Abstract
Impaired wound healing is a major secondary complication of type 2 diabetes that often results in limb loss and disability. Normal tissue repair progresses through discrete phases including hemostasis, inflammation, proliferation, and remodeling. In diabetes, normal progression through these phases is impaired resulting in a sustained inflammatory state and dysfunctional epithelialization in the wound. Due to their plasticity, macrophages play a critical role in the transition from the inflammation phase to the proliferation phase. Diabetes disrupts macrophage function by impairing monocyte recruitment to the wound, reducing phagocytosis, and prohibiting the transition of inflammatory macrophages to an anti-inflammatory state. Diabetes also impedes keratinocyte and fibroblast function during the later phases resulting in impaired epithelialization of the wound. Several recent studies suggest that altered epigenetic regulation of both immune and structural cells in wounds may influence cell phenotypes and healing, particularly in pathologic states, such as diabetes. Specifically, it has been shown that macrophage plasticity during wound repair is partly regulated epigenetically and that diabetes alters this epigenetic regulation and contributes to a sustained inflammatory state. Epigenetic regulation is also known to regulate keratinocyte and fibroblast function during wound repair. In this review, we provide an introduction to the epigenetic mechanisms that regulate tissue repair and highlight recent findings that demonstrate how epigenetic events are altered during the course of diabetic wound healing.
Keywords: Epigenetics, Wound Healing, Type 2 Diabetes
Introduction
Over 30 million people over the age of 18 in the US have diabetes mellitus (1). Additionally, another 86 million live with ‘pre-diabetes’, a condition that can progress to type 2 diabetes (T2D) (1). The overall prevalence of diabetes continues to increase, thereby contributing to increasingly high healthcare costs (1). Diabetes-related healthcare expenditure was 548 billion USD in 2013, accounting for 11% of the total adult healthcare cost, and is projected to exceed 627 billion USD by 2035 (2). Further, it is estimated that approximately 7 million people go undiagnosed thus delaying disease treatment and management (1). Non-healing wounds that result in lower limb amputation, are among the most common complications associated with diabetes and account for more than 200 billion USD annually in healthcare costs and loss of productivity (3). Importantly, amputation is associated with high mortality rates of 16.7% at 12 months and over 50% at 5 years, rates that are worse than many cancers (4).
Impaired wound healing in diabetes is the result of a combination of factors that promote inflammation and disrupt epithelialization and wound closure. Although this process is complex, it has been shown that neutrophils and macrophages recruited to the site of the wound are critical components of the healing process (5–7). During the normal healing process, macrophages are initially pro-inflammatory and then transition to an anti-inflammatory phenotype where they promote tissue repair and transition to the next phase of healing (6, 8, 9). In diabetic wounds, however, this macrophage transition is altered, causing them to remain in a pro-inflammatory state and prevent wound resolution (10–13). The factors driving the macrophage transition are not fully understood but recent evidence suggests that epigenetics plays a role in regulating macrophage function in both normal and diabetic wound healing (11, 14–17). These processes include DNA methylation of CpG islands, and methylation of histone tails.
Additionally, re-epithelialization is delayed in diabetic wounds due to impaired keratinocyte and fibroblast function brought on by hyperglycemia and accumulation of advanced glycation end-products (AGEs) (18–21) and epigenetic regulation has also been implicated in these processes (22–24).
Given the impact on human health, substantial work has focused on understanding the mechanisms by which the cells involved in the wound healing response are regulated and how these mechanisms are disrupted in the setting of diabetes. In this review, we provide a brief overview of epigenetics and diabetic wound healing and highlight the recent advances related to the convergence of these two fields.
Normal wound healing
Phases of wound healing
Wound healing is a dynamic process that occurs in a programmed series of four phases: hemostasis, inflammation, proliferation, and remodeling (25). These steps occur in a linear forward fashion under normal conditions. Following injury, hemostasis occurs and is characterized by the recruitment of platelets and circulating coagulant factors to the wound site to initiate clotting (26). Concurrent with platelet recruitment, injured cells release damage signaling factors, which activate resident macrophages (27–30), as well as damage associated molecular patterns (DAMPs) (31). Together, these stimulate recruitment of polymorphonuclear neutrophils (PMNs) from the vasculature to defend against pathogens (27, 29, 31). As platelets aggregate to the exposed collagen bed, they secrete cytokines and growth factors such as platelet-derived growth factor, that promote clotting and serve to further recruit PMNs (32). Once PMNs begin to migrate to the wound, this initiates the inflammation stage.
Neutrophils dominate during the early part of the inflammation phase; however, their presence is short-lived as they start to wane after 24–48 hours (33). Neutrophils release chemokines that are responsible for recruiting circulating monocytes from the peripheral blood to the wound site. Recruited monocytes that differentiate into macrophages and dendritic cells, along with F4/80+ resident tissue macrophages, carry out the critical steps of the inflammatory phase of wound healing (10, 34). However, the specific roles of recruited monocytes/macrophages, dendritic cells, and tissue macrophages in wound healing are unclear (35). Wound macrophages initially display a “classical” activation state, indicated by expression of pro-inflammatory cytokines such as IL-12, IL-1β, IL-6, TNFα, and iNOS. Pro-inflammatory, or M1-like, macrophages work to clear debris and recruit additional inflammatory cells. As the inflammatory phase progresses, macrophages phagocytose apoptotic cells in the wound and chemokines are released (e.g., CXCL12), promoting the transition from a pro-inflammatory state to one promoting tissue repair and remodeling (36, 37). This occurs as the predominant macrophage phenotype in the wound transitions to an “alternatively activated” state, or M2-like, secreting anti-inflammatory cytokines such as IL-4, IL-10, IL-13, and TGFβ (34, 38). In vivo, macrophages exist along a spectrum and the distinct phenotypes seen in vitro (e.g., M1, M2) are rarely seen (34, 39, 40).
The subsequent proliferation phase is characterized by the recruitment and activation of keratinocytes and fibroblasts. During this phase, growth factors stimulate keratinocytes to reepithelialize the wound (38). During this time, the provisional matrix established by platelets during hemostasis is replaced by granulation tissue. Fibroblasts secrete proteinases and matrix metalloproteases (MMPs) to degrade the provisional matrix while simultaneously secreting collagen and other extracellular matrix (ECM) proteins into the granulation tissue (25).
The final phase of wound healing is the remodeling phase and begins once granulation tissue is present. Here, fibroblasts differentiate into contractile myofibroblasts that contract the wound and the collagen III deposited in the ECM during the proliferation phase is exchanged for collagen I which has a higher tensile strength (41). Once healed, the site is mechanically functional but many structural components, e.g., hair follicles, may not recover and the healed site has, at maximum, ~70% of the original tensile strength (38).
Diabetic foot ulceration and delayed wound healing
The etiology of non-healing diabetic foot ulcerations is multifactorial due to a combination of peripheral neuropathy, peripheral artery disease, and altered immune function (42). These factors work together to predispose the diabetic patient to ulceration and infection. Despite the cause of the wound, it is failure of the wound to heal that is responsible for considerable morbidity and mortality associated with diabetes. Neuropathic edema makes the diabetic foot susceptible to ulceration and infection (43) and ischemia resulting from occlusive arterial disease reduces blood flow (44), both contributing to delayed healing in the diabetic wound. Additionally, recruitment of endothelial progenitor cells is impaired in diabetes due to reduced nitric oxide (NO) production which, ultimately, results in impaired angiogenesis (45). However, the most direct effects on wound healing come from functional alterations in cells activated by the immune response, including platelets, macrophages, neutrophils, endothelial cells, fibroblasts, and keratinocytes; all contributing to a failure to progress through the normal phases of wound healing.
Platelets
Platelets are key constituents of hemostasis and are responsible for some of the earliest events in response to injury, initially forming a platelet plug. As healing progresses, however, the clot must be degraded in order for re-epithelialization to occur. Sustained high glucose and insulin-resistance causes platelets to release high levels of fibrinogen and plasminogen activator inhibitor 1 (PAI-1) making them more adherent to the vascular endothelium and more likely to aggregate (46, 47). Additionally, under diabetic conditions, platelets have been shown to be less responsive to NO released from the vascular endothelium which normally reduces aggregation at the vessel wall (48). Exacerbating this, defects in insulin signaling cause the vascular endothelium to produce less NO (49, 50). Thus, platelet dysfunction in diabetes contributes to development of micro-vascular disease that is well-established in diabetic patients. Hence, in the setting of wound healing, the platelet phenotype induced by diabetes hinders wound healing by reducing coagulation during hemostasis.
Neutrophils
Neutrophils are important components during the inflammatory phase of wound healing, working to clear pathogens. However, sustained recruitment and activation of neutrophils are associated with chronic, non-healing wounds (51–53). Elevated levels of neutrophil-derived proteases are believed to contribute to persistent inflammation and delayed wound healing (51, 52). In addition to proteases, neutrophils release extracellular traps (NETs) composed of decondensed chromatin lined with cytotoxic proteins to kill microbes (53). It has been shown that the protein responsible for NET formation, peptidyl deiminase 4 (PAD4), is elevated in neutrophils from diabetic mice and humans making them more susceptible to NETosis and potentially contributing to persistent inflammation and tissue damage in diabetes (53).
Monocytes/Macrophages
Monocytes are recruited to the wound site very early during the inflammatory phase where they differentiate into macrophages and dendritic cells. Despite the lack of clarity regarding the in vivo definition of these cells, there is substantial evidence that infiltrating monocytes/macrophages are critical for establishing the initial inflammatory phase as well as promoting the transition from a pro-inflammatory to anti-inflammatory environment (6, 8, 11). In diabetic wounds this transition does not occur and macrophages remain in a persistent inflammatory state where they promote the destruction of the surrounding tissue both directly and indirectly by recruiting other pro-inflammatory immune cells (11, 54, 55). Our group recently reported that Ly6CHi monocytes/macrophages, which have been shown to promote inflammation, normally transition to Ly6CLo, which are more regenerative; however, in diabetic wounds, a second wave of Ly6CHi macrophages are recruited during the reparative phase and fail to transition to the anti-inflammatory Ly6CLo cells contributing to a sustained pro-inflammatory environment (34). Furthermore, it has been shown that hyperglycemia and formation of AGEs impede the phagocytic capacity of macrophages ability to clear apoptotic neutrophils thereby promoting a sustained pro-inflammatory state (56, 57). Since the transition from a pro-to anti-inflammatory state is partly stimulated by neutrophil clearance, this results ina higher number of pro-inflammatory macrophages present in the wound. Additionally, studies have shown that monocyte/macrophage infiltration is sustained in diabetic mouse models due to alterations in expression of P-selectin and macrophage chemoattractant protein 1 (MCP-1) (54).
Endothelial cells
Endothelial cells line the luminal surface of blood vessels and are responsible for regulating vaso-constriction and -dilation by expression of vasoactive factors such as endothelial nitric oxide synthase (eNOS) (58). Reduced eNOS expression is associated with peripheral neuropathy and peripheral artery disease and contributes to decreased peripheral blood flow which, in turn, slows wound healing (59). Additionally, endothelial progenitor cells (EPCs) are required for neovascularization of the wound and eNOS stimulates mobilization of EPCs from the bone marrow (45, 60); thus the observed decrease in eNOS also contributes to impaired local angiogenesis during diabetes.
Keratinocytes and Fibroblasts
The late phase of wound healing is carried out by keratinocytes and fibroblasts. In non-healing diabetic wounds, a variety of alterations in keratinocyte function contribute to diminished epithelialization including defective keratinocyte migration and proliferation (61, 62), gap junction abnormalities (63), chronic inflammation and infection (54, 64), reduced angiogenesis (45, 60), oxidative stress (64), and abnormal expression of MMPs (23, 65, 66). The mechanisms associated with these processes have been reviewed elsewhere and we will not list them here (67–69).
Alterations in fibroblast function also contribute to dysfunctional epithelialization and delayed healing in diabetic wounds. These are primarily involved with decreased proliferation, increased apoptosis, and impaired migration to the wound site (20, 21, 70). Alterations in both keratinocytes and fibroblasts can be induced directly by hyperglycemia and AGE formation (18, 19, 22, 71, 72). Moreover, crosstalk between the two cell types is important for re-epithelialization and is dependent on a delicate balance between pro-inflammatory and anti-inflammatory cytokine expression in the wound (73).
Epigenetics and the wound healing response
Despite sharing a common genome, different cell types exhibit specific gene expression profiles that define their function. This is accomplished through epigenetic regulation, which modifies chromatin to activate or silence genes (74). Here, epigenetics is defined as heritable changes in transcription that are not caused by changes in the genetic code. Thus, these modifications are not determined by mutation or permanent changes in nucleotide sequence but are maintained over time and passed through multiple generations of cell division.
There are three main types of epigenetic gene regulation: DNA modification, biochemical modification of histone tails, and ATP-dependent chromatin remodeling (75). These mechanisms are highly interdependent and work in concert with each other to either shut down or promote transcription of specific genes. During normal development, genes are regularly silenced and activated in a cell and/or tissue-specific manner. However, under disease conditions, the epigenetic machinery may often become dysregulated with dire consequences (75). For example, diabetes has been shown to induce changes in the epigenetic machinery that contribute to many of the associated complications. Important in the context of wound healing, epigenetic modifications have been shown to regulate downstream immune mediator expression in monocyte-derived macrophages and other immune cells (76–79). For example, several reports indicate that site-specific histone methylation has a role in macrophage polarization. Macrophage-specific knockout of mixed lineage leukemia 1(Mll1) associates with reduced pro-inflammatory gene expression while the gene absent small and homeotic disks protein 1- like (Ash1l) promotes an anti-inflammatory phenotype by suppressing IL6 and TNFα production (76, 79). Additionally, the H3K27 demethylase, Jumonji domain-containing protein 3 (JMJD3) has been revealed to have roles in activation of both pro- and anti-inflammatory macrophage phenotypes. In, murine macrophages, Jmjd3 can be upregulated by both LPS and IL-4 and drive expression of pro-inflammatory genes or IL-4 target genes, respectively (77). Inhibition of JMJD3 and another H3K27 demethylase, UTX, reduced LPS-induced pro-inflammatory cytokine production in human primary macrophages (80) while induction of IRF4-dependent anti-inflammatory macrophage differentiation was reduced in Jmjd3 knockout mice (81). Thus, JMJD3 may regulate macrophage polarization differently depending on the environmental/tissue-specific context.
Neutrophil activity has also been shown to be epigenetically regulated. Zimmerman et. al. reported that purified human neutrophils maintain the IL-6 promoter in an inactive conformation but promote IL-6 expression after stimulation with the TLR8 agonist R848, corresponding with increased H3K4me3, H3K27ac, and H4ac, all marks of active transcription, at the IL-6 promoter (82). Further, neutrophils from systemic lupus erythematosus (SLE) patients have been reported to have reduced DNA methylation at interferon-regulated genes, suggestive of a role for DNA methylation in regulating the interferon gene program (83).
In addition to immune cells, the functions of other cells involved in wound healing have been shown to be epigenetically regulated (84). For example, JMJD3 is necessary for normal keratinocyte differentiation by promoting expression of differentiation-associated genes (85), while the H4K20 methyltransferase, SETD8, promotes normal keratinocyte proliferation and differentiation (86). Taken together, these reports suggest that epigenetic regulators could prove to be viable therapeutic targets for treating wound healing in diabetes and other conditions.
Epigenetic mechanisms and alterations in the diabetic wound
In the following sections, we will describe the fundamental mechanisms for each of type of epigenetic regulation and outline work showing how these processes are disrupted in diabetic complications and wound healing.
DNA modifications
DNA methylation
There are two different mechanisms by which DNA can be directly modified to modulate gene transcription: DNA methylation and DNA-hydroxy-methylation. DNA methylation is predominantly associated with transcriptional repression and is characterized by transfer of a methyl group to the cytosine ring of DNA by DNA methyltransferases (DNMTs) to form 5-methyl-cytosine (5mC) (87). DNMT3A and DNMT3B deposit de novo methylation marks while DNMT1 is responsible for maintaining these marks since these marks must be re-established with each cell division (88–90). In mammals, the vast majority of DNA methylation in somatic cells occurs at clusters of CpG dinucleotides termed CpG islands, and approximately 40% of genes contain these islands in their promoters (91). Methylation of CpG islands within the promoter can directly silence transcription by impeding transcription factor binding and also interacts with other factors, e.g., histone modifying enzymes, to inhibit transcription (92).
Several studies have shown a role for DNA methylation in diabetic wounds. A report by Shi and colleagues showed that DNMT1 inhibition by 5-aza-cytadine (5-aza-C) promoted M2-like macrophage formation and suppressed inflammation in bone marrow derived macrophages (BMDMs) (15). Interestingly, these mice were also protected against obesity-induced inflammation and insulin-resistance (15). Another study, using both genetic (db/db) and diet-induced obesity (DIO) mouse models of T2D, showed that DNMT1 was elevated in BMDMs and promoted a pro-inflammatory macrophage phenotype (16). Moreover, in this study, DNMT1 knockdown improved wound healing in db/db mice (16). The authors further show that 5-aza-C treatment promoted M2 polarization in vitro. Although the improvement in wound healing could be secondary to systemic effects due to improved obesity and insulin sensitivity, these data suggest that targeting DNMT1 in diabetic macrophages may be a viable therapeutic approach and warrants further investigation. Similar to macrophages, neutrophils in the non-healing wound are characterized by persistent recruitment and activation (51–53). Although reduced DNA methylation has been identified in neutrophils in other inflammatory diseases (83) there is currently insufficient data regarding DNA methylation status of neutrophils in diabetic wound healing.
Although active DNA methylation is known to be important for maintaining proper cell function in renewing epidermal tissue (93), less is known about its role in epithelialization during normal wound healing. Aberrant DNA methylation changes have been demonstrated in human diabetic foot ulcer fibroblasts (24). Surprisingly, instead of increased DNA methylation seen in previous studies, the authors report decreased DNA methylation compared to controls. Pathways affected by the differentially methylated genes were associated highly with wound healing, angiogenesis, and ECM assembly (24). In keratinocytes, the MMP9 promoter is hypomethylated as a result of accumulation of diabetes-dependent AGE-conjugated bovine serum albumin (AGE-BSA) (22, 23, 94). This suggests a potential role for active de-methylation regulating keratinocyte function during diabetic wound healing (see below).
In addition to its role in wound healing, several studies have demonstrated global changes in DNA methylation patterns associated with other diabetic complications. It is believed that DNA methylation plays a key role in maintaining “metabolic memory”, the concept that intensively controlling glucose levels for a number of years can have a long-term effect (24, 95). Importantly, changes in DNA methylation patterns have been found associated with insulin resistance and dysfunctional pancreatic β-cells from diabetic donors (96–99) Volkmar et al. showed that gene promoters were largely hypomethylated in diabetic pancreatic islets and functional analysis showed that subsets of differentially methylated and expressed genes were associated with pathways important for β-cell function (96). Taken together, the role of DNA methylation in diabetes appears to be complex and tissue-specific. Further studies will be necessary to determine how DNA methylation may be targeted in diabetes and, particularly, in wound healing.
DNA hydroxy-methylation
DNA methylation was originally thought to be irreversible and only occurred in the absence of DNMT1 during replication. Recently, however, an active process for DNA demethylation has been discovered whereby 5mC is sequentially oxidized by the Ten-Eleven Translocation (TET) family of enzymes to form 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and finally 5-carboxylcytosine (5caC), which is ultimately removed by base excision repair machinery (100). The 5hmC, 5fC, and 5caC moieties were originally thought to be intermediates in DNA de-methylation to restore gene transcription. However, studies have shown that 5hmC is stably deposited in gene bodies, promoters, and transcription factor binding site across the genome, suggesting that it may have an independent role in controlling gene transcription (100). 5fC and 5caC have also been seen to be stably distributed across the genome albeit at much lower levels (100).
There is evidence showing that DNA de-methylation plays an active role in diabetic wound healing. Yan and colleagues have demonstrated that AGE-BSA, which commonly accumulates as a result of hyperglycemia, retards keratinocyte mobility and proliferation by promoting TET expression which subsequently de-methylates the MMP9 promoter (19, 22, 65, 94, 101). TNFα promotes MMP9 expression in keratinocyte cell lines and is dependent on site-specific de-methylation of the MMP9 promoter (23). This suggests that targeting DNA demethylation machinery may be a promising strategy for treating non-healing diabetic wounds. However, compared to DNA methylation, much less is known regarding the role of DNA demethylation in diabetic wound healing and further study is warranted.
Histone modifications
In eukaryotes, DNA is packaged into repeating units called nucleosomes by wrapping around multimeric histone proteins (102, 103). These nucleosomes are then further arranged in higher order structures to create chromatin fibers (104). In addition to efficiently packaging DNA, higher order chromatin structure acts to regulate gene transcription. When nucleosomes are organized into tightly packed bundles (heterochromatin), transcription is inhibited by barring access of transcriptional machinery (105–107). Conversely, when chromatin is relaxed (euchromatin), the nucleosomes resemble beads on a string and this state is associated with active transcription (107).
Each histone protein is an octamer comprised of 2 sets of H2A, H2B, H3, and H4 proteins with a single histone H1 linker protein between nucleosomes (103). Each histone subunit has an N-terminal “tail” that protrudes away from the surface of the histone octamer creating an exposed surface (108). Here, histone modifying enzymes can methylate, acetylate, phosphorylate, or ubiquitylate specific residues on the histone tail (106). Depending on the modification and the specific residue modified, transcription can be either activated or silenced.
Histone lysine methylation/de-methylation
One highly studied histone modification is methylation. Unlike DNA methylation, which is always associated with transcriptional repression, histone methylation can either promote transcription or silence it depending on the target residue and the number of methyl groups added (106). For example, tri-methylation of lysine 4 on histone H3 (H3K4me3) (109) is a well-known activator of gene transcription while tri-methylation of lysine 27 on H3 (H3K27me3) is associated with transcriptional repression (110). Although H3K27me3 is linked with transcriptional silencing, H3K27 mono-methylation (H3K27me1) has been documented to promote transcription (110). Importantly, both of these marks are involved in macrophage polarization as alternatively activated macrophages have been found to have increased H3K4 methylation and decreased H3K27 methylation in promoters of anti-inflammatory genes (77). Here, an increased level of the H3K27 de-methylase Jmjd3 was shown to be responsible for decreased di- and tri-methylation of H3K27 (77).
Histone H3K4 can be methylated by several different members of the SET domain-containing family of proteins. In particular, MLL1 has been shown to promote expression of inflammatory genes in a NFκB-dependent manner (66, 76, 111). Our group has shown that MLL1 is important for normal tissue repair by catalyzing H3K4me3 deposition at pro-inflammatory genes in macrophages during the inflammation phase of wound healing (14). We further showed that mice bearing a myeloid-specific MLL1 deletion had delayed wound healing and decreased pro-inflammatory cytokine production. Related to diabetic wound healing, our data showed that monocytes isolated from T2D patients exhibit increased MLL1 expression implying that MLL1 expression is dynamically regulated during the transition to the proliferation phase. To date, this is only study imparting MLL1 with a role in diabetic wound healing. Thus, more investigation is necessary to determine whether MLL1 may be a viable therapeutic target.
Our laboratory and others have shown that expression of Jmjd3, the histone demethylase targeting H3K27me3, is increased and H3K27me3 decreased in wound macrophages in a DIO mouse model of diabetes (11, 17). H3K27me3 is a transcriptionally repressive mark that promotes heterochromatin formation in the promoters of genes (112, 113); thus, JMJD3 acts to promote transcription. In a DIO model of diabetes, we found that JMJD3 promotes expression of IL-12 in wound macrophages, and this phenomenon can be reversed by JMJD3 inhibition (11). Similarly, a report by Natoli and colleagues showed that Jmjd3 expression was increased in macrophages in response to inflammatory stimuli and was responsible for regulating developmental genes expression during bone marrow differentiation (17). Studies have shown that IL-6 expression in normal neutrophils is epigenetically regulated in response to TLR activation and is associated with increased H3K4me3, and H3K27ac, and acetylated histone H4 (82). However, there is a lack of data regarding epigenetic regulation in neutrophils during diabetic wound healing warranting further study.
Other studies suggest Jmjd3 expression is required for re-epithelization (114, 115). For example, JMJD3 has been shown to promote keratinocyte migration to the wound site by promoting Notch1 expression (114). Interestingly, Shaw and Martin demonstrated increased Jmjd3 expression in epithelial cells at the leading edge of normal wounds with a concurrent decrease in Ezh2, the HMT responsible for depositing the H3K27 methylation mark (115). Levels of Jmjd3 were upregulated in wounds at day 1 and abated over time. Thus, JMJD3 appears to be tightly regulated in both macrophages and keratinocytes, where higher expression is important early in wound healing but is down-regulated as the wound resolves; where this regulation appears to be absent in the diabetic wound. However, data concerning JMJD3 and EZH2 in keratinocytes has been limited to normal wound conditions. Further study is necessary to determine whether they play a role in the context of diabetic wounds.
Other HMTs have been ascribed with roles processes that could be involved in diabetic wound healing. ASH1L targets histone H3 and has been found to be associated with methylation of K4, K9, K20 and K36 at active gene promoters (116, 117). Ash1l deletion leads to delayed re-epithelialization in normal wounds (118). Despite this, no role for ASH1L has been described in diabetic wound healing. Additionally, another member of the SET domain family of HMTs, SET7, is responsible for mono-methylation of histone H3K4 (H3K4me1) (119). This mark is typically found in enhancer regions of genes where it poises genes for active transcription (120). It has been shown that hyperglycemia increases SET7 expression in vascular endothelial cells and drive expression of pro-inflammatory genes (119).
Taken together, it is clear that histone lysine methylation plays variable roles in wound healing depending on the cell type and the specific histone tail residue modified. Based on limited studies thus far, MLL1 and JMJD3 are promising potential therapeutic targets.
Histone arginine methylation/de-methylation
Histones can also be methylated at arginine residues. Here, arginine is mono- or dimethylated by protein arginine methyl transferases (PRMTs) (121). Arginine has two exposed terminal amino groups and di-methylation can occur either with 2 methyl groups on one amino group (asymmetric) or with both groups mono-methylated (symmetric) (121). Compared to lysine modification, there is much less information regarding histone arginine methylation in diabetic complications, including wound healing. A few studies have shown that PRMTs are involved in insulin secretion in β-cells and some are overexpressed in diabetes (122–124). However, PRMTs can, not only methylate histones, but also non-histone proteins (121). It is currently not clear whether arginine modifications play a histone-dependent or independent role (or both) in diabetic complications. There is evidence that PRMT1 and CARM1/PRMT4 form a complex with NFκB and methylate H3 arginine residues to drive expression of NFκB gene targets (125, 126) suggesting that arginine methylation could be involved in promoting pro-inflammatory responses. However, studies involving histone arginine methylation in diabetes and, in particular, diabetic wound healing are minimal and warrant further investigation.
Histone acetylation/de-acetylation
Another well-characterized modification is histone acetylation. Histone acetylation is carried out by histone acetyl-transferases (HATs) which catalyze the transfer of an acetyl group from acetyl-coenzyme A (Ac-CoA) to the terminal amino group of lysine residues on the histone (127). Lysine’s terminal amino group is positively charged under physiological conditions and interacts with the negatively charged phosphates in the DNA backbone to form a stable complex between the histone and DNA (108). Acetylation of this amino group neutralizes its charge thereby breaking the interaction with DNA and allowing the histone to be repositioned and allow access to transcriptional machinery (128). Thus, histone acetylation is distinct in that it is exclusively associated with transcriptional activation. This process can also be reversed with the removal of the acetyl group by histone deacetylases (HDACs) to shut down gene expression (129, 130).
Given that Ac-CoA is a major byproduct produced during fatty acid metabolism, it seems likely that histone acetylation would play a role in the development of diabetes and potentially be involved in regulating wound healing processes in the context of obesity-induced inflammation. Indeed, histone acetylation has been linked to cellular metabolism and it has been shown that high fat diet can affect acetyl-CoA and histone acetylation levels (131–134). The majority of these studies focus on cancer. However, there are a few reports regarding histone acetylation and diabetes and wound healing. Global decreases in H3K9 and H3K23 acetylation have been reported in diabetic mouse livers (135). In this study, acetylated H3K9 was shown to be decreased in the promoter of Glut2, a glucose transporter important for maintaining glucose homeostasis (136, 137), and could be reversed by treatment with the diabetic inhibitor exendin-4 (135). Related to this, a report showed that exendin-4 can protect against diabetic retinopathy by inducing acetylation of histone H3 at the SOD3 promoter to drive its expression in endothelial cells (138). Further, reports from Spallotta et al. and Melchionna et al. showed that when treated with HDAC inhibitors, mice exhibited increased keratinocyte proliferation and improved wound healing (139, 140). These studies suggest that histone de-acetylation may be a promising therapeutic target. However, aside from these few studies, there is a paucity of information related to histone acetylation in diabetes and diabetic wound healing.
Other histone modifications
Histones can also be phosphorylated and ubiquitylated. These marks have established roles in transcriptional regulation and have been extensively reviewed elsewhere (141–143). However, no work has been done related to these marks in wound healing or diabetes and their roles, at present, remain undefined.
ATP-dependent chromatin remodeling
Aside from histone acetylation, interactions between histones and DNA can also be modulated by remodeling complexes fueled by ATP-hydrolysis. All ATP-dependent remodeling complexes contain an ATPase of the SNF2 family and have been classified into two groups based on their subunits, the SWI2/SNF2 and ISWI groups (144). The activity of each group is similar in that they both utilize ATP. However, while SWI2/SNF2 can be activated by naked DNA as well as nucleosomes (145), ISWI complex must be in contact with nucleosomes containing histones with intact amino-terminal tails (146). The consequence of remodeling is dependent on the context of the nucleosomes at a given promoter and can result in either transcriptional activation or repression (144).
Although ATP-dependent remodeling has been extensively studied, the majority of work in this field has focused on DNA damage/repair in cancer and very little is known about these mechanisms in diabetes or wound healing. Some studies, however, have shown that forced increase of cellular ATP can accelerate wound healing by promoting neovascularization and collagen production (147). The increased ATP levels led to increases in SWI/SNF complex components BRG1 and BRM ATPases and promoted a novel pathway in anti-inflammatory macrophages characterized by increased pro-inflammatory cytokine MCP-1 chemokine production. Related to this, it has been shown that Brg1 deletion impairs keratinocyte terminal differentiation (148) suggesting a more general mechanism for ATP-dependent remodeling.
Conclusions
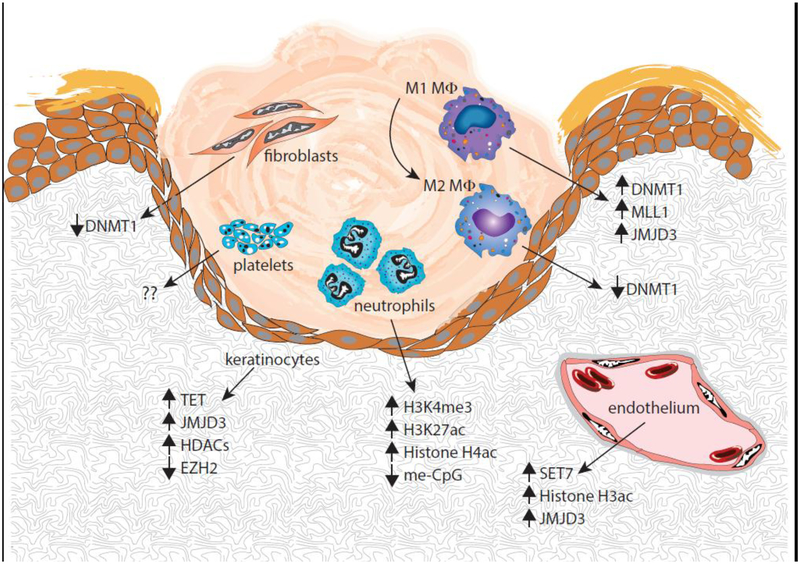
Diabetic wounds are characterized by persistent inflammation secondary to alterations in immune cell function. Here, we have described epigenetic changes that alter immune cell phenotypes as well as other cells important for proper healing (fig. 1). As new mechanisms important for the regulation of healing are discovered, progress towards cell-specific targeted therapies necessary for the treatment of pathologic healing will accelerate.
Figure 1:
Overview of epigenetic mechanisms regulating cells involved in wound healing.
Acknowledgements
All authors have read the journal’s policy on disclosure of potential conflicts of interest have no conflicts of interest to declare.
All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all authors.
Aaron den Dekker is supported by a Research Training Grant through the Michigan Institute for Clinical and Health Research (UL1TR002240)
Abbreviations:
- 5caC
5′-carboxy-cytosine
- 5fC
5′-fluoro-cytosine
- 5hmC
5′-hydroxymethyl-cytosine
- 5mC
5′-methyl-cytosine
- Ac-CoA
Acetyl Coenzyme A
- AGE
Advanced Glycation End-product
- AGE-BSA
Advanced Glycation End-product – Bovine Serum Albumin
- ASH1L
Absent Small and Homeotic Disks Protein 1-like
- BMDM
Bone Marrow-Derived Macrophage
- DAMP
Damage-Associated Molecular Pattern
- DIO
Diet-induced Obesity
- DNMT
DNA Methyltransferase
- ECM
Extracellular Matrix
- eNOS
Endothelial Nitric Oxide Synthase
- H3K4
Histone H3 Lysine 4
- H3K27
Histone H3 Lysine 27
- HAT
Histone Acetyltransferase
- HDAC
Histone De-acetylase
- HMT
Histone Methyltransferase
- JMJD3
Jumonji Domain-containing Protein 3
- LPS
Lipopolysaccharide
- MLL1
Mixed Lineage Leukemia 1
- MCP-1
Macrophage Chemoattractant Protein 1
- MMP
Matrix Metalloprotease
- NET
Neutrophil Extracellular Trap
- NO
Nitric Oxide
- PAD4
Peptidyl Iminase 4
- PAI-1
Plasminogen Activator Inhibitor 1
- PMN
Polymorphonuclear Neutrophils
- SLE
Systemic Lupus Erythematosus
- T2D
Type 2 Diabetes
- TET
Ten Eleven Translocase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 2.Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract. 2011;94(3):322–32. [DOI] [PubMed] [Google Scholar]
- 3.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9. [DOI] [PubMed] [Google Scholar]
- 4.Lamont P, Franklyn K, Rayman G, Boulton AJ. Update on the diabetic foot 2012: the 14th biennial Malvern Diabetic Foot Conference, May 9–11, 2012. Int J Low Extrem Wounds. 2013;12(1):71–5. [DOI] [PubMed] [Google Scholar]
- 5.Theilgaard-Monch K, Knudsen S, Follin P, Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172(12):7684–93. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44(3):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–25. [DOI] [PubMed] [Google Scholar]
- 9.Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol. 2017;199(1):17–24. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher KA, Joshi A, Carson WF, Schaller M, Allen R, Mukerjee S, et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes. 2015;64(4):1420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimball AS, Joshi A, Carson WFt, Boniakowski AE, Schaller M, Allen R, et al. The Histone Methyltransferase MLL1 Directs Macrophage-Mediated Inflammation in Wound Healing and Is Altered in a Murine Model of Obesity and Type 2 Diabetes. Diabetes. 2017;66(9):2459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Cao Q, Yu L, Shi H, Xue B, Shi H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight. 2016;1(19):e87748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Tie G, Wang S, Tutto A, DeMarco N, Khair L, et al. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat Commun. 2018;9(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130(6):1083–94. [DOI] [PubMed] [Google Scholar]
- 18.Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, et al. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003;52(11):2805–13. [DOI] [PubMed] [Google Scholar]
- 19.Zhu P, Yang C, Chen LH, Ren M, Lao GJ, Yan L. Impairment of human keratinocyte mobility and proliferation by advanced glycation end products-modified BSA. Arch Dermatol Res. 2011;303(5):339–50. [DOI] [PubMed] [Google Scholar]
- 20.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162(1):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xuan YH, Huang BB, Tian HS, Chi LS, Duan YM, Wang X, et al. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS One. 2014;9(9):e108182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Yang C, Wang C, Liu D, Lao G, Liang Y, et al. AGE-induced keratinocyte MMP-9 expression is linked to TET2-mediated CpG demethylation. Wound Repair Regen. 2016;24(3):489–500. [DOI] [PubMed] [Google Scholar]
- 23.Ling L, Ren M, Yang C, Lao G, Chen L, Luo H, et al. Role of site-specific DNA demethylation in TNFalpha-induced MMP9 expression in keratinocytes. J Mol Endocrinol. 2013;50(3):279–90. [DOI] [PubMed] [Google Scholar]
- 24.Park LK, Maione AG, Smith A, Gerami-Naini B, Iyer LK, Mooney DJ, et al. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics. 2014;9(10):1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 26.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–48. [DOI] [PubMed] [Google Scholar]
- 27.Jaramillo M, Olivier M. Hydrogen peroxide induces murine macrophage chemokine gene transcription via extracellular signal-regulated kinase- and cyclic adenosine 5’-monophosphate (cAMP)-dependent pathways: involvement of NF-kappa B, activator protein 1, and cAMP response element binding protein. J Immunol. 2002;169(12):7026–38. [DOI] [PubMed] [Google Scholar]
- 28.Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol. 2013;23(5):424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459(7249):996–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, et al. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007;178(11):7376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rani M, Nicholson SE, Zhang Q, Schwacha MG. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns. 2017;43(2):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. [DOI] [PubMed] [Google Scholar]
- 33.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43. [DOI] [PubMed] [Google Scholar]
- 34.Kimball A, Schaller M, Joshi A, Davis FM, denDekker A, Boniakowski A, et al. Ly6C(Hi) Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2018;38(5):1102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, et al. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci Rep. 2017;7(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int J Mol Sci. 2017;18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagesjo E, Ohnstedt E, Mortier A, Lofton H, Huss F, Proost P, et al. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci U S A. 2018;115(8):1895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. [DOI] [PubMed] [Google Scholar]
- 41.Gabbiani G The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–3. [DOI] [PubMed] [Google Scholar]
- 42.Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61(11):2937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward JD, Boulton AJ, Simms JM, Sandler DA, Knight G. Venous distension in the diabetic neuropathic foot (physical sign of arteriovenous shunting). J R Soc Med. 1983;76(12):1011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rayman G, Williams SA, Spencer PD, Smaje LH, Wise PH, Tooke JE. Impaired microvascular hyperaemic response to minor skin trauma in type I diabetes. Br Med J (Clin Res Ed). 1986;292(6531):1295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117(5):1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Undas A, Wiek I, Stepien E, Zmudka K, Tracz W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care. 2008;31(8):1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juhan-Vague I, Roul C, Alessi MC, Ardissone JP, Heim M, Vague P. Increased plasminogen activator inhibitor activity in non insulin dependent diabetic patients--relationship with plasma insulin. Thromb Haemost. 1989;61(3):370–3. [PubMed] [Google Scholar]
- 48.Forstermann U, Mugge A, Alheid U, Haverich A, Frolich JC. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988;62(2):185–90. [DOI] [PubMed] [Google Scholar]
- 49.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87(2):432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes. 2010;59(9):2152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanses F, Park S, Rich J, Lee JC. Reduced neutrophil apoptosis in diabetic mice during staphylococcal infection leads to prolonged Tnfalpha production and reduced neutrophil clearance. PLoS One. 2011;6(8):e23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH, et al. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005;78(4):862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21(7):815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115(2):245–53. [DOI] [PubMed] [Google Scholar]
- 55.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111(5):850–7. [DOI] [PubMed] [Google Scholar]
- 56.Friggeri A, Banerjee S, Biswas S, de Freitas A, Liu G, Bierhaus A, et al. Participation of the receptor for advanced glycation end products in efferocytosis. J Immunol. 2011;186(11):6191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12(4):358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998;47(3):457–63. [DOI] [PubMed] [Google Scholar]
- 60.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–74. [DOI] [PubMed] [Google Scholar]
- 61.Lan CC, Liu IH, Fang AH, Wen CH, Wu CS. Hyperglycaemic conditions decrease cultured keratinocyte mobility: implications for impaired wound healing in patients with diabetes. Br J Dermatol. 2008;159(5):1103–15. [DOI] [PubMed] [Google Scholar]
- 62.Werner S, Breeden M, Hubner G, Greenhalgh DG, Longaker MT. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol. 1994;103(4):469–73. [DOI] [PubMed] [Google Scholar]
- 63.Pollok S, Pfeiffer AC, Lobmann R, Wright CS, Moll I, Martin PE, et al. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J Cell Mol Med. 2011;15(4):861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lan CC, Wu CS, Huang SM, Wu IH, Chen GS. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: new insights into impaired diabetic wound healing. Diabetes. 2013;62(7):2530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu W, Li J, Ren M, Zeng Y, Zhu P, Lin L, et al. Role of the mevalonate pathway in specific CpG site demethylation on AGEs-induced MMP9 expression and activation in keratinocytes. Mol Cell Endocrinol. 2015;411:121–9. [DOI] [PubMed] [Google Scholar]
- 66.Robert I, Aussems M, Keutgens A, Zhang X, Hennuy B, Viatour P, et al. Matrix Metalloproteinase-9 gene induction by a truncated oncogenic NF-kappaB2 protein involves the recruitment of MLL1 and MLL2 H3K4 histone methyltransferase complexes. Oncogene. 2009;28(13):1626–38. [DOI] [PubMed] [Google Scholar]
- 67.Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: Focusing on diabetic wound healing. J Dermatol Sci. 2016;84(2):121–7. [DOI] [PubMed] [Google Scholar]
- 68.Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem. 2008;56(7):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Becker DL, Thrasivoulou C, Phillips AR. Connexins in wound healing; perspectives in diabetic patients. Biochim Biophys Acta. 2012;1818(8):2068–75. [DOI] [PubMed] [Google Scholar]
- 70.Desta T, Li J, Chino T, Graves DT. Altered fibroblast proliferation and apoptosis in diabetic gingival wounds. J Dent Res. 2010;89(6):609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alikhani Z, Alikhani M, Boyd CM, Nagao K, Trackman PC, Graves DT. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem. 2005;280(13):12087–95. [DOI] [PubMed] [Google Scholar]
- 72.Okano Y, Masaki H, Sakurai H. Dysfunction of dermal fibroblasts induced by advanced glycation end-products (AGEs) and the contribution of a nonspecific interaction with cell membrane and AGEs. J Dermatol Sci. 2002;29(3):171–80. [DOI] [PubMed] [Google Scholar]
- 73.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. [DOI] [PubMed] [Google Scholar]
- 74.Barrero MJ, Boue S, Izpisua Belmonte JC. Epigenetic mechanisms that regulate cell identity. Cell Stem Cell. 2010;7(5):565–70. [DOI] [PubMed] [Google Scholar]
- 75.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28(10):1057–68. [DOI] [PubMed] [Google Scholar]
- 76.Carson WFt, Cavassani KA, Soares EM, Hirai S, Kittan NA, Schaller MA, et al. The STAT4/MLL1 Epigenetic Axis Regulates the Antimicrobial Functions of Murine Macrophages. J Immunol. 2017;199(5):1865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114(15):3244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaller M, Ito T, Allen RM, Kroetz D, Kittan N, Ptaschinski C, et al. Epigenetic regulation of IL-12-dependent T cell proliferation. J Leukoc Biol. 2015;98(4):601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia M, Liu J, Wu X, Liu S, Li G, Han C, et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity. 2013;39(3):470–81. [DOI] [PubMed] [Google Scholar]
- 80.Kruidenier L, Chung CW, Cheng Z, Liddle J, Che K, Joberty G, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488(7411):404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11(10):936–44. [DOI] [PubMed] [Google Scholar]
- 82.Zimmermann M, Aguilera FB, Castellucci M, Rossato M, Costa S, Lunardi C, et al. Chromatin remodelling and autocrine TNFalpha are required for optimal interleukin-6 expression in activated human neutrophils. Nat Commun. 2015;6:6061. [DOI] [PubMed] [Google Scholar]
- 83.Coit P, Yalavarthi S, Ognenovski M, Zhao W, Hasni S, Wren JD, et al. Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J Autoimmun. 2015;58:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis CJ, Mardaryev AN, Sharov AA, Fessing MY, Botchkarev VA. The Epigenetic Regulation of Wound Healing. Adv Wound Care (New Rochelle). 2014;3(7):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22(14):1865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Driskell I, Oda H, Blanco S, Nascimento E, Humphreys P, Frye M. The histone methyltransferase Setd8 acts in concert with c-Myc and is required to maintain skin. EMBO J. 2012;31(3):616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187(4173):226–32. [PubMed] [Google Scholar]
- 88.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–57. [DOI] [PubMed] [Google Scholar]
- 89.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–402. [DOI] [PubMed] [Google Scholar]
- 90.Bestor TH, Ingram VM. Two DNA methyltransferases from murine erythroleukemia cells: purification, sequence specificity, and mode of interaction with DNA. Proc Natl Acad Sci U S A. 1983;80(18):5559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takai D, Jones PA. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad Sci U S A. 2002;99(6):3740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li E, Zhang Y. DNA methylation in mammals. Cold Spring Harb Perspect Biol. 2014;6(5):a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463(7280):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou L, Wang W, Yang C, Zeng T, Hu M, Wang X, et al. GADD45a Promotes Active DNA Demethylation of the MMP-9 Promoter via Base Excision Repair Pathway in AGEs-Treated Keratinocytes and in Diabetic Male Rat Skin. Endocrinology. 2018;159(2):1172–86. [DOI] [PubMed] [Google Scholar]
- 95.Chen Z, Miao F, Paterson AD, Lachin JM, Zhang L, Schones DE, et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci U S A. 2016;113(21):E3002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Volkmar M, Dedeurwaerder S, Cunha DA, Ndlovu MN, Defrance M, Deplus R, et al. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J. 2012;31(6):1405–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic beta cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell. 2011;20(4):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao J, Goldberg J, Bremner JD, Vaccarino V. Global DNA methylation is associated with insulin resistance: a monozygotic twin study. Diabetes. 2012;61(2):542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dhawan S, Tschen SI, Zeng C, Guo T, Hebrok M, Matveyenko A, et al. DNA methylation directs functional maturation of pancreatic beta cells. J Clin Invest. 2015;125(7):2851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502(7472):472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu P, Ren M, Yang C, Hu YX, Ran JM, Yan L. Involvement of RAGE, MAPK and NF-kappaB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp Dermatol. 2012;21(2):123–9. [DOI] [PubMed] [Google Scholar]
- 102.Wolffe AP, Hayes JJ. Chromatin disruption and modification. Nucleic Acids Res. 1999;27(3):711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas JO, Kornberg RD. Cleavable cross-links in the analysis of histone-histone associations. FEBS Lett. 1975;58(1):353–8. [DOI] [PubMed] [Google Scholar]
- 104.McGhee JD, Rau DC, Charney E, Felsenfeld G. Orientation of the nucleosome within the higher order structure of chromatin. Cell. 1980;22(1 Pt 1):87–96. [DOI] [PubMed] [Google Scholar]
- 105.Allshire RC, Madhani HD. Ten principles of heterochromatin formation and function. Nat Rev Mol Cell Biol. 2018;19(4):229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–5. [DOI] [PubMed] [Google Scholar]
- 107.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–80. [DOI] [PubMed] [Google Scholar]
- 108.Luger K, Richmond TJ. The histone tails of the nucleosome. Curr Opin Genet Dev. 1998;8(2):140–6. [DOI] [PubMed] [Google Scholar]
- 109.Lauberth SM, Nakayama T, Wu X, Ferris AL, Tang Z, Hughes SH, et al. H3K4me3 interactions with TAF3 regulate preinitiation complex assembly and selective gene activation. Cell. 2013;152(5):1021–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferrari KJ, Scelfo A, Jammula S, Cuomo A, Barozzi I, Stutzer A, et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol Cell. 2014;53(1):49–62. [DOI] [PubMed] [Google Scholar]
- 111.Wang X, Zhu K, Li S, Liao Y, Du R, Zhang X, et al. MLL1, a H3K4 methyltransferase, regulates the TNFalpha-stimulated activation of genes downstream of NF-kappaB. J Cell Sci. 2012;125(Pt 17):4058–66. [DOI] [PubMed] [Google Scholar]
- 112.Morey L, Helin K. Polycomb group protein-mediated repression of transcription. Trends Biochem Sci. 2010;35(6):323–32. [DOI] [PubMed] [Google Scholar]
- 113.Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD. JMJD3 is a histone H3K27 demethylase. Cell Res. 2007;17(10):850–7. [DOI] [PubMed] [Google Scholar]
- 114.Na J, Shin JY, Jeong H, Lee JY, Kim BJ, Kim WS, et al. JMJD3 and NF-kappaB-dependent activation of Notch1 gene is required for keratinocyte migration during skin wound healing. Sci Rep. 2017;7(1):6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shaw T, Martin P. Epigenetic reprogramming during wound healing: loss of polycomb-mediated silencing may enable upregulation of repair genes. EMBO Rep. 2009;10(8):881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gregory GD, Vakoc CR, Rozovskaia T, Zheng X, Patel S, Nakamura T, et al. Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol Cell Biol. 2007;27(24):8466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tanaka Y, Katagiri Z, Kawahashi K, Kioussis D, Kitajima S. Trithorax-group protein ASH1 methylates histone H3 lysine 36. Gene. 2007;397(1–2):161–8. [DOI] [PubMed] [Google Scholar]
- 118.Li G, Ye Z, Shi C, Sun L, Han M, Zhuang Y, et al. The Histone Methyltransferase Ash1l is Required for Epidermal Homeostasis in Mice. Sci Rep. 2017;7:45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okabe J, Orlowski C, Balcerczyk A, Tikellis C, Thomas MC, Cooper ME, et al. Distinguishing hyperglycemic changes by Set7 in vascular endothelial cells. Circ Res. 2012;110(8):1067–76. [DOI] [PubMed] [Google Scholar]
- 120.Local A, Huang H, Albuquerque CP, Singh N, Lee AY, Wang W, et al. Identification of H3K4me1-associated proteins at mammalian enhancers. Nat Genet. 2018;50(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18(3):263–72. [DOI] [PubMed] [Google Scholar]
- 122.Kim DI, Park MJ, Lim SK, Choi JH, Kim JC, Han HJ, et al. High-glucose-induced CARM1 expression regulates apoptosis of human retinal pigment epithelial cells via histone 3 arginine 17 dimethylation: role in diabetic retinopathy. Arch Biochem Biophys. 2014;560:36–43. [DOI] [PubMed] [Google Scholar]
- 123.Porta M, Amione C, Barutta F, Fornengo P, Merlo S, Gruden G, et al. The co-activator-associated arginine methyltransferase 1 (CARM1) gene is overexpressed in type 2 diabetes. Endocrine. 2018. [DOI] [PubMed] [Google Scholar]
- 124.Kim JK, Lim Y, Lee JO, Lee YS, Won NH, Kim H, et al. PRMT4 is involved in insulin secretion via the methylation of histone H3 in pancreatic beta cells. J Mol Endocrinol. 2015;54(3):315–24. [DOI] [PubMed] [Google Scholar]
- 125.Covic M, Hassa PO, Saccani S, Buerki C, Meier NI, Lombardi C, et al. Arginine methyltransferase CARM1 is a promoter-specific regulator of NF-kappaB-dependent gene expression. EMBO J. 2005;24(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hassa PO, Covic M, Bedford MT, Hottiger MO. Protein arginine methyltransferase 1 coactivates NF-kappaB-dependent gene expression synergistically with CARM1 and PARP1. J Mol Biol. 2008;377(3):668–78. [DOI] [PubMed] [Google Scholar]
- 127.Grunstein M Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349–52. [DOI] [PubMed] [Google Scholar]
- 128.Struhl K Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12(5):599–606. [DOI] [PubMed] [Google Scholar]
- 129.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338(1):17–31. [DOI] [PubMed] [Google Scholar]
- 130.Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272(5260):408–11. [DOI] [PubMed] [Google Scholar]
- 131.Carrer A, Parris JL, Trefely S, Henry RA, Montgomery DC, Torres A, et al. Impact of a High-fat Diet on Tissue Acyl-CoA and Histone Acetylation Levels. J Biol Chem. 2017;292(8):3312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20(2):306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao S, Torres A, Henry RA, Trefely S, Wallace M, Lee JV, et al. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep. 2016;17(4):1037–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tu P, Li X, Ma B, Duan H, Zhang Y, Wu R, et al. Liver histone H3 methylation and acetylation may associate with type 2 diabetes development. J Physiol Biochem. 2015;71(1):89–98. [DOI] [PubMed] [Google Scholar]
- 136.Guillam MT, Hummler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17(3):327–30. [DOI] [PubMed] [Google Scholar]
- 137.Seyer P, Vallois D, Poitry-Yamate C, Schutz F, Metref S, Tarussio D, et al. Hepatic glucose sensing is required to preserve beta cell glucose competence. J Clin Invest. 2013;123(4):1662–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yasuda H, Ohashi A, Nishida S, Kamiya T, Suwa T, Hara H, et al. Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells. J Clin Biochem Nutr. 2016;59(3):174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spallotta F, Cencioni C, Straino S, Sbardella G, Castellano S, Capogrossi MC, et al. Enhancement of lysine acetylation accelerates wound repair. Commun Integr Biol. 2013;6(5):e25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Melchionna R, Bellavia G, Romani M, Straino S, Germani A, Di Carlo A, et al. C/EBPgamma regulates wound repair and EGF receptor signaling. J Invest Dermatol. 2012;132(7):1908–17. [DOI] [PubMed] [Google Scholar]
- 141.Cao J, Yan Q. Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Front Oncol. 2012;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rossetto D, Avvakumov N, Cote J. Histone phosphorylation: a chromatin modification involved in diverse nuclear events. Epigenetics. 2012;7(10):1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weake VM, Workman JL. Histone ubiquitination: triggering gene activity. Mol Cell. 2008;29(6):653–63. [DOI] [PubMed] [Google Scholar]
- 144.Vignali M, Hassan AH, Neely KE, Workman JL. ATP-dependent chromatin-remodeling complexes. Mol Cell Biol. 2000;20(6):1899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Quinn J, Fyrberg AM, Ganster RW, Schmidt MC, Peterson CL. DNA-binding properties of the yeast SWI/SNF complex. Nature. 1996;379(6568):844–7. [DOI] [PubMed] [Google Scholar]
- 146.Georgel PT, Tsukiyama T, Wu C. Role of histone tails in nucleosome remodeling by Drosophila NURF. EMBO J. 1997;16(15):4717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kotwal GJ, Sarojini H, Chien S. Pivotal role of ATP in macrophages fast tracking wound repair and regeneration. Wound Repair Regen. 2015;23(5):724–7. [DOI] [PubMed] [Google Scholar]
- 148.Indra AK, Dupe V, Bornert JM, Messaddeq N, Yaniv M, Mark M, et al. Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes unveils two distinct developmental functions of BRG1 in limb morphogenesis and skin barrier formation. Development. 2005;132(20):4533–44. [DOI] [PubMed] [Google Scholar]