Abstract
Objectives
To measure the 24-month impact on continuation, unintended pregnancy, and satisfaction of trying long-acting reversible contraception (LARC) in a population seeking short-acting reversible contraception (SARC).
Study Design
We enrolled 916 women aged 18-29 who were seeking pills or injectables in a partially randomized patient preference trial. Women with strong preferences for pills or injectables, started on those products, while others opted for randomization to LARC or SARC and received their methods gratis. We estimated continuation and unintended pregnancy rates through 24 months. Intent-to-treat principles were applied after method initiation for comparing incidence of unintended pregnancy. We also examined how satisfaction levels varied by cohort and how baseline negative LARC attitudes were associated with satisfaction over time.
Results
Forty-three percent chose randomization, and 57% chose the preference option. Complete loss to follow-up was <2%. The 24-month LARC continuation probability was 64.3% (95% Cl 56.6–70.9), statistically higher than SARC groups (25.5% (randomized) and 40.0% (preference)). The 24-month cumulative unintended pregnancy probabilities were 9.9% (95% Cl: 7.2–12.6) (preference-SARC), 6.9% (95% Cl 3.3–10.6) (randomized-SARC) and 3.6% (95% Cl 1.8–6.4) (randomized-LARC). Statistical tests for comparing randomized groups on unintended pregnancy were mixed: binomial at 24-month timepoint (p=0.02) and logrank survival probabilities (p=0.14 for first pregnancies and p=0.07 when including second pregnancies). LARC satisfaction was high (80% happy/neutral, 73% would use LARC again, 81% would recommend to a friend). Baseline negative attitudes toward LARC (27%) were not clearly associated with satisfaction or early discontinuation.
Conclusions
The decision to try LARC resulted in high continuation rates and substantial protection from unintended pregnancy over 24 months. Despite participants’ initial desires to begin short-acting regimens, they had high satisfaction with LARC. Voluntary decisions to try LARC will benefit large proportions of typical SARC users.
Implications
Even women who do not necessarily view LARC as a first choice may have a highly satisfying experience and avoid unintended pregnancy if they try it.
Keywords: IUD, subdermal contraceptive implant, oral contraceptives, DMPA, LARC, acceptability, contraceptive continuation, adherence, unintended pregnancy
1. Introduction
Long-acting reversible contraception (LARC), consisting of intrauterine devices and the subdermal implant, is the most effective category of reversible family planning. Despite this important attribute, only a small minority of US women (7%) use LARC, whereas 19% use either pills or injectables.[1] Among women using non-permanent contraceptive methods, 17% use LARC, 46% use pills or injectables combined, and 37% use other forms.
Previous research has focused on increasing access to LARC because the barriers are significant (high product cost and lack of trained providers). The most thorough effort ever undertaken in the US to study the impact of removing barriers to LARC resulted in tremendous uptake, interest, and profound reductions in unintended pregnancy and abortions in the St. Louis metropolitan area.[2, 3] However, nationwide, more work is needed to improve access to LARC; product availability ranges from 32% to 56% in office-based facilities and 36% to 60% in Title X-funded clinics.[4]
Even if major barriers to LARC were removed in the US, experts predict that less than one-third of women would choose LARC.[5] Contraindications to LARC play a role, but personal preferences for specific types of contraceptives and aversions to others would also limit uptake. Previous experiences/side effects, trust/fear, knowledge, like/dislike of specific delivery systems (e.g., oral, injection, devices, patch), ease of acquiring, and effectiveness are some influences that come into play. The most commonly cited negative aspects attributed to LARC are irregular bleeding, painful insertion/removal, weight gain, and general aversion to having a device inserted into the body. [6]
We do not know how LARC might meet the needs of a broader population that 1) doesn’t actively seek it out and 2) has some unease or aversions to IUDs and implants. Nearly all measures of contraceptive effectiveness are based upon observational studies, where participants start their preferred method. Those who expressly seek out and choose LARC may be enthusiastic adopters, weary of using short-acting products, and determined to have positive experiences to avoid unintended pregnancy; also, they may be reluctant to seek early removal despite side effects, fears, or other concerns.
Trying LARC, even with some initial doubts or uncertainties, may result in higher than expected personal satisfaction and continued use than short-acting methods. If so, more widespread voluntary uptake of LARC, even in populations without strong inclinations toward LARC, would reduce incidence of unintended pregnancy. In this report, we examine some of these issues and update our 12-month findings[7] by providing extended estimates of LARC effectiveness out to 24 months to understand longer-term impact of participation in this randomized trial. Do randomized LARC users maintain enthusiasm for their method in the second year of use? Potential growing dissatisfaction with LARC in the second year, LARC removals, and unintended pregnancy are fundamental measures to evaluate in this unique research population that agreed to try a long-acting contraceptive.
2. Materials and methods
We described the background, rationale, enrollment, and 12-month follow-up results of this study in previous publications.[7, 8] From December 2011 to December 2013, we enrolled participants in an open-label, partially randomized patient preference trial to compare effectiveness of short-acting reversible contraception (SARC) and LARC. The study was powered to compare continuation rates and included secondary endpoints of unintended pregnancy and satisfaction. The study was conducted at three health centers in North Carolina owned and operated by Planned Parenthood South Atlantic (PPSAT). The study was approved by the federally-registered institutional review board of FHI 360, the Protection of Human Subjects Committee.
We recruited a population 18-29 years of age that was seeking oral contraceptives or the injectable depot medroxyprogesterone acetate (DMPA). We excluded women who came to PPSAT for LARC or previously tried LARC. Together, these strategies created a study population at higher risk of unintended pregnancy and perhaps less favorable towards LARC than a population specifically seeking LARC.
After hearing the study details and options for participation, women agreed to participate by signing the informed consent document and choosing how they wanted to participate. Some chose to continue or start oral contraceptives or DMPA and paid for their services as they would have done in the absence of a study (completely out-of-pocket or covered partially or completely by insurance or financial assistance programs). Others chose to be in the two-arm randomized trial (LARC or SARC) and received a free LARC method or free SARC product for one year. Participants randomized to LARC chose either a subdermal implant, levonorgestrel intrauterine system, or copper IUD. If randomly assigned to SARC, participants chose either oral contraceptives or DMPA. For randomization, we used opaque, sealed, and sequentially ordered envelopes for each health center. No blinding was used for any aspect of the trial. At the time of enrollment, we collected standard sociodemographic/reproductive data, health insurance status, and free product preferences (SARC or LARC). More details on eligibility, enrollment procedures, and how participant characteristics varied by study arm/cohort can be found in the previous publications.[7, 8]
Also at enrollment, we asked participants why they had never tried LARC. Participants provided spontaneous answers. Those who cited any one of six negative aspects of LARC technologies as a reason for not trying LARC previously were categorized as having a negative attitude. The reasons included fear of pain/injury from insertion and/or removal, fear of side effects/health risks, “not sure she would like it,” inconvenience of another clinic visit for removal, modesty regarding IUD insertion, or general dislikes of using an implanted device.
At any time and for any reason, participants were free to switch methods or stop entirely and continue under observation, and LARC participants were informed that they could have the product removed without charge. We did not require follow-up clinic visits, since such visits might artificially influence contraceptive use patterns. We collected data on contraceptive use at spontaneous clinic visits and at six, 12, 18, and 24 months through online questionnaires. Participants received gift cards for completing each questionnaire ($25 for the 6-, 12- and 18-month questionnaires and $75 for the 24-month questionnaire). We asked participants about the main reason for any method switching/discontinuation, incident pregnancies, and pregnancy plans. We also asked participants these verbatim satisfaction questions: 1) Overall, how happy are you (or were you) with the initial method? 2) Would you ever use the method again in the future? and 3) Did you ever recommend the method to a friend/relative? We asked these three satisfaction questions even if the participant discontinued her initial method.
The main endpoints were contraceptive method discontinuation and unintended pregnancy as tallied in three cohorts: preference-SARC, randomized-SARC, and randomized-LARC. The primary comparisons involved the randomized cohorts: SARC versus LARC. Secondary comparisons involved just SARC users (preference versus randomized) to help bridge any results from a randomized trial to an observational cohort.
Analysis
We defined contraceptive discontinuation as the first significant interruption in use of the original method -- a lapse greater than two weeks for SARC and LARC product removal. To provide a broader view of contraceptive satisfaction, intentions, and needs, we also reported subsequent re-starts or method switching. In this analysis, we included only participants who had discontinuation events unrelated to subsequent pregnancy to better evaluate immediate contraceptive actions. We classified pregnancies as intended if the participant wanted the pregnancy at that time or sooner and unintended if the participant stated she did not want a pregnancy at that time or ever and/or had an induced abortion.
Given the over-arching goal of measuring the impact of trying LARC in a population seeking SARC, we applied intent-to-treat principles once the method was initiated. Any unintended pregnancies after method switching or discontinuation were tallied against the initial method. We used the product limit method (Kaplan-Meier method) to estimate the 24-month cumulative crude probabilities of method discontinuation and unintended pregnancy for the cohorts, and for specific contraceptive choices within those cohorts.[9] For statistical comparisons we applied the logrank test (to compare overall patterns as calculated throughout the time period). Second unintended pregnancies were also analyzed. Additionally, we used the crude cumulative probabilities of unintended pregnancy at the 24-month timepoint to compare the groups’ statuses at the very end of the observation period (binomial test); this provides a final analysis on the impact of participants’ initial choices.
As a supporting analysis to control for potential confounding effects that participants’ background factors may have had on the endpoints, we used Cox’s proportional hazards regression[10]. Proportional hazards modeling was used to explore whether LARC and SARC differences in risks of discontinuation and unintended pregnancy were maintained in the randomized cohort, and to determine whether the preference SARC users had similar patterns of discontinuation and unintended pregnancy compared to the randomized cohort. We included only variables that were at least moderately associated with the endpoints (P<=0.1) in the final regression models.
We used Wilcoxon-Mann-Whitney tests, Fisher’s exact tests, or chi square tests of association to identify any significant differences of subjects’ characteristics between cohorts. We used chi square tests to examine associations between method satisfaction at 24 months and cohort, stratified by discontinuation status. Also, we examined how baseline attitudes toward LARC might be associated with satisfaction at 12 and 24 months. We performed statistical analyses using SAS 9.4 (SAS Institute, Cary, NC).
3. Results
A total of 1,092 women were screened for eligibility; of the 916 who remained eligible, 57% (n=524) chose the preference cohort and 43% (n=392) chose the random assignment (Figure 1). Twenty participants in the randomized groups did not receive the intervention and the preference cohort had only two LARC users; the remaining 894 participants formed the cohort for analysis. Ninety-five percent, 92%, and 90% of the cohort completed a 12-month, 18-month, and 24-month interview, respectively. After accounting for 462 clinic visits, only eleven participants (1.2%) did not have any follow-up information.
Figure 1. Participant flow in study.
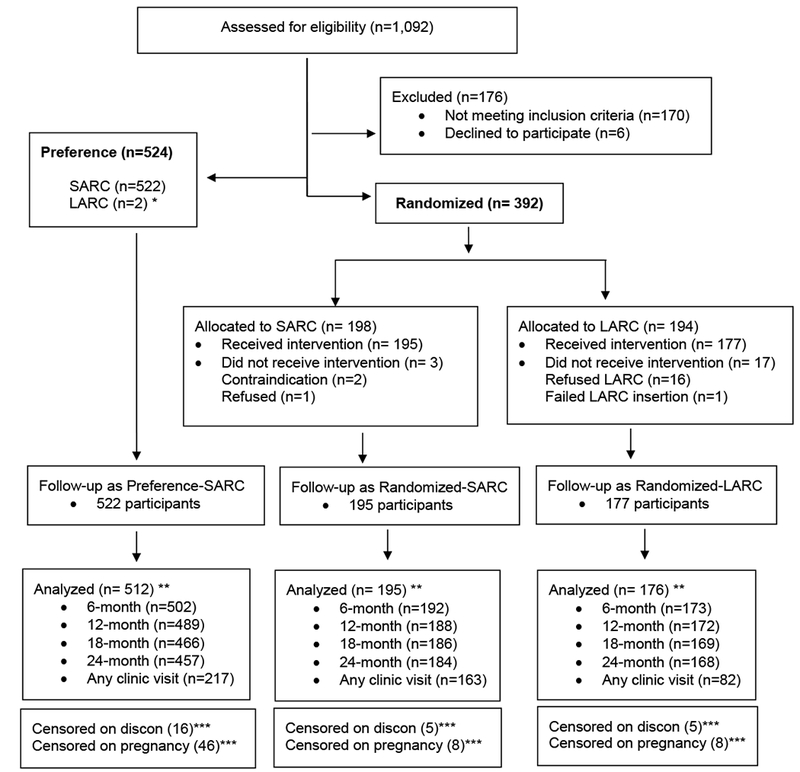
LARC = Long-acting reversible contraception (IUDs and implants)
SARC = Short-acting reversible contraception (oral contraceptives and DMPA)
* excluded from further analysis because of insufficient numbers for comparison to randomized LARC group
** all participants who had some follow-up information for analysis (n=11 completely lost to follow-up)
*** did not experience endpoint at time of last contact and did not have 24-month information
Participants in the preference-SARC, randomized-SARC, and randomized-LARC cohorts were similar in terms of age, marital status, previous abortion, education, pregnancy history, race, ethnicity, and other variables (Table 1). The randomized cohort was less likely to have health insurance than the preference group, the randomized-LARC group was least likely to want more children, and the preference-SARC group had the longest relationships with current partner.
Table 1.
Participant characteristics by study cohort
| Characteristic | Preference SARC (n= 522) n (%) or median (Q1-Q3) | Randomized SARC (n= 195) n (%) or median (Q1-Q3) | Randomized LARC (n= 177) n (%) or median (Q1-Q3) | p-value1 |
|
|---|---|---|---|---|---|
| Randomized Groups | SARC Groups | ||||
| Age | 23 (21-26) | 23 (21-26) | 23 (21-26) | 0.45 | 0.26 |
| Marital status | |||||
| Single | 443 (84.9) | 168 (86.2) | 149 (84.2) | 0.85 | 0.37 |
| Married | 63 (12.1) | 18 (9.2) | 18 (10.2) | ||
| Divorced/Separated | 16 (3.1) | 9 (4.6) | 10 (5.6) | ||
| Months with current partner | 15 (6-36) | 11 (3-25) | 12 (4-36) | 0.24 | <0.01 |
| Race/Ethnicity2 | |||||
| Hispanic | 68 (13.1) | 30 (15.4) | 14 (7.9) | 0.09 | 0.58 |
| Non-Hispanic, white | 269 (51.8) | 105 (53.8) | 111 (62.7) | ||
| Non-Hispanic, black | 124 (23.9) | 44 (22.6) | 34 (19.2) | ||
| All other single and multiple race (non-Hispanic only) | 58 (11.2) | 16 (8.2) | 18 (10.2) | ||
| Education attainment | |||||
| Not complete high school | 20 (3.8) | 7 (3.6) | 9 (5.1) | 0.65 | 0.34 |
| High school | 199 (38.1) | 82 (42.1) | 73 (41.2) | ||
| Post-high school | 102 (19.5) | 26 (13.3) | 30 (16.9) | ||
| College | 157 (30.1) | 66 (33.8) | 57 (32.2) | ||
| Graduate school | 44 (8.4) | 14 (7.2) | 8 (4.5) | ||
| Currently working | 361 (69.2) | 148 (75.9) | 136 (76.8) | 0.83 | 0.08 |
| Health insurance | |||||
| None | 189 (36.2) | 93 (47.7) | 84 (47.5) | 0.93 | 0.01 |
| Private | 266 (51) | 87 (44.6) | 76 (42.9) | ||
| Medicaid | 45 (8.6) | 7 (3.6) | 8 (4.5) | ||
| Other | 22 (4.2) | 8 (4.1) | 9 (5.1) | ||
| Reproductive health | |||||
| Previous unintended pregnancy | 134 (25.7) | 59 (30.3) | 59 (33.3) | 0.52 | 0.22 |
| Ever had an abortion | 122 (23.4) | 53 (27.2) | 53 (29.9) | 0.45 | 0.45 |
| Number of previous pregnancies | |||||
| 0 | 366 (70.1) | 123 (63.1) | 110 (62.1) | 0.70 | 0.10 |
| 1 | 95 (18.2) | 47 (24.1) | 38 (21.5) | ||
| 2 | 33 (6.3) | 14 (7.2) | 18 (10.2) | ||
| 3+ | 28 (5.4) | 11 (5.6) | 11 (6.2) | ||
| Among those previously pregnant: | |||||
| Months since last pregnancy ended | 15 (3-37) | 9 (1-23) | 10 (1-31) | 0.99 | 0.04 |
| Currently menstruating | 98 (18.8) | 34 (17.4) | 35 (19.8) | 0.56 | 0.68 |
| Wants more children | 440 (84.3) | 170 (87.2) | 136 (76.8) | <0.01 | 0.33 |
| Months from today when pregnancy is desired | 60 (36-96) | 60 (48-96) | 60 (48-98) | 0.77 | 0.11 |
| Motivation to opt for randomization | |||||
| To receive free SARC | NA | 32 (16.4) | 9 (5.1) | <0.01 | NA |
| To receive free LARC | 41 (21.0) | 67 (37.9) | |||
| To receive any free method | 122 (62.6) | 101 (57.0) | |||
Preference SARC consisted of 423 oral contraceptives users and 99 DMPA users.
Randomized SARC consisted of 147 oral contraceptive users and 48 DMPA users.
Randomized LARC consisted of 120 Mirena users, 6 ParaGard users, and 51 Implanon/Nexplanon users.
For categorical variables, Exact test was used for any cell number < 5 and Chi-square tests was used for all cells >=5; For continuous variables, Wilcoxon test was used.
Three participants did not report their race/ethnicity.
Reasons for never trying LARC previously varied significantly by cohort (Table 2). Cost of LARC was the predominant reason for never trying LARC among the randomized cohort (cited by 47%) while this reason was only cited by 9% in the preference-SARC cohort. Preference-SARC participants had more negative attitudes (dominated by health concerns) toward LARC than randomized participants (59% versus 32%, respectively).
Table 2.
Reasons for never trying LARC previously, by study cohort
| Reasons for not trying LARC (n) * | Preference SARC (n= 522) n (%) | Randomized SARC (n= 195) n (%) | Randomized LARC (n= 177) n (%) | p-value1 |
|
|---|---|---|---|---|---|
| Randomized Groups | SARC Groups | ||||
| Fear of pain/injury from insertion/removal ** | 148 (28.4) | 35 (17.9) | 30 (16.9) | 0.70 | <0.01 |
| Fear of side effects/health risks ** | 126 (24.1) | 31 (15.9) | 16 (9.0) | 0.12 | 0.02 |
| Modesty issues regarding insertion ** | 20 (3.8) | 3 (1.5) | 1 (0.6) | 0.43 | 0.19 |
| Not sure if she would like it ** | 66 (12.6) | 17 (8.7) | 10 (5.6) | 0.31 | 0.15 |
| Inconvenience of another visit for removal ** | 12 (2.3) | 3 (1.5) | 1 (0.6) | 0.43 | 0.54 |
| Averse to having a device inside the body ** | 30 (5.7) | 7 (3.6) | 1 (0.6) | 0.12 | 0.43 |
| No previous knowledge of any LARC method | 52 (10.0) | 12 (6.2) | 12 (6.8) | 0.81 | 0.11 |
| Too expensive | 46 (8.8) | 83 (42.6) | 91 (51.4) | 0.22 | <0.01 |
| No long-term needs | 44 (8.4) | 8 (4.1) | 7 (4.0) | 0.61 | 0.09 |
| Never in consistent relationship or sexually active | 13 (2.5) | 11 (5.6) | 9 (5.1) | 0.84 | 0.04 |
| Did not know where to get method | 5 (1.0) | 1 (0.5) | 2 (1.1) | 0.58 | 0.57 |
| Prefers to be in control of stopping contraception | 67 (12.8) | 5 (2.6) | 6 (3.4) | 0.70 | <0.01 |
| Likes current method including any health benefits | 55 (10.5) | 13 (6.7) | 12 (6.8) | 0.64 | 0.28 |
| Not sufficiently informed about LARC | 12 (2.3) | 11 (5.6) | 10 (5.6) | 0.66 | 0.03 |
| Has misinformation or misperception on LARC methods | 13 (2.5) | 9 (4.6) | 8 (4.5) | 0.68 | 0.16 |
| Previous provider bias against LARC | 3 (0.6) | 2 (1.0) | 5 (2.8) | 0.24 | 0.51 |
| No time/a hassle/I’m lazy | 5 (1.0) | 2 (1.0) | 3 (1.7) | 0.58 | 0.93 |
| Other | 11 (2.1) | 2 (1.0) | 2 (1.1) | 0.68 | 0.48 |
| Any negative attitude toward LARC technologies ** | 310 (59.4) | 71 (36.4) | 47 (26.6) | 0.04 | <0.01 |
For categorical variables, Exact test was used for any cell number < 5 and Chi-square tests was used for all cells >=5
Individual responses do not sum to 100% since multiple answers were allowed.
Included in any negative attitude summary variable.
In the primary endpoint comparisons among randomized participants, 24-month method continuation probabilities were 25.5% (95% Cl, 22.3 – 28.7) for SARC users and 64.3% (95% Cl, 56.6 –70.9) for LARC users. (p-value<0.001) (Table 3). The 24-month cumulative unintended pregnancy probability was higher for randomized-SARC (6.9%, 95% Cl: 3.3 – 10.6) compared to randomized-LARC (3.6%, 95% Cl: 1.8 – 6.4). Statistical tests for comparing randomized-LARC and randomized-SARC on unintended pregnancy were mixed: binomial at 24-month timepoint (p=0.02) and logrank survival probabilities (p=0.14 for first pregnancies and p=0.07 when including second pregnancies).
Table 3.
Cumulative crude probability of contraceptive method continuation and unintended pregnancy within 24 monthsa
| Preference-SARC (n=522) | Randomized-SARC (n=195) | Randomized-LARC (n=177) | |
|---|---|---|---|
| Number of discontinuing original method | 300 | 142 | 62 |
| Person-years | 654.5 | 225.2 | 274.2 |
| Probability of method continuation (95% CI)b | 40.0 (38.9–41.1) | 25.5 (22.3–28.7) | 64.3 (56.6–70.9) |
| OC only: | OC only: | IUD only: | |
| 45.0 (40.1–49.8) | 31.1 (23.7–38.7) | 65.0 (55.8–72.7) | |
| DMPA only: 18.4 (14.3–22.5) | DMPA only: 9.5 (5.0–14.0) | Implant only: 62.6 (47.9–74.3) | |
| Combining SARC preference and randomized cohorts | OC: 41.3 (37.2–45.4) DMPA: 15.4 (12.3–18.5) |
||
| Reason for method discontinuation [N (%)] | |||
| Wanted to get pregnant | 19 (3.6) | 6 (3.1) | 5 (2.8) |
| Not having sex | 39 (7.5) | 11 (5.6) | 0 (0.0) |
| Side effects | 67 (12.8) | 31 (15.9) | 46 (26.0) |
| Inconvenience of getting more | 34 (6.5) | 18 (9.2) | 0 (0.0) |
| Cost | 27 (5.2) | 7 (3.6) | 0 (0.0) |
| Got pregnant accidentally | 9 (1.7) | 3 (1.5) | 0 (0.0) |
| Forgot to redosec | 40 (7.7) | 20 (10.3) | 0 (0.0) |
| Other | 19 (3.6) | 10 (5.1) | 4 (2.3) |
| IUD expulsion | 0 (0.0) | 0 (0.0) | 6 (3.4) |
| No reasons given | 46 (8.8) | 36 (18.5) | 1 (0.6) |
| Did not discontinue | 222 (42.5) | 53 (27.2) | 115 (65.0) |
| Actions taken after discontinuing original methodd | |||
| Restarted original method | 71 (26.2%) | 29 (22.1%) | 0 (0.0%) |
| Switched to other short-acting method | 96 (35.4%) | 48 (36.6%) | 45 (72.6%) |
| Switched to LARC (or different LARC) | 22 (8.1%) | 25 (19.1%) | 3 (4.8%) |
| Declared no method being used | 74 (27.3%) | 26 (19.9%) | 14 (22.6%) |
| No information reported or missing | 8 (3.0%) | 3 (2.2%) | 0 (0.0%) |
| Number of pregnancies | |||
| Intended | 18 | 5 | 3 |
| Unintendede | 47 | 13 | 6 |
| Person-years | 908.1 | 359.9 | 334.9 |
| Probability of unintended pregnancy (95% CI)f | 9.9 (7.2–12.6) | 6.9 (3.3–10.6) | 3.6 (1.8–6.4) |
| Combining SARC preference and randomized cohorts | OC: 8.5 (6.1–10.8) DMPA: 11.5 (6.0–18.0) |
||
OC = oral contraceptives.
Eleven participants were completely lost to follow-up and are not included.
Between randomized groups: p<.0001; between SARC groups: p=.0013.
Includes forgot to take the pills or get a new injection, forgot to get new pill packs and misplaced pill packs.
Includes only participants who had discontinuation events unrelated to subsequent pregnancy to better evaluate immediate contraceptive actions [preference-SARC (n=271), randomized-SARC (n=131), randomized-LARC (n=62)].
Not included are five repeat unintended pregnancies (three in preference-SARC and two in randomized-SARC).
Based only on first unintended pregnancies. Between randomized groups: p=.14 (log-rank test) and p=.02 (binomial test at 24-month time point); between SARC groups: p=.25 (log-rank test) and p=.06 (binomial test at 24-month time point).
In the secondary comparisons involving only SARC users, the continuation probability was higher in the preference group (40.0% (95% Cl, 38.9 – 41.1)) compared with the randomized group 25.5% (95% Cl, 22.3 – 28.7) p-value=0.001)). However, the SARC-randomized group and SARC-preference group had statistically equivalent probabilities of unintended pregnancy (6.9% (95% Cl:3.3–10.6) and 9.9% (95% Cl:7.2– 12.6), respectively (p-value=0.25). Incidence of intended pregnancy was similar across all groups (p-value>0.05), though the number of events for analysis was small.
Graphically, patterns of product continuation and unintended pregnancy were similar for SARC groups; LARC users had a distinctly different path (Figures 2 and 3). In the LARC cohort, the cumulative effect of product removal over time increased the probability of unintended pregnancy as shown in the second year.
Figure 2. Cumulative crude probability of continuation by study cohort.
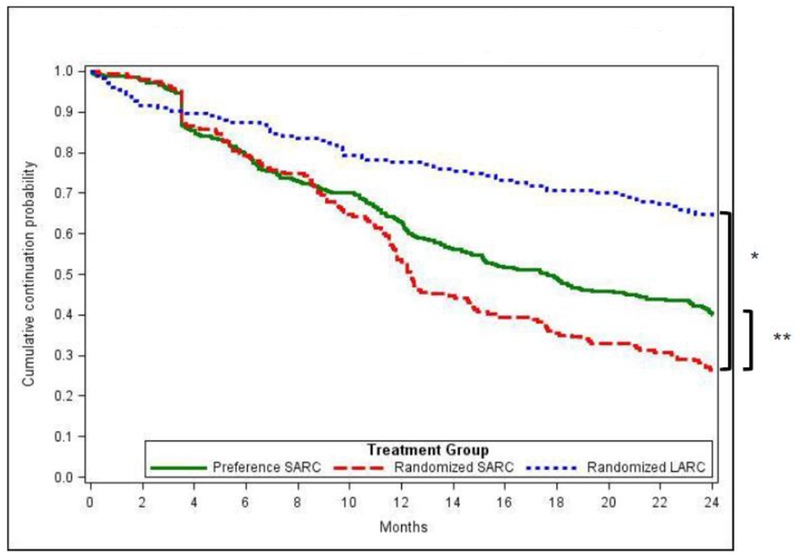
* Randomized groups (primary comparison): P<0.0001 Based on logrank tests
** SARC groups (secondary comparison): P=0.0013 Based on logrank tests
Figure 3. Cumulative crude probability of unintended pregnancy by study cohort.
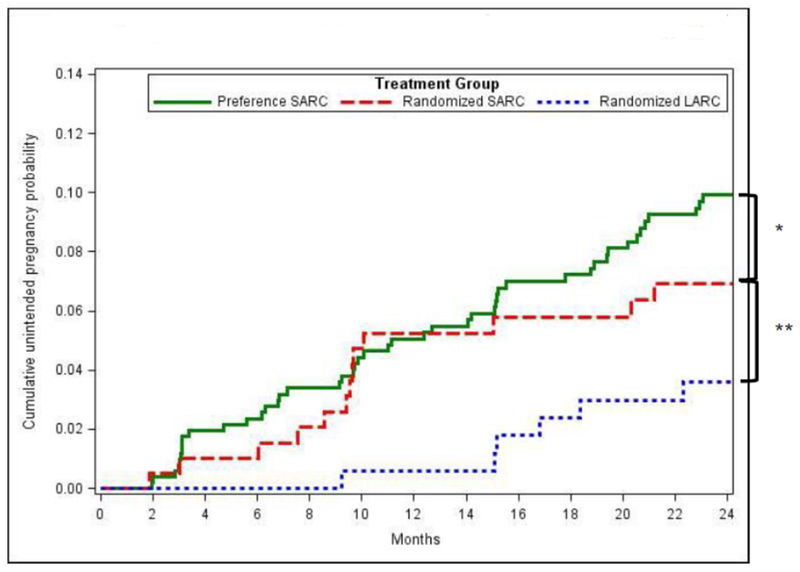
Based only on first unintended pregnancies.
* SARC groups (Secondary comparison): P=0.25 (logrank test), P=0.06 (binomial test at 24-month timepoint)
** Randomized groups (primary comparison): P=0.14 (logrank test), P=0.02 (binomial test at 24-month timepoint)
In the supporting analysis using proportional hazards modeling in the randomized cohort, we controlled for the following factors that met the variable selection criteria: age, Hispanic ethnicity, education, motivation to opt for randomization, and desire for more children. Compared with LARC users, SARC users were more likely to discontinue from the assigned contraception with adjusted hazard ratio (AHR) of 2.8 (95% Cl, 2.0 – 3.8), and also more likely to experience unintended pregnancy but not statistically significant (AHR: 2.1 (95% Cl, 0.8 – 5.5) (data not shown). In comparing the experiences of the preference SARC cohort to the randomized SARC cohort, we controlled for Hispanic ethnicity, education, months with current partner, health insurance, and employment status: the risks of discontinuation in the randomized SARC cohort are statistically higher than the preference SARC cohort (AHR: 1.3 (95% Cl, 1.1 – 1.6)), nevertheless, the risks of unintended pregnancy were statistically similar (AHR: 0.6 (95% Cl, 0.3 – 1.2)) (data not shown).
Reasons for discontinuation varied by cohort (Table 3). Side effects were the predominant reason for all participants; SARC users cited a variety of reasons related to re-dosing challenges (e.g., cost, inconvenience, forgetfulness). About 10% of SARC users said that lack of sexual activity led to discontinuation, while none of the LARC users cited that reason. After stopping use, most users in all groups started SARC. Nineteen percent of randomized-SARC adopted a LARC method, while 8% in the preference group switched to LARC.
After 24 months, happiness levels in the three cohorts were similar in the comparisons using all participants (Table 4). Among the subset of participants still using their original method at 24 months, happiness was high. LARC users who had the product removed were disproportionately unhappy compared to other discontinuers. When participants were asked about using the product in the future and whether they would recommend the product to friends, discontinuers within each cohort were less positive than continuers.
Table 4.
Measures of satisfaction with initial method as assessed at 24 months, by cohort and method discontinuation status
| Totala | Continuing users | Discontinuers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Pref-SARC N=456 | Rand-SARC N=184 | Rand-LARC N=168 | Pref-SARC N=187 | Rand-SARC N=46 | Rand-LARC N=109 | Pref-SARC N=269 | Rand-SARC N=138 | Rand-LARC N=59 |
| Level of happiness with method (% distribution)d | |||||||||
| Happy | 77.6 | 75.0 | 71.4 | 90.3 | 89.6 | 92.7 | 68.2 | 69.9 | 32.2 |
| Neutral | 11.4 | 13.0 | 8.9 | 2.5 | 6.2 | 3.7 | 18.0 | 15.4 | 18.6 |
| Unhappy | 11.0 | 12.0 | 19.6 | 7.2 | 4.2 | 3.7 | 13.8 | 14.7 | 49.2 |
| Would use method again in future (%)b,c,d | 86.2 | 78.7 | 72.6 | 98.5 | 97.9 | 89.0 | 77.0 | 71.9 | 42.4 |
| Recommended that a friend/relative try the method (%)d,e | 79.8 | 79.3 | 80.6 | 89.8 | 88.4 | 92.5 | 72.2 | 76.2 | 58.6 |
Missing data on satisfaction variables: Pref-SARC (n=65), Rand-SARC (n=11), Rand-LARC (n=9).
p<.05 for “Total” column comparisons.
p<.05 for “Continuing users” column comparisons.
p<.05 for “Discontinuing users” column comparisons.
Among those who discussed the topic with a friend or relative (n=402, Pref-SARC; n=169, Rand-SARC; n=165, Rand-LARC).
Negative attitudes toward LARC at baseline were not associated with any satisfaction measures at 12 months (Table 5). However, at 24 months, level of happiness with LARC was distributed differently resulting in a positive association with baseline attitudes (p-value < 0.05); many participants with negative baseline attitudes shifted away from reporting happy or unhappy and instead reported being neutral. The other measures of satisfaction were statistically similar across baseline attitudes. Negative baseline LARC attitudes were not associated with LARC removals over the 24-month period (data not shown).
Table 5.
Satisfaction levels with LARC at12 and 24 months, by negative attitudes toward LARC technologies at baseline
| Baseline attitudes toward LARC technologies | 12-Month measures of satisfaction |
||||||
|---|---|---|---|---|---|---|---|
| Level of happiness (% distribution) |
Would use method again (%) | Recommended that a friend or relative try the method (%)a | N | ||||
| Happy | Neutral | Unhappy | Total | ||||
| No negative | 71.6 | 7.1 | 21.3 | 100.0 | 74.8 | 79.2 | 127 |
| Some negative | 71.1 | 6.7 | 22.2 | 100.0 | 77.8 | 79.5 | 45 |
| Baseline attitudes toward LARC technologies | 24-Month measures of satisfaction |
||||||
|---|---|---|---|---|---|---|---|
| Level of happiness (% distribution)b |
Would use method again (%) | Recommended that a friend or relative try the method (%)* | N | ||||
| Happy | Neutral | Unhappy | Total | ||||
| No negative | 73.4 | 5.6 | 21.0 | 100.0 | 75.0 | 82.8 | 124 |
| Some negative | 65.9 | 18.2 | 15.9 | 100.0 | 65.9 | 74.4 | 44 |
Among those who discussed with a friend/relative.
p<.05.
4. Discussion
This 24-month analysis strengthens and builds upon the results we described previously with 12-month data.[7] Random assignment to LARC and the decision to try a product led to high contraceptive continuation and superior protection from unintended pregnancy, compared to SARC. These findings are noteworthy because our study population was restricted to women seeking short-acting methods, and random assignment reduced bias in measuring and comparing LARC/SARC effectiveness.
The randomized SARC group experienced patterns of contraceptive continuation and unintended pregnancy that were similar to the natural cohort of short-acting users who did not want random assignment (preference SARC group); this lends support for internal validity. Simultaneously, this finding supports external generalizability of the randomized results and completes the overall picture to help validate real-world applicability. We observed some separation of probabilities (preference-SARC versus randomized-SARC) in the second year, likely due to randomized participants no longer receiving free product. Even still, preference-SARC probabilities tracked in a pattern more similar to randomized-SARC.
Our estimated 24-month contraceptive continuation probabilities had similarities and differences compared to estimates from the Contraceptive Choice project (Choice).[11] Notably, both studies found higher continuation rates of LARC relative to SARC. In addition, our preference oral contraceptive users’ continuation rate of 45% was similar to Choice (43%). Our DMPA rate was far lower due to our strict re-injection window of 105 days, while our randomized LARC and randomized oral contraceptive probabilities (64% and 31%, respectively) were also lower than Choice (77% and 43%, respectively). In summary, it appears that our randomized study population had naturally lower continuation rates than Choice; this conclusion is reached by focusing on differences in the LARC estimates (both studies used the same definitions of a discontinuation event for LARC). Our LARC continuation probabilities may be lower than Choice because we recruited women who intended to use SARC methods. Differences in demographics might help explain dissimilar continuation probabilities in the two studies.
We used intent-to-treat principles and always attributed unintended pregnancies to the original method to measure the impact of trying LARC. One important advantage of this approach is that we did not need to second-guess self-reported compliance with the methods. If unintended pregnancy occurred, then the failure was simply attributed to the first-used method. Participants switched contraceptive regimens over time as well, which was of no analytical consequence in our intent-to-treat approach. It should be recognized that our crossovers to LARC (improving effectiveness) and crossovers to SARC (decreasing or maintaining lower effectiveness) probably decreased our ability to measure an impact on unintended pregnancy using the intent-to-treat approach. As explained in our previous publication, a weakness of our effort in the eyes of trialists is that we discontinued participants who did not start the assigned regimen and thus we could only apply intent-to-treat principles after the first dose was used.
Our estimates of reductions in unintended pregnancy from trying LARC might be conservative if applied to other short-acting methods, since the remaining forms of SARC are less effective than pills and DMPA.[12] Reversible contraceptive use in the US is dominated by non-LARC methods (about 82% of reversible method use). Thus, expanded voluntary uptake of LARC, instead of SARC, could have substantial public health impact. While many SARC users might want to try LARC, a large proportion cannot because of access issues (e.g., high cost and lack of trained providers). Our study focused on contraceptive use patterns and behaviors that are independent of access barriers.
This study has limitations. First, it was done in only three clinics in one state. Second, the study was not powered to measure differences in incidence of unintended pregnancy. Third, the follow-up period was only two years. These weaknesses may prevent full generalizability to other environs and introduce some uncertainties about longer-term impact and other endpoints.
The results of this study are highly relevant and reassuring to women who have access to LARC, yet are hesitant to try a product. Our LARC users had no intention of starting LARC when they sought services and a sizeable proportion even had negative attitudes toward the available products. Baseline negative attitudes toward LARC technologies were not associated with dissatisfaction measures at 12 months and only somewhat predictive of dissatisfaction at 24 months. LARC users sought and achieved intended pregnancy as much as both SARC groups; thus, a clinic visit for product removal did not seem to be a barrier to exercising personal fertility goals.
Individuals should only start a method after making informed decisions; the results of this study should be considered in the decision-making process. Prior to the current work, there was little scientific evidence that generally positive experiences of self-selecting LARC users (upon which all effectiveness measures are derived) could even translate to a population of women not actively seeking LARC. Arguably, LARC has been promoted on a presumptuous scientific foundation. The evidence from this report is strong even after two years of follow-up. It should be integrated into applicable counseling situations in ways that guarantee autonomous user-decisions on choice of contraceptive method.
Promoting “LARC first” because of highest contraceptive effectiveness has been criticized because it may affect autonomy of choice and it does not account for personal preferences that may affect satisfaction and well-being.[13] Aversions to invasive procedures and acute pain are natural. Proper counseling on LARC should never dismiss such aversions, but rather shed light on the tradeoffs. Swallowing contraceptive pills is easy, non-invasive, and a highly acceptable delivery system for receiving medications; however, long-term, consistent use is challenging. In these contexts, the results of this study can be explained and fit into tiered[14] and rights-based[15] and patient-centered themes.[16, 17] “A study in North Carolina found that oral contraceptive users, who had no intention of using LARC, gave it a try. As it turned out, they had overall positive experiences and avoided unintended pregnancy, as a whole, much better than if they had just stayed with the pill. Even users with some initial fears and concerns about LARC fared well with their decision.”
In conclusion, our study used a rigorous approach and reduced selection bias to better isolate the impact of trying LARC and validates the findings of the observational cohort study from St. Louis. LARC users reported high satisfaction and the decision to try LARC resulted in high contraceptive effectiveness. These results, combined with our examination of the limited role that initial negative LARC attitudes play in patient satisfaction with LARC, may encourage more women to make voluntary, informed decisions to try LARC.
Acknowledgements
Primary funding for this research study was provided by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD067751. The second year of follow-up was funded by Bayer Healthcare Pharmaceuticals, Inc. In addition, this grant received product donations from Teva Pharmaceuticals, Bayer Healthcare Pharmaceuticals, Inc., and Merck Sharp & Dohme Corp. Finally, an anonymous donor provided additional funding. This study is registered on ClinicalTrials.gov (NCT01299116). The content of this report is solely the responsibility of the authors and does not necessarily represent the views of the funders, FHI360, Planned Parenthood Federation of America, Inc. or Planned Parenthood South Atlantic, Inc.
Primary funding for this research study was provided by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD067751. Funding for extended follow-up as described in this paper was provided by Bayer HealthCare Pharmaceuticals. In addition, this grant received product donations from Teva Pharmaceuticals, Inc. In addition, Bayer HealthCare Pharmaceuticals, Inc., and Merck Sharp & Dohme Corp. Finally, an anonymous donor provided additional funding. This study is registered on ClinicalTrials.gov (NCT01299116).
D.H. has served on Scientific Advisory Boards for Bayer HealthCare Pharmaceuticals, Teva Pharmaceuticals, and OCON Medical. As principal investigator for this research, he received product donations from Bayer HealthCare Pharmaceuticals, Teva Pharmaceuticals, and Merck Sharp & Dohme Corp.
The content of this report is solely the responsibility of the authors and does not necessarily represent the views of the funders, FHI360, Planned Parenthood Federation of America, Inc. or Planned Parenthood South Atlantic, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The remaining authors report no potential conflicts of interest.
References
- [1].Daniels K, Daugherty J, Jones J, Mosher W. Current Contraceptive Use and Variation by Selected Characteristics Among Women Aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015:1–14. [PubMed] [Google Scholar]
- [2].Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. The New England journal of medicine. 2012;366:1998–2007. [DOI] [PubMed] [Google Scholar]
- [3].Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstetrics and gynecology. 2011;117:1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].CDC. Centers for Disease Control Prevention. Contraceptive methods available to patients of office-based physicians and title X clinics — United States, 2009–2010. MMWR Morbidity and mortality weekly report. 2011;60:1–4. [PubMed] [Google Scholar]
- [5].Foster DG, Barar R, Gould H, Gomez I, Nguyen D, Biggs MA. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543–52. [DOI] [PubMed] [Google Scholar]
- [6].Coombe J, Harris ML, Loxton D. What qualities of long-acting reversible contraception do women perceive as desirable or undesirable? A systematic review. Sex Health. 2016. [DOI] [PubMed] [Google Scholar]
- [7].Hubacher D, Spector H, Monteith C, Chen PL, Hart C. Long-acting reversible contraceptive acceptability and unintended pregnancy among women presenting for short-acting methods: a randomized patient preference trial. American journal of obstetrics and gynecology. 2017;216:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hubacher D, Spector H, Monteith C, Chen PL, Hart C. Rationale and enrollment results for a partially randomized patient preference trial to compare continuation rates of short-acting and long-acting reversible contraception. Contraception. 2015;91:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaplan EL, Meier P. Nonparametrics estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- [10].Cox DR. Regression models and life tables. J R Stat Soc [Ser B]. 1972;34:187–220. [Google Scholar]
- [11].O’Neil-Callahan M, Peipert JF, Zhao Q, Madden T, Secura G. Twenty-four-month continuation of reversible contraception. Obstetrics and gynecology. 2013;122:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Trussell J Contraceptive efficacy In: Hatcher R, Trussell J, Nelson A, Cates W, Kowal D, Policar M, editors. Contraceptive Technology. 20th Revised Edition ed. Atlanta, GA: Ardent Media, Inc; 2011. p. 779–863. [Google Scholar]
- [13].Gomez AM, Fuentes L, Allina A. Women or LARC first? Reproductive autonomy and the promotion of long-acting reversible contraceptive methods. Perspectives on sexual and reproductive health. 2014;46:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gavin L, Moskosky S, Carter M, et al. Providing quality family planning services: Recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2014;63:1–54. [PubMed] [Google Scholar]
- [15].World Health Organization. Ensuring human rights in the provision of contraceptive information and services: guidance and recommendations. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- [16].Morse JE, Ramesh S, Jackson A. Reassessing Unintended Pregnancy: Toward a Patient-centered Approach to Family Planning. Obstet Gynecol Clin North Am. 2017;44:27–40. [DOI] [PubMed] [Google Scholar]
- [17].Turok DK. The quest for patient-centered family planning. American journal of obstetrics and gynecology. 2017;216:98–100. [DOI] [PubMed] [Google Scholar]


