of alterations in basal ganglia, thalamus, cerebellum and sensorimotor cortex across different forms of isolated dystonia and hinted to abnormal integration of sensorimotor information flow within the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuitries (e.g. Baker, Andersen, Morecraft, & Smith, 2003; Burciu et al., 2017; Butterworth et al., 2003; Haslinger, Altenmuller, Castrop, Zimmer, & Dresel, 2010; Hu, Wang, Liu, & Zhang, 2006; Islam et al., 2009; Kadota et al., 2010; Pujol et al., 2000; Simonyan & Ludlow, 2010). Additionally, some studies pointed to abnormal sensory processing by primary somatosensory cortex that may contribute to the pathophysiology of dystonia (Dresel, Haslinger, Castrop, Wohlschlaeger, & Ceballos-Baumann, 2006; Haslinger et al., 2010; Simonyan & Ludlow, 2010), whereas others mapped abnormal somatotopy of digit representation in primary somatosensory cortex and putamen in focal hand dystonia (Butterworth et al., 2003; Delmaire et al., 2005; Nelson, Blake, & Chen, 2009). Studies examining the effects of botulinum toxin injections on brain activity in patients with focal dystonia provided largely controversial results, showing either modulated or non-modulated brain activity following the treatment (Ali et al., 2006; Dresel et al., 2011; Haslinger et al., 2005; Nevrly et al., 2018). On the other hand, subclinical abnormalities in sensory discrimination were described as a mediational endophenotype of dystonia (Hutchinson et al., 2013) and linked to alterations in primary somatosensory and middle frontal cortices (Termsarasab et al., 2016). Importantly, differential associations between abnormal sensory discrimination and functional abnormalities were observed depending on the phenotype and genotype of dystonia, including greater cerebellar involvement in familial laryngeal dystonia cases and greater putaminal and cortical sensorimotor inclusion in different phenotypes of laryngeal dystonia (Termsarasab et al., 2016).
Another step forward in understanding the neuroimaging pathophysiology of dystonia came with the development of resting-state functional MRI, which relies on the measurement of low frequency physiological fluctuations in the BOLD signal, reflecting the functional brain organization during various activation states (Biswal, Yetkin, Haughton, & Hyde, 1995; Smith et al., 2009). The resting-state functional MRI approach proved to be useful in circumventing the challenges associated with the implementation of the task-related designs across different forms of dystonia, which exhibit distinct symptoms, thus making the choice of a single, commonly affected task production unfeasible. Furthermore, because the explanation of functional changes across distinct dystonia phenotypes and genotypes may sometimes be ambiguous due to a combination of motor and sensory components, examination of the resting-state activity and connectivity provides a more uniform and coherent understanding of neural alterations.
Resting-state functional MRI studies in focal hand dystonia found decreased connectivity in primary somatosensory region with concomitant increases in the putamen as well as functional decoupling of dorsal premotor cortex from parietal cortex (Delnooz, Helmich, Toni, & van de Warrenburg, 2012; Mohammadi et al., 2012). In cervical dystonia, alterations were found not only in sensorimotor but also in visual and executive control networks (Delnooz, Pasman, Beckmann, & van de Warrenburg, 2013), whereas changes in blepharospasm were related to the default-mode network (Yang et al., 2013). It was further shown that vulnerable connectivity of primary sensorimotor and inferior parietal cortices in laryngeal dystonia is tightly associated with the polygenic risk of dystonia, likely representing an endophenotypic imaging marker of this disorder; genes contributing to the polygenic score are involved in synaptic transmission and neuron development (Battistella, Fuertinger, Fleysher, Ozelius, & Simonyan, 2016; Putzel et al., 2018). Schematic knowledge of functional alterations in dystonia can be viewed in Fig. 4.
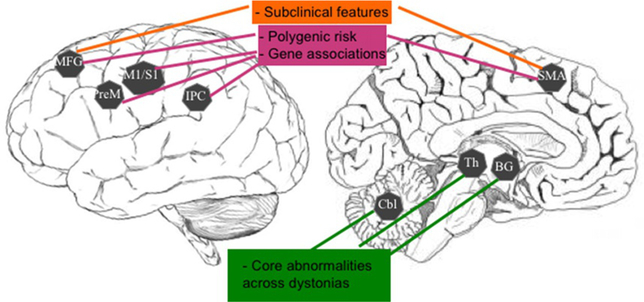
Fig. 4.
Schematic representation of common abnormalities of brain metabolism and activation across different forms of dystonia and their relevance to the pathophysiology of this disorder. MGF, middle frontal gyrus; PreM, premotor cortex; M1/S1, primary sensorimotor cortex; IPC, inferior parietal cortex; SMA, supplementary motor area; BG, basal ganglia; Th, thalamus; Cbl, cerebellum.
Using these findings, a few studies started to probe existing machine learning algorithms in attempt to characterize neural markers of dystonia. Classification algorithms represent a powerful tool for identification of single traits or a combination of features that characterize and separate two or more classes of objects or subjects. Algorithmic classifiers using functional MRI voxel-wise time series have been successfully applied in several neurodegenerative disorders (Fornari, Maeder, Meuli, Ghika, & Knyazeva, 2012; Janousova, Schwarz, & Kasparek, 2015; Yourganov et al., 2014). The first study in dystonia used multivariate classification algorithm of linear discriminant analysis (LDA) based on the measures of abnormal functional resting-state connectivity in primary sensorimotor, premotor and inferior parietal regions, achieving 71% accuracy in classifying laryngeal dystonia and healthy controls (Battistella et al., 2016). It further improved its accuracy in classifying familial vs sporadic laryngeal patients at 81% and remained at the same 71% accuracy level when considering different (adductor and abductor) phenotypes. As such, this study used neuroimaging data to disambiguate focal dystonia from a normal state and differentiate the disorder based not on clinical evaluations of its symptoms but of neuroimaging heterogeneity, opening new avenues to the development of disorder-specific diagnostic biomarkers.
3.3. Dystonia as a Functional Network Disorder
Starting with the positron emission tomography studies in generalized dystonia that showed a presence of the abnormal metabolic network (Eidelberg et al., 1998, 1995; Niethammer et al., 2011), the overall concept of functional alterations not being limited to the basal ganglia, as historically proposed, has evolved into propositions of focal dystonia, too, to represent a functional network disorder (Lehericy, Tijssen, Vidailhet, Kaji, & Meunier, 2013; Neychev, Gross, Lehericy, Hess, & Jinnah, 2011; Quartarone & Hallett, 2013; Ramdhani & Simonyan, 2013; Zoons, Booij, Nederveen, Dijk, & Tijssen, 2011). This concept has been recently experimentally substantiated in a study that used a graph theoretical approach to probe the organization of large-scale functional networks across different forms of focal dystonia. Compared to healthy subjects, patients showed altered network architecture, which was characterized by an abnormal breakdown of the basal gangliathalamo-cerebellar community, a loss of pivotal regions of information transfer (hubs) in the premotor cortex, and a pronounced decline in sensorimotor and inferior parietal cortical connectivity (Battistella, Termsarasab, Ramdhani, Fuertinger, & Simonyan, 2017) (Fig. 5A). These findings pointed to the unified pathophysiological mechanism underlying different forms of dystonia due to common network alterations, while suggesting the concurrent presence of pathophysiologically divergent mechanisms contributing to different forms of dystonia. In line with this assumption, another study using a similar analytic approach mapped marked differences in the topological organization of parietal regions between phenotypically different forms of laryngeal dystonia (Fuertinger & Simonyan, 2017) (Fig. 5B). Moreover, the interface between sporadic genotype and most common adductor phenotype yielded distinct functional communities of interacting regions that were primarily governed by intramodular hub regions. On the other hand, the interface between less common familial genotype and abductor phenotype was associated with numerous long-ranging hub regions and an abnormal integration of left thalamus and basal ganglia.
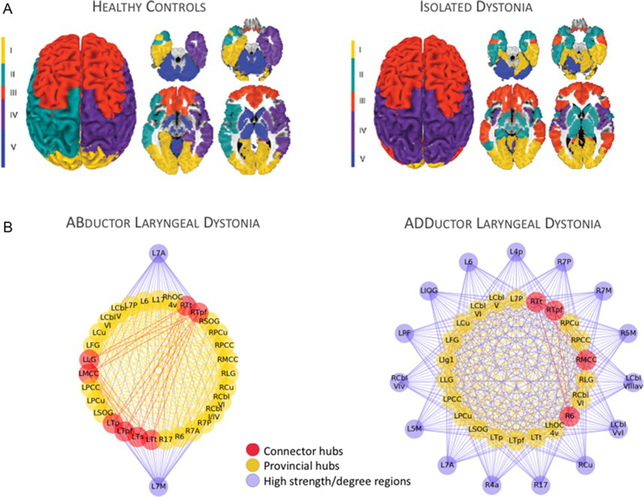
Fig. 5.
(A) Large-scale network organization based on resting-state functional MRI group-averaged networks in healthy subjects and patients with different forms of focal isolated dystonia. This panel shows the regional distribution of neural communities based on the inter-regional coupling. Patients show disintegration of neural communities compared to healthy subjects. (B) Functional architecture of the neural network is influenced by the phenotype of dystonia; an example in abductor vs adductor forms of laryngeal dystonia. The panel depicts phenotype-specific abnormal distribution of connector and provincial hubs (regions of highest information transfer), which, as a result, establish a characteristic pattern of connectivity with non-hub regions across the entire brain. 6/17, area 6/17; 7A/7P, subdivisions of area 7; CbI-I/IV/Cbl-V/Cbl-VI, cerebellar lobules I/IV/V/VI; Cu/PCu, cuneus/precuneus; FG, fusiform gyrus; Ig1, part Ig1 of the insula; LG, lingual gyrus; MCC/PCC, middle/posterior cingulate cortex; SOG, superior orbital gyrus; Tp/Tpf/Ts/Tt, parietal/prefrontal/somatosensory/temporal subdivisions of the thalamus; hOC4v, ventral part of area hOC4; L, left; R, right. Panel (A): Adapted from Battistella, G., Termsarasab, P., Ramdhani, R. A., Fuertinger, S., & Simonyan, K. (2017). Isolated focal dystonia as a disorder of large-scale functional networks. Cerebral Cortex, 27(2), 1203–1215. doi:10.1093/cercor/bhv313. Panel (B): Adapted from Fuertinger, S., & Simonyan, K. (2017). Connectome-wide phenotypical and genotypical associations in focal dystonia. The Journal of Neuroscience, 37(31), 7438–7449. https://doi.org/10.1523/JNEUROSCI.0384-17.2017.
Another aspect of network abnormality is how one region exerts its influences upon another. Although the current analytic methodology, dynamic causal modeling, has severe limitations such as the total number of regions to be realistically explored cannot exceed 4 or 5, rendering examination of whole brain connectivity not feasible, it has nevertheless been useful in showing malfunctioning intracortical connections between primary motor cortex and supplementary motor area as well as abnormal reciprocal excitatory connectivity in the cortico-cerebellar circuitry during performance of a motor task in patients with writer’s cramp (Rothkirch et al., 2018). Future studies should focus on the development of new analytic tools to assess the mechanistic properties of abnormal functional network in dystonia by examining its effective connectivity.
4. NEURORECEPTOR MAPPING STUDIES IN DYSTONIA
4.1. Dopaminergic Neurotransmission
Despite the basal ganglia being at the epicenter of dystonia pathophysiology, until recently the neurochemical underpinning of these abnormalities remained poorly understood. The basal ganglia set the pattern for facilitation of voluntary movements and simultaneous inhibition of competing/interfering movements by balancing excitation and inhibition within the thalamo-cortical circuitry. This is achieved by a synergistic action of the net excitatory direct basal ganglia pathway, which predominantly expresses dopamine D1 family receptors, and the net inhibitory basal ganglia pathway, which predominantly expresses dopamine D2 family receptors (Gerfen, 1992, 2000; Surmeier, Yan, & Song, 1998). Endogenously released striatal dopamine influences direct and indirect pathways both separately and via bridging collaterals between the two pathways, allowing dynamic modulation of thalamo-cortical neurons for physiologically normal facilitation of movement initiated in the motor cortex (Fig. 6A).
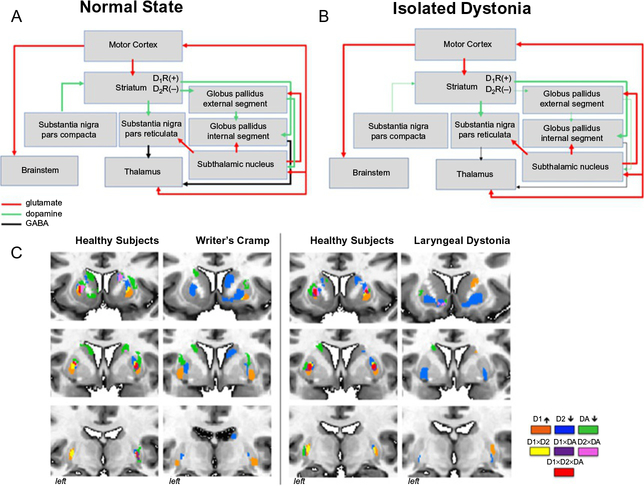
Fig. 6.
Schematic representation of neurotransmission during (A) normal state and (B) in isolated dystonia. Striatal dopaminergic input from the substantia nigra, pars compacta, is weakened; dopaminergic neurotransmission is enhanced via the direct pathway and diminished via the indirect pathway. (C) Topological distribution of striatal dopaminergic function in healthy subjects and patients with writer’s cramp and laryngeal dystonia. Within each patient and control group, a conjunction analysis was used to examine the overlap and distinct distribution between the significant clusters derived from three measures of dopaminergic function: D1 receptor binding; D2 receptor binding, and striatal phasic dopamine release during finger tapping (for the comparison with writer’s cramp) and sentence production (for the comparison with laryngeal dystonia). Healthy subjects have a great degree of overlap between all three measures as well as smaller regions of distinct receptor distribution. This topology is reversed in patients with dystonia, where the overlap is largely diminished. The legend provides the color scheme for overlapping as well as distinct regions of receptor activation and dopamine release. DA, dopamine.
It has been suggested that abnormal dopamine levels may modulate striatal synaptic plasticity in dystonia (Breakefield et al., 2008; Hallett, 2004; Todd & Perlmutter, 1998), while increased D1-mediated excitation and decreased D2 receptor-mediated inhibition may alter the balance between the basal ganglia direct and indirect pathways, cause overall disinhibition of the thalamocortical circuitry, and contribute to dystonia muscle contractions during performance of fine motor tasks (Hallett, 1993).
Using PET or single-photon emission computed tomography with specialized radioligands to target striatal dopamine receptors, decreased striatal dopamine D2 receptor binding at rest was found in patients with both focal and generalized forms of dystonia as well as non-manifesting DYT1 and DYT6 gene carriers (Asanuma et al., 2005; Berman, Hallett, Herscovitch, & Simonyan, 2013; Berger et al., 2007; Carbon et al., 2009; Horie et al., 2009; Horstink et al., 1997; Naumann et al., 1998; Perlmutter et al., 1997; Simonyan, Berman, Herscovitch, & Hallett, 2013). Among these, two studies leveraged the ability of endogenously released phasic dopamine to displace the bound radiotracer in order to assess dopaminergic function during symptomatic and asymptomatic tasks (Berman et al., 2013; Simonyan et al., 2013). It was reported that patients with focal hand dystonia and laryngeal dystonia exhibit abnormally decreased striatal phasic dopamine release during symptomatic task production (finger tapping and speaking, respectively), whereas the levels of striatal dopamine release during asymptomatic tasks (speaking and finger tapping, respectively) are abnormally increased. While there is no apparent neurodegeneration or cell loss within the basal ganglia, experimentally reduced striatal D2 receptor binding was also observed in the dtsz mutant hamster and associated with increased striatal dopamine release during the manifestation of dystonic episodes (Hamann & Richter, 2004; Nobrega, Richter, Tozman, Jiwa, & Loscher, 1996). Decreased neuroreceptor binding at rest is thought to reflect decreased D2 receptor availability due to decreased receptor density and/or increased tonic dopamine levels in the synapses. This contributes to disinhibition within the indirect basal ganglia pathway and leads to an inability to suppress unwanted “nearby” motor contractions during the production of specific actions, a well-established abnormality in isolated dystonia. On the other hand, decreased phasic dopaminergic activity may represent a disorder-specific pathophysiological trait involved in generation of dystonic symptoms, whereas increased dopamine release during unaffected and unrelated motor tasks may be due to compensatory adaptation of the nigrostriatal dopaminergic system.
In terms of involvement of the direct basal ganglia pathway in dystonia, the recent study revealed increased availability of striatal dopamine D1 receptors, suggesting hyperactivity of the direct pathway in patients with focal hand dystonia and laryngeal dystonia (Simonyan, Cho, Hamzehei Sichani, Rubien-Thomas, & Hallett, 2017). This dopaminergic alteration followed a well-known somatotopic organization of the striatum, with changes localized to the striatal hand and larynx representations, respectively. Furthermore, dopaminergic dysfunctions involved both associative (anterior) and sensorimotor (posterior) striatal subdivisions, potentially having direct impact not only on hyperexcitability of motor cortex but also on parietal and prefrontal projection regions, which are responsible for the control of sensorimotor integration and preparation to motor execution and which are known to have abnormal activity and connectivity in dystonia. In fact, these cortical alterations may be a result of propagation of abnormal dopaminergic function via influencing beta oscillations within different striato-cortical loops.
When examining topological distribution of D1 and D2 receptor abnormalities, abnormal segregation of hyperfunctional direct and hypofunctional indirect pathways within the striatum became apparent, with negligible, if any, overlap between the two pathways (Fig. 6C). Moreover, the loss of overlap between the regions of dopamine D1 and D2 receptor availability and phasic dopamine release suggested complex disorganization of a nigro-striatal input.
Overall, these data showed that disorganization of striatal dopaminergic neurotransmission is of a global scale involving both the direct and indirect basal ganglia pathways and representing a common pathophysiological trait in dystonia.
4.2. GABAergic Neurotransmission
A loss of surround inhibition is considered as one of the main pathophysiological features of isolated dystonia (Quartarone & Hallett, 2013). Initial studies employing magnetic resonance spectroscopy to quantify GABAergic function in dystonia patients reported that GABA levels are significantly decreased in sensorimotor cortex and lentiform nucleus in patients with writer’s cramp compared to healthy subjects (Levy & Hallett, 2002). This findings failed a replication in the follow up study that used a somewhat different analytic approach (Herath, Gallea, van der Veen, Horovitz, & Hallett, 2010). Subsequent positron emission studies with [11C] flumazenil radiotracer produced more stable and reproducible results, demonstrating reduced binding of the ligand to GABAA receptors in primary sensorimotor, premotor, anterior cingulate, supplementary motor area, inferior parietal and insular cortex as well as caudate nucleus and cerebellum with some variations of affected regions across different forms of dystonia, including carriers of DYT1 mutation (Berman et al., 2018; Gallea et al., 2018; Garibotto et al., 2011; Simonyan, 2017). This GABAergic deficiency may result from the loss or decreased density of GABAA receptors, abnormal binding properties of these receptors, or decreased GABA synthesis due to a loss of inhibitory interneurons in dystonia. One study showed that decreased GABAA receptor availability in inferior parietal cortex is associated with increased gray matter volume (Simonyan, 2017) and increased brain activity (Gallea et al., 2018). Given its dysfunctional connectivity with sensorimotor regions and an association with the polygenic risk of dystonia (Gallea, Horovitz, Najee-Ullah, & Hallett, 2016; Putzel et al., 2018), inferior parietal cortex may be critical for setting off the disinhibition within the dystonic network.
Taken together, these studies re-evaluated the involvement of the basal ganglia circuitry in the pathophysiology of isolated dystonia as follows (Simonyan et al., 2017). Attenuated and topologically misplaced nigrostriatal dopamine release acts upon upregulated direct basal ganglia pathway and downregulated indirect pathway, which leads to overly excessive excitatory striatal output via the direct pathway in the presence of decreased inhibitory striatal output via the indirect pathway (Fig. 6B). This, collectively, disinhibits the thalamus and propagates to the motor cortex and other sensorimotor cortical regions, potentially underlying dissociations between activity in the striatum and sensorimotor cortex in the development of the dystonia-characteristic cortico-striatal loop.
5. CONTRIBUTION OF NEUROIMAGING TO UNDERSTANDING THE PATHOPHYSIOLOGY OF DYSTONIA
Based on advanced neuroimaging methodologies and analytic techniques, some unifying conclusions about neural alterations in isolated dystonia can be drawn. First, brain changes are not restricted to the basal ganglia; rather, these extend to other subcortical, cerebellar and sensorimotor cortical regions, forming a dysfunctional network with the major impairments within the striato-thalamo-cortical and cerebellar-thalamo-cortical pathways. Second, genotype and phenotype interactions appear to differentially impact brain network disorganization in dystonia, leading to distinct manifestations of the disorder. Third, altered dopaminergic and GABAergic neurotransmission underlies circuit abnormalities by influencing the balance between the basal ganglia direct and indirect pathways, propagating to dis-inhibition and hyperexcitability of cortical regions that are involved not only in the sensorimotor control but also important for the sensorimotor integration and preparation to the movement execution.
The progress in the field of neuroimaging methodologies continues having a direct impact on unraveling dystonic brain disorganization, piece-by-piece. With the further development of advanced imaging methodologies, the studies will be adequately powered and designed to provide yet lacking explanations of whether wide-ranging neural changes are causative, compensatory, or both in isolated dystonia. This, in turn, will be crucial for identification of novel criteria for enhanced and objective diagnosis of dystonia as well as for the development of new therapeutic and neurosurgical approaches to target these aberrations.
REFERENCES
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. (2013). Phenomenology and classification of dystonia: A consensus update. Movement Disorders, 28(7), 863–873. 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SO, Thomassen M, Schulz GM, Hosey LA, Varga M, Ludlow CL, et al. (2006). Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: An H215O PET study. Journal of Speech, Language, and Hearing Research: JSLHR, 49(5), 1127–1146. 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. (2009). Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of Neuroscience, 29(31), 9740–9747. 10.1523/JNEUROSCI.2300-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. (2005). Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology, 64(2), 347–349. 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Asgeirsson H, Jakobsson F, Hjaltason H, Jonsdottir H, & Sveinbjornsdottir S (2006). Prevalence study of primary dystonia in Iceland. Movement Disorders, 21(3), 293–298. 10.1002/mds.20674. [DOI] [PubMed] [Google Scholar]
- Baker RS, Andersen AH, Morecraft RJ, & Smith CD (2003). A functional magnetic resonance imaging study in patients with benign essential blepharospasm. Journal of Neuro-Ophthalmology, 23(1), 11–15. [DOI] [PubMed] [Google Scholar]
- Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, & Simonyan K (2016). Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. European Journal of Neurology, 23(10), 1517–1527. 10.1111/ene.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, & Simonyan K (2017). Isolated focal dystonia as a disorder of large-scale functional networks. Cerebral Cortex, 27(2), 1203–1215. 10.1093/cercor/bhv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger HJ, van der Werf SP, Horstink CA, Cools AR, Oyen WJ, & Horstink MW (2007). Writer’s cramp: Restoration of striatal D2-binding after successful biofeedback-based sensorimotor training. Parkinsonism & Related Disorders, 13(3), 170–173. 10.1016/j.parkreldis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Berman BD, Hallett M, Herscovitch P, & Simonyan K (2013). Striatal dopaminergic dysfunction at rest and during task performance in writer’s cramp. Brain, 136, 3645–3658. Pt. 12. 10.1093/brain/awt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BD, Pollard RT, Shelton E, Karki R, Smith-Jones PM, & Miao Y (2018). GABAA receptor availability changes underlie symptoms in isolated cervical dystonia. Frontiers in Neurology, 9, 188 10.3389/fneur.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S, Battistella G, Huddlestone H, Scharf R, Fleysher L, Rumbach AF, et al. (2017). Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Movement Disorders, 32(4), 560–568. 10.1002/mds.26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Black KJ, Ongur D, & Perlmutter JS (1998). Putamen volume in idiopathic focal dystonia. Neurology, 51(3), 819–824. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Kuster JK, Woodman SC, Kirlic N, Makhlouf ML, Multhaupt-Buell TJ, et al. (2012). Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PLoS One, 7(2), e31654 10.1371/journal.pone.0031654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, de Vries PM, Vincent DJ, Rorden C, Morgan PS, Hurd MW, et al. (2007). Structural white matter abnormalities in patients with idiopathic dystonia. Movement Disorders, 22(8), 1110–1116. 10.1002/mds.21295. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, & Standaert DG (2008). The pathophysiological basis of dystonias. Nature Reviews. Neuroscience, 9(3), 222–234. 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Burciu RG, Hess CW, Coombes SA, Ofori E, Shukla P, Chung JW, et al. (2017). Functional activity of the sensorimotor cortex and cerebellum relates to cervical dystonia symptoms. Human Brain Mapping, 38(9), 4563–4573. 10.1002/hbm.23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth S, Francis S, Kelly E, McGlone F, Bowtell R, & Sawle GV (2003). Abnormal cortical sensory activation in dystonia: An fMRI study. Movement Disorders, 18(6), 673–682. 10.1002/mds.10416. [DOI] [PubMed] [Google Scholar]
- Carbon M, & Eidelberg D (2009). Abnormal structure-function relationships in hereditary dystonia. Neuroscience, 164(1), 220–229. 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, et al. (2004). Microstructural white matter changes in carriers of the DYT1 gene mutation. Annals of Neurology, 56(2), 283–286. 10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- Carbon M, Kingsley PB, Tang C, Bressman S, & Eidelberg D (2008). Microstructural white matter changes in primary torsion dystonia. Movement Disorders, 23(2), 234–239. 10.1002/mds.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Niethammer M, Peng S, Raymond D, Dhawan V, Chaly T, et al. (2009). Abnormal striatal and thalamic dopamine neurotransmission: Genotype-related features of dystonia. Neurology, 72(24), 2097–2103. 10.1212/WNL.0b013e3181aa538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Su S, Dhawan V, Raymond D, Bressman S, & Eidelberg D (2004). Regional metabolism in primary torsion dystonia: Effects of penetrance and genotype. Neurology, 62(8), 1384–1390. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Passingham RE, Warner T, Playford ED, Marsden CD, & Brooks DJ (1995). Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Annals of Neurology, 37(3), 363–372. 10.1002/ana.410370313. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A, Rocca MA, Pagani E, Falini A, Comi G, & Filippi M (2009). Cognitive learning is associated with gray matter changes in healthy human individuals: A tensor-based morphometry study. NeuroImage, 48(3), 585–589. 10.1016/j.neuroimage.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Cheng FB, Wan XH, Feng JC, Ma LY, Hou B, Feng F, et al. (2012). Subcellular distribution of THAP1 and alterations in the microstructure of brain white matter in DYT6 dystonia. Parkinsonism & Related Disorders, 18(8), 978–982. 10.1016/j.parkreldis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB (2004). Synaptic connectivity and neuronal morphology: Two sides of the same coin. Neuron, 43(5), 609–617. 10.1016/j.neuron.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, & Svoboda K (2004). Cortical rewiring and information storage. Nature, 431(7010), 782–788. 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Colosimo C, Pantano P, Calistri V, Totaro P, Fabbrini G, & Berardelli A (2005). Diffusion tensor imaging in primary cervical dystonia. Journal of Neurology, Neurosurgery, and Psychiatry, 76(11), 1591–1593. 10.1136/jnnp.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Aguiar PM, & Ozelius LJ (2002). Classification and genetics of dystonia. Lancet Neurology, 1(5), 316–325. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Krainik A, Tezenas du Montcel S, Gerardin E, Meunier S, Mangin JF, et al. (2005). Disorganized somatotopy in the putamen of patients with focal hand dystonia. Neurology, 64(8), 1391–1396. 10.1212/01.WNL.0000158424.01299.76. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, et al. (2009). Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Archives of Neurology, 66(4), 502–508. 10.1001/archneurol.2009.8. [DOI] [PubMed] [Google Scholar]
- Delnooz CC, Helmich RC, Toni I, & van de Warrenburg BP (2012). Reduced parietal connectivity with a premotor writing area in writer’s cramp. Movement Disorders, 27(11), 1425–1431. 10.1002/mds.25029. [DOI] [PubMed] [Google Scholar]
- Delnooz CC, Pasman JW, Beckmann CF, & van de Warrenburg BP (2013). Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One, 8(5), e62877 10.1371/journal.pone.0062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone JC, Pappas SS, Febo M, Burciu RG, Shukla P, Colon-Perez LM, et al. (2017). Forebrain knock-out of torsinA reduces striatal free-water and impairs whole-brain functional connectivity in a symptomatic mouse model of DYT1 dystonia. Neurobiology of Disease, 106, 124–132. 10.1016/j.nbd.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, & May A (2004). Neuroplasticity: Changes in grey matter induced by training. Nature, 427(6972), 311–312. 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Schneider SA, Fiorio M, Kloppel S, Gambarin M, Tinazzi M, et al. (2009). Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. NeuroImage, 47(4), 1141–1147. 10.1016/j.neuroimage.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, & May A (2003). “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology, 61(9), 1228–1231. [DOI] [PubMed] [Google Scholar]
- Dresel C, Bayer F, Castrop F, Rimpau C, Zimmer C, & Haslinger B (2011). Botulinum toxin modulates basal ganglia but not deficient somatosensory activation in orofacial dystonia. Movement Disorders, 26(8), 1496–1502. 10.1002/mds.23497. [DOI] [PubMed] [Google Scholar]
- Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, & Ceballos-Baumann AO (2006). Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain, 129(Pt. 1), 36–46. 10.1093/brain/awh665. [DOI] [PubMed] [Google Scholar]
- Egger K, Mueller J, Schocke M, Brenneis C, Rinnerthaler M, Seppi K, et al. (2007). Voxel based morphometry reveals specific gray matter changes in primary dystonia. Movement Disorders, 22(11), 1538–1542. 10.1002/mds.21619. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Antonini A, Kazumata K, Nakamura T, Dhawan V, et al. (1998). Functional brain networks in DYT1 dystonia. Annals of Neurology, 44(3), 303–312. 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Przedborski S, et al. (1995). The metabolic topography of idiopathic torsion dystonia. Brain, 118, 1473–1484. Pt. 6. [DOI] [PubMed] [Google Scholar]
- Esmaeli-Gutstein B, Nahmias C, Thompson M, Kazdan M, & Harvey J (1999). Positron emission tomography in patients with benign essential blepharospasm. Ophthalmic Plastic & Reconstructive Surgery, 15(1), 23–27. [DOI] [PubMed] [Google Scholar]
- Etgen T, Muhlau M, Gaser C, & Sander D (2006). Bilateral grey-matter increase in the putamen in primary blepharospasm. Journal of Neurology, Neurosurgery, and Psychiatry, 77(9), 1017–1020. 10.1136/jnnp.2005.087148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini G, Pantano P, Totaro P, Calistri V, Colosimo C, Carmellini M, et al. (2008). Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. European Journal of Neurology, 15(2), 185–189. 10.1111/j.1468-1331.2007.02034.x. [DOI] [PubMed] [Google Scholar]
- Filip P, Gallea C, Lehericy S, Bertasi E, Popa T, Marecek R, et al. (2017). Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Movement Disorders, 32(5), 757–768. 10.1002/mds.26930. [DOI] [PubMed] [Google Scholar]
- Fornari E, Maeder P, Meuli R, Ghika J, & Knyazeva MG (2012). Demyelination of superficial white matter in early Alzheimer’s disease: A magnetization transfer imaging study. Neurobiology of Aging, 33(2), 428.e7–19. 10.1016/j.neurobiolaging.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Fuertinger S, & Simonyan K (2017). Connectome-wide phenotypical and genotypical associations in focal dystonia. The Journal of Neuroscience, 37(31), 7438–7449. 10.1523/JNEUROSCI.0384-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallea C, Herath P, Voon V, Lerner A, Ostuni J, Saad Z, et al. (2018). Loss of inhibition in sensorimotor networks in focal hand dystonia. NeuroImage. Clinical, 17, 90–97. 10.1016/j.nicl.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallea C, Horovitz SG, Najee-Ullah M, & Hallett M (2016). Impairment of a parietopremotor network specialized for handwriting in writer’s cramp. Human Brain Mapping, 37(12), 4363–4375. 10.1002/hbm.23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto V, Romito LM, Elia AE, Soliveri P, Panzacchi A, Carpinelli A, et al. (2011). In vivo evidence for GABA(A) receptor changes in the sensorimotor system in primary dystonia. Movement Disorders, 26(5), 852–857. 10.1002/mds.23553. [DOI] [PubMed] [Google Scholar]
- Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, & Hallett M (2004). Changes in brain anatomy in focal hand dystonia. Annals of Neurology, 55(5), 736–739. 10.1002/ana.20113. [DOI] [PubMed] [Google Scholar]
- Gerfen CR (1992). The neostriatal mosaic: Multiple levels of compartmental organization. Trends in Neurosciences, 15(4), 133–139. [DOI] [PubMed] [Google Scholar]
- Gerfen CR (2000). Molecular effects of dopamine on striatal-projection pathways. Trends in Neurosciences, 23(10 Suppl), S64–S70. [DOI] [PubMed] [Google Scholar]
- Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, et al. (2011). Manual activity shapes structure and function in contralateral human motor hand area. NeuroImage, 54(1), 32–41. 10.1016/j.neuroimage.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Granert O, Peller M, Jabusch HC, Altenmuller E, & Siebner HR (2011). Sensorimotor skills and focal dystonia are linked to putaminal grey-matter volume in pianists. Journal of Neurology, Neurosurgery, and Psychiatry, 82(11), 1225–1231. 10.1136/jnnp.2011.245811. [DOI] [PubMed] [Google Scholar]
- Hallett M (1993). Physiology of basal ganglia disorders: An overview. The Canadian Journal of Neurological Sciences, 20(3), 177–183. [DOI] [PubMed] [Google Scholar]
- Hallett M (2004). Dystonia: Abnormal movements result from loss of inhibition. Advances in Neurology, 94, 1–9. [PubMed] [Google Scholar]
- Hamann M, & Richter A (2004). Striatal increase of extracellular dopamine levels during dystonic episodes in a genetic model of paroxysmal dyskinesia. Neurobiology of Disease, 16(1), 78–84. 10.1016/j.nbd.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Altenmuller E, Castrop F, Zimmer C, & Dresel C (2010). Sensorimotor overactivity as a pathophysiologic trait of embouchure dystonia. Neurology, 74(22), 1790–1797. 10.1212/WNL.0b013e3181e0f784. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, & Ceballos-Baumann AO (2005). “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology, 65(10), 1562–1569. 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- Herath P, Gallea C, van der Veen JW, Horovitz SG, & Hallett M (2010). In vivo neurochemistry of primary focal hand dystonia: A magnetic resonance spectroscopic neurometabolite profiling study at 3T. Movement Disorders, 25(16), 2800–2808. 10.1002/mds.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, & Svoboda K (2006). Experience-dependent and cell-type-specific spine growth in the neocortex. Nature, 441(7096), 979–983. 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Horie C, Suzuki Y, Kiyosawa M, Mochizuki M, Wakakura M, Oda K, et al. (2009). Decreased dopamine D receptor binding in essential blepharospasm. Acta Neurologica Scandinavica, 119(1), 49–54. 10.1111/j.1600-0404.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Ford A, Najee-Ullah MA, Ostuni JL, & Hallett M (2012). Anatomical correlates of blepharospasm. Translational Neurodegeneration, 1(1), 12 10.1186/2047-9158-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Gallea C, Najee-Ullah M, & Hallett M (2013). Functional anatomy of writing with the dominant hand. PLoS One, 8(7), e67931 10.1371/journal.pone.0067931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstink CA, Praamstra P, Horstink MW, Berger HJ, Booij J, & Van Royen EA (1997). Low striatal D2 receptor binding as assessed by [123I]IBZM SPECT in patients with writer’s cramp. Journal of Neurology, Neurosurgery, and Psychiatry, 62(6), 672–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XY, Wang L, Liu H, & Zhang SZ (2006). Functional magnetic resonance imaging study of writer’s cramp. Chinese Medical Journal, 119(15), 1263–1271. [PubMed] [Google Scholar]
- Hutchinson M, Kimmich O, Molloy A, Whelan R, Molloy F, Lynch T, et al. (2013). The endophenotype and the phenotype: Temporal discrimination and adult-onset dystonia. Movement Disorders, 28(13), 1766–1774. 10.1002/mds.25676. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, et al. (2000). The metabolic topography of essential blepharospasm: A focal dystonia with general implications. Neurology, 55(5), 673–677. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, & Hallett M (1999). Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology, 53(1), 96–105. [DOI] [PubMed] [Google Scholar]
- Islam T, Kupsch A, Bruhn H, Scheurig C, Schmidt S, & Hoffmann KT (2009). Decreased bilateral cortical representation patterns in writer’s cramp: A functional magnetic resonance imaging study at 3.0 T. Neurological Sciences, 30(3), 219–226. 10.1007/s10072-009-0045-7. [DOI] [PubMed] [Google Scholar]
- Janousova E, Schwarz D, & Kasparek T (2015). Combining various types of classifiers and features extracted from magnetic resonance imaging data in schizophrenia recognition. Psychiatry Research, 232(3), 237–249. 10.1016/j.pscychresns.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Kadota H, Nakajima Y, Miyazaki M, Sekiguchi H, Kohno Y, Amako M, et al. (2010). An fMRI study of musicians with focal dystonia during tapping tasks. Journal of Neurology, 257(7), 1092–1098. 10.1007/s00415-010-5468-9. [DOI] [PubMed] [Google Scholar]
- Kerrison JB, Lancaster JL, Zamarripa FE, Richardson LA, Morrison JC, Holck DE, et al. (2003). Positron emission tomography scanning in essential blepharospasm. American Journal of Ophthalmology, 136(5), 846–852. [DOI] [PubMed] [Google Scholar]
- Klein C (2014). Genetics in dystonia. Parkinsonism & Related Disorders, 20(Suppl. 1), S137–S142. 10.1016/S1353-8020(13)70033-6. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Agosta F, Sarro L, Tomic A, Kresojevic N, Galantucci S, et al. (2016). Brain structural changes in spasmodic dysphonia: A multimodal magnetic resonance imaging study. Parkinsonism & Related Disorders, 25, 78–84. 10.1016/j.parkreldis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. (1992). Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences of the United States of America, 89(12), 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Tijssen MA, Vidailhet M, Kaji R, & Meunier S (2013). The anatomical basis of dystonia: Current view using neuroimaging. Movement Disorders, 28(7), 944–957. 10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, & Hallett M (2004). Regional cerebral blood flow correlates of the severity of writer’s cramp symptoms. NeuroImage, 21(3), 904–913. 10.1016/j.neuroimage.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Levy LM, & Hallett M (2002). Impaired brain GABA in focal dystonia. Annals of Neurology, 51(1), 93–101. [PubMed] [Google Scholar]
- Magyar-Lehmann S, Antonini A, Roelcke U, Maguire RP, Missimer J, Meyer M, et al. (1997). Cerebral glucose metabolism in patients with spasmodic torticollis. Movement Disorders, 12(5), 704–708. 10.1002/mds.870120513. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA, Zarranz JJ, & Lang AE (1985). The anatomical basis of symptomatic hemidystonia. Brain, 108, 463–483. Pt. 2. [DOI] [PubMed] [Google Scholar]
- Martino D, Di Giorgio A, D’Ambrosio E, Popolizio T, Macerollo A, Livrea P, et al. (2011). Cortical gray matter changes in primary blepharospasm: A voxel-based morphometry study. Movement Disorders, 26(10), 1907–1912. 10.1002/mds.23724. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Beckmann CF, Dengler R, & Munte TF (2012). Changes in resting-state brain networks in writer’s cramp. Human Brain Mapping, 33(4), 840–848. 10.1002/hbm.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Filippini V, & Leggio MG (2002). Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience, 111(4), 863–870. [DOI] [PubMed] [Google Scholar]
- Naumann M, Pirker W, Reiners K, Lange KW, Becker G, & Brucke T (1998). Imaging the pre- and postsynaptic side of striatal dopaminergic synapses in idiopathic cervical dystonia: A SPECT study using [123I] epidepride and [123I] beta-CIT. Movement Disorders, 13(2), 319–323. 10.1002/mds.870130219. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Blake DT, & Chen R (2009). Digit-specific aberrations in the primary somatosensory cortex in writer’s cramp. Annals of Neurology, 66(2), 146–154. 10.1002/ana.21626. [DOI] [PubMed] [Google Scholar]
- Nevrly M, Hlustik P, Hok P, Otruba P, Tudos Z, & Kanovsky P (2018). Changes in sensorimotor network activation after botulinum toxin type A injections in patients with cervical dystonia: A functional MRI study. Experimental Brain Research, 236(10), 2627–2637. 10.1007/s00221-018-5322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neychev VK, Gross RE, Lehericy S, Hess EJ, & Jinnah HA (2011). The functional neuroanatomy of dystonia. Neurobiology of Disease, 42(2), 185–201. 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Carbon M, Argyelan M, & Eidelberg D (2011). Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiology of Disease, 42(2), 202–209. 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega JN, Richter A, Tozman N, Jiwa D, & Loscher W (1996). Quantitative autoradiography reveals regionally selective changes in dopamine D1 and D2 receptor binding in the genetically dystonic hamster. Neuroscience, 71(4), 927–937. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Muenter MD, Melton LJ 3rd, Aronson A, & Kurland LT (1988). Epidemiology of dystonia in Rochester, Minnesota. Advances in Neurology, 50, 361–365. [PubMed] [Google Scholar]
- Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, et al. (2007). Morphometric changes of sensorimotor structures in focal dystonia. Movement Disorders, 22(8), 1117–1123. 10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, & Glynn P (1990). Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magnetic Resonance in Medicine, 14(1), 68–78. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. (1997). The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nature Genetics, 17(1), 40–48. 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Pantano P, Totaro P, Fabbrini G, Raz E, Contessa GM, Tona F, et al. (2011). A transverse and longitudinal MR imaging voxel-based morphometry study in patients with primary cervical dystonia. AJNR. American Journal of Neuroradiology, 32(1), 81–84. 10.3174/ajnr.A2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, et al. (1997). Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. The Journal of Neuroscience, 17(2), 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford ED, Passingham RE, Marsden CD, & Brooks DJ (1998). Increased activation of frontal areas during arm movement in idiopathic torsion dystonia. Movement Disorders, 13(2), 309–318. 10.1002/mds.870130218. [DOI] [PubMed] [Google Scholar]
- Prell T, Peschel T, Kohler B, Bokemeyer MH, Dengler R, Gunther A, et al. (2013). Structural brain abnormalities in cervical dystonia. BMC Neuroscience, 14, 123 10.1186/1471-2202-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Roset-Llobet J, Rosines-Cubells D, Deus J, Narberhaus B, Valls-Sole J, et al. (2000). Brain cortical activation during guitar-induced hand dystonia studied by functional MRI. NeuroImage, 12(3), 257–267. 10.1006/nimg.2000.0615. [DOI] [PubMed] [Google Scholar]
- Putzel GG, Battistella G, Rumbach AF, Ozelius LJ, Sabuncu MR, & Simonyan K (2018). Polygenic risk of spasmodic dysphonia is associated with vulnerable sensorimotor connectivity. Cerebral Cortex, 28(1), 158–166. 10.1093/cercor/bhw363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quallo MM, Price CJ, Ueno K, Asamizuya T, Cheng K, Lemon RN, et al. (2009). Gray and white matter changes associated with tool-use learning in macaque monkeys. Proceedings of the National Academy of Sciences of the United States of America, 106(43), 18379–18384. 10.1073/pnas.0909751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, & Hallett M (2013). Emerging concepts in the physiological basis of dystonia. Movement Disorders, 28(7), 958–967. 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, & Simonyan K (2014). What’s special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Movement Disorders, 29(9), 1141–1150. 10.1002/mds.25934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdhani RA, & Simonyan K (2013). Primary dystonia: Conceptualizing the disorder through a structural brain imaging lens. Tremor and Other Hyperkinetic Movements (New York, N. Y.), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkirch I, Granert O, Knutzen A, Wolff S, Govert F, Pedersen A, et al. (2018). Dynamic causal modeling revealed dysfunctional effective connectivity in both, the cortico-basal-ganglia and the cerebello-cortical motor network in writers’ cramp. NeuroImage. Clinical, 18, 149–159. 10.1016/j.nicl.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K (2017). Inferior parietal cortex as a hub of loss of inhibutuib and maladaptive plasticity. In Paper presented at the Annual Meeting of Americal Academy of Neurology, Boston. [Google Scholar]
- Simonyan K, Berman BD, Herscovitch P, & Hallett M (2013). Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. The Journal of Neuroscience, 33(37), 14705–14714. 10.1523/JNEUROSCI.0407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Cho H, Hamzehei Sichani A, Rubien-Thomas E, & Hallett M (2017). The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain, 140(12), 3179–3190. 10.1093/brain/awx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, & Ludlow CL (2010). Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: An fMRI study. Cerebral Cortex, 20(11), 2749–2759. 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, & Ludlow CL (2012). Abnormal structure-function relationship in spasmodic dysphonia. Cerebral Cortex, 22(2), 417–425. 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, et al. (2008). Focal white matter changes in spasmodic dysphonia: A combined diffusion tensor imaging and neuropathological study. Brain, 131(Pt. 2), 447–459. 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, & Rubenstein JL (2005). Patterning and plasticity of the cerebral cortex. Science, 310(5749), 805–810. 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Yan Z, & Song WJ (1998). Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. Advances in Pharmacology, 42, 1020–1023. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mizoguchi S, Kiyosawa M, Mochizuki M, Ishiwata K, Wakakura M, et al. (2007). Glucose hypermetabolism in the thalamus of patients with essential blepharospasm. Journal of Neurology, 254(7), 890–896. 10.1007/s00415-006-0468-5. [DOI] [PubMed] [Google Scholar]
- Termsarasab P, Ramdhani RA, Battistella G, Rubien-Thomas E, Choy M, Farwell IM, et al. (2016). Neural correlates of abnormal sensory discrimination in laryngeal dystonia. NeuroImage. Clinical, 10, 18–26. 10.1016/j.nicl.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD, & Perlmutter JS (1998). Mutational and biochemical analysis of dopamine in dystonia: Evidence for decreased dopamine D2 receptor inhibition. Molecular Neurobiology, 16(2), 135–147. 10.1007/BF02740641. [DOI] [PubMed] [Google Scholar]
- Ulug AM, Vo A, Argyelan M, Tanabe L, Schiffer WK, Dewey S, et al. (2011). Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proceedings of the National Academy of Sciences of the United States of America, 108(16), 6638–6643. 10.1073/pnas.1016445108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL (2002). Pathophysiology of dystonia: A neuronal model. Movement Disorders, 17(Suppl. 3), S49–S62. [DOI] [PubMed] [Google Scholar]
- Vo A, Sako W, Dewey SL, Eidelberg D, & Ulug AM (2015). 18FDG-microPET and MR DTI findings in Tor1a+/− heterozygous knock-out mice. Neurobiology of Disease, 73, 399–406. 10.1016/j.nbd.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Vo A, Sako W, Niethammer M, Carbon M, Bressman SB, Ulug AM, et al. (2015). Thalamocortical connectivity correlates with phenotypic variability in dystonia. Cerebral Cortex, 25(9), 3086–3094. 10.1093/cercor/bhu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh JL, Kuster JK, Levenstein JM, Makris N, Multhaupt-Buell TJ, Sudarsky LR, et al. (2016). Thalamic volume is reduced in cervical and laryngeal dystonias. PLoS One, 11(5), e0155302 10.1371/journal.pone.0155302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Luo C, Song W, Chen Q, Chen K, Chen X, et al. (2013). Altered regional spontaneous neuronal activity in blepharospasm: A resting state fMRI study. Journal of Neurology, 260(11), 2754–2760. 10.1007/s00415-013-7042-8. [DOI] [PubMed] [Google Scholar]
- Yourganov G, Schmah T, Churchill NW, Berman MG, Grady CL, & Strother SC (2014). Pattern classification of fMRI data: Applications for analysis of spatially distributed cortical networks. NeuroImage, 96, 117–132. 10.1016/j.neuroimage.2014.03.074. [DOI] [PubMed] [Google Scholar]
- Zeuner KE, Knutzen A, Granert O, Gotz J, Wolff S, Jansen O, et al. (2015). Increased volume and impaired function: The role of the basal ganglia in writer’s cramp. Brain and Behavior: A Cognitive Neuroscience Perspective, 5(2), e00301 10.1002/brb3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoons E, Booij J, Nederveen AJ, Dijk JM, & Tijssen MA (2011). Structural, functional and molecular imaging of the brain in primary focal dystonia—A review. NeuroImage, 56(3), 1011–1020. 10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]