Abstract
Background:
Polycyclic aromatic hydrocarbons (PAHs) are environmental pollutants formed from incomplete combustion of organic matter; some PAHs are carcinogens. Smoking, diet, and other activities contribute to exposure to PAHs. Exposure data to PAHs among combustible tobacco product users (e.g. cigarette smokers) exist; however, among non-combustible tobacco products users (e.g., e-cigarette users), such data are rather limited.
Objectives:
We sought to evaluate exposure to PAHs among participants in Wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) Study based on the type of tobacco product (combustible vs non-combustible), and frequency and intensity of product use.
Methods:
We quantified seven PAH urinary biomarkers in 11,519 PATH Study participants. From self-reported information, we categorized 8327 participants based on their use of tobacco products as never-tobacco user (never user, n = 1700), exclusive current established combustible products user (combustible products user, n = 5767), and exclusive current established non-combustible products user (non-combustible products user, n = 860). We further classified tobacco users as exclusive cigarette user (cigarette user, n = 3964), exclusive smokeless product user (SLT user, n = 509), and exclusive e-cigarette user (e-cigarette user, n = 280). Last, we categorized frequency of product use (everyday vs some days) and time since use (last hour, within 3 days, over 3 days). We calculated geometric mean (GM) concentrations, and evaluated associations between tobacco product user categories and PAH biomarkers concentrations.
Results:
Combustible products users had significantly higher GMs of all biomarkers than non-combustible products users and never users; non-combustible products users had significantly higher GMs than never users for four of seven biomarkers. For all biomarkers examined, cigarette users had the highest GMs compared to other tobacco-product users. Interestingly, GMs of 2-hydroxyfluorene, 3-hydroxyfluorene and Σ2,3-hydroxyphenanthrene were significantly higher in SLT users than in e-cigarette users; 3-hydroxyfluorene and 1-hydroxypyrene were also significantly higher in e-cigarette and SLT users than in never users. Everyday cigarette and SLT users had significantly higher GMs for most biomarkers than some days’ users; cigarette and SLT users who used the product in the last hour had significantly higher GMs of most biomarkers than other occasional cigarette or SLT users respectively. By contrast, everyday e-cigarette users’ GMs of most biomarkers did not differ significantly from those in some days’ e-cigarette users; we did not observe clear trends by time of last use among e-cigarette users.
Conclusions:
Users of tobacco products had higher PAH urinary biomarker concentrations compared to never users, and concentrations differed by type and frequency of tobacco product use.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants formed from incomplete combustion of organic matter (Stogiannidis and Laane, 2015). People can be potentially exposed to PAHs from numerous sources including fuels such as oil, gas, coal, wood (Hu et al., 2011; Choosong et al., 2014; Rodrigues et al., 2014; Bencsath et al., 2015; Negri et al., 2016; Roy et al., 2017), diet (Rose et al., 2015), and smoking cigarettes (Li et al., 2008; McAdam et al., 2013; Slezakova et al., 2014; Zhang et al., 2015). Some PAHs induce tumors in animals and are carcinogenic to humans (ATSDR, 1995; IARC, 2010). For example, benzo[a]pyrene induces malignant lesions in animal studies (Hyunok Choi et al., 2010). Urinary concentrations of PAH metabolites, specifically monohydroxylated PAHs (OH-PAHs), have been used as biomarkers of human exposure to PAHs including naphthalene, fluorene, phenanthrene and pyrene (Li et al., 2008; Jongeneelen et al., 1985; Jongeneelen et al., 1988).
Smoking is the leading cause of preventable disease, disability, and death in the United States (CDC, 2018a; DHHS, 2014). In 2009, the U.S. Food and Drug Administration (FDA) obtained authority through the Family Smoking Prevention and Tobacco Control Act (FDA, 2018) to regulate manufacturing, marketing and distribution of tobacco products to protect public health. To help monitor the impact of these regulatory actions, FDA partnered with the National Institute on Drug Abuse at the National Institutes of Health to launch the Population Assessment of Tobacco and Health (PATH) Study (Hyland et al., 2017) to evaluate the impact of tobacco use on public health.
In the United States, general population exposures to PAHs have been assessed through the National Health and Nutrition Examination Survey (NHANES) since the early 2000s (Li et al., 2008; CDC, 2017), and these data have been used in addressing various health risks (Scinicariello and Buser, 2014; Liu et al., 2016; Farzan et al., 2016; Jain, 2016). However, assessment of exposure to PAHs among tobacco product users in NHANES is limited to cigarette smokers (St Helen et al., 2012). Information on the extent of PAHs exposure among users of non-combustible tobacco products (e.g., e-cigarettes, smokeless tobacco products) is limited to relatively small studies (Goniewicz et al., 2017; Hecht et al., 2015; Shahab et al., 2017), even though use of these products may be on the rise, particularly among adolescents and young adults (CDC, 2018b).
To address these gaps, we used PATH Study Wave 1 data to evaluate exposure to PAHs among never users and tobacco product users, including users of combustible and non-combustible tobacco products. We report reference ranges of seven OH-PAH urinary biomarkers: 1hydroxynaphthalene, 2-hydroxynaphthalene, 2-hydroxyfluorene, 3-hydroxyfluorene, 1-hydroxyphenanthrene, the sum of 2-hydroxyphenanthrene and 3-hydroxyphenanthrene (Σ2,3-hydroxyphenanthrene), and 1-hydroxypyrene by tobacco product user groups.
2. Materials and methods
2.1. Study design and participants
Data used in this current analysis are from Wave 1 of the PATH Study, collected from September 12, 2013 to December 15, 2014. Recruitment employed address-based, area-probability sampling, using an in-person household screener to select youth (ages 12–17) and adults. Adult tobacco users, young adults ages 18 to 24, and African Americans were oversampled relative to population proportions. A stratified probability sample of 11,522 adults who completed the Wave 1 adult interview and provided a spot urine for the planned analyses was selected for biomarker analysis from a diverse mix of tobacco product user groups. Estimates for this group of adults can be described as representative of never, current, and recent former (within 12 months) users of tobacco products in the U.S. civilian, noninstitutionalized adult population at the time of Wave 1. Specific details of the PATH study design, participant recruitment, and collection and handling of biological specimens have been presented previously (Hyland et al., 2017). The study was conducted by Westat and approved by the Westat Institutional Review Board. For this study, we present results for 11,519 samples which had quantifiable levels of OH-PAHs.
2.2. Quantitation of PAH biomakers in urine
The PATH Study measured urinary concentrations of seven OHPAHs using enzymatic hydrolysis, online solid phase extraction, and high performance liquid chromatography isotope dilution tandem mass spectrometry (online SPE-HPLC-MS/MS) as previously described (Wang et al., 2017). In brief, sulfate and glucuronide conjugates of OH-PAHs were enzymatically hydrolyzed from 100 μL urine, followed by online solid phase extraction and quantification by LC-MS/MS with 13C isotope–labeled internal standards. The inter- and intra-day precision of the method varied from 5.2 to 16.7%, depending on the analyte and concentration. The limits of detection (LOD) were 0.06 μg/L (1-hydroxynaphthalene), 0.09 μg/L (2-hydroxynaphthalene), 0.008 μg/L (2-hydroxyfluorene, 3-hydroxyfluorene), 0.009 μg/L (1-hydroxyphenanthrene), 0.01 μg/L (Σ2,3-hydroxyphenanthrene), and 0.07 μg/L (1-hydroxypyrene). The analytical measurements followed strict quality control/quality assurance protocols, including participation in quality assessment schemes to demonstrate the method accuracy and precision. Furthermore, along with study samples, each analytical run included spiked quality control materials and reagent blanks to assure the accuracy and reliability of the data. Details of the analytical procedure used are available at https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/PAH_H_MET_Aromatic_Hydrocarbons.pdf.
2.3. Tobacco product user groups and specific product user groups
Participants completed the Wave 1 Adult Interview and provided detailed information about the tobacco product use. This information was used to determine whether they were current established users (see definitions in Table 1 (Hyland et al., 2017)) of a combustible product (i.e., cigarette, traditional cigar, cigarillo, filtered cigar, hookah, pipe) or a non-combustible product (i.e., e-cigarette, smokeless tobacco, snus pouches, dissolvable tobacco). In addition, participants reported frequency (i.e., some days, everyday) and last time of tobacco product use. Self-reported and biomarker data are known to be highly correlated (West et al., 2007). Furthermore, previous research suggest the validity of self-reported data among PATH participants (Tourangeau et al., 2018).
Table 1.
Definition of tobacco products user groups and specific product user groups for the present study (Hyland et al., 2017).
| Definitiona | |
|---|---|
| 1a: Tobacco user groups | |
| Never-tobacco user (never user) (n = 1700) | Has never used any tobacco products |
| Exclusive current established combustible products user (combustible products user) (n = 5767) | 1. Has ever used at least one of the combustible products: cigarette, traditional cigar, cigarillo, filtered cigar, hookah, and pipe. |
| 2. Has used > 100 cigarettes in their entire life or fairly regularly used one or more of the following products: cigar, hookah, and pipe. | |
| 3. Now use everyday or some days. | |
| 4. Not current established users for any non-combustible products (answer “No” for each noncombustible products from questionnaire) (missing will not be included) | |
| Exclusive current established non-combustible products user (noncombustible products user) (n = 860) | 1. Has ever used at least one of the non-combustible products: e-cigarette, smokeless tobacco, snus pouches, and dissolvable tobacco. |
| 2. Has used fairly regularly. | |
| 3. Now use everyday or some days. | |
| 4. Not current established users for any combustible products (answer “No” for each combustible products from questionnaire) (missing will not be included). | |
| Mixed dual user (dual user) (n = 1258) | Users who meet definition 1–3 for both combustible products user and non-combustible products user |
| Others (other user) (n = 1934) | Users who do not meet the above definitions for never user, combustible products user, noncombustible products user, or dual user |
| 1b: Specific product user group | |
| Exclusive current established cigarette user (cigarette user) (n = 3964) (subset of combustible products user) | 1. Includes only cigarettes. |
| 2. Has ever used a cigarette | |
| 3. Has used > 100 of the cigarettes in their entire life | |
| 4. Now use everyday or some days | |
| 5. Currently does not use any other products | |
| Exclusive current established smokeless users (SLT user) (n = 509) (subset of non-combustible products user) | 1. Includes moist snuff, dip, spit, chewing tobacco, loose snus and pouched snus |
| 2. Has ever used a smokeless product | |
| 3. Has used fairly regularly | |
| 4. Now use everyday or some days | |
| 5. Currently does not use any other products | |
| Exclusive current established e-cigarette users (e-cigarette user) (n = 280) (subset of non-combustible products user) | 1. Has ever used a e-cigarette product |
| 2. Has used fairly regularly | |
| 3. Now use everyday or some days | |
| 4. Currently does not use any other products | |
| Never-tobacco user (never user) (n = 1700) | Has never used any tobacco products |
To belong to a given user group category, a person must meet all conditions included under the definition ofthe category.
Based on participants’ self-report, we categorized participants into three main tobacco user groups (Table 1a: Tobacco user group): never-tobacco user (never user; n = 1700), exclusive current established combustible products user (combustible products user; n = 5767) and exclusive current established non-combustible products user (noncombustible products user; n = 860). We categorized participants who reported being both combustible products user and non-combustible products user as dual-group user (n = 1258), and participants not included in the above user groups (including former smokers/users) as other user (n = 1934). We provided only descriptive statistical data for dual-group user and other user in the total population estimates in the Supplemental material. Participants who used more than one product were considered exclusive current established users if all products were in the same group (combustible or non-combustible).
We further defined four specific product user groups (Table 1b: Specific product user groups): exclusive current established cigarette user (cigarette user), exclusive current established smokeless product user (SLT user), exclusive current established e-cigarette user (e-cigarette user), and never user as one specific product reference user group.
2.4. Statistical evaluation
We used SAS (version 9.4; SAS Institute Inc., Cary, NC) and SUDAAN (version 11; Research Triangle Institute, Research Triangle Park, NC) for all statistical analyses. SAS and SUDAAN incorporate the appropriate sample weights to account for the complex design of the PATH Study. Further information on the weighting procedure can be obtained from the PATH Study Biomarker Restricted Use File User Guide (available at https://doi.org/10.3886/ICPSR36840.v1). The variance estimate was a balanced repeated replication with Fay’s method (Fay = 0.3), and statistical significance was set at p < 0.05. The Bonferroni adjustment was used for multiple testing, and the pvalues were multiplied by the number of comparisons in the descriptive analyses whereas the alphas for the adjusted model based confidence interval (CI) were divided by the number of comparisons. Data were weighted and all estimates produced are representative of never users, recent former users, and current users of tobacco products in the civilian, non-institutionalized U.S. population at the time of PATH Study Wave 1.
We defined four major racial/ethnic groups based on self-reported data: non-Hispanic white, non-Hispanic black, Hispanic, and Asian and Other (which included multiracial). We stratified age, reported in years at the last birthday, into four groups: 18–24 years, 25–34 years, 35–54 years, and 55 years and older.
We calculated the weighted frequency of detection for each biomarker. We also calculated the geometric mean (GM) and distribution percentiles for both the volume-based (in μg/L for 1-hydroxynaphthalene and 2-hydroxynaphthalene, and ng/L for the remaining biomarkers), and creatinine-corrected (in μg/g creatinine for 1-hydroxynaphthalene and 2-hydroxynaphthalene, and ng/g creatinine for the other biomarkers; see Supplemental tables) concentrations by tobacco product user group, age, sex, and race/ethnicity group. For concentrations below the LOD, as recommended for the analysis of NHANES data we used a value equal to the LOD divided by the square root of 2 (Hornung and Reed, 1990). We only calculated creatinine corrected concentrations in the descriptive analyses and included the log10 transformed creatinine as a covariate in the regression model for the 11,266 samples (weighted percentage, 98.5%) with normal (10–370 mg/dL) creatinine levels (Boeniger et al., 1993).
For each biomarker, we conducted weighted univariate analysis using one way ANOVA to compare GMs for the three main user groups: combustible products user, non-combustible products user, and never user. Due to skewed distributions, we used log10 transformed OH-PAH concentrations as the dependent variable. Of note, although the log10 tranformed biomarker distribution was not perfectly normal, the absolute values of skewness and kurtosis for each log10 transformed biomarker concentrations were between 0.025 and 0.5, and 0.25–1.4, respectively, all within the normal distribution range. The three-level user group was the independent variable. We also performed another analysis by adding the log10 transformed creatinine as a covariate to adjust for urinary dilution (Barr et al., 2005).
We also used weighted ANCOVA to evaluate the log10 transformed OH-PAH concentrations and their relationship with the three-level to-bacco main user groups adjusting by log10(creatinine), race/ethnicity, sex, and age, by calculating the covariate-adjusted GM ratios where never user was the reference group. The higher GM ratio, the more significant is the difference between the GMs in a given specific tobacco user group compared to the reference never user group. This approach allowed both the urinary biomarker concentration to be appropriately adjusted for urinary creatinine and the statistical significance of other variables in the model to be independent of effects of creatinine concentration.
Similarly, we also calculated GMs for the four-level specific product user groups (cigarette user, e-cigarette user, SLT user, and never user). We conducted the weighted univariate one way ANOVA and multiple regression ANCOVA analyses of the log transformed OH-PAH concentration by these four-level user groups, adjusted by log10(creatinine), race/ethnicity, sex, and age. We also stratified the analyses based on frequency of product use (everyday or some days) and by the last time the product was used (within the last hour, within the past three days, more than three days ago).
3. Results
3.1. Biomarkers of exposure by tobacco user groups
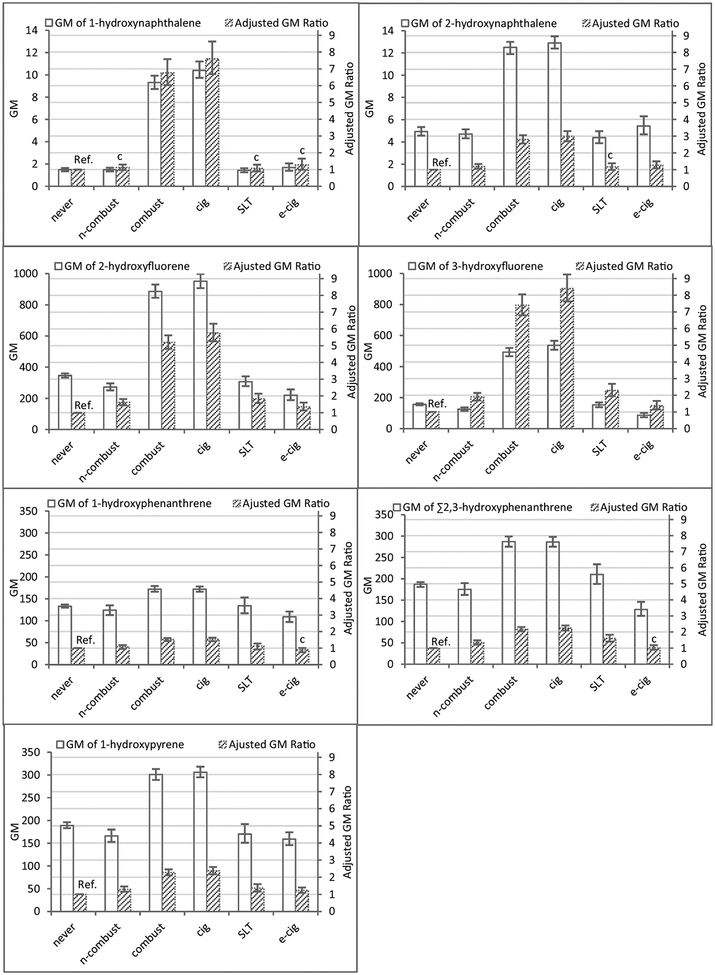
We report data for seven urinary OH-PAH metabolites from 11,519 (unweighted) participants in Wave 1 of the PATH Study. The percentage of missing data ranged from 0% to 0.24%, depending on the biomarker. Weighted detection frequencies for six of the seven OH-PAH were > 99%; the weighted detection frequency was lowest for 1-hydroxypyrene (89.6%; Supplemental Table 1S–A). For our primary analysis, the number of participants were 1700 (never user), 5767 (combustible products user), and 860 (non-combustible products user), and their demographic characteristics are shown in Supplemental Table 1SB. For each tobacco user group, we calculated GMs and 95% CIs of the seven biomarkers (Fig. 1, Supplemental Table 2S). GMs and selected percentiles by demographic categories and all tobacco user groups (both creatinine uncorrected and corrected) are provided in Supplemental Table 3S-A and 3S-B. For all biomarkers, combustible products users had significantly higher GMs than non-combustible products users and never users (Fig. 1, Supplemental Table 2S and Table 4S). On the other hand, non-combustible products users had significantly higher GMs than never users for most biomarkers (Fig. 1, Supplemental Table 2S and Table 4S), with the exception of 1-hydroxynaphthalene (p = 1.00), 2-hydroxynaphthalene (p = 1.00) and 1-hydroxyphenanthrene (p = 0.14). The ANOVA comparison by user groups after adjusting by log10 creatinine did not alter the results (data not shown). Similarly, although use of the Bonferroni method for multiple testing may increase type II error, it did not seem to appreciably affect the results of the comparisons (data not shown).
Fig. 1.
Geometric mean (GM) biomarker concentrations (95% CI)a and adjusted geometric mean ratios (95% CI) from ANCOVA model (never user as reference)b,c by tobacco user groupsd and specific tobacco-product user groupse, PATH Study Wave 1 (2013–2014).
aGM concentration in μg/L for 1-hydroxynaphthalene and 2-hydroxynaphthalene; in ng/L for the rest of the biomarkers; error bars display the 95% confidence intervals.
bAdjusted for log10(creatinine), race/ethnicity, sex, and age.
cRelative standard error (RSE) > 30% (CDC, 2002).
dThree main tobacco user groups: never user (never), non-combustible products user (n-combust), and combustible products user (combust) are mutually exclusive.
eFour specific tobacco-product user groups: never user (never), e-cigarette user (e-cig), SLT user (SLT), and cigarette user (cig) are mutually exclusive.
From the ANCOVA model results (Fig. 1, Supplemental Table 5S), compared to never users, the adjusted GM ratio (95% CI) for combustible products users was highest for 3-hydroxyfluorene (7.40 (6.80–8.05)), followed by 1-hydroxynaphthalene (6.76 (6.03–7.58)), and 2-hydroxyfluorene (5.20 (4.81–5.62)); for 1-hydroxypyrene, one of the most frequently used PAH exposure biomarkers, the adjusted GM ratio was 2.28 (2.11–2.46). For non-combustible products users, all adjusted GM ratios were much smaller than the ratios between combustible products users and never users. 3-Hydroxyfluorene and 2-hydroxyfluorene had the highest ratios [1.90 (1.69–2.14) and 1.62 (1.45–1.82), respectively]. For 1-hydroxynaphthalene, the relative standard error (RSE) was 52.45%, exceeding the 30% value above which estimates are considered unreliable (CDC, 2002).
3.2. Biomarkers of exposure by specific type of tobacco product
Fig. 1 also presents GMs (95% CI) of the seven OH-PAHs by specific tobacco-product user groups: cigarette user, SLT user, e-cigarette user, and never user. For all biomarkers examined, cigarette user had GMs significantly higher than any of the other three specific tobacco-product user groups (Fig. 1, Supplemental Tables 2S and 4S). Compared to ecigarette user, SLT user had significantly higher GMs of 2-hydroxyfluorene, 3-hydroxyfluorene, and Σ2,3-hydroxyphenanthrene. Furthermore, SLT user, compared to never user, had significantly higher GMs of four biomarkers (2-hydroxyfluorene, 3-hydroxyfluorene, Σ2,3-hydroxyphenanthrene, 1-hydroxypyrene). Last, e-cigarette user had significantly higher GMs than never user for 3-hydroxyfluorene and 1hydroxypyrene.
In adjusted ANCOVA model analysis (Fig. 1, Supplemental Table 5S), compared to never user, cigarette user had adjusted GM ratios (95% CI) (e.g., 5.77 (5.27–6.31) and 8.10 (7.63–9.24) for 2-hydroxyfluorene and 3-hydroxyfluorene, respectively) significantly higher than SLT user (e.g., 1.83 (1.57–2.13) for 2-hydroxyfluorene and 2.29 (1.95–2.69) for 3-hydroxyfluorene) or e-cigarette user (e.g., 1.35 (1.13–1.61) for 2-hydroxyfluorene and 1.38 (1.15–1.66) for 3-hydroxyfluorene) regardless of biomarker; the RSE were also lowest among cigarette user for all biomarkers. In contrast, compared to never user, the adjusted GM ratios for SLT user or e-cigarette user were relatively small and with relatively high RSE. Of note, the relatively small sample size (< 50) for e-cigarette and SLT user may have contributed to a few estimates having relatively large RSE. Also, the large RSE for the GM ratio may relate, at least in part, to the fact that the GM ratio was close to one, which resulted in a rather small denominator for the RSE on the log scale, and hence large RSE. Large RSEs should be interpreted with caution.
3.3. Biomarkers of exposure by frequency of tobacco product use
We further analyzed OH-PAH concentrations among cigarette user and other users according to smoking frequency (everyday vs some days) and last time used (Table 2, Supplemental 6S). Cigarette user’s GM concentrations of all biomarkers were significantly higher in everyday user than in some day user. In addition, GMs of all OH-PAH biomarkers were highest in cigarette user who smoked in the last hour, followed by those who last smoked within 3 days, and those who last smoked > 3 days ago. However, the GMs of 1-hydroxyphenanthrene in cigarette user who last smoked within 3 days or > 3 days ago did not differ significantly (p = 0.51).
Table 2.
Geometric mean biomarker concentrations (95% confidence intervals) of different specific tobacco-product user groups by frequency and last time used, PATH Study Wave 1 (2013–2014)a.
| Biomarker | Specific Product type | Frequency of usage | When last use | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Everyday | Some days | Last hour | Within-3-day | Over-3-day | |||||||
| GM(95% CI) | N | GM(95% CI) | N | GM(95% CI) | N | GM(95% CI) | N | GM(95% CI) | N | ||
| 1-Hydroxynaphthalene | Cigarette | 13.2(12.3–14.2) | 3223 | 3.29(2.93–3.69) | 740 | 14.2(13.2–15.3) | 2759 | 6.22(5.52–7.02) | 969 | 1.69(1.37–2.10) | 233 |
| SLT | 1.42(1.23–1.64) | 398 | 1.46(1.12–1.91)† | 111 | 1.42(1.19–1.7) | 329 | 1.38(1.16–1.64) | 139 | 1.69(1.03–2.78)† | 40 | |
| E-cigarette | 1.53(1.24–1.90) | 187 | 2.14(1.46–3.14) | 93 | 1.39(1.10–1.75)† | 156 | 2.35(1.41–3.92)b | 72 | 1.92(1.20–3.08)† | 51 | |
| 2-Hydroxynaphthalene | Cigarette | 14.5(13.9–15.2) | 3221 | 7.31(6.80–7.86) | 740 | 15.0(14.3–15.7) | 2757 | 10.2(9.33–11.1) | 969 | 5.23(4.54–6.02) | 233 |
| SLT | 4.6(3.97–5.33) | 398 | 3.75(3.01–4.67) | 111 | 4.78(4.02–5.68) | 329 | 3.68(3.17–4.26) | 139 | 4.03(2.74–5.92)† | 40 | |
| E-cigarette | 4.88(4.15–5.74) | 186 | 7.02(5.01–9.85) | 93 | 4.75(4.03–5.59) | 156 | 6.83(4.92–9.48) | 71 | 5.91(3.70–9.46) | 51 | |
| 2–Hydroxyfluorene | Cigarette | 1147(1098–1199) | 3223 | 382(353–413) | 740 | 1224(1167–1283) | 2760 | 623(575–674) | 968 | 223(196–253) | 233 |
| SLT | 336(301–375) | 398 | 227(179–287) | 111 | 359(313–411) | 329 | 235(199–277) | 139 | 222(142–349)† | 40 | |
| E-cigarette | 197(167–233) | 187 | 285(203–401) | 93 | 192(163–225) | 156 | 257(167–397) | 72 | 262(182–376) | 51 | |
| 3-Hydroxyfluorene | Cigarette | 665(636–696) | 3224 | 189(172–208) | 740 | 707(672–743) | 2760 | 355(325–389) | 969 | 91.4(78.4–107) | 233 |
| SLT | 170(152–189) | 398 | 108(83.3–140) | 111 | 181(159–207) | 329 | 118(97.8–142) | 139 | 96.4(59.0–157)† | 40 | |
| E-cigarette | 76.5(63.5–92) | 187 | 118(82.1–170) | 93 | 72.5(60–87.7) | 156 | 108(69.1–169) | 72 | 108(71.8–163) | 51 | |
| 1-Hydroxyphenanthrene | Cigarette | 183(176–190) | 3224 | 127(115–141) | 740 | 189(181–197) | 2760 | 141(133–149) | 969 | 118(92.1–150) | 233 |
| SLT | 144(124–168) | 398 | 102(83.5–125) | 111 | 150(126–180) | 329 | 107(89.8–128) | 139 | 109(76.9–155)† | 40 | |
| E-cigarette | 102(90.3–116) | 187 | 126(99.2–160) | 93 | 99(87.6–112) | 156 | 123(92.7–163) | 72 | 121(89.7–164) | 51 | |
| Σ2,3-Hydroxyphenanthrene | Cigarette | 315(301–330) | 3224 | 179(165–194) | 740 | 333(319–348) | 2760 | 213(199–229) | 969 | 140(117–169) | 233 |
| SLT | 231(205–261) | 398 | 150(123–183) | 111 | 242(211–278) | 329 | 165(140–194) | 139 | 151(105–218)† | 40 | |
| E-cigarette | 118(102–136) | 187 | 158(123–203) | 93 | 113(98.1–131) | 156 | 153(110–213) | 72 | 146(108–198) | 51 | |
| 1-Hydroxypyrene | Cigarette | 333(321–346) | 3224 | 203(188–220) | 740 | 344(329–360) | 2760 | 247(233–262) | 969 | 170(154–188) | 233 |
| SLT | 180(158–206) | 398 | 140(119–164) | 111 | 184(158–214) | 329 | 149(132–170) | 139 | 140(103–190)† | 40 | |
| E-cigarette | 150(135–166) | 187 | 185(154–224) | 93 | 144(128–162) | 156 | 188(151–233) | 72 | 171(137–212) | 51 | |
GM concentration in μg/L for 1-hydroxynaphthalene and 2-hydroxynaphthalene; in ng/L for the rest of the biomarkers.
Estimate should be interpreted with caution because it has low precision. It is based on a denominator sample size of < 50, or the coefficient of variation of the estimate or its complement is larger than 30% (CDC, 2002).
Everyday SLT user had significantly higher GMs than some days SLT user for all biomarkers with the exception of 1-hydroxynaphthalene (p = 0.86) and 2-hydroxynaphthalene (p = 0.14). In addition, SLT user who used the product in the last hour had significantly higher GMs of most OH-PAH biomarkers (except for 1-hydroxynaphthalene and 2hydroxynaphthalene) than those who had used the product within 3 days. SLT user who had used the product in the last hour had significantly higher GM for 3-hydroxyfluorene (p = 0.047) than those who had last used over three days previously. We observed no significant differences in GMs for all biomarkers for SLT user who used the product within 3 days or > 3 days.
Of note, we observed no significant differences in GM of most biomarkers (Table 2) in everyday and some days e-cigarette users. We also observed no statistically significant differences regardless of the last time the product was used.
4. Discussion
The PATH Study quantified seven OH-PAHs in 11,519 (unweighted) urine samples collected from a tobacco product user sample of persons 18 years of age and older who participated in Wave 1 of the PATH Study between 2013 and 2014, with the corresponding sampling weight representing the U.S. population. The PATH Study analysis detected six OH-PAH biomarkers in over 99% of the sample who provided biospecimens, confirming widespread exposure to naphthalene, fluorene, phenanthrene, and pyrene in the general U.S. adult population, consistent with data from NHANES (CDC, 2017).
To increase our understanding of the contribution of tobacco product use and exposure to PAHs, we investigated urinary concentrations of monohydroxylated PAH biomarkers in three mutually exclusive tobacco user groups: combustible products user, non-combustible products user, and never user. First, the OH-PAH biomarker concentrations and concentration ranges among combustible products user and never user were consistent with those reported for a subsample of adult smokers and non-smokers, respectively, in NHANES 2011–2012 (CDC, 2017). More importantly, as expected, because PAHs form during incomplete combustion, combustible products users had significantly higher GM concentrations of all OH-PAH biomarkers evaluated than non-combustible products users or never users. However, although noncombustible products users had significantly higher GM concentrations of the biomarkers of fluorene, phenanthrene, and pyrene compared to never users, GMs of the two naphthalene biomarkers between these two user groups, did not differ significantly.
We used adjusted GM ratios as suggestive indicators of the selectivity of the biomarkers to assess PAH exposure associated with tobacco use. 2-Hydroxyfluorene and 3-hydroxyfluorene, which correlated well with each other as expected for two metabolites of the same parent compound, fluorene (Li et al., 2008), were two of the three biomarkers with the highest adjusted GM ratios both among combustible products user and non-combustible products user. 1-Hydroxynaphthalene also had relatively high GM ratios, particularly for combustible products user, but the RSE was larger than for the fluorene biomarkers. Of note, 1-hydroxynaphthalene, a non-specific metabolite of naphthalene, is also a known metabolite of carbaryl (1-naphthyl-N-methylcarbamate), a major active pesticide ingredient (Maroni et al., 2000; Meeker et al., 2007). Recent exposure to carbaryl may contribute, at least in part, to the concentrations of 1-hydroxynaphthalene detected among PATH Study participants (Maroni et al., 2000; Meeker et al., 2007). We believe that the lack of specificity of 1-hydroxynaphthalalene diminishes its suitability as a biomarker of naphthalene exposure from tobacco-use only. 1-Hydroxypyrene, a commonly used OH-PAH biomarker (Jongeneelen et al., 1988; Siwinska et al., 1999; Yamano et al., 2014; Jongeneelen, 2014) also had higher GM ratios in combustible products users than in non-combustible products users, but the RSE, particularly among non-combustible products users, was considerably larger than for the two fluorene biomarkers. Taken together, these results suggest that 2-hydroxyfluorene, 3-hydroxyfluorene, and, to some extent, 1-hydroxypyrene might be sensitive and specific biomarkers for the purposes of assessing exposure to PAHs from use of tobacco products, particularly combustible products.
Because PAHs form during combustion and use of non-combustible products does not involve combustion, the much lower GMs and adjusted GM ratios (vs never user) of OH-PAH biomarkers among noncombustible products user (including e-cigarette user and SLT user) relative to combustible products user were expected.
GMs of 2-hydroxyfluorene, 3-hydroxyfluorene, Σ2,3-hydroxyphenanthrene, and 1-hydroxypyrene were significantly higher for SLT users compared to never users. Smokeless tobacco products can contain PAHs (McAdam et al., 2013; Hoffmann et al., 1987; Stepanov et al., 2008; Stepanov et al., 2010; Hearn et al., 2013), which could explain the above differences in biomarkers concentrations between exclusive users of smokeless tobacco and never users. GMs of 3-hydroxyfluorene and 1-hydroxypyrene were also significantly higher among e-cigarette users than never users. However, because PAHs have not been consistently detected in e-cigarette aerosols (Oh and Kacker, 2014; O’Connell et al., 2015; Lisko et al., 2015; Rawlinson et al., 2017), PAH metabolites in e-cigarette users may relate to other activities (e.g., diet (Rose et al., 2015)) instead of use of tobacco products.
For specific product user groups, we evaluated the OH-PAH biomarkers concentrations based on frequency of product use and by the last time used. Cigarette users who smoked everyday had significantly higher GMs of all biomarkers than cigarette users who did not smoke as often, and cigarette users who smoked most recently had the highest GMs. These data are consistent with the relatively short half-life (2.5–6.1 h) of PAHs in humans (Li et al., 2012) and their rapid urinary excretion. SLT users who used the product everyday also had significantly higher GMs for most biomarkers (except 1-hydroxynaphthalene and 2-hydroxynaphthalene) than occasional users; persons who used the product within the last hour also had the highest concentrations of these biomarkers. The much lower concentrations of OH-PAHs among e-cigarette users and the non-persistent nature of the biomarkers likely explain the lack of apparent frequency-related concentration trend for these biomarkers in this group of users.
5. Conclusions
In summary, we present the first representative PAHs exposure data in never and select (including current) users of both combustible and non-combustible tobacco products in the U.S. adult general population between 2013 and 2014. The almost universal detection of six of the seven OH-PAH biomarkers evaluated and the similar GM concentrations of all seven biomarkers to those reported before among adults participating in NHANES 2011–2012 confirm the widespread exposure of the U.S. population to PAHs. Users of combustible tobacco products had significantly higher concentrations of all OH-PAHs than users of non-combustible tobacco products and never users; concentrations of some PAH biomarkers were significantly higher in users of smokeless tobacco products and e-cigarettes than in never users. The reported OHPAH concentration differences by type and frequency of tobacco product use highlight the importance of evaluating the potential health impact from PAH exposure from the use of these products. Lastly, the PATH Study Wave 1 data can establish a baseline to identify PAH exposure trends as tobacco use behavior may change over time.
Supplementary Material
Acknowledgments
Funding
This project is supported with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, and the Center for Tobacco Products, Food and Drug Administration, Department of Health and Human Services, under a contract to Westat (Contract No. HHSN271201100027C) and through an interagency agreement between the Center for Tobacco Products, FDA and the Centers for Disease Control and Prevention.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2018.11.068.
Disclaimer
The views and opinions expressed in this report are those of the authors and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies. MLG received a research grant from Pfizer and served as a member of an advisory committee to Johnson & Johnson, manufacturer of smoking cessation medications.
References
- ATSDR, 1995. Toxicological profile for polycyclic aromatic hydrocarbons. Agency for Toxic Substances and Disease Registry. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf. [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL, 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect. 113 (2), 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsath FA, Benner RA Jr., Abraham A, Wang Y, El Said KR, Jester EL, et al. , 2015. Screening for petrochemical contamination in seafood by headspace solidphase microextraction gas chromatography-mass spectrometry. Anal. Bioanal. Chem 407 (14), 4079–4090. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J, 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J 54 (10), 615–627. [DOI] [PubMed] [Google Scholar]
- CDC, 2002. NHANES 1999–2000 Addendum to the NHANES III analytic guidelines. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/1999-2000/guidelines1.pdf. [Google Scholar]
- CDC, 2017. Fourth national report on human exposure to environmental chemicals. Available at: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume2_Jan2017.pdf. [PubMed] [Google Scholar]
- CDC, 2018a. Smoking & Tobacco Use. Office on Smoking and Health (OHS), Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion; Available at: https://www.cdc.gov/tobacco/basic_information/ecigarettes/index.htm. [Google Scholar]
- CDC, 2018b. Smoking & Tobacco Use. Electronic Cigarettes. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion; Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm. [Google Scholar]
- Choosong T, Phakthongsuk P, Tekasakul S, Tekasakul P, 2014. Urinary 1-hydroxypyrene levels in workers exposed to polycyclic aromatic hydrocarbon from rubber wood burning. Saf. Health Work 5 (2), 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS, 2014. The Health Consequences of Smoking, 50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General, Rockville, MD: Available at: https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf. [Google Scholar]
- Farzan SF, Chen Y, Trachtman H, Trasande L, 2016. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003–2008. Environ. Res 144 (Pt A), 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P, Benowitz NL, 2017. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob. Res 19 (2), 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2018. Family Smoking Prevention and Tobacco Control Act, Public Law 111–31 [H.R. 1256 U.S. Food and Drug Administration, Silver Spring, MD: Available at: https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm262084.htm. [Google Scholar]
- Hearn BA, Renner CC, Ding YS, Vaughan-Watson C, Stanfill SB, Zhang L, et al. , 2013. Chemical analysis of Alaskan Iq’mik smokeless tobacco. Nicotine Tob. Res 15 (7), 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, et al. , 2015. Evaluation of toxicant and carcinogen metabolites in the urine of ecigarette users versus cigarette smokers. Nicotine Tob. Res 17 (6), 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Adams JD, Lisk D, Fisenne I, Brunnemann KD, 1987. Toxic and carcinogenic agents in dry and moist snuff. J. Natl. Cancer Inst 79 (6), 1281–1286. [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5 (1), 46–51. [Google Scholar]
- Hu SW, Chan YJ, Hsu HT, Wu KY, Changchien GP, Shie RH, et al. , 2011. Urinary levels of 1-hydroxypyrene in children residing near a coal-fired power plant. Environ. Res 111 (8), 1185–1191. [DOI] [PubMed] [Google Scholar]
- Hyland A, Ambrose BK, Conway KP, Borek N, Lambert E, Carusi C, et al. , 2017. Design and methods of the population assessment of tobacco and health (PATH) study. Tob. Control 26 (4), 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyunok Choi RH, Komulainen Hannu, Delgado Saborit Juana M., 2010. Polycyclic aromatic hydrocarbons. In: WHO Guidelines for Indoor Air Quality: Selected Pollutants. World Health Organization, Geneva: Available at: https://www.ncbi.nlm.nih.gov/books/NBK138705/. [PubMed] [Google Scholar]
- IARC, 2010. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 92. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. Available at: http://monographs.iarc.fr/ENG/Monographs/vol92/mono92.pdf. [PMC free article] [PubMed] [Google Scholar]
- Jain RB, 2016. Association between polycyclic aromatic hydrocarbons and thyroid function among males and females: data from NHANES 2007–2008. Int. J. Environ. Health Res 26 (4), 405–419. [DOI] [PubMed] [Google Scholar]
- Jongeneelen FJ, 2014. A guidance value of 1-hydroxypyrene in urine in view of acceptable occupational exposure to polycyclic aromatic hydrocarbons. Toxicol. Lett 231 (2), 239–248. [DOI] [PubMed] [Google Scholar]
- Jongeneelen FJ, Anzion RB, Leijdekkers CM, Bos RP, Henderson PT, 1985. 1Hydroxypyrene in human urine after exposure to coal tar and a coal tar derived product. Int. Arch. Occup. Environ. Health 57 (1), 47–55. [DOI] [PubMed] [Google Scholar]
- Jongeneelen FJ, Anzion RB, Scheepers PT, Bos RP, Henderson PT, Nijenhuis EH, et al. , 1988. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann. Occup. Hyg 32 (1), 35–43. [DOI] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. , 2008. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res 107 (3), 320–331. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, et al. , 2012. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem. Res. Toxicol 25 (7), 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH, 2015. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in E-cigarette cartridges and refill solutions. Nicotine Tob. Res 17 (10), 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xu C, Jiang ZY, Gu A, 2016. Association of polycyclic aromatic hydrocarbons and asthma among children 6–19 years: NHANES 2001–2008 and NHANES 2011–2012. Respir. Med 110, 20–27. [DOI] [PubMed] [Google Scholar]
- Maroni M, Colosio C, Ferioli A, Fait A, 2000. Biological monitoring of pesticide exposure: a review. Introduction. Toxicology 143 (1), 1–118. [DOI] [PubMed] [Google Scholar]
- McAdam KG, Faizi A, Kimpton H, Porter A, Rodu B, 2013. Polycyclic aromatic hydrocarbons in US and Swedish smokeless tobacco products. Chem. Cent. J 7 (1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R, 2007. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J. Expo. Sci. Environ. Epidemiol 17 (4), 314–320. [DOI] [PubMed] [Google Scholar]
- Negri AP, Brinkman DL, Flores F, Botté ES, Jones RJ, Webster NS, 2016. Acute ecotoxicology of natural oil and gas condensate to coral reef larvae. Sci. Rep 6, 21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell G, Colard S, Cahours X, Pritchard JD, 2015. An assessment of indoor air quality before, during and after unrestricted use of E-cigarettes in a small room. Int. J. Environ. Res. Public Health 12 (5), 4889–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh AY, Kacker A, 2014. Do electronic cigarettes impart a lower potential disease burden than conventional tobacco cigarettes? Review on E-cigarette vapor versus tobacco smoke. Laryngoscope 124 (12), 2702–2706. [DOI] [PubMed] [Google Scholar]
- Rawlinson C, Martin S, Frosina J, Wright C, 2017. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J. Chromatogr. A 1497, 144–154. [DOI] [PubMed] [Google Scholar]
- Rodrigues EG, Smith K, Maule AL, Sjodin A, Li Z, Romanoff L, et al. , 2014. Urinary polycyclic aromatic hydrocarbon (OH-PAH) metabolite concentrations and the effect of GST polymorphisms among US Air Force personnel exposed to jet fuel. J. Occup. Environ. Med 56 (5), 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, Holland J, Dowding A, Petch SR, White S, Fernandes A, et al. , 2015. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem. Toxicol 78, 1–9. [DOI] [PubMed] [Google Scholar]
- Roy D, Seo YC, Sinha S, Bhattacharya A, Singh G, Biswas PK, 2017. Human health risk exposure with respect to particulate-bound polycyclic aromatic hydrocarbons at mine fire-affected coal mining complex. Environ. Sci. Pollut. Res. Int 10.1007/s11356-017-9202-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Scinicariello F, Buser MC, 2014. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001–2006). Environ. Health Perspect 122 (3), 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, et al. , 2017. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann. Intern. Med 166 (6), 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwinska E, Mielzynska D, Bubak A, Smolik E, 1999. The effect of coal stoves and environmental tobacco smoke on the level of urinary 1-hydroxypyrene. Mutat. Res 445 (2), 147–153. [DOI] [PubMed] [Google Scholar]
- Slezakova K, Castro D, Delerue-Matos C, Morais S, Pereira MOC, 2014. Levels and risks of particulate-bound PAHs in indoor air influenced by tobacco smoke: a field measurement. Environ. Sci. Pollut. Res. Int 21 (6), 4492–4501. [DOI] [PubMed] [Google Scholar]
- St Helen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P 3rd, Benowitz NL, 2012. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem. Res. Toxicol 25 (4), 952–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS, 2008. New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. In: Nicotine & Tobacco Research, 12 ed. pp. 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Villalta PW, Knezevich A, Jensen J, Hatsukami D, Hecht SS, 2010. Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem. Res. Toxicol 23 (1), 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogiannidis E, Laane R, 2015. Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. Rev. Environ. Contam. Toxicol 234, 49–133. [DOI] [PubMed] [Google Scholar]
- Tourangeau R, Yan T, Sun H, Hyland A, Stanton CA, 2018. Population assessment of tobacco and health (PATH) reliability and validity study: selected reliability and validity estimates. Tob. Control 10.1136/tobaccocontrol-2018054561. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wang Y, Meng L, Pittman EN, Etheredge A, Hubbard K, Trinidad DA, et al. , 2017. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 409 (4), 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Zatonski W, Przewozniak K, Jarvis MJ, 2007. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol. Biomark. Prev 16 (4), 820–822. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Hara K, Ichiba M, Hanaoka T, Pan G, Nakadate T, 2014. Urinary 1hydroxypyrene as a comprehensive carcinogenic biomarker of exposure to polycyclic aromatic hydrocarbons: a cross-sectional study of coke oven workers in China. Int. Arch. Occup. Environ. Health 87 (7), 705–713. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hou H, Chen H, Liu Y, Wang A, Hu Q, 2015. Quantification of 16 polycyclic aromatic hydrocarbons in cigarette smoke condensate using stable isotope dilution liquid chromatography with atmospheric-pressure photoionization tandem mass spectrometry. J. Sep. Sci 38 (22), 3862–3869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.