Abstract
Objective:
The purpose of this article is to review the etiopathogenesis, molecular cytogenetics, histopathology, clinical features and multimodality imaging features of Desmoid Fibromatosis (DF). Recent advances in the management of Desmoid Fibromatosis will also be discussed.
Conclusion:
Desmoid Fibromatosis is a rare soft tissue neoplasm with a high incidence of local recurrence. Imaging plays an important role in the diagnosis and management of this disease.
Keywords: Desmoid Fibromatosis, CT, MR, Ultrasound
Introduction
Desmoid fibromatosis (DF) is a locally aggressive, deep seated connective tissue malignancy developing in musculoaponeurotic tissues. It is also known as aggressive fibromatosis, deep fibromatosis, musculoaponeurotic fibromatosis and desmoid tumor. DF is a rare tumor, with reported incidence of 2–4 per million population and account for 0.03% of all neoplasms [1, 2]. DF most commonly develops between the age of 15 and 60 years, and tends to be more common in females [3]. DF may affect any site but is commonly seen in the extremities, abdominal wall and abdominal mesentery [4]. Although DF lacks metastatic potential, it has a high propensity for recurrence. Therefore, DF has now been classified as “intermediate, locally aggressive” tumor in the WHO classification of soft tissue tumors. In the past, DF was typically managed with surgical resection. However, recent advances in the molecular cytogenetics and tumor biology of DF have led to a paradigm shift in the management of this condition.
In this article, we illustrate the multimodality imaging features of DF. We also review the etiopathogenesis, molecular cytogenetics, histopathology & clinical features of DF and discuss the recent advances in the management.
Etiopathogenesis
The etiopathogenesis of DF is not yet clear but is believed to be multifactorial [4–8]. DF may be sporadic or familial in nature. Trauma, pregnancy and use of oral contraceptives have been implicated in the etiopathogenesis [4–8]. DF has a high proclivity to develop at sites of surgical scar, especially in those after cesarean section and intra-abdominal resections (Fig. 1). While pregnancy and use of oral contraceptives have been shown to be associated with development of DF, the exact role of hormonal influence is not fully understood.
Fig. 1-.
18-year-old-woman with abdominal wall desmoid fibromatosis
(A) Axial contrast enhanced CT shows a 3.5 cm well defined lobulated, moderately enhancing, soft tissue mass (open arrow) in the left abdominal wall involving the rectus muscle. The patient had history of abdominal surgery and this mass is seen adjacent to the midline surgical scar (closed arrow).
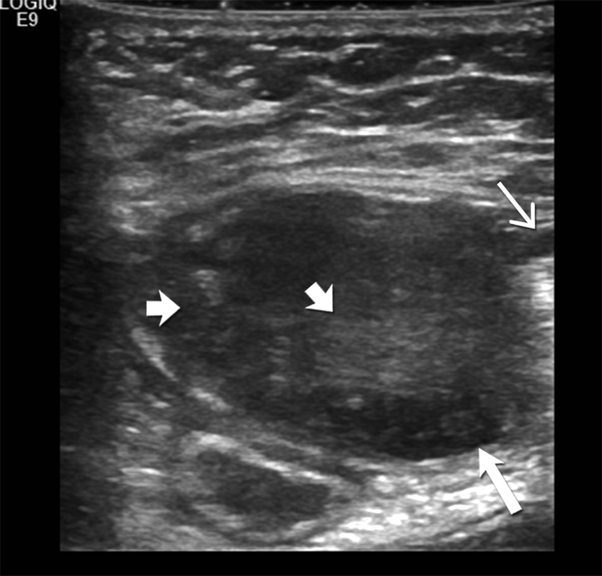
(B)Transverse ultrasound image of the left abdominal wall shows a 3.5 cm heteregenous hypoechoic mass mass (large open arrow) in the left abdominal wall involving the rectus muscle. Hyperechoic foci (short open arrows) are seen within the mass, likley reflecting fibrous collagenous bands. Note the sun-burst like linear extension seen along the fascial plane (closed arrow).
Although the vast majority of these tumors are sporadic, DF may also be hereditary. Familial DF develops predominantly in patients with familial adenomatosis polyposis (FAP) (Fig.2). Patients with FAP have 1000 fold higher risk of developing DF [9]. Studies report that DF may be seen in 5–16 % of FAP [2, 6, 9, 10]. The sporadic tumors occur more frequently in the extra-abdominal location (Fig 3–5). In contrast, majority of the FAP-associated DF develop intra-abdominally within the mesentery and/ or in the abdominal wall (Fig 2, 6–9). Further, FAP-associated DF tends to be larger, multifocal and occur more commonly in the younger patients [2, 6, 9, 10].
Fig. 2 -.
31-year-old man with mesenteric and abdominal wall desmoid fibromatosis
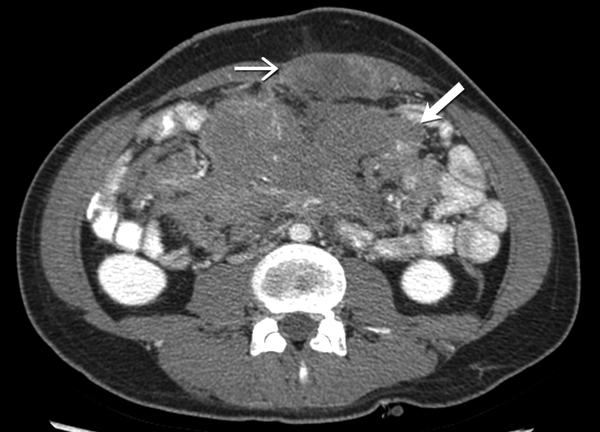
Axial contrast enhanced CT shows a large, heterogeneous, mild to moderately enhancing infiltrative soft tissue mass (open arrow) in the mesentery encasing the mesenteric vessels. There is also a lobulated hypovascular soft tissue mass (closed arrow) in the left abdominal wall. Patient had history of familial adenomatosis polyposis. Biopsy of the abdominal wall mass confirmed the diagnosis of desmoid fibromatosis.
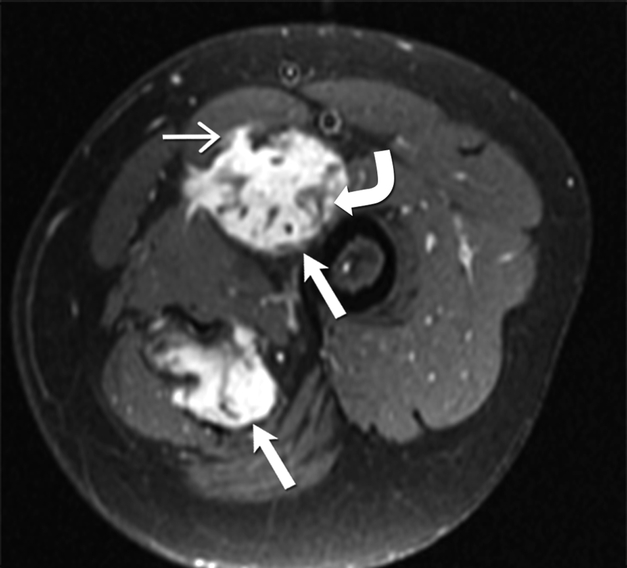
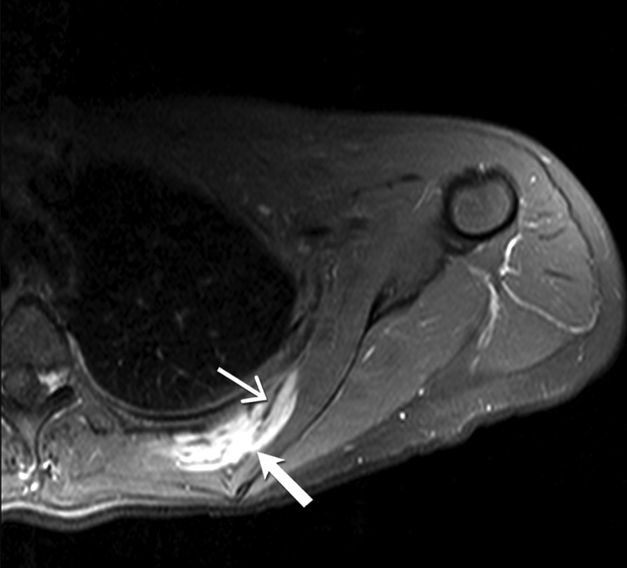
Fig 3 -.
24-year-old-man with desmoid fibromatosis.
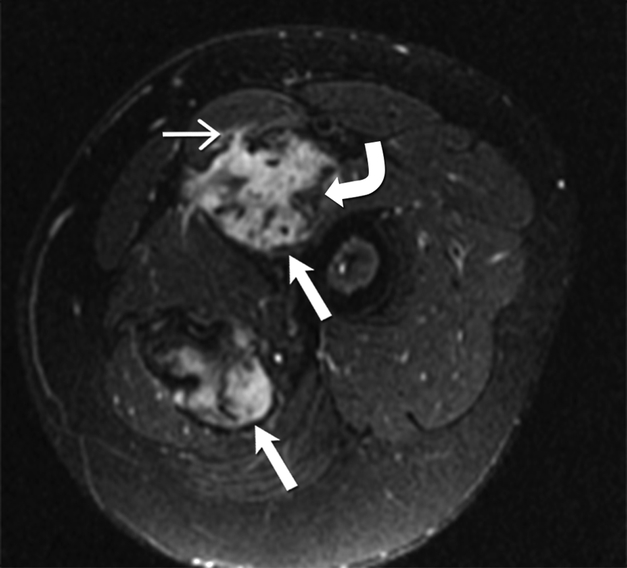
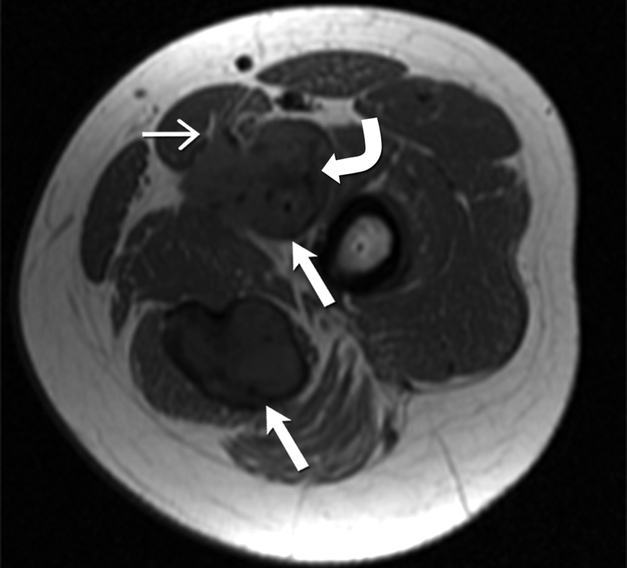
(A) Axial T2 weighted MR of left thigh shows two heterogeneous high signal intensity soft tissue masses (straight open arrows) in the intermuscular compartment in the left thigh. Bands of linear low T2 signal intensity are seen within the tumor (curved arrow), reflecting the presence of collagen and low cellularity. Linear extensions are also seen extending from the periphery of the mass along the fascia (straight closed arrow).
(B)Axial T1 weighted MR of the left thigh shows two heterogeneous soft tissue masses (straight open arrows) in the intermuscular compartment in the left thigh, which are isointense to the skeletal muscle. Bands of linear low T1 signal intensity are seen within the tumor (curved arrow), reflecting the presence of collagen and low cellularity. Linear extensions are also seen extending from the periphery of the mass along the fascia (straight closed arrow).
(C) Axial post contrast T1 weighted MR of the left thigh shows two heterogeneous enhancing soft tissue masses (straight open arrows) in the intermuscular compartment in the left thigh. Avid enhancement within the mass reflects areas of high cellularity within the tumor. However, non-enhancing linear bands of low signal intensity are seen within the tumor (curved arrow), reflecting the presence of collagen bands. Linear extensions are also seen extending from the periphery of the mass along the fascia (straight closed arrow).
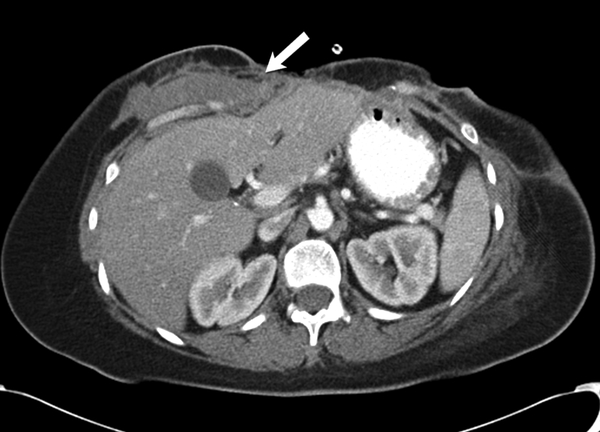
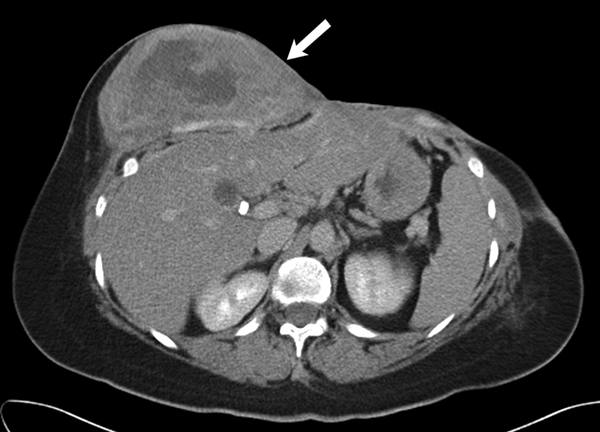
Fig 5 -.
36 year old female with recurrent desmoid fibromatosis
(A) Axial contrast enhanced CT of the chest shows a hypovascular infiltrative mass (arrow) in the right lower anterior chest wall. Patient had undergone two surgeries in the past for this recurrent tumor, as well as radiation therapy. The tumor had recurred for the third time locally in the site of surgical bed.
(B) Axial contrast enhanced CT performed 18 months after the initial scan shows that the heterogeneous mass (arrow) had enlarged significantly and failed multiple lines of systemic therapy including tamoxifen, sulindac, and Adriamycin based chemotherapy. Given the increasing pain in this region and failed response to systemic therapy, surgical resection was performed
Fig. 6-.
47-year-old man with familial adenomatosis polyposis
(A) Axial contrast enhanced CT of the abdomen shows enhancing circumferential rectal tumor (arrow in A). Biopsy confirmed rectal adenocarcinoma. Patient underwent low anterior resection and diverting ileostomy.
(B) Axial CT scan of the abdomen performed 4 months after surgery shows a 2 cm soft tissue mass in the right abdominal wall near the ileostomy surgical scar. Biopsy of the abdominal wall mass confirmed the diagnosis of desmoid fibromatosis.
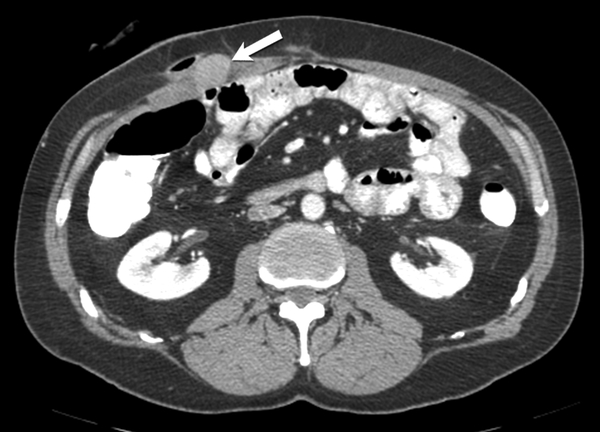
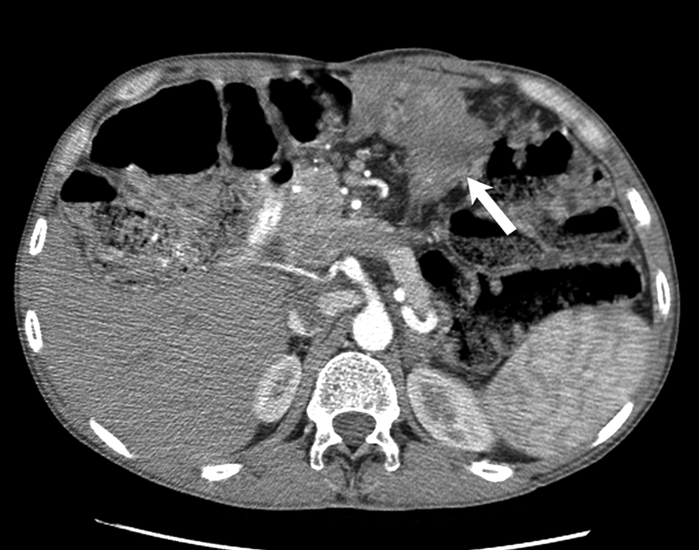
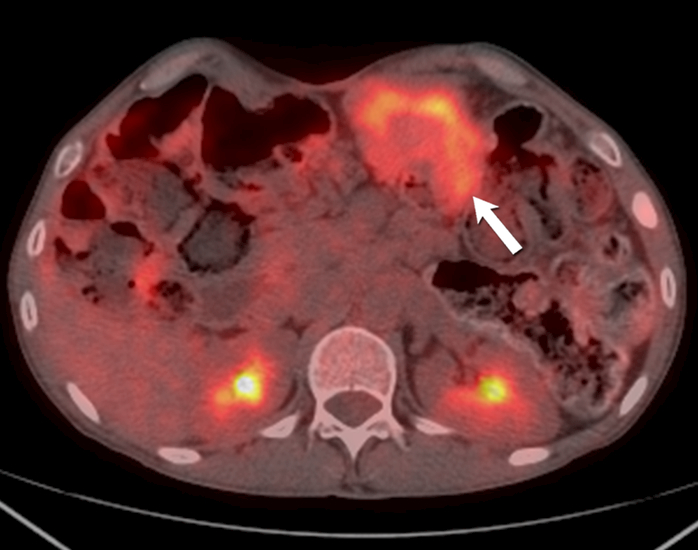
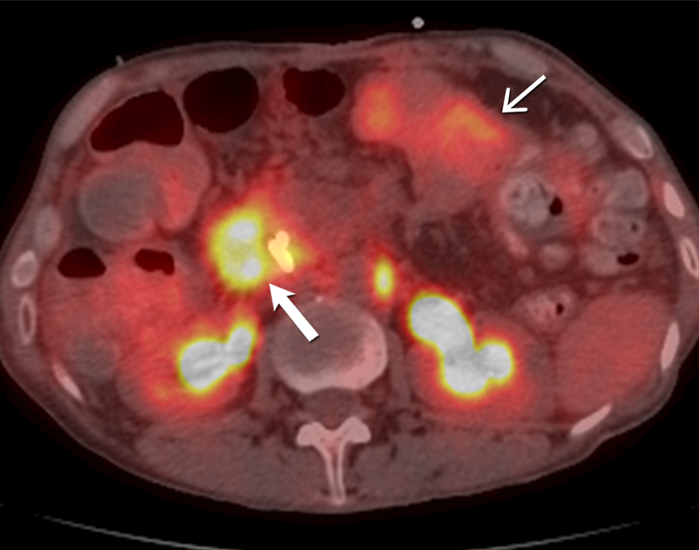
Fig. 9 -.
51-year-old- man with familial adenomatosis polyposis and desmoid fibromatosis
(A) Axial contrast enhanced CT of abdomen shows large infiltrative mesenteric mass (arrow).
(B) Axial fused PET CT shows only mild FDG uptake (SUVmax 4.2) in the mesenteric desmoid fibromatosis (arrow). Patient was asymptomatic and this was monitored during routine follow up for familial adenomatosis polyposis. Three years later, patient developed rapidly progressive metastatic rectal adenocarcinoma.
(C) Axial contrast enhanced CT of abdomen performed 3 years later shows a new heterogeneous mass (arrow) involving the periampullary and duodenal region. In the interval period, patient had been diagnosed with metastatic rectal adenocarcinoma. Biopsy of the duodenal/ periampullary mass confirmed metastatic adenocarcinoma.
(D) Axial fused PET CT of the abdomen shows markedly FDG avid (SUVmax 9.6) metastatic adenocarcinoma involving the periampullary and duodenal region (open arrow). In contrast, note the mesenteric desmoid fibromatosis (close arrow) has only mild FDG uptake (SUVmax 4.4).
Molecular Cytogenetics
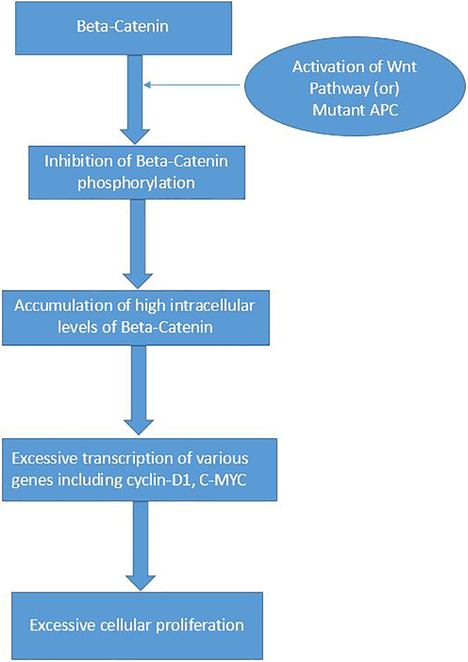
Awareness of the molecular cytogenetics of DF has provided an insight in the pathogenesis of both the familial and sporadic DF. Wnt/ β-catenin pathway is believed to play a key role in the pathogenesis of DF (Fig 10) [5, 11]. β-catenin is an important molecule with multiple cellular functions including serving as cell adhesion molecule in adherens junctions in the mesenchyme cells. Intra-cellular levels of β-catenin are regulated by the adenomatous polyposis coli (APC) gene and the Wnt pathway [5]. APC gene plays a key role in the phosphorylation and subsequent proteasomal degradation of the β-catenin. On the other hand, Wnt pathway inhibits this APC-dependent phosphorylation of β-catenin (Fig 10) [11]. Besides APC, another important gene complex playing a vital role in the downstream regulation and degradation of the β-catenin is the catenin beta 1 (CTNNB1) gene [5,11]. Genetic alterations in the APC and CTNNB1 gene are thought to be vital events resulting in the hereditary and sporadic DF.
Fig. 10 -.
The Wnt-Beta-Catenin pathway in Desmoid Fibromatosis
β-catenin molecule is normally located in the adherens junctions. Adenomatosis Polyposis Coli (APC) gene complex regulates the phosphorylation of β-catenin and its subsequent degradation in the proteasomes. When there is activation of Wnt (by its binding with its ligand) or there is mutation in the APC gene, it prevents the APC induced phosphorylation of the β-catenin. This results in excessive intracellular accumulation of β-catenin, which translocates to the nucleus and causes significant activation and transcription of various genes including Cyclin-D1 and C-MYC. In turn, this leads to a cascade of events resulting in excessive cell proliferation.
All patients with FAP syndrome inherit a single mutant defective allele of the APC gene; DF develops in FAP when the second allele also becomes defective due to somatic inactivation such as mutation 3’ in codon 1444 of the APC gene (Knudson’s two-hit hypothesis). Non-functional APC gene results in excessive accumulation of intra-cellular β-catenin; high levels of β-catenin may cause over stimulation of various genes such as cyclin-D1 and c-myc, resulting in a cascade of events leading to excessive cell proliferation & differentiation, with resultant DF tumor development in FAP.
Sporadic DF is associated with a high incidence (85%) of mutations in CTNNB1 gene, which may lead to uninhibited activation of Wnt pathway, leading to accumulation of excessive cytoplasmic β-catenin, ultimately resulting in development of the tumor [12]. Trisomy 8 and trisomy 20 has also been reported in sporadic DF [13]. Mutations in other genes such as AKT1, BRAF and TP53 have also been reported in DF [14].
Better understanding of the molecular cytogenetics of DF has also enhanced our knowledge of the molecular biology and tumor behavior of this rare condition. For example, in patients with FAP-related DF, presence of mutation in 5’ of codon 400 in APC gene is associated with a significantly better prognosis compared to mutation 3” of codon 1400 [15]. Similarly, S45 mutation of CTNNB1 mutation has been associated with a higher risk of local recurrence in sporadic DF, compared to other types of mutation in beta-catenin gene [16, 17]. Studies analyzing gene-expression profiles in DF have also identified specific gene-expression signatures which can help predict progression fee survival and time to recurrence [18, 19].
Unravelling cytogenetics of DF may also pave way for improved management of this tumor. For example, recent studies indicate that targeting Wnt/ β-catenin pathway and Notch pathway with PF-03084014, a potent γ secretase inhibitor, may be a potential alternative therapy for managing DF [20]. Further, tumor genotype may also be useful to predict response to therapy and treatment efficacy. For example, CTNNB1 mutation status may help to identify patients who are most likely to benefit from targeted therapy with imatinib [21]. Similarly, identification of CTNNB1 S45 mutation status may be helpful in predicting treatment efficacy of meloxicam in DF [22].
Histopathology
Histopathologically, DF is characterized by proliferation of uniform spindle cells resembling myofibroblasts, in the background of abundant collagenous stroma and vascular network [23]. Histological features such as hyperchromatasia and atypia are absent. Tumor cells are similar to the myofibroblasts seen during the proliferative stage of wound healing. Cells may have nuclei containing euchromatin or heterochromatin [24]. Large amounts of myxoid stroma may be seen in some tumors, especially those that develop in mesentery. On immunohistochemistry, DF stains positive for nuclear B-catenin, vimentin, cyclooxygenase 2, tyrosine kinase PDGFRb, androgen receptor and estrogen receptor beta but negative for desmin, S-100, h-caldesmon, CD34 and c-KIT [25].
Clinical features
The clinical presentation in DF is highly variable and may be influenced by the tumor location [26, 27]. Most DF in the abdominal wall and extra-abdominal locations may present as painless mass. However, larger lesions and those adjacent to neurovascular structures may be associated with pain and functional impairment (Fig 4, 5) [26, 27]. Intra-abdominal DF can result in various complications such as bowel obstruction, hydronephrosis and rarely intestinal perforation [28–30] (Fig 7).
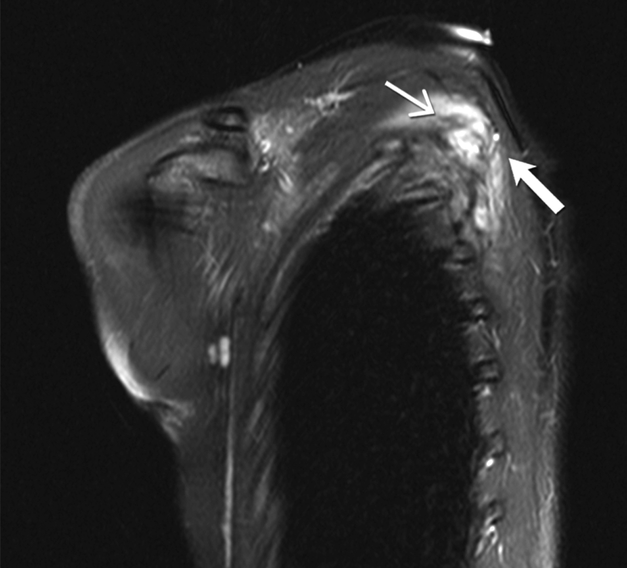
Fig. 4 -.
27-year-old woman with desmoid fibromatosis.
(A) Coronal fat suppressed T2 weighted image of the left scapula shows an infiltrative 10 × 6 × 3 cm mass (open arrow) lying between the left subscapularis and left posterior ribs. Linear low signal intensity bands ( closed arrow) are seen within the mass, consistent with band sign
(B) Axial fat suppressed post contrast enhanced T1 weighted image of the left scapula shows an infiltrative enhancing mass (open arrow) lying between the left subscapularis and left posterior ribs. Linear non—enhancing low signal intensity bands ( closed arrow) are seen within the mass, consistent with band sign
(C) Sagittal fat suppressed post contrast enhanced T1 weighted image of the left scapula shows an infiltrative enhancing mass (open arrow) lying between the left subscapularis and left posterior ribs. Linear non-enhancing low signal intensity bands (closed arrow) are seen within the mass, consistent with band sign. Patient had significant pan in this left shoulder and scapular region, and hence this was surgically resected.
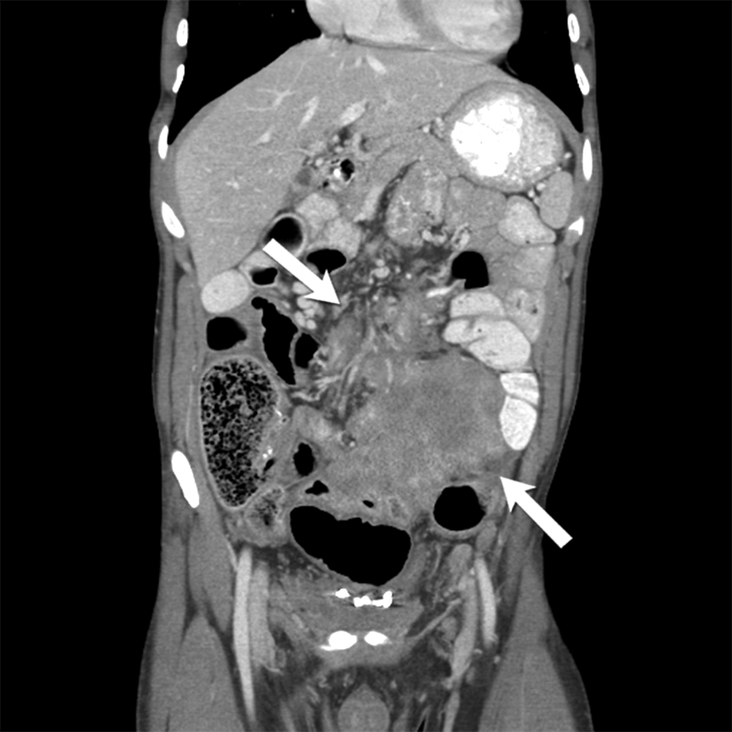
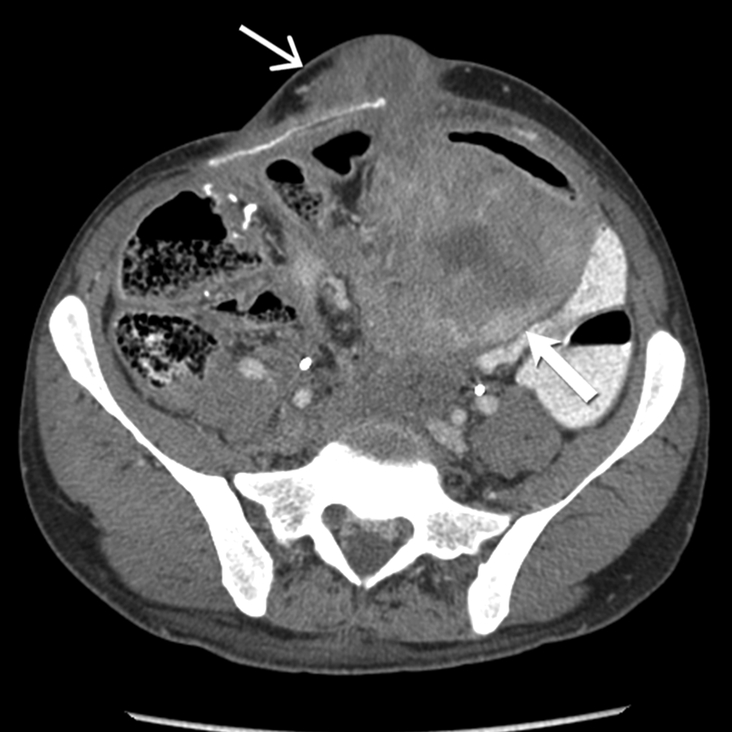
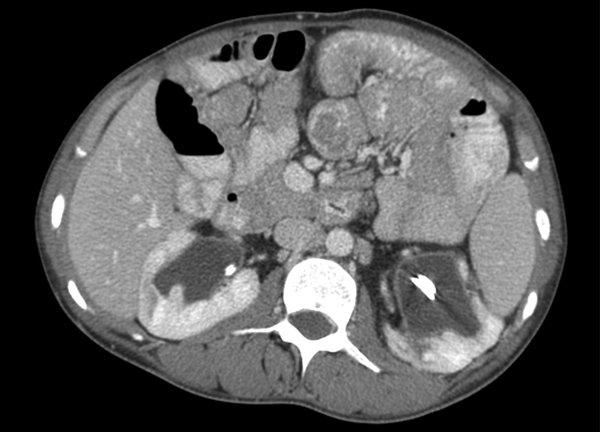
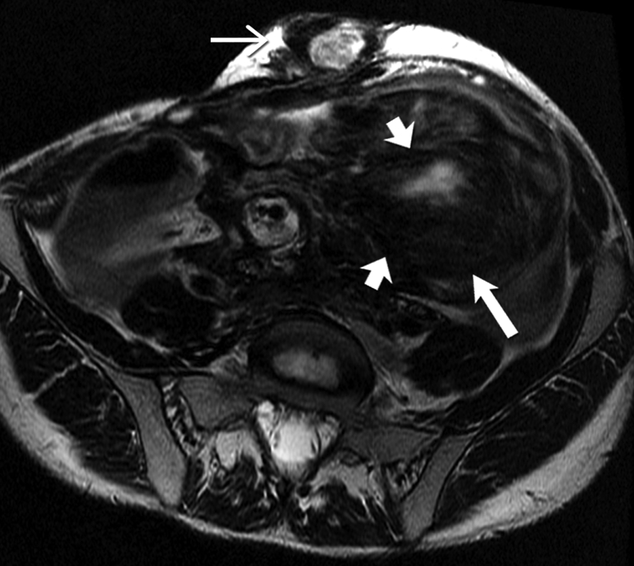
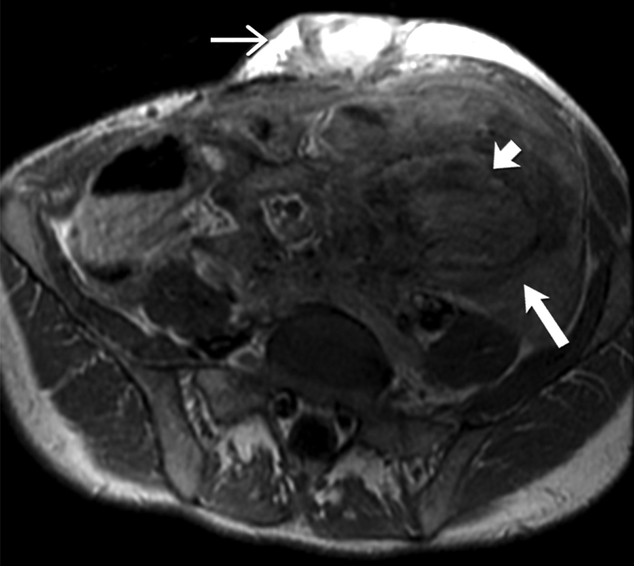
Fig 7-.
26-year-old man with familial adenomatosis polyposis and desmoid fibromatosis
(A) Coronal contrast enhanced T1 weighted image shows a large infiltrative mass in the root of mesentery encasing the major vascular structures and infiltrating the adjacent bowel loops. Mildly dilated small bowel loops are seen. Patient had partial small bowel obstruction.
(B) Axial contrast enhanced CT of the abdomen shows the heterogeneous mesenteric tumor (open arrow). Patient had history of rectal cancer which was treated with low anterior resection and had also undergone prior mesh repair of ventral hernia. Note the heterogeneous abdominal wall mass (close arrow) seen along the site of surgical scar, adjacent to abdominal wall mesh.
(C) Axial contrast enhanced CT of the upper abdomen shows bilateral hydronephrosis secondary to ureteric obstruction by the infiltrative mesenteric mass. Bilateral ureteral stents are in situ.
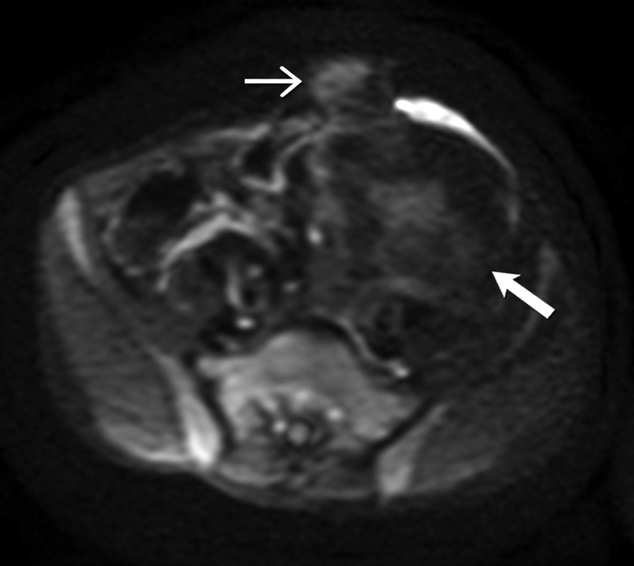
(D) Axial T2 weighted MR of the abdomen shows heterogeneous mesenteric mass (long open arrow) with numerous linear bands (short open arrow) of low signal intensity and central areas of high signal intensity. The abdominal wall mass (close arrow) shows similar signal intensity characteristics
(E) Axial post contrast enhanced T1 weighted MR of the abdomen shows enhancement within the cellular areas of the heterogeneous mesenteric mass (long open arrow). Non-enhancing linear bands of low signal intensity reflecting collagen bands (short open arrow) are also seen within the tumor. The abdominal wall mass (close arrow) shows similar enhancement characteristics.
(F) Axial high B value diffusion weighted MR of the abdomen shows only mild restricted diffusion in the central portions of the mesenteric tumor (open arrow) and abdominal wall mass (close arrow) corresponding to the cellular areas of the tumor. In general, MR signal intensity characteristics of desmoid fibromatosis reflect the histological and cellular components of the tumor. Highly cellular areas of the tumor tend to be T2 hyperintense, demonstrate avid enhancement and are associated with restricted diffusion. In contrast, collagen bands and fibrous, hypocellular portions of the tumor tend to be T2 hypointense, demonstrate poor enhancement and do not restrict diffusion.
Imaging features
Multimodality imaging including ultrasound, computed tomography (CT) and magnetic resonance (MR) play a key role in the diagnosis, staging and follow up of DF [31–34]. Ultrasound is helpful for delineating the tumor, especially DF occurring in the abdominal wall and extremities (Fig. 1). DF can present as well defined or poorly defined infiltrative heterogeneous solid mass [33, 35–37]. Tumors have variable echogenicity depending upon the amount of collagen, fibrosis and cellular components within the lesion [36]. A recent study reported that presence of stellar-type configuration with multiple irregular sun-burst like extensions along fascial planes may be a useful diagnostic feature of superficial extra-abdominal DF but larger prospective studies would be helpful to validate this finding [35].
CT is commonly used for imaging DF and is particularly helpful for intra-abdominal lesions. Tumors occurring within the abdominal cavity tend to most commonly involve the mesentery, although other sites may be affected (Fig. 2, 7). Intra-abdominal tumors may present as well defined soft tissue masses with variable attenuation, or occur as poorly defined infiltrative lesions (30–32). Similar to ultrasound, the CT appearance of the tumor may be dictated by the amount of collagenous and myxoid contents. In general, myxoid portions of the tumor tend to be hypodense compared to skeletal muscle, while collagenous and fibrotic components can be isodense or hyperdense (30–32). DF typically enhances following intravenous contrast administration; however, the degree of enhancement is usually mild to moderate, owing to the presence of varying myxoid and collagenous material within the tumor [33, 38]. Necrosis is typically absent [34]. CT can provide critical information required for treatment planning, including relationship of the tumor to the major vessels and adjacent organs (Fig. 7). Complications such as bowel obstruction, bowel ischemia and hydronephrosis are readily identified on CT [38].
Given its excellent soft tissue resolution, MR is very useful for imaging DF, especially the extra-abdominal lesions occurring in the extremities, head and neck, abdominal and chest wall (Fig. 3, 4, 8). MR may also be useful for monitoring mesenteric DF, particularly in those with allergy to iodinated contrast media as well as in young patients in whom it is desirable to reduce radiation exposure (Fig. 7). MR characteristics of DF depend upon the histological components of the tumor (Fig 3–4, 7–8) [32, 39–41]. Fibrotic and collagenous portions of DF typically demonstrate low signal intensity on T2 weighted sequences and demonstrate mild to moderate enhancement, especially on the delayed phase post contrast enhanced images; in contrast, prominent cellular stroma and myxoid matrix in DF manifests as heterogeneous T2 hyperintense areas and demonstrate moderate to intense enhancement following intravenous contrast administration. Presence of linear, non-enhancing, T1- and T2-hypointense bands seen within the tumor (band sign) is reported to a characteristic MR finding seen in 60–90% of DF (Fig. 4) [39]. However, it should be noted that band sign is not pathognomonic of DF as other musculoskeletal soft tissue tumors such as malignant fibrous histiocytoma may also demonstrate this imaging feature [39, 40]. Fascial tail sign refers to the presence of a linear infiltrative border extending from the tumor along the fascial plane and may be seen in up to 83 % of DF (Fig. 3–4) [39]. A recent study reported that the mean apparent diffusion coefficient (ADC) of DF was significantly higher than the malignant soft tissue sarcomas, implying that diffusion weighted imaging may be helpful in differentiating DF from the malignant tumors [42].
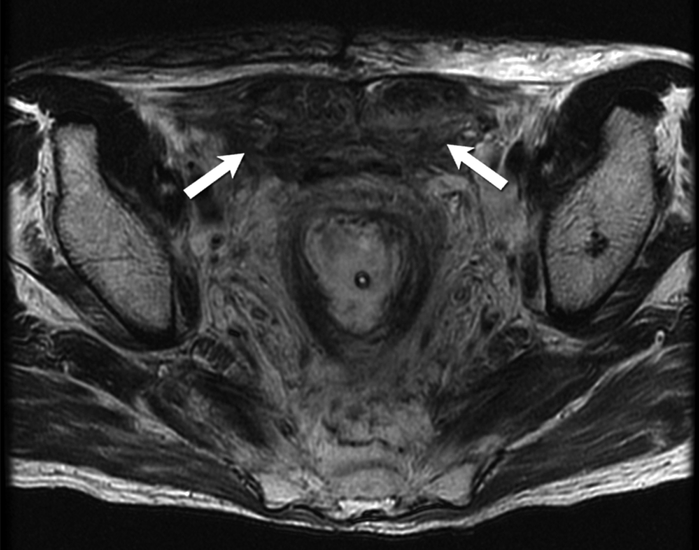
Fig. 8-.
48-year-old man with familial adenomatosis polyposis and abdominal wall desmoid fibromatosis Axial T2 weighted MR image shows heterogeneous fusiform bilateral abdominal wall masses (arrows) containing T2-hyperintense and T2-hypointense areas.
CT and MR imaging features can aid decision on patient management. The relationship of the tumor to the adjacent structures (especially the major neurovascular structures and vital organs) should be carefully evaluated and reported as this can help decide the feasibility of surgery. Also, CT and MR play a critical role in the follow up as these tumors tend to have a high recurrence rate. Furthermore, imaging is important in evaluating response to non-surgical therapy. Conventionally, response to therapy is evaluated using tumor response criteria RECIST 1.1, which is based on decrease in tumor size. However, this can significantly underestimate response to therapy, especially when systemic targeted therapies are used. Hence alternate tumor response criteria has been proposed in DF which takes into account changes in tumor size as well as interval changes in tumor attenuation (on CT) and changes in T2 signal intensity and degree of tumor enhancement ( on MR), following systemic therapy (31). Even in the absence of change in tumor size, reduction in CT attenuation, decrease in T2 signal intensity and decrease in enhancement of the tumor are reported to be findings suggestive of modified response to therapy (31). These imaging findings may reflect response in the form of increased fibrous component and decreased active inflammatory component within DF.
The role of fluoro-de-oxy-glucose positron emission tomography (FDG PET/ CT) in DF is not yet fully defined. There is limited literature regarding the utility of PET CT in DF, with most of the current evidence coming from small case series and case reports [43–47]. DF tends to demonstrate mild FDG avidity (Fig. 9) [43–47]. Few small studies indicate that PET CT may be useful for monitoring efficacy of systemic therapy in DF as reduction in FDG uptake correlated with pathological features such as fibrosis and decrease in tumor mitosis [44, 47]. Larger, prospective studies would be required for validation before PET CT can be used in the routine clinical practice for DF.
Differential Diagnoses
Abdominal DF: Various pathological processes can result in abdominal wall masses include infection, inflammation, endometriosis, hematoma and neoplasm [48, 49]. Clinical history and imaging features are often helpful in narrowing the differential diagnoses [Table 1].
Table 1.
Differential diagnosis of Abdominal wall DF (45,46)
| Condition | Helpful clinical and imaging features |
|---|---|
| DF | Female patient in child-bearing age group, prior history of pregnancy, abdominal surgery such as cesarean section, use of oral contraceptives, slow growing mass, heterogeneous signal intensity on MR with low T2 signal intensity areas corresponding to collagen and fibrosis |
| Endometriosis | Mass along the cesarean scar, classic cyclical symptoms with size and pain increasing during menstrual period, co-existing pelvic endometriosis, CT may show homogenous infra-umbilical nodule with linear strands extending peripherally from the central nodule (gorgon sign), heterogeneous MR features depending upon age of blood products |
| Hematoma | History of trauma, anticoagulation therapy, coagulopathies; presence of high attenuation mass on non-contrast CT, lack of enhancement, |
| Injection granuloma | History of subcutaneous injections in abdominal wall, soft tissue lesion with foci of air, calcification, fluid etc. |
| Abscess | Relevant clinical history, characteristic physical exam findings ( erythema, warmth, tenderness etc.), heterogeneous hypoechoic lesion with increased Doppler flow along the periphery, hypodense fluid containing mass with thick enhancing peripheral rim on CT |
| Arteriovenous malformations | Vascular lesion showing flow voids on MR, presence of phleboliths |
| Lipomatous tumors ( including lipoma, atypical lipomatous tumors and liposarcoma) | Presence of fat in the lesion seen in ultrasound/ CT/ MR |
| Neurogenic tumors | History of neurofibroma, fusiform mass seen along the course of the nerve, avid enhancement, target sign on T2 weighted sequences |
| Lymphoma | History of lymphoma, homogenous soft tissue mass with moderate enhancement, presence of adenopathy above and below the diaphragm, splenomegaly etc. |
| Other malignant mesenchymal neoplasms | Biopsy is typically required for definitive diagnosis |
| Metastases | History of primary malignancy elsewhere in the body, |
Mesenteric DF: Mesenteric DF typically occurs in patients with history of FAP. However, colorectal cancers in FAP patients can result in mesenteric metastases, mimicking DF. Further, it has to be noted that DF may develop in the mesentery even in patients without a known history of FAP. In such circumstances, other disease processes which can result in mesenteric masses should be considered in the differential diagnoses, including carcinoid tumor, lymphoma, retractile sclerosing mesenteritis, gastrointestinal stromal tumor and mesenteric metastases [Table 2] [34].
Table 2.
Differential diagnosis of mesenteric DF (32)
| Condition | Helpful imaging features |
|---|---|
| Carcinoid tumor | Presence of stellate, calcified mesenteric mass causing desmoplastic reaction, co-existing hypervascular small bowel tumor and hepatic metastases |
| Mesenteric lymphoma | Presence of mesenteric mass encasing the adjacent vessels and organs but without any significant mass effect or obstructive symptoms (despite its large size |
| Carcinomatosis involving mesentery | Involvement at other peritoneal compartments, co-exiting primary tumors such as ovarian or gastrointestinal malignancies |
| Exophytic GIST | Localization of tumor to gastrointestinal tract, presence of significant tumor necrosis/ cystic degeneration or hemorrhage, hypervascular tumor enhancement |
Extra-abdominal DF: Various soft tissue tumors may mimic extra-abdominal DF occurring in extremities, head and neck and trunk. Although the imaging features described previously can help to narrow the differential diagnoses and point towards a preliminary diagnosis of DF, definitive diagnosis requires histopathological confirmation.
Management
Traditionally, DF was managed by surgical resection. However, surgical resection of these tumors may be associated with significant morbidity and mortality. Resection of tumors located close to major neurovascular structures might result in significant functional impairment. Also, optimal removal of intra-abdominal mesenteric DF might warrant extensive surgery, including resection of significant portions of bowel, major vascular structures and adjacent abdominal organs, which may lead to significant postoperative complications. Furthermore, DF has a high incidence of local recurrence (20–39%) [50].
In view of the unpredictable tumor behavior and the significant morbidity associated with surgery, a more conservative, step-wise approach is currently preferred in the management of DF [7, 8, 51]. Newly diagnosed, asymptomatic DF may be managed with “wait and see” approach. Studies have shown that patients with asymptomatic DF may be initially managed with active surveillance as a significant percentage of these tumors show long term stability without treatment [8, 52]. In one study involving 83 DF patients placed on active surveillance, the 5 year progression free survival (PFS) was 50% and the median time to progression (TTP) was 14 months [8]. Another study reported that 16 out of 27 patients placed on active surveillance had stable disease during a median follow up of 52 months, with a TTP of 19 months [52]. Further, some of the DF may undergo spontaneous regression. Bonvalot et al. reported that 29 out of 102 patients (28%) with abdominal wall DF had spontaneous tumor regression [53]. Similarly, another study reported 20% spontaneous regression rate in extra-abdominal wall DF managed non-surgically [54].
However, treatment should be offered to patients who decline observation. Also, tumors showing interval increase in size and/ or causing symptoms (including those resulting in cosmetic disfigurement) may require active management. Factors associated with a higher risk of post-operative recurrence include large tumor size, younger patient age, and location of the tumors in the extremities and mesentery [55, 56]. In general, optimal resection with negative surgical margin should be the primary goal of the surgery for best oncological outcome [55, 57, 58]. However, some studies reported no significant difference in the 5 year PFS in patients with positive versus negative surgical margin [56, 59, 60]. Given these conflicting findings, it is currently unclear if patients with positive surgical margin warrant 2nd look surgery or can be managed conservatively.
Radiotherapy may be useful for unresectable or recurrent DF as well as in patients who are at high risk for surgery [61–64]. Further, adjuvant radiotherapy following surgery may also be considered in DF, especially those with positive surgical margins [64]. A recent meta-analysis reported that combination of surgery and radiotherapy had a lower local failure rate compared to surgery alone [65]. However, it has to be noted that radiation therapy is also associated with significant morbidity and hence careful risk versus benefit analysis should be performed and therapeutic doses kept to as low as reasonably possible [65, 66]. Another treatment option includes radiofrequency tumor ablation, although this is not well established.
Systemic therapy also plays an important part in the management of DF. Various therapeutic agents including anti-estrogenic drugs (tamoxifen and toremifene), non-steroidal anti-inflammatory drugs (meloxicam, indomethacin, sulindac and celecoxib), cytotoxic chemotherapy (doxorubicin, methotrexate and vinblastine) and tyrosine kinase inhibitors (imatinib, sunitinib, pazopanib, sorafenib, sirolimus) have been reported to be useful for achieving disease stability in DF [4, 21, 55, 67–69]. Given the high toxicity profile of some of these agents, patients should be closely monitored for any potential treatment induced complications [55, 70].
In summary, DF is a rare, locally aggressive soft tissue tumor with variable tumor behavior. DF may develop at any site but is particularly more common in the extremities, abdominal wall and mesentery. Management of desmoid tumors mandates multidisciplinary approach including watchful waiting, surgery, radiation and systemic therapy. Multimodality imaging including ultrasound, CT and MR is useful in the diagnosis, evaluation of treatment response and surveillance of these tumors.
Acknowledgments
Sources of funding: Supported by the NIH/NCI under award number P30 CA016672
Footnotes
Conflicts of Interest: None declared
Disclosures: None (All the authors confirm that there are no relevant disclosures)
Publisher's Disclaimer: Submission Declaration: All the authors confirm that this manuscript has not been published previously, and that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Contributor Information
Dhakshina moorthy Ganeshan, Division of Diagnostic Imaging, Body Imaging section, Unit 1473, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, Texas 77030-4009, Telephone: 404-983-1983, Fax: 713-745-1151,.
Behrang Amini, Division of Diagnostic Imaging, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, Texas 77030-4009.
Paul Nikolaidis, Northwestern University, Feinberg School of Medicine, 676 N. St. Clair St., Chicago, IL 60611.
Matthew Assing, Department of Radiology, Stanford University School of Medicine, 300 Pasteur Drive, Stanford, CA 94305-5105.
Raghunandan Vikram, Division of Diagnostic Imaging, Unit 1473, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, Texas, 77030-4009.
References
- 1.Sakorafas GH, Nissotakis C, Peros G. Abdominal desmoid tumors. Surg Oncol 2007; 16:131–142 [DOI] [PubMed] [Google Scholar]
- 2.Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, Dekkers OM, Hogendoorn PC, Vasen HF. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 2011; 129:256–261 [DOI] [PubMed] [Google Scholar]
- 3.de Camargo VP, Keohan ML, D’Adamo DR, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 2010; 116:2258–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiore M, MacNeill A, Gronchi A, Colombo C. Desmoid-Type Fibromatosis: Evolving Treatment Standards. Surg Oncol Clin N Am 2016; 25:803–826 [DOI] [PubMed] [Google Scholar]
- 5.Martinez Trufero J, Pajares Bernad I, Torres Ramon I, Hernando Cubero J, Pazo Cid R. Desmoid-Type Fibromatosis: Who, When, and How to Treat. Curr Treat Options Oncol 2017; 18:29. [DOI] [PubMed] [Google Scholar]
- 6.Koskenvuo L, Ristimaki A, Lepisto A. Comparison of sporadic and FAP-associated desmoid-type fibromatoses. J Surg Oncol 2017; [DOI] [PubMed] [Google Scholar]
- 7.Kasper B, Baumgarten C, Bonvalot S, et al. Management of sporadic desmoid-type fibromatosis: a European consensus approach based on patients’ and professionals’ expertise - a sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer 2015; 51:127–136 [DOI] [PubMed] [Google Scholar]
- 8.Fiore M, Rimareix F, Mariani L, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 2009; 16:2587–2593 [DOI] [PubMed] [Google Scholar]
- 9.Gurbuz AK, Giardiello FM, Petersen GM, et al. Desmoid tumours in familial adenomatous polyposis. Gut 1994; 35:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallen T, Wilson M, Morlan B, Lindor NM. Desmoid tumors -- a characterization of patients seen at Mayo Clinic 1976–1999. Fam Cancer 2006; 5:191–194 [DOI] [PubMed] [Google Scholar]
- 11.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 2009; 10:468–477 [DOI] [PubMed] [Google Scholar]
- 12.Le Guellec S, Soubeyran I, Rochaix P, et al. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: a study of 260 desmoid tumors and 191 potential morphologic mimics. Mod Pathol 2012; 25:1551–1558 [DOI] [PubMed] [Google Scholar]
- 13.Qi H, Dal Cin P, Hernandez JM, et al. Trisomies 8 and 20 in desmoid tumors. Cancer Genet Cytogenet 1996; 92:147–149 [DOI] [PubMed] [Google Scholar]
- 14.Meazza C, Belfiore A, Busico A, et al. AKT1 and BRAF mutations in pediatric aggressive fibromatosis. Cancer Med 2016; 5:1204–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Church J, Xhaja X, LaGuardia L, O’Malley M, Burke C, Kalady M. Desmoids and genotype in familial adenomatous polyposis. Dis Colon Rectum 2015; 58:444–448 [DOI] [PubMed] [Google Scholar]
- 16.Colombo C, Miceli R, Lazar AJ, et al. CTNNB1 45F mutation is a molecular prognosticator of increased postoperative primary desmoid tumor recurrence: an independent, multicenter validation study. Cancer 2013; 119:3696–3702 [DOI] [PubMed] [Google Scholar]
- 17.Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 2008; 173:1518–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salas S, Brulard C, Terrier P, et al. Gene Expression Profiling of Desmoid Tumors by cDNA Microarrays and Correlation with Progression-Free Survival. Clin Cancer Res 2015; 21:4194–4200 [DOI] [PubMed] [Google Scholar]
- 19.Dufresne A, Paturel M, Alberti L, et al. Prediction of desmoid tumor progression using miRNA expression profiling. Cancer Sci 2015; 106:650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang H, Braggio D, Lee YJ, et al. Targeting the Notch pathway: A potential therapeutic approach for desmoid tumors. Cancer 2015; 121:4088–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasper B, Gruenwald V, Reichardt P, Bauer S, Hohenberger P, Haller F. Correlation of CTNNB1 Mutation Status with Progression Arrest Rate in RECIST Progressive Desmoid-Type Fibromatosis Treated with Imatinib: Translational Research Results from a Phase 2 Study of the German Interdisciplinary Sarcoma Group (GISG-01). Ann Surg Oncol 2016; 23:1924–1927 [DOI] [PubMed] [Google Scholar]
- 22.Hamada S, Futamura N, Ikuta K, et al. CTNNB1 S45F mutation predicts poor efficacy of meloxicam treatment for desmoid tumors: a pilot study. PLoS One 2014; 9:e96391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller E, Castagnaro M, Yandel D, Wolfe H, Alman BA. Molecular genetic and immunohistochemical analysis of the tumor suppressor genes Rb and P53 in plamar and agressive fibromatosis. Diagnostic Molecular Pathology 1996; 5:194–200 [DOI] [PubMed] [Google Scholar]
- 24.Misemer BS, Skubitz AP, Carlos Manivel J, et al. Expression of FAP, ADAM12, WISP1, and SOX11 is heterogeneous in aggressive fibromatosis and spatially relates to the histologic features of tumor activity. Cancer Med 2014; 3:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotiligam D, Lazar AJ, Pollock RE, Lev D. Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol 2008; 23:117–126 [DOI] [PubMed] [Google Scholar]
- 26.Stoeckle E, Coindre JM, Longy M, et al. A critical analysis of treatment strategies in desmoid tumours: a review of a series of 106 cases. Eur J Surg Oncol 2009; 35:129–134 [DOI] [PubMed] [Google Scholar]
- 27.Chew C, Reid R, O’Dwyer PJ. Evaluation of the long term outcome of patients with extremity desmoids. Eur J Surg Oncol 2004; 30:428–432 [DOI] [PubMed] [Google Scholar]
- 28.Abdalla S, Wilkinson M, Wilsher M, Uzkalnis A. An atypical presentation of small bowel obstruction and perforation secondary to sporadic synchronous intra-abdominal desmoid tumours. Int J Surg Case Rep 2016; 20:147–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos FG, Martinez CA, Novaes M, Nahas SC, Cecconello I. Desmoid tumors: clinical features and outcome of an unpredictable and challenging manifestation of familial adenomatous polyposis. Fam Cancer 2015; 14:211–219 [DOI] [PubMed] [Google Scholar]
- 30.Xhaja X, Church J. Small bowel obstruction in patients with familial adenomatous polyposis related desmoid disease. Colorectal Dis 2013; 15:1489–1492 [DOI] [PubMed] [Google Scholar]
- 31.Braschi-Amirfarzan M, Keraliya AR, Krajewski KM, et al. Role of Imaging in Management of Desmoid-type Fibromatosis: A Primer for Radiologists. Radiographics 2016; 36:767–782 [DOI] [PubMed] [Google Scholar]
- 32.Walker EA, Petscavage JM, Brian PL, Logie CI, Montini KM, Murphey MD. Imaging features of superficial and deep fibromatoses in the adult population. Sarcoma 2012; 2012:215810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casillas J, Sais GJ, Greve JL, Iparraguirre MC, Morillo G. Imaging of intra- and extraabdominal desmoid tumors. Radiographics 1991; 11:959–968 [DOI] [PubMed] [Google Scholar]
- 34.Winant AJ, Vora A, Ginter PS, Levine MS, Brylka DA. More than just metastases: a practical approach to solid mesenteric masses. Abdom Imaging 2014; 39:605–621 [DOI] [PubMed] [Google Scholar]
- 35.Milos RI, Moritz T, Bernathova M, et al. Superficial desmoid tumors: MRI and ultrasound imaging characteristics. Eur J Radiol 2015; 84:2194–2201 [DOI] [PubMed] [Google Scholar]
- 36.Lou L, Teng J, Qi H, Ban Y. Sonographic appearances of desmoid tumors. J Ultrasound Med 2014; 33:1519–1525 [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Tang J, Luo Y. Sonographic diagnosis of fibromatosis. J Clin Ultrasound 2008; 36:330–334 [DOI] [PubMed] [Google Scholar]
- 38.Einstein DM, Tagliabue JR, Desai RK. Abdominal desmoids: CT findings in 25 patients. AJR Am J Roentgenol 1991; 157:275–279 [DOI] [PubMed] [Google Scholar]
- 39.Dinauer PA, Brixey CJ, Moncur JT, Fanburg-Smith JC, Murphey MD. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics 2007; 27:173–187 [DOI] [PubMed] [Google Scholar]
- 40.Walker EA, Fenton ME, Salesky JS, Murphey MD. Magnetic resonance imaging of benign soft tissue neoplasms in adults. Radiol Clin North Am 2011; 49:1197–1217, vi [DOI] [PubMed] [Google Scholar]
- 41.Kamali F, Wang WL, Guadagnolo BA, et al. MRI may be used as a prognostic indicator in patients with extra-abdominal desmoid tumours. Br J Radiol 2016; 89:20150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oka K, Yakushiji T, Sato H, et al. Usefulness of diffusion-weighted imaging for differentiating between desmoid tumors and malignant soft tissue tumors. J Magn Reson Imaging 2011; 33:189–193 [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Koo HJ, Lim S, et al. Desmoid-Type Fibromatosis of the Thorax: CT, MRI, and FDG PET Characteristics in a Large Series From a Tertiary Referral Center. Medicine (Baltimore) 2015; 94:e1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasper B, Dimitrakopoulou-Strauss A, Pilz LR, Strauss LG, Sachpekidis C, Hohenberger P. Positron emission tomography as a surrogate marker for evaluation of treatment response in patients with desmoid tumors under therapy with imatinib. Biomed Res Int 2013; 2013:389672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhandari S, Taylor NJ, Sinha A, et al. Can combined 18F-FDG-PET and dynamic contrast-enhanced MRI predict behavior of desmoid tumors in patients with familial adenomatous polyposis? Dis Colon Rectum 2012; 55:1032–1037 [DOI] [PubMed] [Google Scholar]
- 46.Sohn MH, Jeong YJ, Lim ST, Kim DW, Jeong HJ, Yim CY. F-18 FDG PET/CT Findings of Spontaneous Mesenteric Fibromatosis in a Patient with Gardner’s Syndrome. Nucl Med Mol Imaging 2011; 45:156–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu S, Nair N, Banavali S. Uptake characteristics of fluorodeoxyglucose (FDG) in deep fibromatosis and abdominal desmoids: potential clinical role of FDG-PET in the management. Br J Radiol 2007; 80:750–756 [DOI] [PubMed] [Google Scholar]
- 48.Yarmish G, Sala E, Goldman DA, et al. Abdominal wall endometriosis: differentiation from other masses using CT features. Abdom Radiol (NY) 2017; 42:1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bashir U, Moskovic E, Strauss D, et al. Soft-tissue masses in the abdominal wall. Clin Radiol 2014; 69:e422–431 [DOI] [PubMed] [Google Scholar]
- 50.Wang YF, Guo W, Sun KK, et al. Postoperative recurrence of desmoid tumors: clinical and pathological perspectives. World J Surg Oncol 2015; 13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gronchi A, Colombo C, Le Pechoux C, et al. Sporadic desmoid-type fibromatosis: a stepwise approach to a non-metastasising neoplasm--a position paper from the Italian and the French Sarcoma Group. Ann Oncol 2014; 25:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol 2011; 29:3553–3558 [DOI] [PubMed] [Google Scholar]
- 53.Bonvalot S, Ternes N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 2013; 20:4096–4102 [DOI] [PubMed] [Google Scholar]
- 54.Colombo C, Miceli R, Le Pechoux C, et al. Sporadic extra abdominal wall desmoid-type fibromatosis: surgical resection can be safely limited to a minority of patients. Eur J Cancer 2015; 51:186–192 [DOI] [PubMed] [Google Scholar]
- 55.Yao X, Corbett T, Gupta AA, et al. A systematic review of active treatment options in patients with desmoid tumours. Curr Oncol 2014; 21:e613–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg 2013; 258:347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He XD, Zhang YB, Wang L, et al. Prognostic factors for the recurrence of sporadic desmoid-type fibromatosis after macroscopically complete resection: Analysis of 114 patients at a single institution. Eur J Surg Oncol 2015; 41:1013–1019 [DOI] [PubMed] [Google Scholar]
- 58.Zeng WG, Zhou ZX, Liang JW, et al. Prognostic factors for desmoid tumor: a surgical series of 233 patients at a single institution. Tumour Biol 2014; 35:7513–7521 [DOI] [PubMed] [Google Scholar]
- 59.Gronchi A, Casali PG, Mariani L, et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol 2003; 21:1390–1397 [DOI] [PubMed] [Google Scholar]
- 60.Merchant NB, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 1999; 86:2045–2052 [PubMed] [Google Scholar]
- 61.Santti K, Beule A, Tuomikoski L, et al. Radiotherapy in desmoid tumors : Treatment response, local control, and analysis of local failures. Strahlenther Onkol 2017; 193:269–275 [DOI] [PubMed] [Google Scholar]
- 62.Kriz J, Eich HT, Haverkamp U, et al. Radiotherapy is effective for desmoid tumors (aggressive fibromatosis) - long-term results of a German multicenter study. Oncol Res Treat 2014; 37:255–260 [DOI] [PubMed] [Google Scholar]
- 63.Karabulut S, Keskin S, Ekenel M, et al. The clinical effect of a positive surgical margin and adjuvant postoperative radiotherapy in the treatment of resectable desmoid tumors. Mol Clin Oncol 2013; 1:1061–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol 1999; 17:158–167 [DOI] [PubMed] [Google Scholar]
- 65.Wood TJ, Quinn KM, Farrokhyar F, Deheshi B, Corbett T, Ghert MA. Local control of extra-abdominal desmoid tumors: systematic review and meta-analysis. Rare Tumors 2013; 5:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keus RB, Nout RA, Blay JY, et al. Results of a phase II pilot study of moderate dose radiotherapy for inoperable desmoid-type fibromatosis--an EORTC STBSG and ROG study (EORTC 62991–22998). Ann Oncol 2013; 24:2672–2676 [DOI] [PubMed] [Google Scholar]
- 67.Fiore M, Colombo C, Radaelli S, et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer 2015; 51:2800–2807 [DOI] [PubMed] [Google Scholar]
- 68.Chugh R, Wathen JK, Patel SR, et al. Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res 2010; 16:4884–4891 [DOI] [PubMed] [Google Scholar]
- 69.Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol 2003; 14:181–190 [DOI] [PubMed] [Google Scholar]
- 70.Li W, Zhou Y, Li Q, Tong H, Lu W. Intestinal perforation during chemotherapeutic treatment of intra-abdominal desmoid tumor in patients with Gardner’s syndrome: report of two cases. World J Surg Oncol 2016; 14:178. [DOI] [PMC free article] [PubMed] [Google Scholar]