Abstract
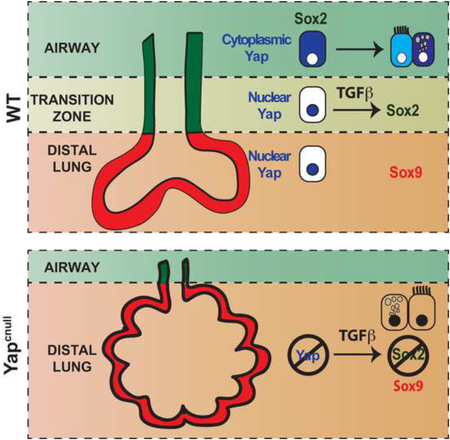
How epithelial progenitor cells integrate local signals to balance expansion with differentiation during organogenesis is still little understood. Here we provide evidence that the Hippo pathway effector Yap is a key regulator of this process in the developing lung. We show that when epithelial tubules are forming and branching a nucleocytoplasmic shift of Yap marks the boundary between the airway and the distal lung compartments. At this transition zone, Yap specifies a transcriptional program that controls Sox2 expression and ultimately generates the airway epithelium. Without Yap, epithelial progenitors are unable to properly respond to local TGFβ-induced cues and control levels and distribution of Sox2 to form airways. Yap levels and subcellular localization also markedly influence Sox2 expression and differentiation of adult airway progenitors. Our data reveal a role for the Hippo-Yap pathway in integrating growth factor-induced cues in the developing and adult lung potentially key for homeostasis and regeneration-repair.
Keywords: Hippo pathway, Yap, airway epithelium, lung development, stem cell, epithelial differentiation, progenitor cell
Graphical Abstract
INTRODUCTION
Organ growth and tissue homeostasis rely on the coordinated proliferation and differentiation of progenitor cell populations. Coordination of these events is challenging in developing organs, such as the lung, in which epithelial progenitors organize into complex branching structures to form the conducting airways in proximal regions, and the future gas-exchange region at distal sites. Inputs from multiple signaling pathways, including those induced by Fibroblast Growth Factor (FGF), Transforming Growth Factor-beta (TGFβ), Wnt, and Notch are essential in this process (Cardoso and Lu, 2006; Morrisey and Hogan, 2010). Yet, how these signals integrate to control lung growth, pattern formation and differentiation is still poorly understood.
The transcriptional regulator Yap (Yes-associated protein) has emerged as a key modulator of developmental signals that ultimately influence cell proliferation, apoptosis and cell fate in development and cancer. Yap exerts its function largely through TEA-domain (Tead) family transcription factors, which are expressed in multiple tissues. Yap activity depends on its nuclear or cytoplasmic localization regulated by the Hippo pathway. This pathway comprises a highly conserved group of serine/threonine kinases that regulates Yap localization through phosphorylation of conserved serine residues on Yap. The core Hippo pathway is composed of Mst1 and Mst2 kinases, which together with Ww45 (Sav1) and Mob1A/B activate the Lats1 and Lats2 kinases to promote Yap phosphorylation. Inactivation of Hippo pathway kinases results in nuclear localization and enhanced transcriptional activity of Yap (Mauviel et al., 2011; Tremblay and Camargo, 2012; Varelas et al., 2010; 2008).
Yap in the nucleus drives the expansion of stem and progenitor cell populations, while cytoplasmic retention of phosphorylated Yap plays a major role in cell contact inhibition of proliferation and in modulating the activity of different signaling pathways. Hippo pathway signaling is important when transitioning from a proliferative to a quiescent state, ensuring proper differentiation in different biological contexts. Uncontrolled nuclear Yap activity is associated with cellular overgrowth and cancer (Camargo et al., 2007; Habbig et al., 2011; Pan, 2010).
Components of the Hippo pathway have been examined in the lung and some were implicated in lung development. Systemic knockout of Taz (Transcription-Activator with PDZ-Binding domain), a Yap paralog, results in no overt developmental defects in the lung. Taz null mice, however, show an abnormal alveolar enlargement postnatally that resembled human pulmonary emphysema (Mitani et al., 2009). Disruption of Mst1/2 expression in the developing lung epithelium also result in an alveolar defect phenotype, which appear prenatally and are characterized by immature Type II pneumocytes and reduced number of type I pneumocytes (Chung et al., 2013). Accumulation of less differentiated epithelial progenitors due to increased Yap transcriptional activity had been previously reported in mice lacking Mst1/2 selectively in the liver and intestinal epithelium (Zhou et al., 2011a). However, Mst1/2 ablation in the lung had no significant impact on Yap. Thus, the role of Yap in the lung remained elusive. Little is known of where and when Yap is expressed and whether it functions in lung development. The early lethality of systemic Yap knockout has precluded the analysis of phenotypes in the lung (Morin-Kensicki et al., 2006).
Here we report an essential role for Yap and the control of its phosphorylation and nuclear-cytoplasmic distribution in specification and differentiation of the airway epithelium. We show that Yap is required for the expression of Sox2 in the progenitors that will give rise to the airways, allowing these cells to respond to TGFβ and initiate a program of airway epithelial cell fate. Moreover we found that, later, Yap phosphorylation is crucial for differentiation of the epithelial progenitors into the distinct cellular phenotypes of the developing and the adult airways. Our results reveal a major role for the Hippo-Yap pathway in integrating growth factor-induced cues during airway morphogenesis and differentiation.
RESULTS
Yap is differentially distributed in distinct populations of epithelial progenitors of the developing lung
To gain insights into the role of Yap in the lung, we investigated the developmental pattern of Yap mRNA expression in embryonic lungs from E10.5 (secondary bud formation, initiation of branching morphogenesis) to E18.5 (saccular stage). In situ hybridization (ISH) showed expression throughout the stages examined, with prominent expression at earlier developmental stages. Yap transcripts were ubiquitously distributed in all layers but appeared to be strongest in the epithelium of the developing airways and distal buds (Fig. 1A).
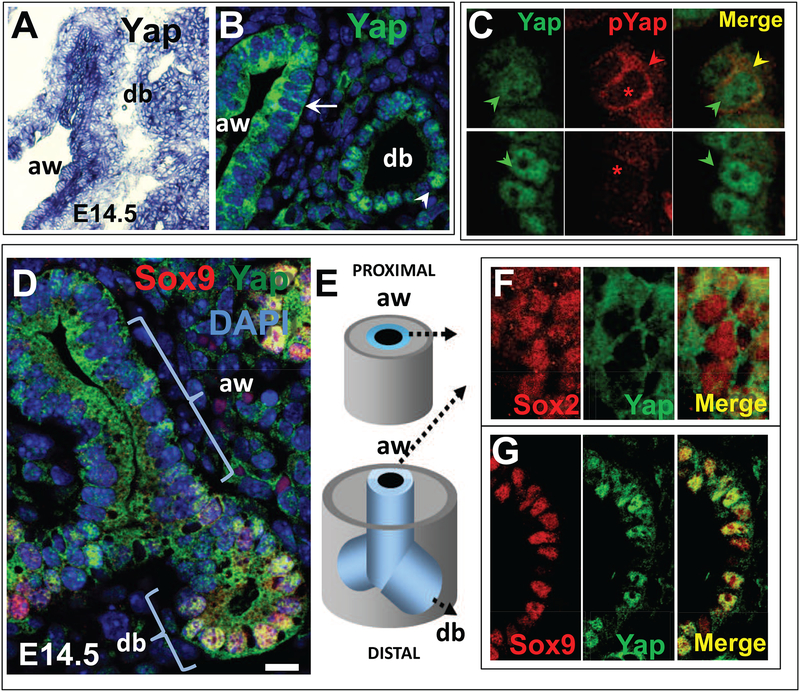
Figure 1. Differential activation of Yap in distinct populations of epithelial progenitors of the developing lung.
(A) In situ hybridization (ISH) of Yap showing widespread distribution of signals in airways (aw) and distal buds (db) of E14.5 mouse lung. (B) Immunofluorescence (IF) and confocal analysis of Yap (total) shows distinct nuclear (arrowhead, db) and cytoplasmic (arrow, aw) signals. DAPI (in blue) highlights nuclear staining. (C) Comparison of the pattern of Yap (total) and phosphorylated Yap (pYAP, S112-Yap phosphorylation) in E12.5 lung confirms pYap staining in the epithelium to be essentially cytoplasmic (red and yellow arrowheads); nuclear signals (green arrowheads in Yap staining) are absent in pYap-stained sections (asteriks). (D-G) Double IF analysis of Yap with markers of distal bud (Sox9) or airway (Sox2) cell fate during branching morphogenesis depicting overlapping Yap nuclear signals typically seen in Sox9-labeled distal buds largely absent in Sox2-labeled forming airways. The Yap cytoplasmic pattern is seen the vast majority of airway epithelial progenitors throughout the P-D axis of the lung (diagram in E; F-G). Scale bar in D, 12μm.
The subcellular localization of Yap is controlled by its phosphorylation on several conserved residues, including a key Serine residue (Ser112 in Mus musculus, homologous to Ser127 in human). This promotes Yap binding to 14–3–3 proteins and subsequent cytoplasmic sequestration. Given the ubiquitous distribution of Yap mRNA, we examined whether we could identify distinct patterns of Yap protein subcellular localization in the developing lung. We analyzed lung sections by immunofluorescence (IF) using antibodies against total (Yap; Santa Cruz) or S112-phosphorylated Yap (pYap; Cell Signaling). IF analysis of total Yap revealed a markedly distinct subcellular distribution in the epithelial tubules with nuclear Yap restricted to progenitors of distal buds and cytoplasmic Yap present in the airway epithelial progenitors (Fig. 1B). A comparison of the staining patterns of total Yap and phosphorylated Yap (pYap) revealed a pattern that mirrored Yap cytoplasmic signals and suggested that cytoplasmic Yap is in its phosphorylated form, as reported in other contexts (Pan, 2010) (Fig. 1C).
The distal identity of the epithelial progenitors expressing nuclear Yap was confirmed by co-labeling with Sox9 in distal buds (Perl et al., 2005). Cytoplasmic Yap was confirmed in the epithelial regions proximal to these buds which co-expressed Sox2 (Que et al., 2009). Sox2 and cytoplasmic Yap mark cells that during branching morphogenesis are committed to an airway fate and later differentiate into specific cellular phenotypes of the conducting airways (Fig. 1D–G). This pattern of Yap localization was consistently observed as new generations of airways developed with no obvious gradient along the proximal-distal (P-D) axis (Fig. 1D). The distinct nuclear-cytoplasmic localization shift suggested that Yap could be regulating specific cellular events in epithelial progenitors at the transition of the distal to the nascent airway compartments.
Yap expression is required for airway morphogenesis
The presence of nuclear Yap in nascent distal buds suggested that the local activation of a Yap-mediated transcriptional program could be crucial for expansion of the distal lung compartment during morphogenesis. To test this hypothesis, we generated mice in which the Yap gene was conditionally removed from the developing lung epithelium. Yap mutants (Yap f/f) were crossed to mice expressing Cre recombinase from the Sonic hedgehog (Shh) promoter (Shh-Cre) to delete Yap in the foregut endoderm and the respiratory epithelium from the earliest developmental stages (Harris et al., 2006). Conditional deletion of both Yap alleles in Yap f/f; Shh-Cre/+ (Yapcnull hereafter) was lethal at birth.
Mutant embryos were slightly smaller than their littermates but no other obvious gross abnormality was apparent (Fig. 2A). By contrast, lungs from Yapcnull were highly hypoplastic and showed severe disruption in branching morphogenesis resulting in dilated cyst-like structures (Fig. 2B). Histological examination revealed that these structures occupied most of the lung and were lined by a cuboidal epithelium. By contrast, areas with tall cylindrical epithelium characteristic of proximal airways were greatly reduced (Fig. 2F,G,J). Efficient targeting of the lung epithelium by the Shh-Cre driver was confirmed in the Yapcnull mutants by the dramatic loss of Yap expression selectively in the epithelium, contrasting with the preserved signals in the mesenchyme (Fig. 2C–D).
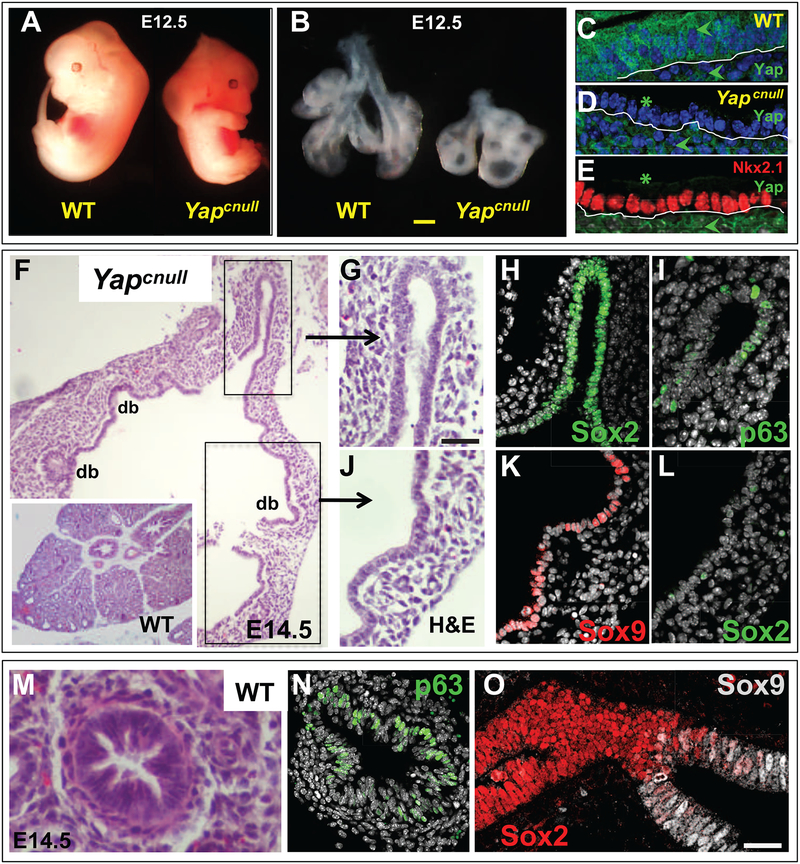
Figure 2. Yap is required for airway progenitor cell fate and epithelial morphogenesis in the developing lung.
(A-B) E12.5 Yapcnull mouse embryos are slightly smaller than WT but have highly hypoplastic lungs with cystic structures (lungs dissected at the level of the carina). (C) Yap expression (arrowheads) in both epithelium and mesenchyme of WT E12.5 lungs; (D-E) selective disruption of Yap in E12.5 Yapcnull lung epithelium (asteriks) with preserved signals in the mesenchyme (arrowhead). Nkx2–1 expression confirms that Yapcnull epithelium maintained its lung identity. (F-L) Histological analysis showing aberrant epithelial morphogenesis of E14.5 Yapcnull lungs compared to WT (H&E: F,G,J) with abnormal expansion of the distal compartment (Sox9 positive, Sox2 negative: J,K,L) and great reduction in the airway compartment (Sox2, p63 positive): Yapcnull (G-I). (M-O) WT: H&E, p63 and Sox9-Sox2 IF. DAPI (in grey: D, H). Scale bars in B, 360 μm, G, 80 μm, O, 35 μm.
IF analysis of Sox9 revealed strong signals throughout the epithelium of the dilated structures of mutant lungs, indicating that the loss of Yap led to an aberrant expansion of the distal epithelial progenitors. The strong Nkx2–1 (Ttf1) signals throughout the P-D axis confirmed that the Yap-deficient epithelium maintained its lung cell identity (Kimura et al., 1999) (Fig. 2E). IF for Sox2 showed signals in only few cells of the most proximal epithelial tubules. The local expression of p63 in these cells confirmed their proximal identity and suggested that the expansion of the distal compartment occurred at the costs of the proximal progenitors that normally give rise to airways (Fig. 2G–O). These observations were confirmed by assessing Sox2 expression by quantitative real-time PCR (qPCR), and by quantitating the proportion of Sox2-expressing cells compared to Sox9 (Suppl. Fig. 1A–C).
A more detailed analysis of the Yapcnull lung phenotype during the early stages of branching morphogenesis (E10.5–14.5) revealed that trachea and primary buds were grossly unaffected, and that multiple buds appeared to be arising from the distal cystic structures. Remarkably these buds expressed Sox9, Bmp4 and Sftpc (surfactant-associated-protein C), markers typically associated with the developing distal epithelial buds, as observed in controls (Fig. 3A–H). These suggested that in the absence of Yap the distal lung progenitors were capable of expanding and responding to morphogenetic cues required for distal bud formation. Thus, the Yap nuclear localization and transcriptional activity in the distal epithelium is not necessarily linked with the ability of distal epithelial progenitors to emerge and proliferate. Despite the overall reduction in lung size, analysis of the cell cycle markers Ki67 and PCNA in the Yapcnull epithelium showed patterns similar to that found in the WT distal lung epithelium (Suppl. Fig. 2A–B). No differences in cleaved-Caspase3 expression were observed between control and Yapcnull tissues, indicating that apoptotic cell death does not account for the phenotype (Suppl. Fig. 2B).
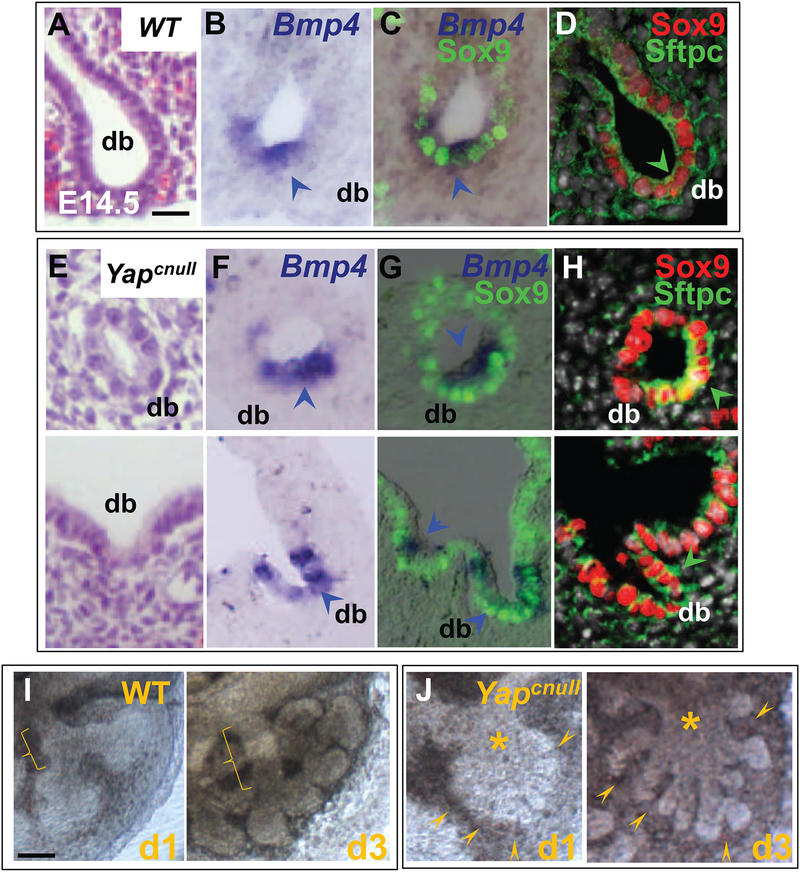
Figure 3: Yap deficiency does not prevent induction of distal buds (db).
IF analysis of Sox9, Sftpc and Bmp4 ISH (arrowhead) in E14.5 WT (A-D) and Yapcnull lungs (E-H). (J) Lung epithelial morphogenesis in E12.5 explants cultured for 3 days showing formation of typical distal buds and tube-like airway structures (bracket) in WT lungs. (K) By contrast in Yapcnull lungs cultures distal buds are induced (arrowheads) but airway structures do not form (asterisks). DAPI (in grey: D,H). Scale bars in E, 30 μm.
To better understand how bud formation and branching differed between Yapcnull and WT mice we isolated E11.5 lung explants and followed epithelial development ex vivo under defined conditions for 3 days (Fig. 3I–J). Consistent with our observations in vivo, the loss of Yap did not affect bud initiation, as multiple buds were found arising from the distal epithelium. However, in contrast to WT, the newly formed buds from the Yapcnull explants did not organize into tubule-like airway structures. Instead, buds arising from Yapcnull explants continued expanding and did not form airways. Yap may restrict the expansion of distal progenitors following the initiation of branching morphogenesis, a step that is essential for the proper formation of airways.
Yap is transiently activated in airway epithelial progenitors and regulates Sox2 expression.
The aberrant P-D patterning of the Yapcnull lungs suggested that Yap is required to induce a program of airway epithelial cell fate in areas proximal to the growing buds. Sox2 is the earliest known marker for the specification of airway epithelial progenitors and is essential for the development of the lineages of the entire airway epithelium (Gontan et al., 2008; Tompkins et al., 2009; Wang et al., 2013). IF analysis of E12.5-E14.5 WT lungs indicated that Sox2 expression is initiated precisely at the region where Yap shifts its localization from the nucleus to the cytoplasm, a region that we have termed the transition zone (tz) (Fig. 4A–D). High resolution imaging of this region showed cells displaying both Yap and Sox2 in the nucleus (Fig. 4C–D). Nuclear Yap is known to regulate the expression of Sox2 in mouse embryonic stem cells and in neuronal differentiation (Cao et al., 2008; Lian et al., 2010). Thus, we examined the possibility that Sox2 might be a transcriptional target of Yap in airway progenitors.
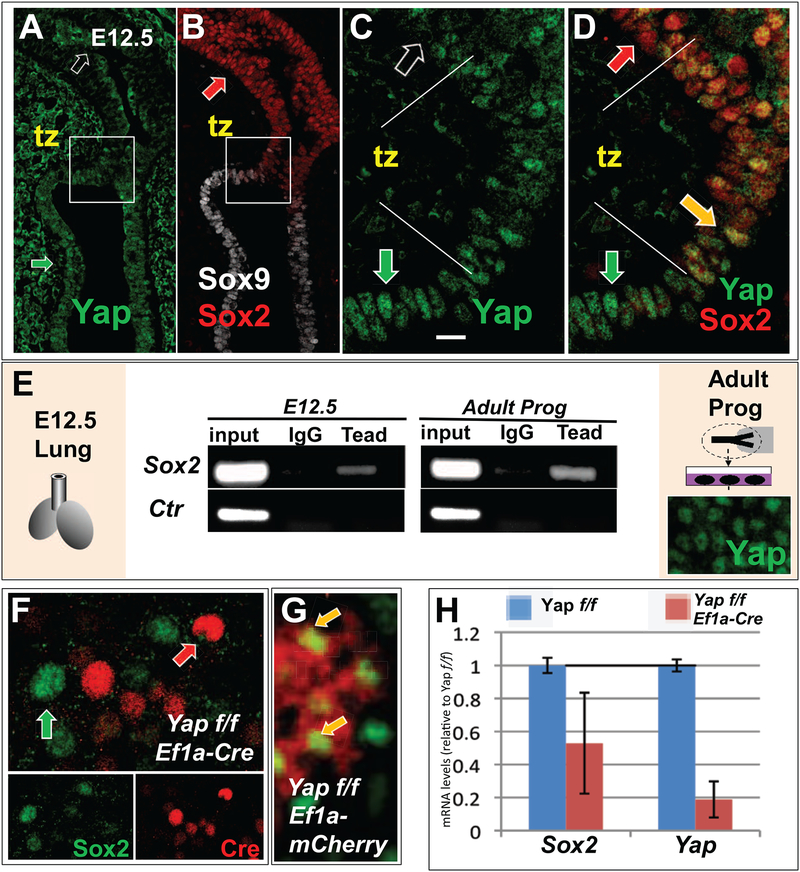
Figure 4. Yap is transiently activated in airway epithelial progenitors and regulates Sox2 expression.
(A-B) IF staining of (A) Yap, (B) Sox2 and Sox9 in E12.5 WT lungs reveals a transition zone (tz; boxed area enlarged in (C,D)) in the epithelium between the distal bud and forming airways where both Yap and Sox2 are expressed in the nucleus (D, yellow arrow). Regions flanking the tz show non-overlapping nuclear expression of the same signals (red and green arrows: Sox2 and Yap, respectively; clear arrow: non-nuclear Yap). (E) ChIP-PCR of Sox2 (top) and an unrelated genomic control Fabp4 enhancer region (Ctr) using Tead1–4 antibody and cells from freshly isolated E12.5 lungs (left) or epithelial progenitors from adult mouse trachea expanded in culture (right; note nuclear Yap expression by IF). (F-G) When these cells are isolated from adult Yap f/f tracheas (as in (E)) and transduced with Ef1a-Cre, Yap is deleted in Cre-expressing cells, which fail to express Sox2 (note non-overlapping Sox2-Cre pattern in (F)). This contrasts with Yap f/f transduced with Ef1a-mCherry control cultures (yellow arrow: overlapping signals in (G)). (H) qPCR shows that Yap deletion in Ef1a-Cre-transduced Yap f/f cultures leads to decreased Sox2 expression. Scale bar in C, 20μm.
Yap is recruited to DNA through interactions with transcription factors, the best described of which are the family of Teads (Tead1–4). In silico promoter analysis at the Sox2 locus revealed several Tead-binding sequences upstream of the Sox2 transcriptional start site (not shown). We investigated whether Tead transcription factors reside on the Sox2 promoter in airway progenitors of embryonic lungs, which we showed to express Sox2 and Yap. For this, we performed chromatin immunoprecipitation (ChIP) analysis in homogenates of WT 12.5 lungs using a Tead antibody that recognizes all four Tead family members (Beyer et al., 2013). As shown in Fig. 4B and extensively reported by others, Sox2 is expressed in the embryonic lung exclusively in the developing airway epithelium (Morrisey and Hogan, 2010). PCR analysis of the ChIP samples from E12.5 lung using Sox2 specific primers confirmed that Tead transcription factors are enriched at the Sox2 promoter in these cells (Fig. 4E).
We next asked whether the recruitment of Teads to the Sox2 promoter was restricted to the embryonic lung, or whether such enrichment was also present in adult airway progenitors. Primary airway progenitor cells were isolated from mouse adult tracheas (n=3) and expanded in culture to confluence, as reported (You et al., 2002). ChIP analysis showed strong recruitment of Teads to the Sox2 promoter in these samples, consistent with our observations in E12.5 lungs. Importantly, IF analysis of Yap in these cells indicated strong nuclear Yap localization (Fig. 4E), suggesting that Yap-Tead complexes regulate Sox2 expression. To test whether nuclear Yap controls Sox2 expression, we isolated adult airway epithelial progenitors from Yap f/f mice and deleted Yap by transducing these cells with a lentivirus expressing Cre recombinase from an EF1a-promoter (EF1a-Cre), or with a control EF1a-mCherry (AddGene). qPCR analysis showed Sox2 expression decreased by approximately 50% upon the loss of Yap (Fig. 4H). Moreover IF analysis for Cre or Sox2 revealed that Yap f/f cells expressing Cre (deleted Yap) lost Sox2 expression (Fig. 4F–G). Our data support a hypothesis that nuclear Yap activates a transcriptional program at the transition zone that induces Sox2 expression and leads to the specification of airway epithelial cell precursors.
Epithelial progenitors require Yap to properly respond to TGFβ-induced cues in developing airways.
The aberrant phenotype of Yapcnull mice suggested that mechanisms normally restricting expansion of distal progenitors and fostering formation of the airway compartment are profoundly altered in the absence of Yap. Multiple studies indicate key roles for Wnt, TGFβ, and BMP in the control of P-D specification in the developing lung (Lebeche et al., 1999; Shu et al., 2005; Weaver et al., 1999).
TGFβ signaling is known to restrict budding and foster the development of the airway compartment. When airways are forming, TGFβl accumulates in the mesenchyme associated with the stalks of branching tubules, thereby activating signals in the mesenchyme and neighboring epithelial cells to restrict airway budding (Heine et al., 1990). We explored the possibility that the loss of Yap could interfere with the TGFβ effects in the lung epithelium. Sites of activity of TGFβ signaling can be identified in the developing lung mesenchyme during this process by examining the expression of Tgfbi (TGFβ-induced gene, or Bigh3) mRNA., Tgfbi is a well-characterized TGFβ target gene that can be used to indirectly map regions where endogenous TGFβ ligand is available and active in branching airways (Lu et al., 2004).
ISH of Tgfbi in E14.5 WT lungs confirmed the mesenchymal pattern of distribution previously reported (Fig 5A–B). Interestingly, Tgfbi was expressed in the mesenchymal region associated with the transition zone, between the distal and the airway compartment. In E14.5 Yapcnul1 lungs Tgfbi continued to be restricted to the subepithelial mesenchyme; however, the domain of Tgfbi expression extended widely into the mesenchyme of the enlarged Sox9-labeled distal buds (Fig. 5C–F). The strong Tgfbi expression in the mesenchyme indicated that TGFβ ligand was available in the Yapcnull mutants and capable of activating target genes locally at the presumptive sites of stalk formation. However, the defective epithelial patterning of Yapcnull lungs raised the possibility that the epithelium was unable to properly respond to TGFβ without Yap. This was further suggested by qPCR analysis of E14.5 lungs showing in Yapcnul1 decreased mRNA levels of Smad7, an epithelial target of TGFβ in the lung and kidney (Li et al., 2002; Zhao et al., 2000) (Suppl. Fig. 3G).
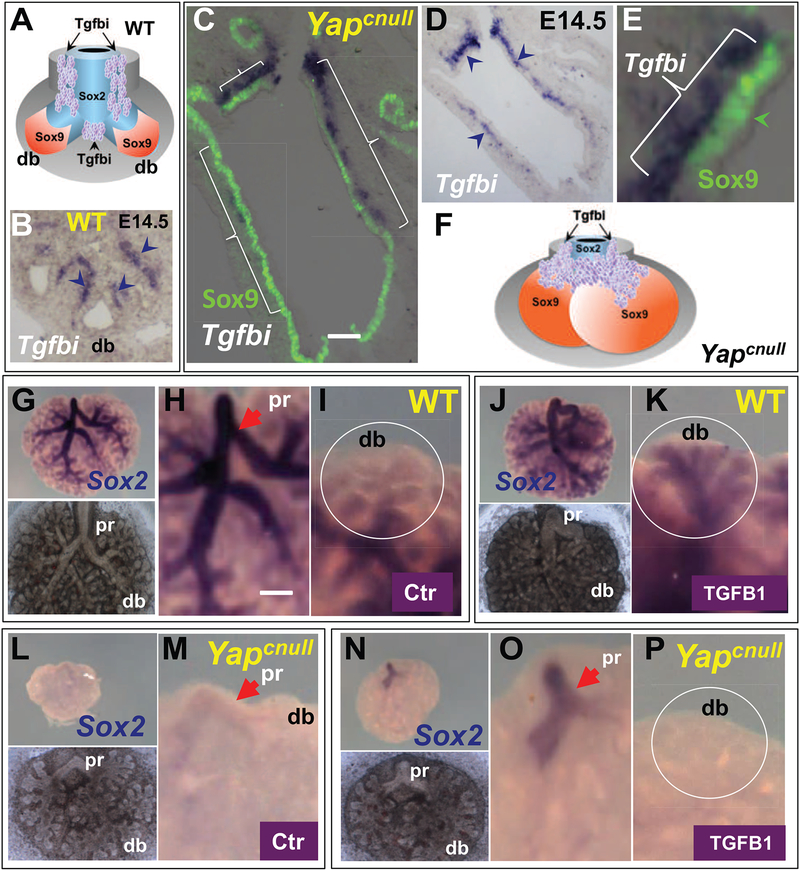
Figure 5. Epithelial progenitors require Yap to properly respond to TGFβ-induced cues in developing airways.
(A-B) Tgfbi expression pattern in E14.5 WT lung by ISH showing enriched signals (arrowheads) in the stalk of distal buds (db) and interbud regions of forming airways. (C-E) Double ISH/IF in E14.5 Yapcnull lung shows Tgfbi signals extended distally in the mesenchyme associated with the Sox9-labeled distal epithelium of the mutant lungs (arrowheads in (D) and brackets in (C) and (E)). (F) Diagrammatic representation of the Tgfbi pattern in the abnormally expanded distal regions of Yapcnull lungs (compare with (A)). (G-P) E12.5 lung explants from WT (G-K) and Yapcnull (L-P) mice cultured for 3 days in Control (G-I; L-M) or TGFβ1-containing (J-K; N-P) media. Whole mount ISH of Sox2 showing strong signals in airways of WT (arrow; pr, proximal) that are nearly abolished in Yapcnull lungs (H,M). Treatment with recombinant TGFβ1 extends the Sox2 domain to the distal buds (db) in WT but not in Yapcnull lungs, (circled area in (I, K, M, P)), although proximal (pr) regions that already expressed Sox2 (main bronchi) show slightly increased signals. Whole mount images of cultured lungs are depicted in bottom panel of (G, J, L). Scale bars in C and H: 72 μm, 100 μm, respectively.
Since we could not ensure that the levels of endogenous TGFβ in Yapcnull tissues were sufficient to elicit the proper epithelial patterning responses, we examined whether this could be achieved with supplementation of exogenous TGFβl in embryonic lung explant cultures. ISH analysis of WT lung explants growing in control conditions confirmed the pattern of Sox2 staining throughout the airway epithelium with no signals in distal buds, as previously reported in vivo (Fig. 5G–I). Recombinant TGFβ1 (5ng/ml) inhibited branching morphogenesis and led to a dramatic expansion of the Sox2 domain to the most distal epithelium (Fig. 5J–K). Yapcnull lungs cultured in control medium displayed the aberrant phenotype shown in Fig. 3I–J, suggestive of the inability to properly form airways during branching morphogenesis. ISH of the Yapcnull lung explants revealed strong downregulation of Sox2 expression in proximal airways (main bronchi) and no signals in the other epithelial tubules that developed subsequently in culture (Fig. 5L–M). The phenotype was consistent with that of Yapcnull E14.5 lungs in vivo (Fig. 2B, F–I). Treatment of Yapcnull lung explants with recombinant TGFβ1 (5ng/ml) led to stronger Sox2 signals in the proximal airways, but failed to induce expression beyond this domain as TGFβ1 did in WT lungs (Fig. 5N–P). qPCR analysis of Yapcnull cultured lungs showed that recombinant TGFβ1 was unable to elicit the increase in Sox2 expression seen in TGFβ1-treated WT cultures (Suppl. Fig. 3H). We concluded that in the absence of Yap, epithelial progenitors are not able to properly respond to TGFβ-induced cues to control levels and the spatial domain of Sox2 expression.
We considered the possibility that the failure to form the airway compartment in Yapcnull lungs might also have resulted from hyperactive Wnt signaling in the epithelium. Canonical Wnt is known to expand the distal lung domain, marked by Wnt targets, such as Sox9 and Bmp4 (Shu et al., 2005). If so, restricting Wnt activation in Yap-deficient lungs could presumably foster the appearance of the program of airway progenitors in response to local signals. To test whether hyperactive Wnt signaling contributes to the patterning defects of Yap-deficient lungs, we antagonized Wnt with recombinant Dkk1 in lung explant cultures from WT and Yapcnull. Analysis of WT lungs after 3 days growing in the presence of Dkk1 showed truncation of distal buds, as previously reported (De Langhe et al., 2005), and extended Sox2 expression to the periphery of the explant (Suppl. Fig. 3A–D). Thus, restricting Wnt signaling fosters distal epithelial progenitors to acquire an airway fate. In Yapcnull lungs Dkk1 was unable to elicit a similar response; lungs were slightly smaller and Sox2 expression remained restricted to the main bronchi suggesting that in these mutants, in spite of an environment that represses distal epithelial fate, airway progenitors do not form beyond the initial primary buds/main bronchi. Although modest, an increase in Sox2 signals in proximal airways of Dkk1-treated Yapcnull lungs suggested that in this compartment Wnt signaling could potentially contribute to the regulation of Sox2. ISH and qPCR analysis of the Wnt target Axin2 in E14.5 lungs also showed no evidence of increased expression in Yapcnull mutants (Suppl. Fig. 3E–H).
Yap is required to generate airway epithelial cell phenotypes
As the airway epithelium transitions from undifferentiated to a differentiated state, the levels and dynamics of Yap subcellular localization also change with time. At E14.5 epithelial progenitors commit to secretory and ciliated cell fate lineages, as recognized by expression of secretoglobin Scgb3a2 and Foxj1, respectively. IF analysis of Yap indicated that both secretory and ciliated cell precursors continued to display strong pancytoplasmic signals (Fig. 6A–B). As late markers of differentiation are detected in secretory (Scgb1a1) and multiciliated (a-Tub-labeled multicilia) cells, Yap signals remain strong in secretory cells, while they become weaker and restricted to the apical region in ciliated cells. This pattern is maintained to adulthood (Fig. 6D and data not shown).
Figure 6. Yapcnull epithelium is unable to differentiate during lung development.
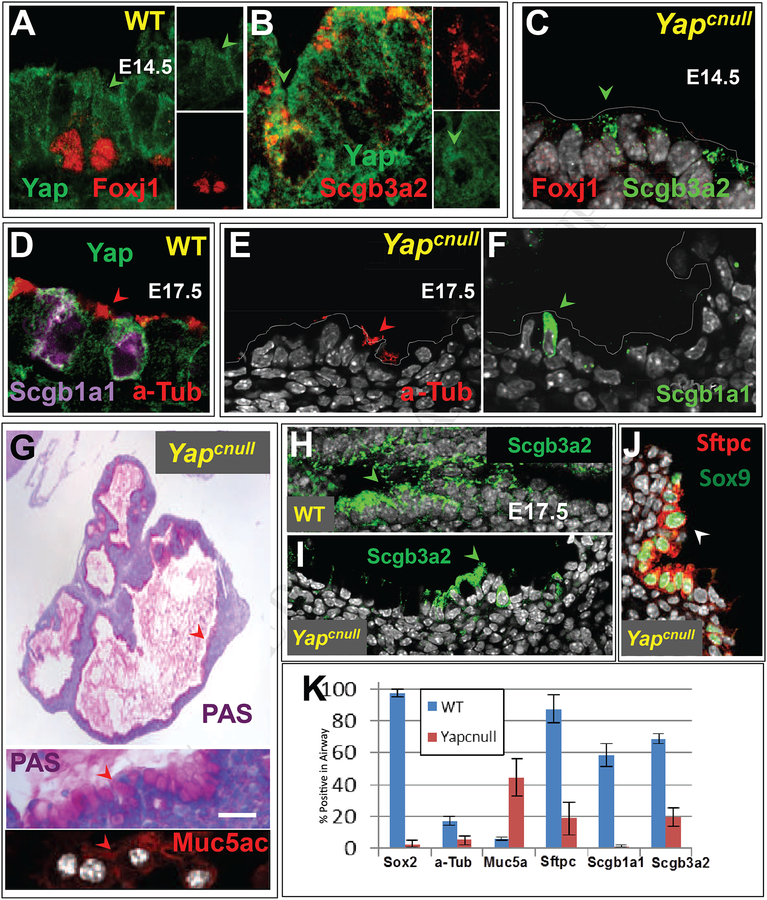
(A-B) Double IF of E14.5 WT lungs showing Yap pancytoplasmic signals (arrowheads) in ciliated (Foxj1) and secretory (Scgb3a2) cell precursors. (C) In E14.5 Yapcnull lungs no Foxj1 and only residual Scgb3a2 expression is present in the epithelium. (D) In E17.5 WT airways Yap-expressing cells undergoing differentiation express a-Tub and Scgb1a1 in ciliated and Clara cells, respectively (E-F). (H-I) By contrast E17.5 Yapcnull lungs show nearly no a-Tub, Scgb3a2 or Scgb1a1 staining and have abundant PAS and Muc5ac positive goblet-like cells in the epithelium. (J) Only rare clusters of double-labeled Sox9-Sftpc cells are present in E17.5 Yapcnull lungs. (K) Morphometric analysis showing the relative number of epithelial cells labeled with each differentiation marker in Yapcnull and WT lungs (mean ± SEM; n=3); Yap deficiency is associated with decreased expression of distal and proximal differentiation markers but increased Muc5ac expression. Scale bar, G, 40 μm.
We reasoned that if the loss of Yap prevented airway progenitors from forming, the subsequent acquisition of cellular phenotypes typical of airways should be also impaired. Indeed, analysis of Yapcnull lungs at E14.5 showed no Foxj1 and only scattered Scgb3a2-expressing cells (Fig. 6C). To determine whether this phenotype represented a developmental delay, we assessed embryos at a later stage, when airway branching is normally completed and epithelial differentiation is advanced in WT lung. E17.5 Yapcnull lungs were highly hypoplastic and showed multiple cyst-like structures. While in WT late markers of differentiation were abundantly expressed in the airway epithelium, only rare a-Tub, Scgb3a2 or Scgb1a1-labeled cells were found in Yapcnull lungs (Fig. 6D–F, H–I)
Interestingly, Periodic acid–Schiff (PAS) staining revealed widespread mucin in the epithelial cells lining the cyst-like structures and in the lumen. Strong expression of Muc5ac confirmed that the vast majority of the epithelium acquired a goblet cell-like phenotype (Fig. 6G). This phenotype is strikingly similar to that of Sox2-deficient mice (Que et al., 2007). Indeed, IF analysis of E17.5 lungs showed Sox2 expression nearly undetected in these mutants. At this stage Sox9 and Sftpc signals were restricted to scattered epithelial cells lining these cysts (Fig. 6J). Quantitative analyses confirmed these observations (Fig. 6K). Nkx2–1 staining indicated that, in spite of this aberrant differentiation profile, the Yapcnull epithelium preserved its lung identity (data not shown).
The nearly absence of differentiated cell phenotypes in Yapcnull airways strongly supports the idea that precursors of these cells could not be specified in the absence of Yap. Thus, Yap deficiency from early developmental stages prevents subsequent steps of differentiation, fostering the acquisition of a pathological goblet cell-like phenotype.
Yap is required for differentiation of adult airway epithelial progenitors
Next, we asked whether airway epithelial progenitors in the mature lung also required Yap to self-renew and to generate differentiated cell phenotypes. Basal cells are the main epithelial progenitor of the adult murine trachea and a major component of the progenitor cell pool of the mature extrapulmonary and proximal intrapulmonary airways (Rock and Hogan, 2011).
Airway progenitors isolated from adult murine tracheas can be expanded in vitro as monolayers and, upon reaching confluence, these cells can be induced to differentiate if subjected to an air-liquid interface (ALI) condition (You et al., 2002). qPCR analysis of these cultures revealed that immediately prior to induction of ALI (day 0), Yap mRNA was highly expressed in these undifferentiated cells. Yap levels decline in subsequent days as cells start to differentiate (Fig. 7A), suggesting a potential role for Yap in maintaining their progenitor state.
Figure 7. Yap regulates differentiation of adult airway epithelial progenitors.
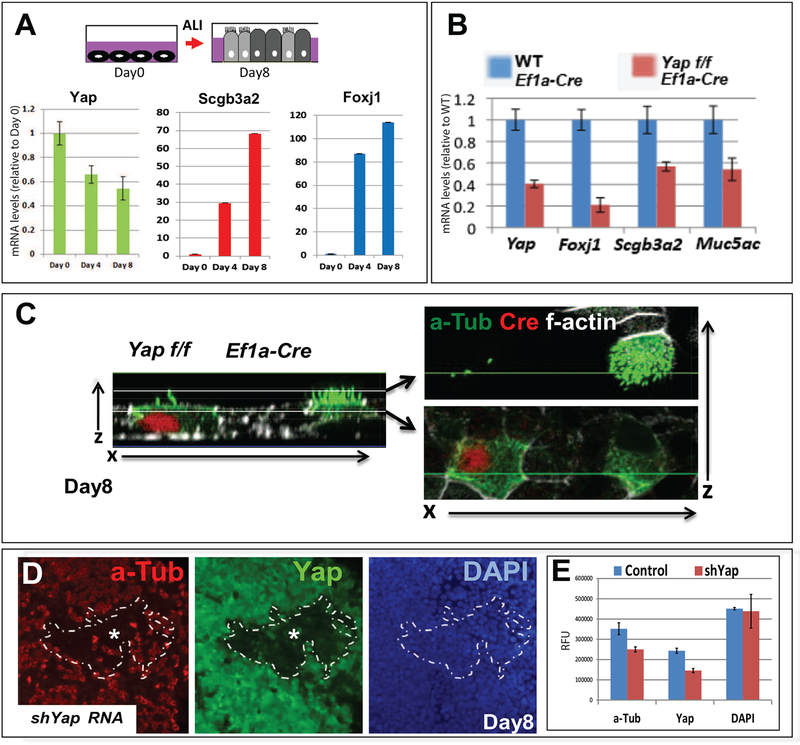
(A) Diagram depicting differentiation of epithelial progenitors isolated from adult murine tracheas after cultured to confluence (day 0) and subjected to air-liquid-interface (ALI) for 8 days. qRT-PCR shows decreasing Yap mRNA and increasing Scgb3a2 and Foxj1 as cells differentiate (mean ± SEM n=3 samples per time point). (B) Disruption of Yap through lentiviral gene transduction of Ef1a-Cre in cultured airway epithelial progenitors from adult Yap f/f tracheas leads to decreased expression of Foxj1, Scgb3a2 and Muc5ac compared to WT cultures. (C) a-Tub (green)/Cre (red) double IF and confocal analysis (x/z axis) of day 8 Yap f/f cultures infected with Ef1a-Cre show impaired formation of multicilia in cells expressing Cre recombinase (compare with abundant a-Tub-labeled cilia in Cre-negative cell). (D) Airway progenitors transduced with a short hairpin UBC-shYAP construct and cultured in ALI conditions for 8 days. Areas of efficient transduction are demarcated by the dotted line as cells (DAPI-labeled) expressing little to no Yap and a-Tub and with a significant decrease in number of a-Tub-labeled cells. (E) Morphometric analysis confirms the significant decrease in number of cells labeled with Yap and a-Tub within the dotted area (knockdown) compared to surrounding control areas. Mean ± SEM; values normalized by DAPI (similar in both groups).
To investigate the role of Yap in these cells, adult progenitors were isolated from Yap f/f tracheas and transduced with EF1a-Cre lentivirus (Tokushige et al., 1997). qPCR and IF analysis confirmed efficient disruption of Yap and showed impaired differentiation in Yap deficient cells (Fig. 7B–C, data not shown). This was evident by a significant decrease in expression of ciliated (Foxj1) and secretory cell (Scgb3a2, Muc5a) markers, and by the loss of apical acetylated alpha-Tubulin (a-Tub) structures that mark multi-cilia. Interesting, postnatal disruption of Yap inhibited mucous cell differentiation, as found by the decreased number Muc5ac-expressing cells in Yapf/f-EF1aCre compared to control cultures. This contrasted the overabundance of mucous cells that were seen when Yap was inhibited prenatally (Fig. 6G). The effect on mucous cells can be ascribed to the timing of Sox2 inhibition by Yap inactivation either during development (Suppl. Fig. 1A) or in adult progenitors (Fig. 4H), consistent with the previously reported effect of inactivating Sox2 expression during early embryonic or postnatal life (Que et al. 2009; Tompkins et al. 2009). The role for Yap in adult progenitor differentiation was also independently demonstrated in wild type cells transduced with a lentivirus carrying a short hairpin shRNA targeting Yap (shYap). IF analysis after 8 days of differentiation in ALI-conditions demonstrated that cell clusters lacking Yap had little to no a-Tub or Scgb3a2 expression (Fig. 7D and not shown). Quantitative image analysis confirmed the significant decrease in a-Tub in shYap-transduced regions.
Changes in Yap localization mark adult airway progenitors transitioning to a differentiated state, and reveals a crucial role for cytoplasmic Yap in differentiation.
We found that during branching morphogenesis Yap was expressed transiently in the nucleus of Sox2-labeled airway epithelial progenitors (transition zone). Differentiation however was only observed once Yap shifted to the cytoplasm in these cells. We investigated whether this pattern was also observed in the differentiation of adult airway progenitors. We analyzed sections of 8-week old mouse tracheas for the expression and localization of Yap in basal cells, the main adult progenitor of trachea. IF analysis of Krt5 and p63 identified these cells and showed that Yap was strongly expressed, but interestingly, under these homeostatic conditions Yap was localized predominantly in the cytoplasm (Fig. 8A).
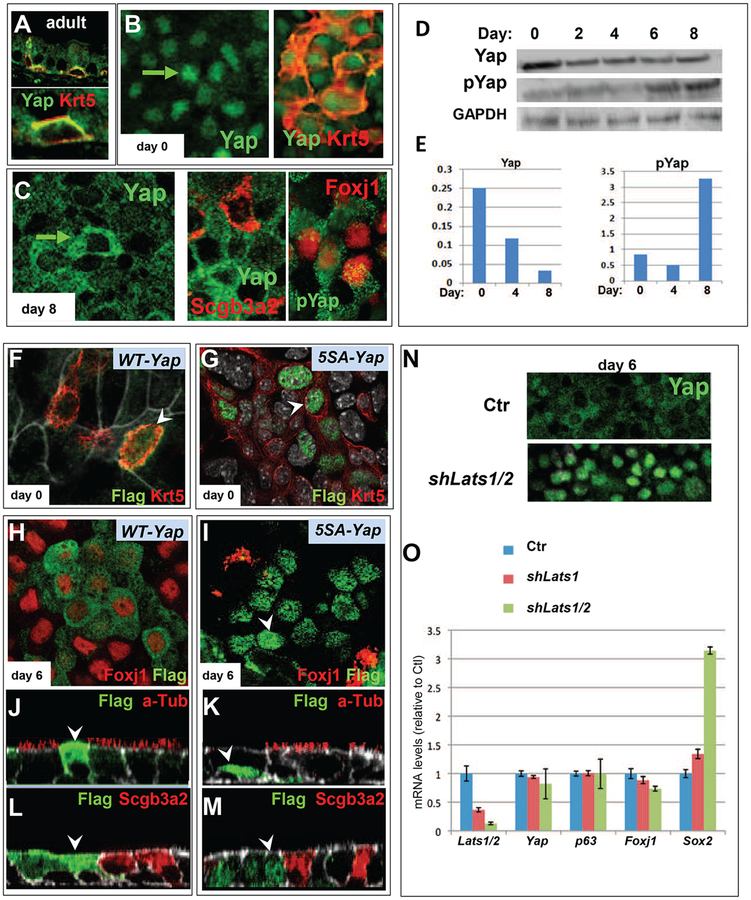
Figure 8. Nucleocytoplasmic translocation and phosphorylation of Yap in differentiation of adult airway progenitors.
(A) Double IF of Krt5 and Yap in adult WT trachea shows that under steady-state conditions airway progenitors (basal cells) express Yap in the cytoplasm. (B) Disrupting homeostasis by isolation and expansion of these cells to confluence in vitro (arrow in day0) leads to nuclear localization of Yap (right: note Krt5 and Yap in distinct cellular compartments). (C) Dramatic shift of Yap localization from the nucleus to the cytoplasm as airway progenitors differentiate into Scgb3a2 and Foxj1-expressing cells (right: cytoplasmic pYAP staining confirmed with specific antibody). (D) Western blot and densitometric analysis of day 0–8 ALI cultures using antibodies against Yap (total) or pYap and GAPDH shows a decrease in total Yap with time and reveals that the Yap nucleocytoplasmic shift during differentiation is accompanied by an increase in pYap. (F-M) Lentiviral gene transduction of adult airway progenitors transduced at the time of plating with CMV-Flag-WT-Yap (F,H,J,L) or a CMV-Flag-5SA-Yap mutant (G, I, K, M). Double IF analysis using antibodies against Flag (arrowheads) and a cell type marker in cultures prior to differentiation (ALI day 0: F-G) shows WT-Yap in both nucleus and cytoplasm of Krt5-labeled cells while 5SA-Yap is restricted to the nucleus. At day 6 WT-Yap (H, J, L) shows anti-Flag signals predominantly in the cytoplasm and express Foxj1 but not a-Tub or Scgb3a2 while day 6 5SA-Yap shows anti-Flag signals in the nucleus of cells expressing none of the differentiation markers (N) Lentiviral transduction of airway epithelial progenitors with an UBC-shLats1/2 construct showing strong Yap staining (IF) in the nucleus of ALI day 6 culture, contrasting with the predominant cytoplasmic Yap of day 6 control. (O) Real time PCR of Day 6 adult airway epithelial cultures transduced with UBC-shLats1 or UBC-shLats1/2 showing decreasing levels of Lats associated with a marked increase in Sox2 and a decrease in Foxj1 without significant changes in Yap or p63 mRNAs (Mean ± SEM of 3 experiments).
To perturb homeostasis we isolated and induced these cells to expand and differentiate in culture using the ALI conditions. IF analysis showed that during expansion Yap signals were essentially in the nucleus of the Krt5-labeled airway progenitors. Thus, altering the status of a basal cell from resting homeostasis to conditions that trigger expansion, shifts Yap into the nucleus. Remarkably, as these adult progenitors differentiate in ALI, Yap shifted from the nucleus to the cytoplasm (Fig. 8B–C). Immunoblot analysis of total Yap and pYap in cell lysates isolated from undifferentiated to a differentiated state (day 8 ALI-cultured cells) revealed that the cytoplasmic shift was accompanied by an increase in the pYap/Yap ratio (Fig. 8D–E). This occurred despite of the overall decline in Yap both at protein and mRNA levels as cells differentiate (Fig. 8D–E; Fig. 7A). Thus, altering the status of a basal cell from a resting to an activated one triggers cytoplasmic shuttling of Yap to the nucleus, presumably to activate cellular functions that are dependent on a Yap transcriptional program
These observations suggested that Yap levels, subcellular localization and phosphorylation status may have a key role in modulating the progenitor versus differentiation status of these cells. To test this idea, we examined the consequences of maintaining high levels of nuclear Yap during differentiation under ALI-conditions.
Cells were isolated and transduced with lentivirus carrying FLAG-tagged versions of a WT or 5SA-Yap, a phosphodeficient Yap in which all serines targeted by Lats1/2 kinases for phosphorylation sites have been mutated, rendering this mutant resistant to cytoplasmic retention (Bin Zhao et al., 2007). Immunofluorescence analysis with anti-FLAG and antibodies for various cell fate markers indicated key differences in cells expressing Yap-5SA or WT-Yap. In the undifferentiated state (day 0) marked by Krt5 expression, 5SA-Yap was nuclear and WT-Yap was initially present in both compartments (Fig. 8F–G). By day 6 of ALI-culture, cells transduced with WT-Yap exhibited cytoplasmic Yap, similar to endogenous Yap, and several Foxj1-labeled cells. By contrast, 5SA-Yap transduction led to prominent nuclear Yap and no overlap with Foxj 1 expression, indicating that nuclear Yap prevented differentiation of these progenitor cells (Fig. 8H–I). Although Yap-WT cells committed to a ciliated cell program, ciliogenesis was severely impaired, as shown by the nearly absence of a-Tub-labeling in WT-Yap-expressing cells. No Scgb3a2 expression was found in either 5SA or WT-transduced cells, indicating disruption of secretory cell differentiation by high Yap expression (Fig 8J–M). The data suggested that maintaining high levels of Yap even if properly targeted to the cytoplasm interferes with differentiation. Thus, proper differentiation requires regulation of both subcellular compartmentalization and precise control of the levels of Yap.
While informative, interpretation of these experiments was limited by the presence of both endogenous and exogenous Yap and the potential non-physiological effects of exogenous Yap. Thus, we took a similar approach to prevent serine phosphorylation of endogenous Yap in these cells using lentiviral-mediated inhibition of the Hippo pathway kinases Lats1 and Lats2. Airway epithelial progenitors were transduced with lentivirus that expresses control shRNA or shRNA that targets either Lats1 alone (shLats1) or both Lats1 and Lats2 (shLats1/2). IF of Yap in day 6 cultures transduced with shLats1/2 showed strong Yap staining in the nucleus, as expected (Hao et al., 2008), contrasting with the largely cytoplasmic Yap of day 6 controls (Fig. 8N). qPCR analysis confirmed the decrease in Lats1 and Lats2 expression as well as the decrease in Foxj1 mRNA (Fig. 8O). Levels of Yap or p63 transcripts were unaltered suggesting that the phenotype in unlikely to involve major changes in Yap stability or changes in the progenitor cell pool. Remarkably, we observed a major increase in Sox2 expression following Lats1/2 knockdown (Fig. 8O). Such observations are consistent with nuclear Yap activating a transcriptional program that includes Sox2, and that maintains the progenitor state of these cells as previously reported in ES cells (Lian et al., 2010). However, sustained nuclear Yap maintains high levels of these targets thereby preventing differentiation. Indeed, studies in Sox2-GFP mice show enriched expression in phenotypically immature basal cells in the adult epithelium of various origins (Arnold et al., 2011).
Taken together, our data provide evidence that dynamic nuclear-cytoplasmic changes in Yap localization and upstream regulators, such as Lats1/2 are key in modulating the behavior of airway progenitors cells during differentiation. Given the critical roles in P-D patterning and in adult stem cell regulation, our data implicate Yap as a regulator of lung branching and patterning that integrates growth factor-induced cues, placing the Hippo-Yap pathway at the nexus of lung morphogenesis.
DISCUSSION
Here we provide evidence of a regulatory mechanism involving interactions of the Hippo pathway effector Yap with Sox2 and TGFβ in controlling epithelial progenitor cell fate and morphogenesis during airway development. We found that a nucleocytoplasmic shift of Yap marks the boundary between the distal (Sox9-expressing) and the airway (Sox2-expressing) compartments. At this transition zone, Yap specifies a unique transcriptional program that controls the expression of Sox2, enabling the initiation of the progenitor cell program that forms the airways during branching morphogenesis. This program is key to generate the airway epithelial compartment and its branched tubular structures. We found that Yap is required to render epithelial progenitors responsive to TGFβ-induced signaling in the developing airways. Furthermore, we show that, later, Yap remains a prominent regulator of airway epithelial differentiation, and that proper subcellular localization of Yap is crucial for generation of secretory and ciliated cells.
Loss of Sox2-expressing airway progenitors and disruption of branching morphogenesis have recently been reported in mice deficient in histone deacetylases 1 and 2 (Hdac1/2) in the developing lung epithelium (Wang et al., 2013). The phenotype resembled that of the Yapcnull lungs but, unlike Yap-deficient lungs, Hdac1/2 mutants exhibited enhanced Bmp4 expression and increased apoptosis. We found that the distal epithelial progenitors of Yap-deficient lungs are still able to respond to signaling cues from the mesenchyme and induce distal buds that typically express Sox9, Sfptc, Bmp4. Thus, our observations suggest that despite similar defects in developmental progression, Yap directs alternative signals to those of Hdac1/2.
Our analysis of Yapcnull lungs did not provide strong evidence of aberrant Wnt epithelial activation and showed that Dkk1 was unable to prevent the abnormal expansion of distal cells. Thus, hyperactivation of Wnt signaling is unlikely to be the primary defect causing this phenotype. Our data rather suggested that endogenous Yap works in concert with TGFβ during induction of the airway progenitor cell fate program. This program involves induction of Sox2. Yap deletion resulted in reduced Sox2 levels, whereas enhanced nuclear Yap levels promoted Sox2 expression. It is intriguing that nuclear Yap only overlaps with Sox2 in a very limited region, the transition zone. This transition zone encompasses an area well known to express abundant TGFβ1 ligand in the surrounding mesenchyme, therefore available to activate signaling locally in both layers when airways are forming (Heine et al 1990). Indirect evidence of dynamic TGFβ activity in this region was suggested by the strong local expression of the TGFβ target Tgfbi in the mesenchyme adjacent to the epithelium at the transition zone. We found that Yap-deficient progenitors were refractory to exogenous TGFβ and consequently were unable to respond to the TGFβ patterning cues.
Given that TGFβ is a strong activator of Sox2 in the airway epithelium, our data support a model in which nuclear Yap-Tead complexes cooperate with TGFβ-induced signals to activate a transcriptional program that includes Sox2 to induce airway epithelial cell fate. Consistent with this model, Tead transcription factors reside at the Sox2 promoter, and recent data show that Yap, Tead, and Smads assemble into functional transcriptional complexes (Beyer et al., 2013). Supporting evidence for our model includes: a) nuclear Yap localization coinciding with the emergence of Sox2 expression at the transitional zone, b) decreased Sox2 expression with disruption of Yap in cultured airway progenitors that express Yap in the nucleus, c) ChIP analysis of Tead revealing its enrichment at the Sox2 promoter in airway progenitor cells and in E12.5 lungs, and, d) induced nuclear Yap levels as a result of Lats1/2 kinase depletion, promoting Sox2 expression. Nevertheless, we cannot discard the possibility that cytoplasmic Yap, acting through currently unknown downstream transcription factors, contributes to promote Sox2 expression in proximal airways
Our results highlight the importance of Yap subcellular localization in patterning the lung epithelium, consistent with recent studies showing the importance of Yap localization in organs, such as the skin and intestine (Pan, 2010). Notably, the cytoplasmic Yap in the lung does not rapidly translate into advanced progenitor differentiation. Pancytoplasmic Yap was present throughout the P-D axis of the developing airways as early as E11.5-E12, well before the features of epithelial differentiation appear. Thus, in the embryonic lung cytoplasmic Yap is associated with the emergence of the progenitors of the developing airways. Our data suggest that these progenitors are specified in a “transition zone” as they acquire Sox2 expression and are primed by other factors to initiate a program of differentiation. In the adult lung, where repopulation of the airway epithelium arises from an already specified Sox2-expressing progenitor, nucleocytoplasmic shift is more directly linked to the acquisition of a differentiated phenotype
There is currently no report describing a similar nuclear-cytoplasmic shift in Taz localization in the developing lung. The roles of Taz appear to be restricted to the distal (alveolar) lung compartment (Mitani et al., 2009). TAZ is known to be overexpressed and acts as an oncogene in non-small cell lung carcinoma. By contrast, Yap has been more consistently detected in the airway-derived HBE (human bronchial epithelial) cells (Zhou et al., 2011b). A better understanding of the mechanisms regulated by Taz and Yap is likely to provide important insights into the pathogenesis of human conditions affecting the airway and the distal lung.
MATERIALS AND METHODS
Mouse Models.
Developmental and adult expression studies were performed on CD1 mice (Charles River). Yapf/f mice were provided by Dr. Fernando Camargo (Harvard University). Yapcnull mice were generated by crossing Yapf/f mice with Yapf+;Shh-Cre/+ mice. Genotyping of the Yap mutant mice was conducted as previously described (Schlegelmilch et al., 2011).
RT-PCR.
Quantitative real time (qRT)-PCR was performed as described (Taso et al., 2009). RNA was extracted with Trizol (Life Technologies, 15596–26) or (RNAeasy kit; Qiagen) and reverse transcribed using random hexamers or Oligo(DT) primers (SuperScript III kit; Invitrogen). The following primers (Applied Bio systems) were used: Sox2 (mm00488369_s1), Lats1 (mm01191886_m1), Tgfbi (mm00493634_m1), Yap (mm01143263_m1), Trp63 (mm0495788_m1), Scgb1a1 (mm00442046_m1), Foxj1 (mm00807215_m1), Krt5 (mm01305291_g1), Scgb3a2 (mm00504412_m1), Smad7 (mm00484742_m1), Axin2 (mm00443610_m 1), Bmp4 (mm00432087_m1), Nkx2.1 (mm00447558_m1), Sox9 (mm00448840_m1), and b-Actin (control, 4352341E). PCR reactions were performed using Taq-Man Fast Advanced Master Mix (Applied Biosystems, 4444556) and samples were analyzed on a Step-One Plus instrument (Applied Biosystems).
In Situ Hybridization (ISH), Immunofluorescence (IF) and Imaging.
(ISH) Embryonic and adult lungs were fixed overnight in 4% paraformaldehyde in PBS at 4 °C and processed for frozen sections. T7-linked and T3-linked gene-specific primers were used for PCR and subsequent riboprobe synthesis: Yap: Forward-5’ AATTAACCCTCACTAAAGGGGTCTTTGAGTGCTTCTAGA 3’ Reverse-5’TAATACGACTCACTATAGGGTGGATCATCCATCTGCTCAA 3’; The Axin2 probe template is the “Tong DNA #94” (Axin2 nucleotides 1–2397). The antisense and sense Axin2 riboprobes were made by linearizing this plasmid with NotI or SalI and transcribing with T3 or T7 RNA polymerase, respectively. Bmp4 and Tgfbi biotin-UTP-labeled (Roche) riboprobes were synthesized (MAXIscript kit; Ambion) and hybridization was performed in 5–7 um sections as described previously described (Lu et al., 2004) with the following modification in the concentrations of labeling reagents: DIG-UTP-labeled probes (0.2 ng/mL), alkaline phosphatase-conjugated anti-DIG antibody (Roche; 1:2,500), peroxidase-conjugated anti-DIG Fab fragment (Roche; 1:100–1:500). BM Purple (Roche) was used as a chromogenic substrate.
(IF) was performed using the following antibodies acetylated alpha-tubulin (Sigma; 1:2,000), goat anti-Scgb1a1 (Santa Cruz; 1:1,500), and mouse anti-Foxj1 (eBioscience; 1:300), rabbit anti-Sox2 (AbCam; 1:100) goat anti Sox9 (R&D; 1:50), mouse anti-Yap (Santa Cruz; 1:100), rabbit ant-phospho-Yap (Cell Signaling Technologies; 1:50), rabbit anti-Cre (Cell Signaling; 1:100), mouse anti-Cre (Millipore; 1:100), rabbit anti-Scgb3a2 (gift of Kimura Lab, 1:2000). Retrieval before immunolabeling was performed with Low pH Antigen Unmasking Solution (Vector Laboratories,) in a microwave oven. We used secondary antibodies conjugated to Alexa Fluor-488, −568, or −647 (Invitrogen; 1:300). Images were acquired on a Nikon Labophoto 2 microscope equipped with a Nikon Digital Sight DS-Ri1 charge-coupled device camera or on a Zeiss LSM710 metaconfocal laser-scanning microscope.
Whole Lung Organ Cultures.
Embryonic lungs were dissected from WT and Yapcnull mice and cultured for 3 days in BGjb 1% inactivated fetal calf serum medium (control) or media containing human recombinant TGFβ1 (5–20ng/ml, R&D Systems) or DKK1 (5–20μg/ml, R&D Systems) as described previously (Chen et al. 2009; De Langhe et al 2005).
Whole-mount in situ hybridization (WMISH)
WMISH was performed in a 96-well plate dishes as previously described (Lu et al 2004). Briefly, digoxigenin (DIG)-labeled riboprobes (Maxiscript kit, Ambion) were generated from plasmids carrying Sox2 cDNA. Specimens were digested with proteinase K (Qiagen), prehybridized (1 hour, 70°C) in buffer containing 50% formamide, 5SSC, 1% donkey serum, 50 mg/ml yeast RNA and heparin followed by overnight hybridization with DIG-labeled RNA probes, and another overnight incubation with anti-DIG alkaline phosphatase conjugate (Roche) at 4°C. Signal was visualized with BM Purple substrate (Roche Diagnostics).
Isolation and culture of mouse adult airway progenitor cells in air-liquid interface (ALI):
Primary airway progenitor cells were isolated from tracheas of adult (6 −10 week) WT and Yap f/f mice, expanded and subsequently differentiated in air-liquid interface cultures in Transwell plates (Costar) as previously detailed (You, et al 2002).
Lentivirus production and concentration:
Recombinant lentiviruses were made by Lipofectamine transfection of 50% confluent 293T cells with lentiviral transfer vector, packaging plasmid pAX2 DNA, and pMD2G-VSVG envelope plasmid DNA. Transfer vectors used were: pLKO.1 shCTL, pLKO.1 shLATS1/2, pLVX-Puro CTL, pLVX-Puro 3F-Yap5SA, and pHAGE EF1a-Cre-w. The recombinant viruses were harvested by collecting the media at 48h post-transfection and concentrated by ultracentrifugation.
Chromatin Immunoprécipitation (ChIP)
ChIP was performed as previously described (Lian et al 2010). Briefly, primary airway epithelial cells or homogenized mouse lungs were fixed (1% formaldehyde, 10 mins,) and resuspended in 1% SDS, 10mM EDTA, 50mM Tris-HCL, with a protease inhibitor cocktail (Thermo Scientific). Sonication (Branson Dismembrator) was performed to achieve chromatin fragment around 500bp. IP was carried out using equal amounts of chromatin using Tead1–4 antibody, or IgG, overnight at 4°C with protein A/G magnetic beads (Milipore) for enrichment. PCR was performed using Yap1 (Lian et al 2010) and control (Fabp4 Forward - TGGGTGGTGACTTCCTGCTG, Reverse - AGAGCCATGCGGATTCTTGG) Primers.
Supplementary Material
Highlights.
Yap regulates proximal-distal patterning of the developing lung.
Yap specifies a transcriptional program crucial to generate airways
Yap is required to render airway progenitors responsive to TGFβ and to induce Sox2
Yap phosphorylation is key for airway differentiation during embryonic and adult life
ACKNOWLEDGEMENTS
We are indebt to Dr. Fernando Camargo (Harvard University) for providing us with the Yapf/f mice and to Shioko Kimura (NIH) for the Scgb3a2 antibody. We thank Jun Qian and Anne Hinds for their technical support and Dr. Hector Marquez for help with the ChIP-PCR protocol and for scientific discussion. This work was funded by grants from the March of Dimes Foundation (5-FY11–578) and Concern Cancer Foundation to X.V, and NIH-NHLBI P01 HL047049 and R01 HL105971–01 to W.V.C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, and Hochedlinger K (2011). Sox2+ Adult Stem and Progenitor Cells Are Important for Tissue Regeneration and Survival of Mice. Cell Stem Cell 9, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer TA, Weiss A, Khomchuk Y, Huang K, Ogunjimi AA, Varelas X, and Wrana JL (2013). Switch Enhancers Interpret TGF-β and Hippo Signaling to Control Cell Fate in Human Embryonic Stem Cells. Cell Rep. 5, 1–14 [DOI] [PubMed] [Google Scholar]
- Zhao Bin, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, and Brummelkamp TR (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Current Biology 17, 2054–2060. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, and Gage FH (2008). YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes & Development 22, 3320–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso WV, and Lu J (2006). Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611–1624. [DOI] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lu J, and Cardoso WV (2007). Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134, 2969–2979. [DOI] [PubMed] [Google Scholar]
- Chung C, Kim T, Kim M, Song H, Kim TS, Seo E, Lee SH, Kim H, Kim SK, Yoo G, et al. (2013). Hippo-Foxa2 signaling pathway plays a role in peripheral lung maturation and surfactant homeostasis. Proceedings of the National Academy of Sciences 110, 7732–7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe SP, Sala FG, Del Moral P-M, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, and Bellusci S (2005). Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Developmental Biology 277, 316–331. [DOI] [PubMed] [Google Scholar]
- Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, and Rottier R (2008). Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Developmental Biology 317, 296–309. [DOI] [PubMed] [Google Scholar]
- Habbig S, Bartram MP, Müller RU, Schwarz R, Andriopoulos N, Chen S, Sägmüller JG, Hoehne M, Burst V, Liebau MC, et al. (2011). NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. J Cell Biol 193, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, and Yang X (2008). Tumor suppressor LATS1 is a negative regulator of oncogene YAP. Journal of Biological Chemistry 283, 5496–5509. [DOI] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, and Sun X (2006). Dicer function is essential for lung epithelium morphogenesis. Proc. Natl. Acad. Sci. U.S.a. 103, 2208–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine UI, Munoz EF, Flanders KC, Roberts AB, and Sporn MB (1990). Colocalization of TGF-beta 1 and collagen I and III, fibronectin and glycosaminoglycans during lung branching morphogenesis. Development 109, 29–36. [DOI] [PubMed] [Google Scholar]
- Kimura S, Ward JM, and Minoo P (1999). Thyroid-specific enhancer-binding protein/thyroid transcription factor 1 is not required for the initial specification of the thyroid and lung primordia. Biochimie 81, 321–327. [DOI] [PubMed] [Google Scholar]
- Lebeche D, Malpel S, and Cardoso WV (1999). Fibroblast growth factor interactions in the developing lung. Mechanisms of Development 86, 125–136. [DOI] [PubMed] [Google Scholar]
- Li JH, Zhu HJ, Huang XR, and Lai KN (2002). Smad7 inhibits fibrotic effect of TGF-beta on renal tubular epithelial cells by blocking Smad2 activation. J. Am Soc Nephrol 13, 1464–1472 [DOI] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel M, Goldstein L, Abujarour R, et al. (2010). The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes & Development 24, 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Qian J, Izvolsky KI, and Cardoso WV (2004). Global analysis of genes differentially expressed in branching and non-branching regions of the mouse embryonic lung. Developmental Biology 273, 418–435. [DOI] [PubMed] [Google Scholar]
- Mauviel A, Nallet-Staub F, and Varelas X (2012). Integrating developmental signals: a Hippo in the (path) way. Oncogene. 31, 1743–1756 [DOI] [PubMed] [Google Scholar]
- Mitani A, Nagase T, Fukuchi K, Aburatani H, Makita R, and Kurihara H (2009). Transcriptional Coactivator with PDZ-binding Motif Is Essential for Normal Alveolarization in Mice. American Journal of Respiratory and Critical Care Medicine 180, 326–338. [DOI] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O’Neal W, and Milgram SL (2006). Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell. Biol 26, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, and Hogan B (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Developmental Cell. 18, 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D (2010). The Hippo Signaling Pathway in Development and Cancer. Developmental Cell 19, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A-KT, Kist R, Shan Z, Scherer G, and Whitsett JA (2005). Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis 41, 23–32. [DOI] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, and Hogan BL (2009). Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136, 1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, and Hogan BLM (2011). Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu. Rev. Cell Dev. Biol 27, 493–512. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. (2011). Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, et al. (2005). Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Developmental Biology 283, 226–239. [DOI] [PubMed] [Google Scholar]
- Tokushige K, Moradpour D, Wakita T, and Geissler M (1997). Comparison between cytomegalovirus promoter and elongation factor-1 a promoter-driven constructs in the establishment of cell lines expressing hepatitis C virus. Journal of Virological. 64, 73–80. [DOI] [PubMed] [Google Scholar]
- Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, and Whitsett JA (2009). Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE 4, e8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay AM, and Camargo FD (2012). Hippo signaling in mammalian stem cells. Seminars in Cell & Developmental Biology 23, 818–826. [DOI] [PubMed] [Google Scholar]
- Tsao P-N, Vasconcelos M, Izvolsky KI, Qian J, Lu J, and Cardoso WV (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, and Gregorieff A (2010). The Hippo pathway regulates Wnt/β-catenin signaling. Developmental Cell. 18, 579–591 [DOI] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, and Wrana JL (2008). TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10, 837–848. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tian Y, Morley MP, Lu MM, DeMayo FJ, Olson EN, and Morrisey EE (2013). Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Developmental Cell 24, 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M, Yingling JM, Dunn NR, and Bellusci S (1999). Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 126, 4005–4015 [DOI] [PubMed] [Google Scholar]
- You Y, Richer E, and Huang T (2002). Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 283, L1315–L1321 [DOI] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, and Pan D (2010). The Merlin/NF2 Tumor Suppressor Functions through the YAP Oncoprotein to Regulate Tissue Homeostasis in Mammals. Developmental Cell 19, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Shi W, Chen H, and Warburton D (2000). Smad7 and Smad6 differentially modulate transforming growth factor beta-induced inhibition of embryonic lung morphogenesis. Journal of Biological Chemistry 275, 23992–23997. [DOI] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, et al. (2011a). Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proceedings of the National Academy of Sciences 108, E1312–E1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hao Y, Liu N, Raptis L, Tsao MS, and Yang X (2011b). TAZ is a novel oncogene in non-small cell lung cancer. Oncogene 30, 2181–2186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.