Abstract
Thyroid hormone (TH) action is of clinical interest in treating demyelinating diseases of the central nervous system (CNS). Two amide prodrugs of sobetirome, a potent thyroid hormone agonist, were previously shown to significantly improve CNS selective distribution of the parent drug through hydrolysis in the CNS by fatty acid amide hydrolase (FAAH). This concept is elaborated upon here with a series of 29 amide prodrugs targeting FAAH. We identify that conservative aliphatic modifications such as the N-methyl (4), N-ethyl (5), N-fluoroethyl (15), and N-cyclopropyl (18) substantially favor selective CNS distribution of the parent drug in mice. Additionally, lead compounds exhibit moderate to good rates of hydrolysis at FAAH in vitro suggesting both enzymatic and physicochemical properties are important parameters for optimization. Both 4 and 15 were orally bioavailable while retaining appreciable CNS parent drug delivery following an oral dose. The pharmacokinetic parameters of 4 over 24 h postdose (i.v. and p.o.) were determined.
Keywords: Prodrug, sobetirome, thyroid hormone, remyelination, CNS, FAAH
The blood–brain barrier (BBB) represents a unique challenge for CNS drug development as it can significantly restrict therapeutic exposure from systemic circulation. Demyelinating diseases represent one class of therapeutically underserved CNS disorders with multiple sclerosis (MS), an autoinflammatory demyelinating disease, representing the most common demyelinating disorder. Current approved therapies for MS are mechanistically anti-inflammatory and do not promote repair to damaged myelin.1 Thyroid hormone (T3, 1) action has garnered significant attention for remyelination therapy as T3 is important in both developmental myelination as well as myelin repair.2,3 Unlike T3, the clinical stage thyroid hormone agonist (thyromimetic) sobetirome has a suitable therapeutic index for clinical use due to receptor- and tissue-specific action.4 While sobetirome is unique in that it5,6 and close analogues7 are the only known thyromimetics to distribute to the CNS, efforts to develop sobetirome analogues with better CNS distribution have led to a new class of amide prodrugs termed sobetiramides.8 Both the primary amide Sob-AM1 (3, Figure 1) and N-methylamide Sob-AM2 (4, Figure 1) were shown to deliver significantly more sobetirome to the CNS compared to a systemic dose of sobetirome. This improvement in CNS exposure was coupled with a concomitant decrease in peripheral exposure of sobetirome. This reversal from peripheral to central drug action makes the sobetiramides promising remyelination drug candidates with selective tissue action.
Figure 1.
Structure of thyroid hormone, the clinical stage thyromimetic sobetirome, and sobetiramides.
From mechanistic studies of the unique CNS distribution of sobetiramides, we established that Sob-AM1 and Sob-AM2 are selectively hydrolyzed by fatty acid amide hydrolase (FAAH) and that FAAH activity is required for the selective CNS delivery of sobetirome from Sob-AM2.8 FAAH catalyzes the hydrolysis of a diverse array of fatty acid amide signaling molecules including the endocannabinoid ananadamide (AEA),9 the hypnotic oleamide (OEA),10 the anti-inflammatory N-palmitoylethanolamine (PEA),11 and N-arachidonoyltaurine.12 Structure–activity relationship (SAR) studies have shown that FAAH is also capable of hydrolyzing a diverse set of synthetic arachidonoyl amides including substituted derivatives of ethylamine and aniline as well as synthetic luciferin derivatives.13,14 The pharmacology of FAAH inhibition has been of significant clinical interest for antinociception and has been extensively reviewed.15−17 However, as a pharmacological substrate for FAAH, the scope of how existing SAR studies apply to the sobetiramide scaffold remains to be explored.
Here we report a small library of sobetiramides (Figure 1, Table 1) designed to probe the structural requirements of FAAH-mediated CNS delivery. The compound library consists of simple aliphatic amides (5–8, 20), substituted ethylamines (9–17), a variety of anilines and aryl amines (21–26), and several polar derivatives (27–31). The compounds were synthesized from sobetirome in a direct manner from readily available starting materials in one or two steps. In certain cases (5–6, 14), prodrug synthesis was accomplished in a protecting group-free manner from sobetirome identical to the report for 3 and 4 (Scheme 1, Method A). The remaining sobetiramides were prepared from O-benzyl sobetirome (32, Scheme 1, Methods B–E) using common amide bond coupling reactions followed by deprotection via transfer hydrogenation or treatment with boron trichloride.
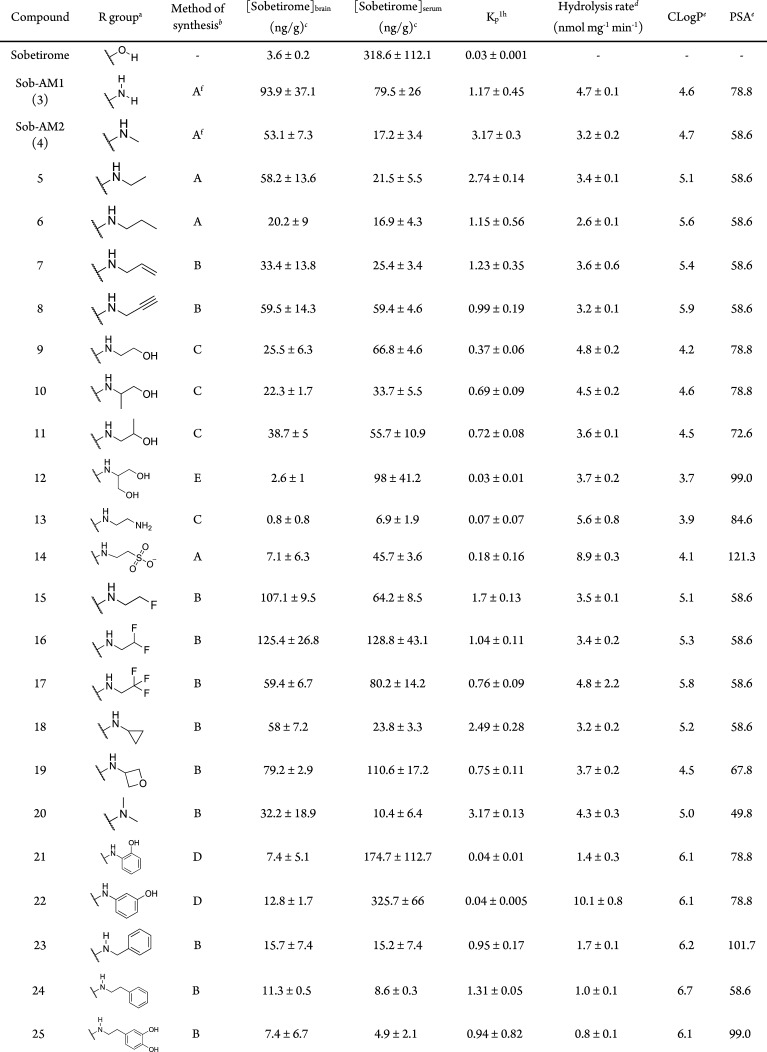
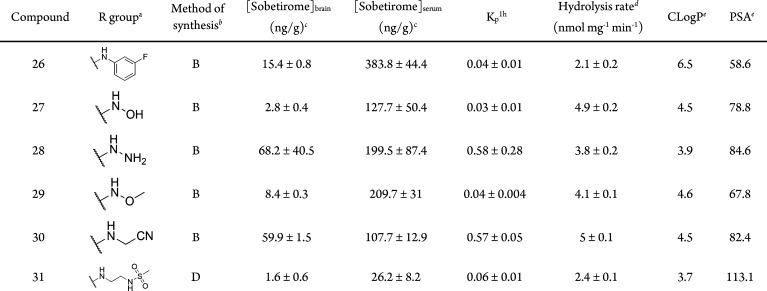
Table 1. Structure, Tissue Distribution, FAAH Hydrolysis Rates, and Physicochemical Parameters of the Library of Sobetiramide Prodrugs.
Concentrations expressed as ng sobetirome per gram tissue; data points represent mean ± SEM and n = 3.
Kinetics of prodrug hydrolysis in COS-7 cell homogenate overexpressing human FAAH, substrate concentration was 100 μM, data points represent mean ± SEM, and n = 3.
Calculated using Canvas (Schrödinger).
Previously published in ref (8)
Scheme 1. Synthesis of the Sobetiramide Library.
Reagents and conditions: Method A: R1 = H, (i) MeOH, H2SO4, (ii) MeOH, amine. Method B: R1 = Bn, (i) CDI, THF, (ii) BCl3, DCM. Method C: R1 = Bn, (i) oxalyl chloride, DCM, (ii) amine, (iii) Pd/C, TES. Method D: R1 = Bn, (i) EDC, HOBT, DIEA, (ii) Pd/C, TES. Method E: R1 = Bn, (i) DCC, NHS, (ii) amine. Note: compound 20 is a secondary amide (−NR2)
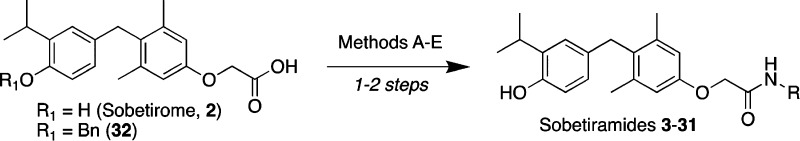
The sobetiramide library was initially screened using a 1 h tissue distribution experiment in male mice. The 1 h time point was selected because it approximates the Tmax of sobetirome brain concentration observed previously with related sobetirome prodrugs.8,18,19 Mice were dosed systemically (i.p.) with equimolar doses of test compounds, and brain and blood were collected at 1 h postdose. Using LC–MS/MS, the concentration of the parent drug sobetirome hydrolyzed from the prodrug was quantified in both the brain and serum (Table 1). Brain-to-serum concentration ratios (Kp1h) were calculated from these 1 h time points as a measure of central vs peripheral selectivity of distribution. A majority of tested sobetiramides show substantially elevated levels of sobetirome in the brain compared to an equimolar dose of sobetirome. Importantly, several derivatives including ethylamine 5, propargylamine 8, fluoroethylamine 15, and difluoroethylamine 16 show comparable levels of sobetirome in the brain to those delivered by Sob-AM1 (3) and Sob-AM2 (4). With Kp1h near or above unity, these prodrugs deliver sobetirome preferentially to the CNS at the 1 h time point. Combined with our previous results with Sob-AM1 and Sob-AM2, these data suggest that small, nonpolar modifications lead to favorable CNS distribution of sobetirome from sobetiramides.
As the sobetiramides were designed to include the amine components of known FAAH substrates, it is possible that the increased CNS distribution results from higher rates of FAAH-catalyzed hydrolysis. To test this hypothesis, compounds were screened in an in vitro hydrolysis assay using a cell homogenate containing expressed FAAH as previous described for Sob-AM1 and Sob-AM2.8 Interestingly, the two prodrugs with the highest rates of cleavage in the in vitro enzymatic assay were the taurine derivative 14 (8.9 ± 0.3 nmol mg–1 min–1) and 3-hydroxyaniline 22 (10.1 ± 0.8 nmol mg–1 min–1). The remaining aromatic derivatives including the 2-hydroxyaniline demonstrated low rates of hydrolysis (21, 24–26) while most of the other aliphatic and polar derivatives showed intermediate kinetics in this range similar to 4 (3.2 ± 0.2 nmol mg–1 min–1). One possibility is that the charged nature of 14 precludes successful penetration of the BBB. Compound 22 demonstrated similar levels of serum sobetirome compared to an equimolar dose of sobetirome itself suggesting that this sobetiramide is cleaved by other hydrolases in addition to FAAH in vivo.
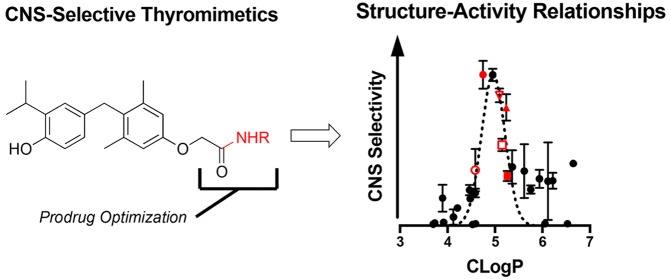
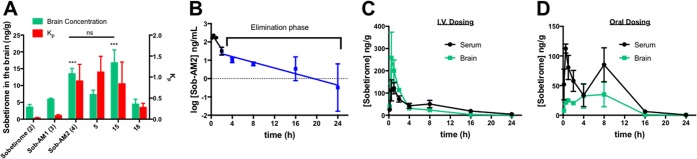
Comparing FAAH hydrolysis rates with Kp1h (Figure 2A) reveals that the prodrugs with the highest CNS selectivity all have similar rates of in vitro hydrolysis (∼3–5 nmol mg–1 min–1). This suggests that adequate enzyme kinetics is necessary but not sufficient to account for sobetirome tissue distribution in vivo.
Figure 2.
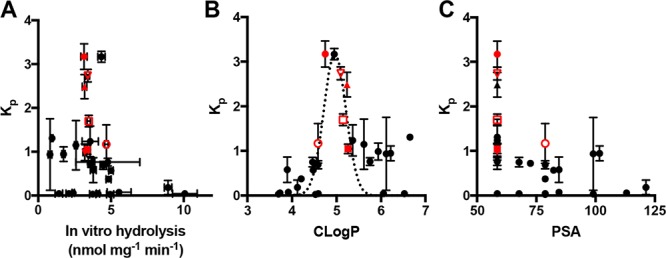
Comparative plots Kp1h verse (A) in vitro hydrolysis in cell homogenate overexpressing FAAH, (B) prodrug CLogP, and (C) PSA. For panel B, a Gaussian curve was fit to better illustrate the local maximum. A set of lead prodrugs from the in vivo screen are denoted by red ○ (Sob-AM1, 3), red ● (Sob-AM2, 4), red □ (15), red ■ (16), red ▲ (18), and red ▽ (5).
Compound physicochemical properties can play an outsized role in determining CNS drug penetration due to the unique nature of the BBB.20 It has previously been shown by a number of QSAR studies that properties such as charged state, CLogP, polar surface area (PSA), and rotatable bonds can help predict whether a drug will be able to passively diffuse through the BBB. In the case of the sobetiramides, the complete SAR likely contains components favoring both enzymatic hydrolysis and passive diffusion through the BBB. To examine this relationship, physicochemical parameters were calculated for the sobetiramides (Table 1). When the relationship between Kp1h and CLogP is plotted (Figure 2B), a bell-shaped response with an apparent maximum is found around CLogP ≈ 5. While higher than average for approved CNS drugs, this value is consistent with the recommended range for CNS penetrating compounds.21−23 PSA is another important parameter that correlates with known drug penetration of the brain. A plot of prodrug PSA values vs Kp1h (Figure 2C) shows that the better brain penetrating prodrugs have smaller PSA values. This preference for low PSA is supported by QSAR analysis of approved CNS compounds.22 It has recently been determined that drug molecules featuring PSA values ≤90 Å2 are optimal for BBB penetration, a claim that applies to the sobetiramides in this study.24 While CLogP and PSA are two of the relevant properties affecting brain penetration, these factors could explain why lead compounds similar to 4 and 15 were selected from the in vivo screen, and more polar prodrugs with similar or better hydrolysis rates at FAAH did not perform well in vivo. This suggests that FAAH-targeted prodrugs must be optimized for enzymatic activity while also retaining CNS drug-like physicochemical properties.
As oral dosing is generally the preferred route of administration in clinical development, it is important to understand the CNS distribution of sobetirome from oral dosing of sobetiramide prodrugs. To this end, the tissue distribution of sobetirome from a subset of prodrugs was determined 1 h following oral gavage. Compared with an equimolar dose of sobetirome, both Sob-AM2 (4) and 15 show statistically significant improvement in total sobetirome in the brain and Kp1h (Figure 3A).18 Interestingly, the remainder of the prodrugs tested shows attenuated or nonsignificant improvements over sobetirome itself. These differences could be attributable to a more pronounced effect in first-pass metabolism for these prodrugs. With Sob-AM2 showing favorable properties in the 1 h time point, we aimed to fully characterize Sob-AM2 in a 24 h distribution experiment. Pharmacokinetic parameters for sobetirome and Sob-AM2 are summarized in Tables 1, 2, and S1. Sob-AM2 has an elimination half-life (t1/2) of 8.8 h, similar to that of sobetirome (t1/2 = 7.2 h).18 Exposure of sobetirome over a 24 h period following a systemic dose of Sob-AM2 shows a Tmax of ∼30 min (Figure 3C). The Kp of sobetirome from Sob-AM2 over the 24 h exposure (Kp0–24h = 0.44) is well within the range of typical CNS-active compounds.25 When mice are dosed orally, total exposure in the brain is lower, and the Kp0–24h is approximately half that compared to i.v. administration suggesting that more sobetirome is liberated in the periphery from orally administered Sob-AM2 compared to intravenous administration. It is known that FAAH is expressed in both the intestinal tract as well as the liver making it likely that FAAH-mediated cleavage of orally delivered Sob-AM2 occurs before Sob-AM2 crosses these first-pass barriers into systemic circulation, which could account for the additional peripheral prodrug cleavage observed from oral administration.26
Figure 3.
Tissue distribution of lead prodrugs from oral dosing and 24 h pharmacokinetic analysis of Sob-AM2. (A) Distribution from dose (p.o., 9.15 μmol/kg) of sobetirome or lead prodrugs followed by tissue collection 1 h postdose. (B) Serum concentration of Sob-AM2 following single dose (9.15 μmol/kg, i.v.) over 24 h. The elimination phase for Sob-AM2 is highlighted as blue. (C) Brain and serum distribution of sobetirome from a single dose of Sob-AM2 (9.15 μmol/kg, i.v.) over 24 h postdose. (D) Same as that in panel C but dosed by oral gavage (p.o., 9.15 μmol/kg). Data points represent mean ± SEM and n = 3.
Table 2. Summary of Pharmacokinetic and Pharmacodynamic Properties of Sobetirome and Sob-AM2.
| propertiesa | sobetiromeb | Sob-AM2 |
|---|---|---|
| oral bioavailability, F (%) | 92 | 19.5c |
| terminal half-life (h) | 7.2 | 8.8c |
| clearance, Cl (mL/min/kg) | 8 | 91.7c |
| brain AUC0→24h (i.v., ng·h·g–1) | 694d | |
| Kp0–24h (i.v., ng·h·g–1) | 0.82d | |
| brain AUC0→24h (p.o., ng·h·g–1) | 378d | |
| Kp0–24h (p.o., ng·h·g–1) | 0.44d |
From 24 h areas under the curve.
From ref (16).
Calculated from quantified Sob-AM2 concentrations in serum.
Calculated from quantified sobetirome concentrations in tissue.
We previously described how both sobetirome and an ester prodrug demonstrate a secondary maximal peak following oral dosing, which is consistent with the enterohepatic circulation observed with thyroid hormone.18,27 It was hypothesized that glucuronidation or other reversible secondary metabolites were responsible for this phenomenon. A similar secondary peak is also observed following oral dosing of Sob-AM2 (Figure 3D). Defining the role of FAAH and other Phase I and Phase II metabolic pathways could provide valuable insight in explaining the pharmacokinetics of these compounds.
With such a small chemical alteration differentiating prodrug from parent drug, it is necessary to characterize prodrug activity at the thyroid hormone receptor (TR) target. Prior thyromimetic SAR suggests it is important to have an anionic carboxylate at this position for high-affinity TR binding.28 In cellular transactivation assays with either human TRα or human TRβ, Sob-AM2 was found to have weak agonist activity that was less potent than that of either sobetirome or T3 (Figure 4, Table S2). Because Sob-AM2-induced TR activation decreased at higher concentrations creating a bell-shaped profile, it is not clear whether this is partial agonist activity or agonist activity blunted by cytotoxicity at high Sob-AM2 concentrations. Interestingly, the activity was unchanged by FAAH inhibitor PF-3845 suggesting that it is arising from Sob-AM2 itself and not FAAH-mediated cleavage of Sob-AM2 providing sobetirome to the cells.29 Regardless, this weak activity occurs at substantially higher concentrations than sobetirome activity and can be considered negligible for in vivo TR target engagement.
Figure 4.
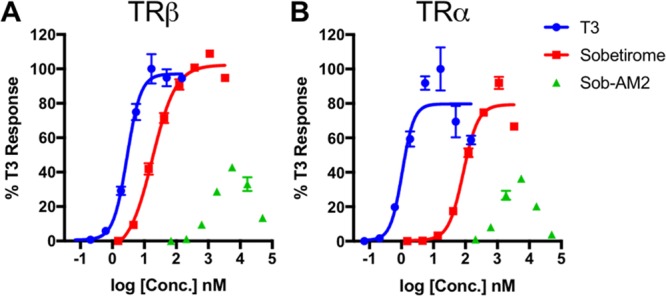
Dose response of T3 (1), sobetirome (2), and prodrug Sob-AM2 (4) in a plate-based luciferase assay for on-target thyroid receptor isoform activation (A, TRα; B, TRβ). Data points represent mean ± SEM and n = 3.
In conclusion, our data suggest that the sobetiramide strategy is effective in delivering useful levels of sobetirome into the brain from a systemic dose compared to a systemic dose of the parent drug itself. The SAR demonstrates a clear preference for a “less-is-more” approach with small, nonpolar amide modifications being more productive for CNS distribution in vivo. As it is likely that FAAH-targeted prodrugs could be developed for additional drug scaffolds beyond sobetirome, this work may define at least some of the structural requirements needed for BBB penetration and FAAH activity. Recently, Sob-AM2 has been shown to be effective in delivering sobetirome to the brain to treat a mouse model of MCT8-deficiency (Allan–Herndon–Dudley syndrome), a condition of congenital CNS hypothyroidism characterized by profound cognitive impairment.30 As such, this FAAH-targeted prodrug CNS delivery strategy is providing useful tools for studying thyroid hormone action in the CNS and potentially new therapeutic agents for CNS diseases that would benefit from CNS thyroid hormone action.
Acknowledgments
We thank Lisa Bleyle (OHSU Bioanalytical Shared Resource/Pharmacokinetics Core) for technical assistance with LC–MS/MS, Prof. Martin Kaczocha (Stony Brook) for the pcDNA4-FAAH plasmid, and Prof. Michael Cohen (OHSU) for the COS-7 line.
Glossary
ABBREVIATIONS
- TH
thyroid hormone
- SAR
structure–activity relationship
- FAAH
fatty-acid amide hydrolase
- CNS
central nervous system
- BBB
blood–brain barrier
- MS
multiple sclerosis
- AEA
anandamide
- OEA
oleamide
- PEA
N-palmitoylethanolamine
- i.p.
intraperitoneal
- i.v.
intravenous
- p.o.
per os
- Kp1h
brain-to-serum concentration ratio 1 h post dose
- Kp0–24h
brain-to-serum exposure ratio over 24 h postdose
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00501.
General methods, synthetic procedures and compound characterization, in vivo pharmacology, and in vitro assays (PDF)
Author Contributions
§ These authors contributed equally. J.M.M., S.J.F., and T.S.S. wrote the manuscript. J.M.M., S.J.F., T.S.S., and D.B. developed the concept. J.M.M., S.J.F., and Tp.B. synthesized the compounds. J.M.M., S.J.F., Tp.B, Tn.B, and H.S.S performed and optimized in vivo experiments. All authors analyzed results. All authors have given approval to the final version of the manuscript.
This work was supported by the OHSU Laura Fund for Innovation in Multiple Sclerosis, the National Institutes of Health (DK 052798 to T.S.S.; F32GM125127 to J.M.M.; T32DK007680 to S.J.F.), and the National Multiple Sclerosis Society (RG 5199A4/1 to D.B.).
The authors declare the following competing financial interest(s): T.S.S., J.M.M., S.J.F., Tp.B., and Tn.B. are inventors on a licensed patent application claiming central nervous system-penetrating prodrugs of sobetirome and their uses. T.S.S. and D.B. are founders of Llama Therapeutics. The remaining authors have nothing to disclose.
Supplementary Material
References
- Compston A.; Coles A. Multiple sclerosis. Lancet 2008, 372 (9648), 1502. 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Baxi E. G.; Schott J. T.; Fairchild A. N.; Kirby L. A.; Karani R.; Uapinyoying P.; Pardo-Villamizar C.; Rothstein J. R.; Bergles D. E.; Calabresi P. A. A selective thyroid hormone beta receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia 2014, 62 (9), 1513. 10.1002/glia.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Ma Z.; Qin H.; Yao Z. Thyroid Hormone Potentially Benefits Multiple Sclerosis via Facilitating Remyelination. Mol. Neurobiol. 2016, 53 (7), 4406. 10.1007/s12035-015-9375-z. [DOI] [PubMed] [Google Scholar]
- Baxter J. D.; Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat. Rev. Drug Discovery 2009, 8 (4), 308. 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- Trost S. U.; Swanson E.; Gloss B.; Wang-Iverson D. B.; Zhang H.; Volodarsky T.; Grover G. J.; Baxter J. D.; Chiellini G.; et al. The thyroid hormone receptor-β-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 2000, 141 (9), 3057. 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- Takahashi N.; Asano Y.; Maeda K.; Watanabe N. In vivo evaluation of 1-benzyl-4-aminoindole-based thyroid hormone receptor β agonists: importance of liver selectivity in drug discovery. Biol. Pharm. Bull. 2014, 37 (7), 1103. 10.1248/bpb.b13-00915. [DOI] [PubMed] [Google Scholar]
- Devereaux J.; Ferrara S. J.; Banerji T.; Placzek A. T.; Scanlan T. S. Increasing Thyromimetic Potency through Halogen Substitution. ChemMedChem 2016, 11 (21), 2459. 10.1002/cmdc.201600408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinig J. M.; Ferrara S. J.; Banerji T.; Banerji T.; Sanford-Crane H. S.; Bourdette D.; Scanlan T. S. Targeting Fatty-Acid Amide Hydrolase with Prodrugs for CNS-Selective Therapy. ACS Chem. Neurosci. 2017, 8 (11), 2468. 10.1021/acschemneuro.7b00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt B. F.; Giang D. K.; Mayfield S. P.; Boger D. L.; Lerner R. A.; Gilula N. B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384 (6604), 83. 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt B. F.; Prospero-Garcia O.; Siuzdak G.; Gilula N. B.; Henriksen S. J.; Boger D. L.; Lerner R. A. Chemical characterization of a family of brain lipids that induce sleep. Science 1995, 268 (5216), 1506. 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Lambert D. M.; Vandevoorde S.; Jonsson K. O.; Fowler C. J. The palmitoylethanolamide family: a new class of anti-inflammatory agents?. Curr. Med. Chem. 2002, 9 (6), 663. 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- Saghatelian A.; Trauger S. A.; Want E. J.; Hawkins E. G.; Siuzdak G.; Cravatt B. F. Assignment of Endogenous Substrates to Enzymes by Global Metabolite Profiling. Biochemistry 2004, 43 (45), 14332. 10.1021/bi0480335. [DOI] [PubMed] [Google Scholar]
- Lang W.; Qin C.; Lin S.; Khanolkar A. D.; Goutopoulos A.; Fan P.; Abouzid K.; Meng Z.; Biegel D.; Makriyannis A. Substrate Specificity and Stereoselectivity of Rat Brain Microsomal Anandamide Amidohydrolase. J. Med. Chem. 1999, 42 (5), 896. 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- Mofford D. M.; Adams S. T.; Reddy G. S. K. K.; Reddy G. R.; Miller S. C. Luciferin amides enable in vivo bioluminescence detection of endogenous fatty acid amide hydrolase activity. J. Am. Chem. Soc. 2015, 137 (27), 8684. 10.1021/jacs.5b04357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H. Recent advances in the discovery and evaluation of fatty acid amide hydrolase inhibitors. Expert Opin. Drug Discovery 2010, 5 (10), 961. 10.1517/17460441.2010.513378. [DOI] [PubMed] [Google Scholar]
- Roques B. P.; Fournie-Zaluski M. C.; Wurm M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat. Rev. Drug Discovery 2012, 11 (4), 292. 10.1038/nrd3673. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discovery 2018, 17 (9), 623. 10.1038/nrd.2018.115. [DOI] [PubMed] [Google Scholar]
- Ferrara S. J.; Meinig J. M.; Placzek A. T.; Banerji T.; McTigue P.; Hartley M. D.; Sanford-Crane H. S.; Banerji T.; Bourdette D.; Scanlan T. S. Ester-to-amide rearrangement of ethanolamine-derived prodrugs of sobetirome with increased blood-brain barrier penetration. Bioorg. Med. Chem. 2017, 25 (10), 2743. 10.1016/j.bmc.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek A. T.; Ferrara S. J.; Hartley M. D.; Sanford-Crane H. S.; Meinig J. M.; Scanlan T. S. Sobetirome prodrug esters with enhanced blood-brain barrier permeability. Bioorg. Med. Chem. 2016, 24 (22), 5842. 10.1016/j.bmc.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic Z. CNS drug design: balancing physicochemical properties for optimal brain exposure. J. Med. Chem. 2015, 58 (6), 2584. 10.1021/jm501535r. [DOI] [PubMed] [Google Scholar]
- Hitchcock S. A.; Pennington L. D. Structure-brain exposure relationships. J. Med. Chem. 2006, 49 (26), 7559. 10.1021/jm060642i. [DOI] [PubMed] [Google Scholar]
- Pajouhesh H.; Lenz G. R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2 (4), 541. 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. Moving beyond Rules: The Development of a Central Nervous System Multiparameter Optimization (CNS MPO) Approach To Enable Alignment of Druglike Properties. ACS Chem. Neurosci. 2010, 1 (6), 435. 10.1021/cn100008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. A.; Pennington L. D. Structure-brain exposure relationships. J. Med. Chem. 2006, 49 (26), 7559. 10.1021/jm060642i. [DOI] [PubMed] [Google Scholar]
- Doran A.; Obach R. S.; Smith B. J.; Hosea N. A.; Becker S.; Callegari E.; Chen C.; Chen X.; Choo E.; et al. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: Evaluation using the MDR1A/1B knockout mouse model. Drug Metab. Dispos. 2005, 33 (1), 165. 10.1124/dmd.104.001230. [DOI] [PubMed] [Google Scholar]
- Wei B. Q.; Mikkelsen T. S.; McKinney M. K.; Lander E. S.; Cravatt B. F. A Second Fatty Acid Amide Hydrolase with Variable Distribution among Placental Mammals. J. Biol. Chem. 2006, 281 (48), 36569. 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- Rutgers M.; Heusdens F. A.; Bonthuis F.; de Herder W. W.; Hazenberg M. P.; Visser T. J. Enterohepatic circulation of triiodothyronine (T3) in rats: importance of the microflora for the liberation and reabsorption of T3 from biliary T3 conjugates. Endocrinology 1989, 125 (6), 2822. 10.1210/endo-125-6-2822. [DOI] [PubMed] [Google Scholar]
- Jorgensen E. C. Structure activity relationships of thyroxine analogs. Pharmacol. Ther., Part B 1976, 2 (4), 661. 10.1016/0306-039X(76)90073-8. [DOI] [PubMed] [Google Scholar]
- Ahn K.; Johnson D. S.; Mileni M.; Beidler D.; Long J. Z.; McKinney M. K.; Weerapana E.; Sadagopan N.; Liimatta M.; et al. Discovery and Characterization of a Highly Selective FAAH Inhibitor that Reduces Inflammatory Pain. Chem. Biol. 2009, 16 (4), 411. 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barez-Lopez S.; Hartley M. D.; Grijota-Martinez C.; Scanlan T. S.; Guadano-Ferraz A. Sobetirome and its Amide Prodrug Sob-AM2 Exert Thyromimetic Actions in Mct8-Deficient Brain. Thyroid 2018, 28 (9), 1211. 10.1089/thy.2018.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.