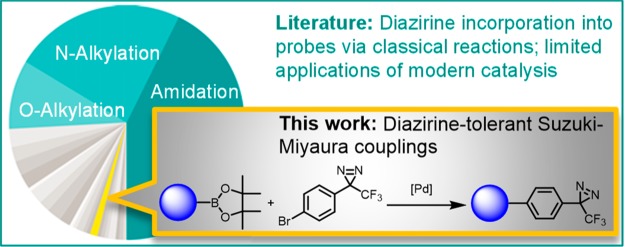
Abstract
Access to high quality photoaffinity probe molecules is often constrained by synthetic limitations related to diazirine installation. A survey of recently published photoaffinity probe syntheses identified the Suzuki–Miyaura (S–M) coupling reaction, ubiquitous in drug discovery, as being underutilized to incorporate diazirines. To test whether advances in modern cross-coupling catalysis might enable efficient S–M couplings tolerant of the diazirine moiety, a fragment-based screening approach was employed. A model S–M coupling reaction was screened under various conditions in the presence of an aromatic diazirine fragment. This screen identified reaction conditions that gave good yields of S–M coupling product while minimally perturbing the diazirine reporter fragment. These conditions were found to be highly scalable and exhibited broad scope when applied to a chemistry informer library of 24 pharmaceutically relevant aryl boron pinacol esters. Furthermore, these conditions were used to synthesize a known diazirine-containing probe molecule with improved synthetic efficiency.
Keywords: Diazirine, photoaffinity, Suzuki−Miyaura, photochemistry, high-throughput experimentation
Diazirine-based photoaffinity labeling (PAL) reagents are an important class of chemical probes.1 These compounds are widely used owing to their rapid decomposition upon exposure to 365 nm light to generate N2 and short-lived, reactive carbenes that undergo C–H, N–H, and O–H insertion to form stable covalent adducts with proximal binding partners.2 This reactivity has proven useful in myriad applications in biological systems including target deconvolution of phenotypic screening hits and assessment of in-cell target engagement.3 Despite their prominence, diazirines have been synthesized only by a limited set of methods,4 imposing significant constraints on the design of diazirine-containing PAL probes.
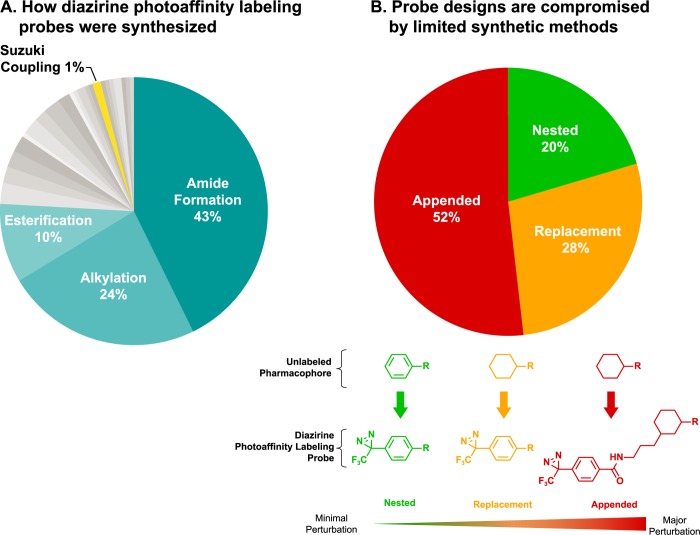
The impact of limited synthetic methods on chemical probe design is illustrated in Figure 1, based on an extensive literature survey.5Figure 1A shows that approximately three-quarters of diazirine probes have been prepared by amide bond formation, alkylation, and esterification using diazirine-containing synthons. These routes utilize reliable reactions, but because they do not take advantage of modern synthetic methods, they constrain diazirine incorporation to few positions within probes. Figure 1B shows the distribution of diazirine probes among the categories defined by James Hill and Avril Robertson in their recent review of diazirine synthetic chemistry:4 Nested, Replacement, or Appended. These terms refer to diazirine placement within the structure of the pharmacophore on which the diazirine probe is based, with Nested diazirines conferring minimal structural perturbation, Appended conferring major structural perturbation, and Replacement conferring a moderate degree of perturbation. Figure 1B shows that most diazirines fall under the Appended or Replacement categories, a probe design result that is unfavorable since structural perturbations often lead to significant changes in intrinsic potency, physicochemical properties, and membrane permeability.4 Because diazirine PAL studies are extremely useful for investigating novel biology in intact cells, diazirine probes should exhibit minimal structural and physicochemical divergence from unlabeled parent compounds. Figure 1B also indicates that most diazirine probes are Appended, an arrangement in which the reactive carbene is placed at some considerable distance from the parent pharmacophore; this contributes to low labeling efficiency and increases the risk of false negatives since the carbene is projected away from the biological binding partner.6
Figure 1.
Published diazirine PAL probes (n = 212) were analyzed according to (A) reaction used for diazirine incorporation and (B) category of diazirine placement in probe versus unlabeled parent pharmacophore.4 As described by Hill and Robertson,4 the categories Nested, Replacement, and Appended represent minimal, moderate, and major perturbations of structure and physicochemical properties, respectively. Despite their advantages, Nested diazirines are the least common found in the literature. Chemical structures in part B are adapted from ref (4).
Convinced that available synthetic methods were compromising diazirine PAL probe design across a broad range of studies, we investigated expanding the range of methods for their incorporation. A particularly notable finding in Figure 1A is the near absence of metal-catalyzed cross coupling reactions, which are so useful they have become ubiquitous across medicinal chemistry,7 natural products total synthesis,8 and chemical manufacturing.9 Potential reasons for this absence have been noted: “there are several obstacles to consider, namely, the diazirine thermal instability and the incompatibility with several metallic reagents.”4 Despite these challenges, we undertook a study of Pd-catalyzed cross coupling of diazirine-containing aryl halides and boronic acids to form biaryls, i.e., the Suzuki–Miyaura (S–M) reaction, because of its well-established utility.
Several characteristics drew our attention to biaryl synthesis. Biaryls are found in many biologically important compounds,10 are consistently reported as hits in phenotypic screens,11 and have been designed into DNA-encoded libraries.12 Structural studies show that most biaryls in small molecule ligand–protein complexes are within 5 Å of the protein.13 Although biaryls have well-described liabilities,14 this structural class remains important and valuable for drug discovery15 and is likely to remain abundant in compound screening collections for the foreseeable future.
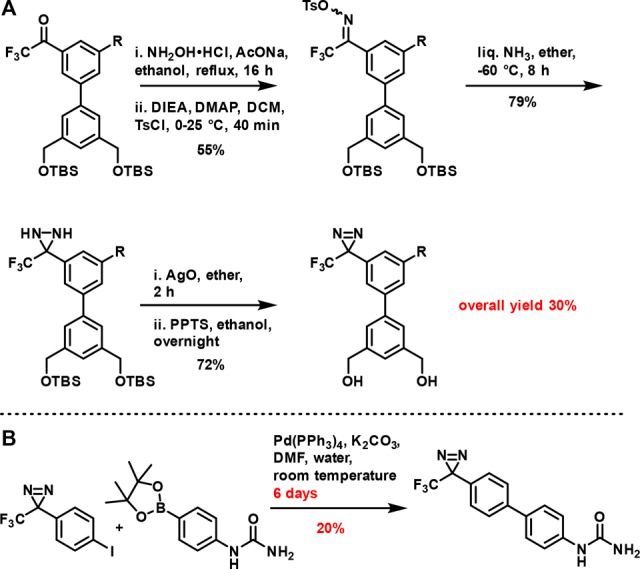
Because biaryls are prominent in biologically active compounds, the scarcity of published syntheses of biaryls bearing nested diazirines16−18 suggests synthetic access is a problem. The few previously reported syntheses (see Scheme 1 for examples) either use a 3–4-step reaction sequence to build the diazirine from a biaryl carbonyl compound17 or employ cross coupling reactions with low yields and very long reaction times (e.g., several days).18 Both provide low to moderate yields and require significant time. As noted,4 thermal stability of the diazirine is one factor limiting application of metal-catalyzed reactions; another is that diazirine itself is a competent cross-coupling partner.19
Scheme 1. Example Syntheses of Diazirine PAL Probes Bearing Nested Diazirines via (A) Multistep Synthesis17 or (B) Suzuki Coupling18.
The ideal S–M method for diazirine chemical probe synthesis could be described by the following criteria: the method should be suitable for the synthesis of biaryls containing nested diazirines, should allow incorporation of the diazirine late in the synthetic sequence, tolerate a wide range of functionality typically present in drug-like compounds, and be operationally simple. The approach to identify such S–M conditions consisted of (1) an initial fragment-based robustness screen to identify S–M conditions that give high cross-coupling efficiency while not degrading aryl diazirines followed by (2) a survey of pharmaceutically relevant substrates to define the scope and limitations of the method.
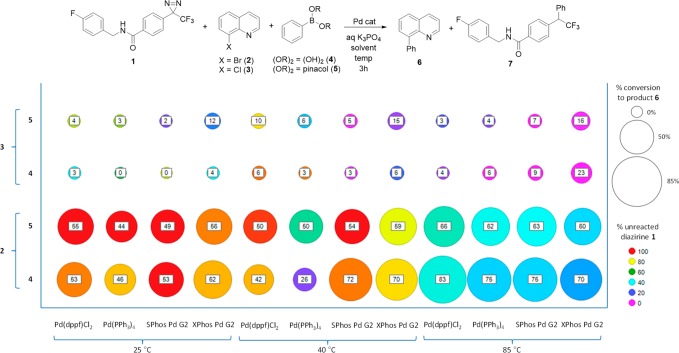
The fragment-based robustness test developed by Glorius and co-workers can be a useful tool for determining a functional group’s potential for interfering in a given transformation.20 Inspired by this approach, we sought to assess the compatibility of the diazirine moiety with typical palladium-catalyzed S–M cross coupling conditions. Thus, we synthesized compound 1 for use as a spectator in the cross coupling of a quinoline halide (2, 3) and a phenyl boronic acid derivative (4, 5) (Figure 2). The ability of 1 to exhibit both a UV signal and the appropriate mass ion on the mass spectrometer facilitated its detection throughout our screening. The cross coupling reaction was screened across solvents, catalysts, and temperatures.21 Screening wells lacking catalyst and base provided a control for the thermal stability assessment of 1 (see SI for details). As stated earlier, the propensity for the diazirine to participate directly in the coupling was an anticipated competitive reaction. Indeed, in some instances the product of direct coupling between 1 and the boronate species was observed to produce 7. The formation of 7 occurred in large part at high temperatures (Figure 2), in agreement with published results.19 The totality of these screening results indicated a preferred temperature of 40 °C for this reaction, which both preserved the diazirine and limited its participation in the competitive side reaction. Additionally, that temperature was adequate for the SPhos G2 Pd catalyst to yield an efficient cross-coupling of 2 and 5. Lastly, the screening results indicated that the bromoquinoline 2 was a more competent coupling partner, leading to a higher conversion efficiency than the chloroquinoline 3, which is in-line with established trends.22
Figure 2.
Screening results for the Suzuki–Miyaura cross-coupling of 8-haloquinolines 2 and 3 with phenylboronic acid derivatives 4 and 5 at three different temperatures (25, 40, and 85 °C) with four different Pd catalysts in the presence of diazirine-containing spectator 1. Size of circle proportionate to the conversion to desired product 6 as determined by HPLC UV area % (value given inside circle). Color of each circle indicates the mol % diazirine 1 remaining after 3 h. Warmer colors and larger circles represent more favorable reaction outcomes.
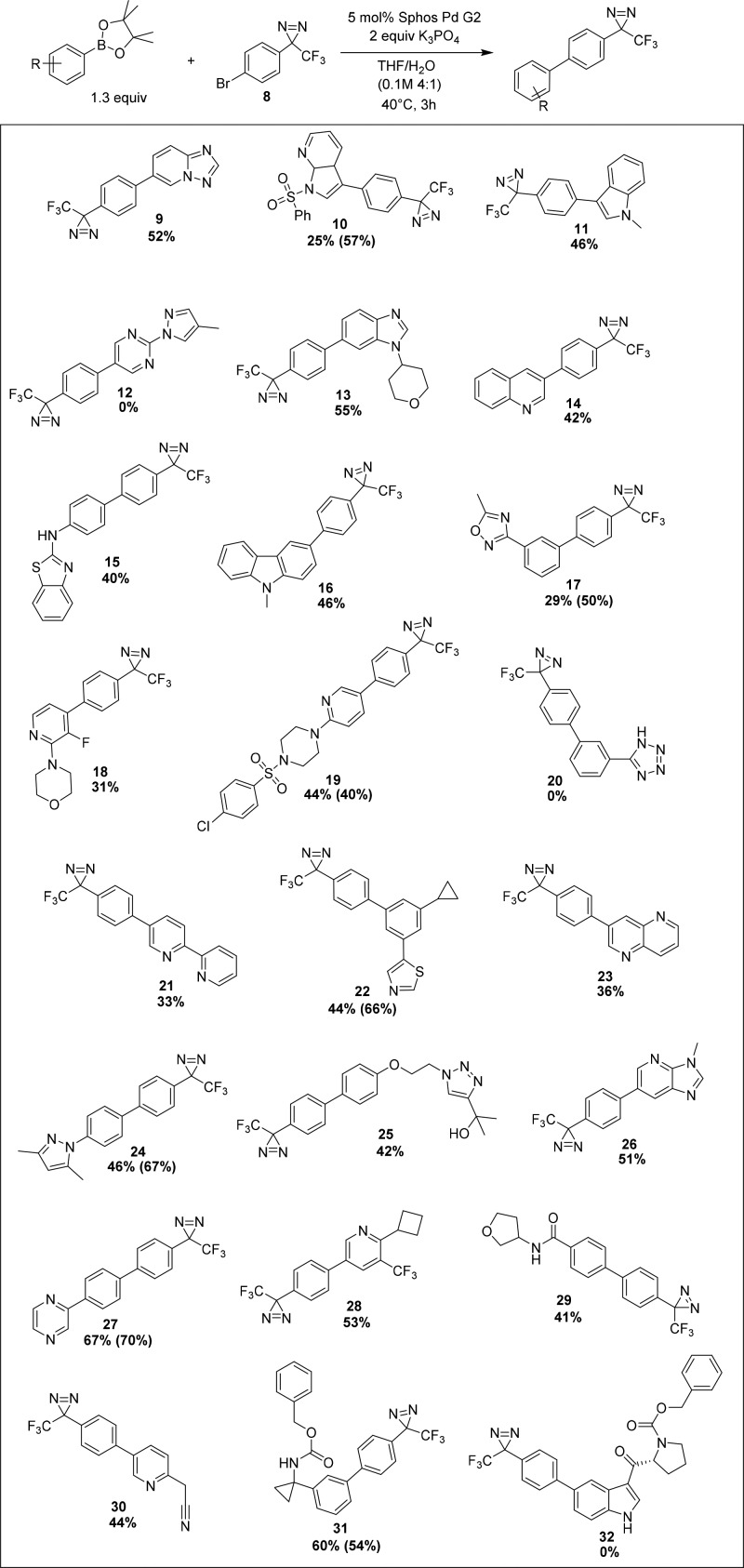
After the identification of S–M conditions suitable for maintaining a stable diazirine moiety, we next turned toward evaluating the ability of a diazirine-containing aryl halide to participate as a partner in the cross coupling as a means of enabling nested probe synthesis. Diazirine-containing aryl bromide 8 was prepared in manner similar to Bender et al.23 and screened across an informer set of pinacol boronate esters.24 This standardized set of complex molecules is an effective way to evaluate the generality of a chemical transformation in the context of pharmaceutically relevant structures. As summarized in Scheme 2, the optimal S–M conditions gave useful yields with 21 out of the 24 informer boronates, a comparable result to that reported in the literature using a heterocyclic bromide that did not contain a diazirine.24 The average yield across the entire set of 24 informer substrates was 40%, and a variety of saturated and unsaturated heterocyclic ring systems were tolerated. Substrates bearing hydrogen bond donors were successfully coupled, including aromatic amine 15, alcohol 25, and amide 29; however, some substrates proved challenging (e.g., 12, 20, and 32). Gratifyingly, when representative examples were repeated on larger scale, the resulting isolated yields tracked well with the original screening results. Overall, the SPhos G2 Pd/K3PO4 system exhibited excellent tolerance to pharmaceutically relevant functional groups and structural motifs. To further examine the scope of this reaction, the pinacol boronate informer compound used to generate entry 25 was reacted with 3-(3-bromophenyl)-3-(trifluoromethyl)-3H-diazirine (33) under the standard conditions. The successful production of the corresponding meta-substituted analog 34 confirmed these conditions were applicable beyond p-halophenyldiazirine synthons.
Scheme 2. Results for the Suzuki–Miyaura Cross Coupling of 8 with an Informer Library of Pinacol Boronate Esters.
Yields listed are based on 19F NMR spectroscopic analysis of 0.010 mmol scale screening reactions. Isolated yields of 0.1–0.4 mmol scale reactions are listed in parentheses (see SI for details).
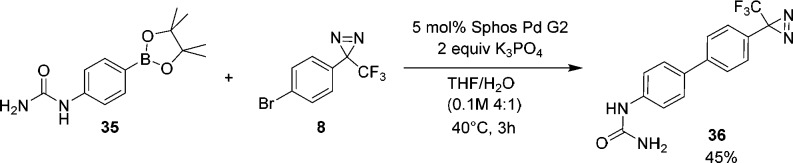
Encouraged by the results from the chemistry informer library, we sought to apply these reaction conditions to the synthesis of a known PAL probe containing a biaryl motif. GSK-3 (36) is an ATP-competitive KSP inhibitor that has been used to elucidate the allosteric binding mode for this class of compounds.18 Luo and co-workers employed a room temperature S–M coupling using a para-iodo-phenyl diazirine (Scheme 1) to synthesize 36, using Pd(PPh3)4 as catalyst over a six-day time period. We were intrigued to see if we could improve upon their 20% isolated yield using the SPhos Pd G2/40 °C conditions. Indeed, as shown in Scheme 3 the newer protocol provided a significant boost in isolated yield (45% vs 20%) with a greatly shortened reaction time (3 h vs 6 d) using a less reactive aryl bromide coupling partner.
Scheme 3. Application of Optimized Suzuki–Miyaura Conditions toward the Synthesis of GSK-3 (36).
In conclusion, we have identified an efficient and robust set of Suzuki–Miyaura coupling conditions that allow for the direct incorporation of the diazirine moiety into biaryl motifs. These conditions have shown good scope of applicability across a range of pharmaceutically relevant functional groups and structural types as judged by their performance against a (hetero)aryl boronate chemistry informer library. In addition, these conditions have provided improved access to the known PAL probe GSK-3. The availability of diazirine-tolerant S–M coupling conditions should enable the synthesis of new Nested PAL probe designs with better efficiency than existing protocols. Ultimately, we hope that this work will help to address some of the current gaps and limitations in diazirine synthesis, thus enabling the discovery of novel tool compounds for elucidation of fundamental questions of ligand–target interactions.
Acknowledgments
We thank Allyson Debes (MRL Library) and David Carroll (CAS) for help with the literature survey. Sebastian Schneider and Scott Johnson assisted with the PDB search. We thank Mycah Uehling, Sean Bowen, Qi Gao, and James Small for NMR analyses and Rong-Sheng Yang and Nina Jarrah for HRMS analyses. We thank Iván Cornella-Taracido for inspiring discussions. N.I. is supported through the Merck & Co., Inc., Kenilworth, NJ, USA, Postdoctoral Research Fellow Program.
Glossary
ABBREVIATIONS
- PAL
photoaffinity labeling
- S–M
Suzuki–Miyaura reaction
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00403.
Literature survey search strategy; Synthesis of starting materials and product standards, S–M coupling conditions screen, thermal stability of diazirine 1, screen with ArBpin chemistry informer library, general procedure for preparative S–M cross coupling of diazirine 8 with selected members of Ar-Bpin chemistry informer library, compound characterization, and spectroscopic data (PDF)
Literature survey search results (XLSX)
Author Present Address
# Department of Process Chemistry, Takeda Pharmaceuticals International Co., 35 Landsdowne St., Cambridge, MA 02139.
Author Contributions
⊥ These authors contributed equally. The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Schmitz E.; Habisch D.; Stark A. Diazirines as Carbene Precursors. Angew. Chem., Int. Ed. Engl. 1963, 2, 548–548. 10.1002/anie.196305481. [DOI] [Google Scholar]; Dubinsky L.; Krom B. P.; Meijler M. M. Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 2012, 20, 554–570. 10.1016/j.bmc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- Liu M. T. H. The thermolysis and photolysis of diazirines. Chem. Soc. Rev. 1982, 11, 127–140. 10.1039/cs9821100127. [DOI] [Google Scholar]
- Das J. Aliphatic Diazirines as Photoaffinity Probes for Proteins: Recent Developments. Chem. Rev. 2011, 111, 4405–4417. 10.1021/cr1002722. [DOI] [PubMed] [Google Scholar]; Smith E.; Collins I. Photoaffinity labeling in target- and binding-site identification. Future Med. Chem. 2015, 7, 159–183. 10.4155/fmc.14.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. R.; Robertson A. A. B. Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis. J. Med. Chem. 2018, 61, 6945. 10.1021/acs.jmedchem.7b01561. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for a description.
- Fleming S. A. Chemical reagents in photoaffinity labeling. Tetrahedron 1995, 51, 12479–12520. 10.1016/0040-4020(95)00598-3. [DOI] [Google Scholar]
- Brown D. G.; Boströ J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?. J. Med. Chem. 2016, 59, 4443–4458. 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- Taheri Kal Koshvandi A.; Heravi M. M.; Momeni T. Current Applications of Suzuki–Miyaura Coupling Reaction in The Total Synthesis of Natural Products: An update. Appl. Organomet. Chem. 2018, 32, e4210 10.1002/aoc.4210. [DOI] [Google Scholar]
- Magano J.; Dunetz J. R.. Transition Metal-Catalyzed Couplings in Process Chemistry: Case Studies From the Pharmaceutical Industry; Wiley-VCH: Weinheim, 2013. [Google Scholar]
- For example, biaryls are found in 6% of drugs approved since 1980 (ChEMBL), aand many chemical probes. For example, 22% of NIH MLSCN probes contain biaryl substructures and 43% of ChemicalProbes.org curated probe list contains biaryls. See; Gaulton A.; Hersey A.; Nowotka M.; Bento A. P.; Chambers J.; Mendez D.; Mutowo P.; Atkinson F.; Bellis L. J.; Cibrián-Uhalte E.; Davies M.; Dedman N.; Karlsson A.; Magariños M. P.; Overington J. P.; Papadatos G.; Smit I.; Leach A. R. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. 10.1093/nar/gkw1074. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oprea T. I.; Bologa C. G.; Boyer S.; Curpan R. F.; Glen R. C.; Hopkins A. L.; Lipinski C. A.; Marshall G. R.; Martin Y. C.; Ostopovici-Halip L.; Rishton G.; Ursu O.; Vaz R. J.; Waller C.; Waldmann H.; Sklar L. A. A crowdsourcing evaluation of the NIH chemical probes. Nat. Chem. Biol. 2009, 5, 441–447. 10.1038/nchembio0709-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recent examples:; Ramirez C. N.; Ozawa T.; Takagi T.; Antczak C.; Shum D.; Graves R.; Holland E. C.; Djaballah H. Validation of a High-Content Screening Assay Using Whole-Well Imaging of Transformed Phenotypes Recent examples. Assay Drug Dev. Technol. 2011, 9, 247–261. 10.1089/adt.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kraft R.; Kahn A.; Medina-Franco J. L.; Orlowski M. L.; Baynes C.; López-Vallejo F.; Barnard K.; Maggiora G. M.; Restifo L. L. A cell-based fascin bioassay identifies compounds with potential anti-metastasis or cognition-enhancing functions. Dis. Models & Mech. 2013, 6, 217–235. 10.1242/dmm.008243. [DOI] [PMC free article] [PubMed] [Google Scholar]; Namkung W.; Park J.; Seo Y.; Verkman A. S. Novel Amino-Carbonitrile-Pyrazole Identified in a Small Molecule Screen Activates Wild-Type and ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator in the Absence of a cAMP Agonist. Mol. Pharmacol. 2013, 84, 384–392. 10.1124/mol.113.086348. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ng P. S.; Manjunatha U. H.; Rao S. P. S.; Camacho L. R.; Ma N. L.; Herve M.; Noble C. G.; Goh A.; Peukert S.; Diagana T. T.; Smith P. W.; Kondreddi R. R. Structure activity relationships of 4-hydroxy-2-pyridones: A novel class of antituberculosis agents. Eur. J. Med. Chem. 2015, 106, 144–156. 10.1016/j.ejmech.2015.10.008. [DOI] [PubMed] [Google Scholar]; Wittmann C.; Reisch M.; Shah A. H.; Kronfuss E.; Mikut R.; Liebel U.; Grabher C. A Zebrafish Drug-Repurposing Screen Reveals sGC-Dependent and sGC-Independent Pro-Inflammatory Activities of Nitric Oxide. PLoS One 2015, 10, e0137286 10.1371/journal.pone.0137286. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ursu A.; Illich D. J.; Takemoto Y.; Porfetye A. T.; Zhang M.; Brockmeyer A.; Janning P.; Watanabe N.; Osada H.; Vetter I. R.; Ziegler S.; Scholer H. R.; Waldmann H. Epiblastin A Induces Reprogramming of Epiblast Stem Cells Into Embryonic Stem Cells by Inhibition of Casein Kinase 1. Cell Chem. Biol. 2016, 23, 494–507. 10.1016/j.chembiol.2016.02.015. [DOI] [PubMed] [Google Scholar]; Wang P.; Zhang F.; He Q.; Wang J.; Shiu H. T.; Shu Y.; Tsang W. P.; Liang S.; Zhao K.; Wan C. Flavonoid Compound Icariin Activates Hypoxia Inducible Factor-1α in Chondrocytes and Promotes Articular Cartilage Repair. PLoS One 2016, 11, e0148372 10.1371/journal.pone.0148372. [DOI] [PMC free article] [PubMed] [Google Scholar]; Baragaña B.; Norcross N. R.; Wilson C.; Porzelle A.; Hallyburton I.; Grimaldi R.; Osuna-Cabello M.; Norval S.; Riley J.; Stojanovski L.; Simeons F. R. C.; Wyatt P. G.; Delves M. J.; Meister S.; Duffy S.; Avery V. M.; Winzeler E. A.; Sinden R. E.; Wittlin S.; Frearson J. A.; Gray D. W.; Fairlamb A. H.; Waterson D.; Campbell S. F.; Willis P.; Read K. D.; Gilbert I. H. Discovery of a Quinoline-4-carboxamide Derivative with a Novel Mechanism of Action, Multistage Antimalarial Activity, and Potent in Vivo Efficacy. J. Med. Chem. 2016, 59, 9672–9685. 10.1021/acs.jmedchem.6b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Franklin G. J.; DeLorey J. L.; Centrella P. A.; Mataruse S.; Clark M. A.; Skinner S. R.; Belyanskaya S. Design and Synthesis of Biaryl DNA-Encoded Libraries. ACS Comb. Sci. 2016, 18, 625–629. 10.1021/acscombsci.6b00078. [DOI] [PubMed] [Google Scholar]
- Of the 21280 ligands <600 Da in PDB protein complex structures, 3791 (18%) contain a biaryl, and of these, 98% place the biaryl within 5Å of the protein. See Supporting Information for more detail.
- For instance, biaryls contribute to increased fraction of sp2 hybridized carbons, which is associated with higher risk of drug development attrition, reduced solubility, increased lipophilicity, and risk of off-target binding. See; Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; Ritchie T. J.; Macdonald S. J. F. The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design?. Drug Discovery Today 2009, 14, 1011–1020. 10.1016/j.drudis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- For example, 11 biaryl-containing drugs have been approved since 2014 spanning a range of molecular mechanisms and indications: Telotristat ethyl, Rucaparib, Lumacaftor, Erismodegib, Selexipag, Eluxadoline, Sacubitril, Osimertinib, Tedizolid phosphate, Netupitant, and Vorapaxar.
- Protasova I.; Bulat B.; Jung N.; Brasë S. Synthesis of Diaziridines and Diazirines via Resin-Bound Sulfonyl Oximes. Org. Lett. 2017, 19, 34–37. 10.1021/acs.orglett.6b03252. [DOI] [PubMed] [Google Scholar]; Derbré S.; Lecat-Guillet N.; Pillon F.; Ambroise Y. Synthesis and evaluation of photoreactive probes to elucidate iodide efflux in thyrocytes. Bioorg. Med. Chem. Lett. 2009, 19, 825–827. 10.1016/j.bmcl.2008.12.008. [DOI] [PubMed] [Google Scholar]; Topin A. N.; Gritsenko O. M.; Brevnov M. G.; Gromova E. S.; Korshunova G. A. Synthesis of a new Photo-Cross-Linking Nucleoside Analogue Containing an Aryl(Trifluoromethyl)Diazirine Group: Application for Eco RII and Mva I Restriction-Modification Enzymes. Nucleosides Nucleotides 1998, 17, 1163–1175. 10.1080/07328319808004229. [DOI] [Google Scholar]
- Babu Kumar A; Anderson J. M.; Manetsch R. Design, synthesis and photoactivation studies of fluorous photolabels. Org. Biomol. Chem. 2011, 9, 6284–6292. 10.1039/c1ob05748k. [DOI] [PubMed] [Google Scholar]
- Luo L.; Parrish C. A.; Nevins N.; McNulty D. E.; Chaudhari A. M.; Carson J. D.; Sudakin V.; Shaw A. N.; Lehr R.; Zhao H.; Sweitzer S.; Lad L.; Wood K. W.; Sakowicz R.; Annan R. S.; Huang P. S.; Jackson J. R.; Dhanak D.; Copeland R. A.; Auger K. R. ATP-competitive inhibitors of the mitotic kinesin KSP that function via an allosteric mechanism. Nat. Chem. Biol. 2007, 3, 722–726. 10.1038/nchembio.2007.34. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Wu G.; Yan C.; Lu K.; Li H.; Zhang Y.; Wang J. Microwave-Assisted, Pd(0)-Catalyzed Cross-Coupling of Diazirines with Aryl Halides. Org. Lett. 2010, 12, 5580–5583. 10.1021/ol102434v. [DOI] [PubMed] [Google Scholar]; Wu G.; Zhao X.; Ji W.; Zhanga Y.; Wang J. Metal-free oxidative cross-coupling of diazirines with arylboronic acids. Chem. Commun. 2016, 52, 1961–1963. 10.1039/C5CC08855K. [DOI] [PubMed] [Google Scholar]
- Collins K. D.; Glorius F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 2013, 5, 597–601. 10.1038/nchem.1669. [DOI] [PubMed] [Google Scholar]; Collins K. D.; Glorius F. Intermolecular Reaction Screening as a Tool for Reaction Evaluation. Acc. Chem. Res. 2015, 48, 619–627. 10.1021/ar500434f. [DOI] [PubMed] [Google Scholar]; Collins K. D.; Glorius F. Employing a robustness screen: rapid assessment of rhodium(III)-catalysed C–H activation reactions. Tetrahedron 2013, 69, 7817–7825. 10.1016/j.tet.2013.05.068. [DOI] [Google Scholar]; Collins K. D.; Ruhling A.; Lied F.; Glorius F. Rapid Assessment of Protecting-Group Stability by Using a Robustness Screen. Chem. - Eur. J. 2014, 20, 3800–3805. 10.1002/chem.201304508. [DOI] [PubMed] [Google Scholar]; Collins K. D.; Glorius F. Application of a robustness screen for the evaluation of synthetic organic methodology. Nat. Protoc. 2014, 9, 1348–1353. 10.1038/nprot.2014.076. [DOI] [PubMed] [Google Scholar]
- For a recent example of application of high-throughput experimentation methods to S–M couplings, see:; Jaman Z.; Mufti A.; Sah S.; Avramova L.; Thompson D. H. High Throughput Experimentation and Continuous Flow Validation of Suzuki-Miyaura Cross-Coupling Reactions. Chem. - Eur. J. 2018, 24, 9546–9554. 10.1002/chem.201801165. [DOI] [PubMed] [Google Scholar]
- Littke A. F.; Fu G. C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem., Int. Ed. 2002, 41, 4176–4211. . [DOI] [PubMed] [Google Scholar]
- Bender T.; Huss M.; Wieczorek H.; Grond S.; von Zezschwitz P. Convenient Synthesis of a [1-14C]Diazirinylbenzoic Acid as a Photoaffinity Label for Binding Studies of V-ATPase Inhibitors. Eur. J. Org. Chem. 2007, 2007, 3870–3878. 10.1002/ejoc.200700194. [DOI] [Google Scholar]
- Kutchukian P.; Dropinski J.; Dykstra K.; Li B.; DiRocco D.; Streckfuss E.; Campeau L. C.; Cernak T.; Vachal P.; Davies I.; Krska S.; Dreher S. Chemistry informer libraries: a chemoinformatics enabled approach to evaluate and advance synthetic methods. Chem. Sci. 2016, 7, 2604–2613. 10.1039/C5SC04751J. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.