Important Compound Classes
Title
Bridged piperidine derivatives
Patent Application Number
WO 2018060300A1
Publication Date
April 5th, 2018
Priority Application
EP16191512.9
Priority Date
September 29th, 2016
Inventors
Bartels, B.; Jakob-Roetne, R.; Linberg, A.; Neidhart, W.; Ratni, H.; Reutlinger, M.; Sarie, J. C.; Vastakaite, G.
Assignee Company
Pharmascience, Inc.
Disease Area
Alzheimer’s disease
Biological Target
γ-Secretase
Summary
Despite decades of research, effective therapies for Alzheimer’s disease remain elusive. The current U.S. patient population exceeds 5 million and is expected to rise to 16 million by 2050 (https://www.alz.org/facts/). This progressive neurodegenerative disorder has been linked to β-amyloid plaques and neurofibrillary tangles in the cortical and subcortical regions of the brain. Further, the formation of these features is associated with the degeneration and loss of neurons. They contain β-amyloid and tau proteins, respectively. It has been hypothesized that prevention or elimination of this material from the brain will arrest and possibly reverse the progression of Alzheimer’s disease. β-Amyloid plaques are formed from the Aβ42 protein, a cleavage product of the amyloid precursor protein (APP). Initial β-secretase mediated cleavage of APP produces a soluble fragment (β-APP) and a membrane bound portion (C-99). γ-Secretase then cleaves C-99, which produces Aβ42, a protein that forms insoluble aggregates that are the main component of β-amyloid plaques. In theory, inhibition of either β-secretase or γ-secretase would prevent the formation of Aβ42, prevent the formation of the associated plaques, and arrest Alzheimer’s disease progression. The present application discloses a series of compounds that selectively inhibit γ-secretase and are potentially useful for the treatment of Alzheimer’s disease.
Definitions
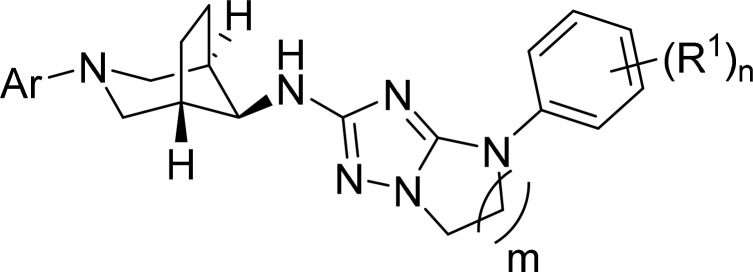
R1 is hydrogen, lower alkyl, lower alkyl substituted by halogen, halogen, lower alkoxy or lower alkoxy substituted by halogen; Rl may be the same or different, if n = 2 or 3;
n is 1, 2, or 3;
m is 1, 2, or 3;
Ar is a five- or six-membered heteroaryl group,
selected from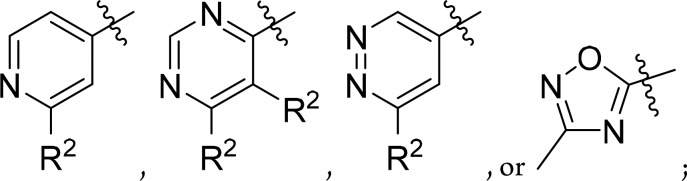
wherein
R2 is hydrogen, lower alkyl, lower alkyl substituted by halogen, halogen or lower alkoxy;
R3 is hydrogen or halogen;
–()m– is −(CH2)m–.
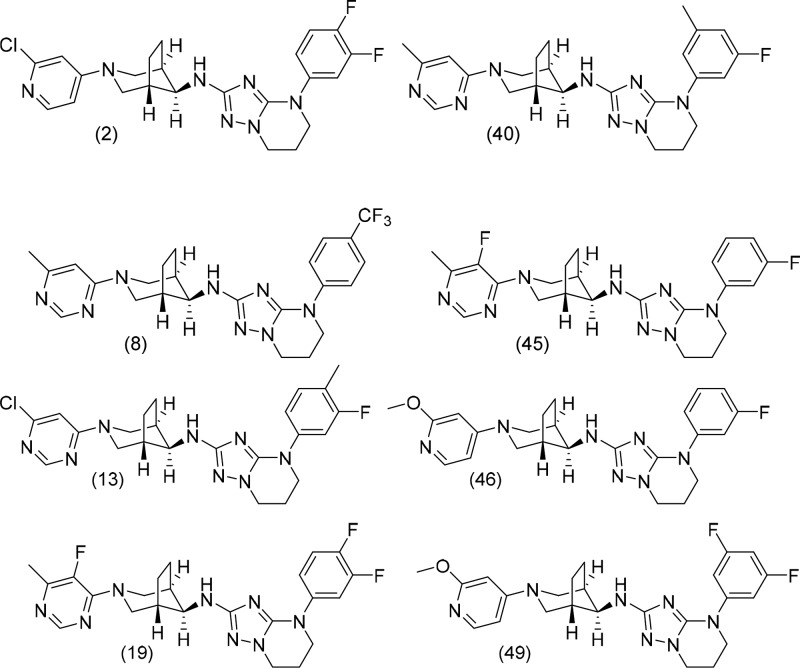
Key Structures
Biological Assay
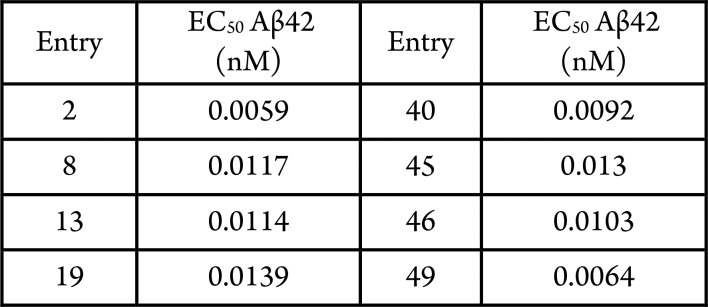
Cellular γ-secretase assay with quantification of secreted Aβ42 by the means of an AlphaLisa assay kit (Human Amyloid beta 1–42 Kit: Cat# AL203C, PerkinElmer).
Biological Data
Claims
Nineteen total claims.
Thirteen composition of matter claims.
One process claim.
Five method of use claims.
Recent Review Articles
-
1.
Johnson D. S.; Pettersson M.. γ-Secretase modulators as Aβ42-lowering pharmacological agents to treat Alzheimer’s disease. Topics in Medicinal Chemistry 2017, 24, 87–118.
-
2.
Kumar D.; Ganeshpurkar A.; Kumar D.; Modi G.; Gupta S. K.; Singh S. K.. Secretase inhibitors for the treatment of Alzheimer’s disease: Long road ahead. European Journal of Medicinal Chemistry 2018, 148, 436–452.
-
3.
Tan Y.; Zhang Q.; Wong S. G.; Hua Q.. Anti-Alzheimer therapeutic drugs targeting γ-secretase. Current Topics in Medicinal Chemistry 2016, 16 ( (5), ), 549–557.
The author declares no competing financial interest.