Abstract
Background:
Opioid agonist therapies with methadone are associated with higher levels of adherence to antiretroviral therapy (ART), yet no studies have explored factors associated with optimal ART levels in HIV-positive patients on methadone maintenance treatment (MMT), including explanatory pathways using mediation analysis.
Setting:
Participants included 121 HIV-positive, methadone-maintained patients who reported HIV-risk behaviors and were taking ART.
Methods:
Participants were assessed using an audio-computer assisted self-interview (ACASI). Multivariable logistic regression was used to identify significant correlates and Process macro to test the explanatory pathway (i.e., mediational effect) for optimal ART adherence.
Results:
Among 121 participants, almost 40% reported sub-optimal adherence to ART. Optimal ART adherence was significantly associated with being virally suppressed (adjusted odds ratio (aOR) =6.470, p=0.038), higher motivation to adhere to ART (aOR=1.171, p=0.011), and lower anticipated HIV-related stigma (aOR=0.384, p=0.015). Furthermore, results revealed an indirect effect of motivation on the relationship between HIV stigma and ART adherence (Effect=−0.121, p=0.043), thus supporting the mediation effect.
Conclusion:
Our findings underscore the complexities surrounding ART adherence, even in patients on MMT. These findings provide insights on how to more effectively intervene to optimize HIV treatment outcomes, including HIV treatment-as-prevention (TasP) initiatives, in methadone-maintained patients.
Keywords: ART adherence, HIV, methadone, opioid use disorder, HIV-related stigma, HIV risk behavior
Introduction
Treatment of human immunodeficiency virus (HIV) infection using antiretroviral therapy (ART) has improved steadily since the advent of potent combination therapy. The administration of combination antiretroviral therapies has been shown to suppress plasma HIV-1 RNA to undetectable levels, and transformed HIV infection from being life-threatening into a chronic, manageable condition.1,2 Over the past year, a growing body of literature has provided even stronger evidence of the effectiveness of HIV treatment in viral suppression and, in turn, preventing the sexual transmission of HIV. This evidence greatly contributed to an increasing global consensus toward HIV treatment-as-prevention (TasP) initiatives as a means of preventing new HIV infections and HIV-associated morbidity and mortality.3–6
International and U.S. guidelines now recommend universal ART for people living with HIV (PLWH). Once prescribed ART, however, PLWH must optimally adhere to treatment to achieve viral suppression, which in turn improves individual outcomes and reduces HIV transmission to others. Sub-optimal ART adherence is associated with virological failure and the emergence of antiretroviral resistance.1,7–9 In the context of HIV-positive people who use drugs (PWUD), the provision of optimal HIV treatment has become a major challenge. Prior studies have shown that HIV-positive PWUD are less likely to have access to and receive regular HIV care, and have lower levels of adherence, which all leads to poor treatment outcomes.10–15 Thus, tailoring intervention approaches to address their specific needs could help optimize HIV treatment outcomes and prevention efforts.
In a recent decade, opioid agonist therapies like methadone maintenance treatment (MMT) or buprenorphine, have been shown to be highly effective in improving treatment outcomes. A recent systematic review among opioid dependent HIV-positive patients suggests that being maintained on opioid agonist therapies is associated with being prescribed ART and achieving optimal adherence and viral suppression levels.16 PLWH on MMT, however, are highly diverse and beyond the benefit of methadone on HIV TasP efforts. In the broader literature, several factors have been associated with optimal ART adherence.15,17–23 Despite substantial research in this area, prior studies have neither examined theoretically informed correlates of ART adherence nor explored the possible mechanism for optimal adherence among HIV-positive opioid dependent individuals within drug treatment settings. Identifying subgroups at risk for sub-optimal adherence would provide new insight to guide tailored and more effective HIV TasP strategies in this population.
Methods
Study setting and procedures
Data for this secondary analysis was derived from the Holistic Health for HIV (3H+) project, a randomized controlled trial designed to improve HIV risk reduction and medication adherence among high-risk HIV-positive PWUD. The study design has been described previously.24 Briefly, participants were recruited from community-based addiction treatment programs and HIV clinical care settings within the greater New Haven, Connecticut, using clinic-based advertisements and flyers, word-of-mouth, and direct referral from counselors. Interested individuals who met inclusion criteria and who provided informed consent were administered the baseline survey using an audio computer-assisted self-interview (ACASI) program. Baseline data collected prior to randomization was analyzed. All participants were paid for their time to complete the survey. The study protocol was approved by the Institutional Review Boards at the University of Connecticut and Yale University, and received board approval from the methadone clinic. Clinical trial registration is available at www.ClinicalTrials.gov (NCT01741311).
Participants
We recruited 133 participants between September 2012 and January 2018. Individuals were eligible if they were: i) ≥18 years; ii) confirmed HIV-positive status; iii) reported drug- (i.e., sharing of injection equipment) or sex-related (i.e., condomless sex) risk behavior (past 6 months); iv) met DSM-V criteria for opioid use disorder and stable on methadone (dose ~ 80 mg); v) able to understand, speak, and read English; vi) not actively suicidal, homicidal, or psychotic; vii) able to provide informed consent. Only those individuals who reported taking ART in the past month were included in the analysis (N=121).
Measures
The dependent variable was optimal ART adherence in the past month, measured using an empirically validated, self-report visual analog scale (VAS).25 In this method, participants were asked to indicate the percentage of ART medication taken as directed in the previous month by pointing along a continuous line between 0 – 100%. Optimal ART adherence was defined as adherence of 95% or greater.9
Covariates included in the analysis were based on prior research on adherence as well as findings from other studies conducted within drug treatment settings. Data collected included participant characteristics, including age, gender, sexual orientation, ethnicity, marital status, educational status, employment status, income, living status, methadone dose, HIV diagnosis duration, and ART status. Other variables included participant health status variables including the most recent viral load (VL) and CD4 count. These variables were extracted from their medical record. Viral suppression was defined as clinic-recorded HIV-1 RNA test value <200 copies/mL and high CD4 as CD4 count ≥500 cells/mm.3,26,27
Motivation to adhere to ART was measured using an 18-item, validated scale. For example, “How strong is your intention to take all of your ART medications as directed by your health care provider in the next month?”, “When you were growing up, how important was it to members of your family to take medications as prescribed?”) Responses were indicated on a 4-point Likert scale ranging from not at all (0) to extremely (3), with higher values indicating greater motivation to adhere to ART (α=0.72).
Data collection also included measures of the information-motivation-behavioral skills (IMB) model constructs related to HIV risk reduction,28 including: (a) Information – HIV risk-related knowledge (range: 0 – 4); (b) Motivation - readiness to change and intentions to change HIV risk behavior (range: 0 – 32); and (c) Behavioral Skills - risk reduction skills (range: 0 – 16).
HIV-related stigma included measures of internalized, anticipated, and enacted HIV stigma assessed using a validated HIV stigma Mechanism Measure.29 Internalized HIV stigma (α=0.91) was measured with 6 items including “I feel ashamed of having HIV.” Anticipated HIV stigma (α=0.90) was measured with 9 items including “Healthcare workers will treat me with less respect.” Enacted HIV stigma (α=0.91) was measured with 9 items including “Family members have avoided me.” Items were rated on 5-point Likert-type scales. Items were averaged to create composite scores with higher scores indicating greater stigma.
Disclosure of HIV status was defined as having any sex where HIV status to the partners was disclosed in the past six months. Serostatus disclosure to partners was measured by asking, “In the past six months, did you have sex with anyone who you told your HIV status sometime before you had sex?” Responses were reported using a ‘yes’ or ‘no’. Participants were also asked about their knowledge of partner’s HIV status in the past 30 days.
The HIV risk assessment, adapted from NIDA’s Risk Behavior Assessment 30 was used to measure several aspects of HIV risk behaviors in the past 30 days, including a measurement of “any” high risk behavior (sexual or drug-related) as well as measurements of event-level (i.e., partner-by-partner) behaviors.
Data analyses
We computed descriptive statistics, including frequencies and percentages for categorical variables, and means and standard deviations for continuous variables. After conducting bivariate analyses to examine significant associations with the dependent variable (i.e., ART adherence), we conducted multivariable logistic regression analyses on those bivariate associations found to be significant at p<0.10. Additionally, we examined the interactive effect of pairs of variables in the main effects model to determine the moderated effect on optimal ART adherence. Stepwise forward entry and backward elimination methods both showed the same results in examining the independent correlates (p<0.05) expressed as adjusted odds ratios (aOR) and their 95% confidence intervals. Model fit was assessed using a Hosmer and Lemeshow Test.31
Next, we incorporated a mediation model to examine the explanatory pathway through which significant correlates, based on multivariable logistic regression, influence optimal ART adherence. We utilized the SPSS PROCESS macro developed by Hayes (2013) which utilized the logistic regression model to test the mediational effect. As outlined by Baron and Kenny (1986), this macro estimates all paths designated in mediation models (Figure 1). The indirect effect was calculated as the product of the beta coefficients from two regression models (a*b). Additionally, the test of the mediational process was further guided by the Sobel test,32 and the bootstrap method.33 Bias corrected bootstrap confidence intervals (95%) were calculated to estimate indirect effects using 5,000 iterations, and an indirect effect was determined to be significant if the confidence interval did not include 0.33 The following covariates were included in our mediation model: age, gender, sexual orientation, ethnicity, marital status, education, and income. Estimates were evaluated for statistical significance based on p< 0.05. All analyses were conducted using SPSS version 23.34
Figure 1:
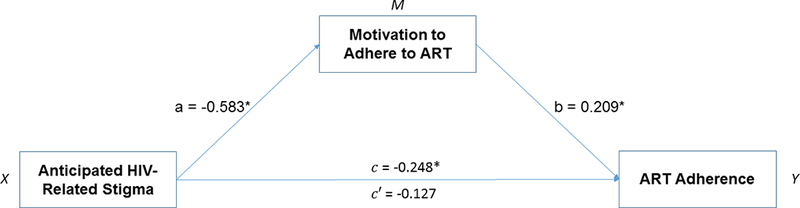
Statistical model of the mediational process
Note. Controlled for age, gender, sexual orientation, ethnicity, marital status, education, and income; PrEP = Pre-exposure prophylaxis, NCI = Neurocognitive impairment.
Results
Participant characteristics
Table 1 summarizes participant characteristics. The mean age of participants was 49.4 (±8.3) years and over half of the participants (53.7%) reported cocaine use in the past 30 days. In our sample, 63.6% had achieved optimal adherence and 85.4% were virally suppressed. Self-reported HIV risk behaviors were highly prevalent among participants.
Table 1:
Characteristics of participants and HIV transmission risk behaviors, stratified by level of ART adherence
| Variables | Entire Sample (N = 121) | Optimal ART Adherence e | OR f (95% CI g) | p | |||
|---|---|---|---|---|---|---|---|
| Frequency | % | No (n = 44) | Yes (n = 77) | ||||
| Characteristics of participants | |||||||
| Age: Mean (±SD) a | 49.4 (±8.3) | 49.4 (7.8) | 49.4 (8.6) | 1.000 (0.957, 1.046) | 0.987 | ||
| Gender | |||||||
| Male | 73 | 60.3 | 24 (19.8) | 49 (40.5) | - | - | |
| Female | 48 | 39.7 | 20 (16.5) | 28 (23.1) | 0.686 (0.323, 1.457) | 0.326 | |
| Heterosexual sexual orientation | |||||||
| No | 26 | 21.5 | 11 (9.1) | 15 (12.4) | - | - | |
| Yes | 95 | 78.5 | 33 (27.3) | 62 (51.2) | 1.378 (0.568, 3.339) | 0.478 | |
| Ethnicity | |||||||
| Non-White | 86 | 71.1 | 26 (21.5) | 60 (49.6) | - | - | |
| White | 35 | 28.9 | 18 (14.9) | 17 (14.0) | 0.409 (0.183, 0.917) | 0.030 | |
| Currently married | |||||||
| No | 107 | 88.4 | 40 (33.1) | 67 (55.4) | - | - | |
| Yes | 14 | 11.6 | 4 (3.3) | 10 (8.3) | 1.493 (0.439, 5.075) | 0.521 | |
| High school graduate | |||||||
| No | 51 | 42.1 | 13 (10.7) | 38 (31.4) | - | - | |
| Yes | 70 | 57.9 | 31 (25.6) | 39 (32.2) | 0.430 (0.196, 0.945) | 0.036 | |
| Employed | |||||||
| No | 116 | 95.9 | 42 (34.7) | 74 (61.2) | - | - | |
| Yes | 5 | 4.1 | 2 (1.7) | 3 (2.5) | 0.851 (0.137, 5.301) | 0.863 | |
| Income level | |||||||
| < $10,000 | 104 | 86.0 | 40 (33.1) | 64 (52.9) | - | - | |
| ≥ $10,000 | 17 | 14.0 | 4 (3.3) | 13 (10.7) | 2.031 (0.619, 6.665) | 0.242 | |
| Living with family/friends | |||||||
| No | 70 | 57.9 | 27 (22.3) | 43 (35.5) | - | - | |
| Yes | 51 | 42.1 | 17 (14.0) | 34 (28.1) | 1.256 (0.590, 2.673) | 0.554 | |
| Methadone Dose: Mean (±SD) a | 65.2 (±39.8) | 67.6 (35.9) | 63.8 (42.2) | 0.998 (0.988, 1.007) | 0.622 | ||
| HIV diagnosis duration (Years): Mean (±SD) a | 14.9 (±9.3) | 12.3 (10.0) | 16.4 (8.7) | 1.050 (1.006, 1.095) | 0.024 | ||
| Virally suppressed b | n = 103 | ||||||
| No | 15 | 14.6 | 11 (10.7) | 4 (3.9) | - | - | |
| Yes | 88 | 85.4 | 30 (29.1) | 58 (56.3) | 5.317 (1.560, 18.123) | 0.008 | |
| High CD4 count c | n = 105 | ||||||
| No | 52 | 49.5 | 22 (21.0) | 30 (28.6) | - | - | |
| Yes | 53 | 50.5 | 20 (19.0) | 33 (31.4) | 1.210 (0.554, 2.644) | 0.633 | |
| HIV risk reduction-related | |||||||
| Information: Mean (±SD) | 3.7 (±0.6) | 3.9 (0.2) | 3.7 (0.7) | 0.474 (0.184, 1.225) | 0.123 | ||
| Motivation: Mean (±SD) | 27.4 (±3.9) | 26.6 (4.0) | 27.8 (3.9) | 1.082 (0.985, 1.188) | 0.099 | ||
| Behavioral skills: Mean (±SD) | 9.8 (±3.8) | 9.5 (3.5) | 10.0 (3.9) | 1.038 (0.942, 1.144) | 0.452 | ||
| Motivation to adhere to ART: Mean (±SD) | 30.1 (±6.8) | 29.0 (6.4) | 31.0 (7.0) | 1.742 (1.086, 2.102) | 0.039 | ||
| HIV-related Stigma: Mean (±SD) | |||||||
| Internalized HIV Stigma | 2.3 (±1.0) | 2.5 (0.8) | 2.2 (1.1) | 0.768 (0.540, 1.093) | 0.142 | ||
| Anticipated HIV Stigma | 1.8 (±0.8) | 2.0 (0.7) | 1.7 (0.8) | 0.615 (0.385, 0.982) | 0.042 | ||
| Enacted HIV Stigma | 1.8 (±0.8) | 1.9 (0.8) | 1.7 (0.8) | 0.784 (0.511, 1.203) | 0.265 | ||
| Disclosed HIV status | |||||||
| No | 44 | 36.4 | 18 (14.9) | 26 (21.5) | - | - | |
| Yes | 77 | 63.6 | 26 (21.5) | 51 (42.1) | 1.358 (0.632, 2.916) | 0.433 | |
| Knowledge of partner’s HIV status | |||||||
| No | 72 | 59.5 | 26 (21.5) | 46 (38.0) | - | - | |
| Yes | 49 | 40.5 | 18 (14.9) | 31 (25.6) | 0.973 (0.485, 2.069) | 0.944 | |
| HIV transmission risk behaviors | |||||||
| Duration of drug use: Mean (±SD) | 24.9 (±9.7) | 23.5 (10.0) | 25.8 (9.6) | 1.025 (0.986, 1.065) | 0.216 | ||
| Current cocaine use d | |||||||
| No | 56 | 46.3 | 18 (14.9) | 38 (31.4) | - | - | |
| Yes | 65 | 53.77 | 26 (21.5) | 39 (32.2) | 0.711 (0.336, 1.502) | 0.371 | |
| Ever injected illicit drug | |||||||
| No | 13 | 10.7 | 6 (5.0) | 7 (5.8) | - | - | |
| Yes | 108 | 89.3 | 38 (31.4) | 70 (57.9) | 1.579 (0.495, 5.035) | 0.440 | |
| Injected illicit drug d | n = 108 | ||||||
| No | 54 | 50.0 | 14 (13.0) | 40 (37.0) | - | - | |
| Yes | 54 | 50.0 | 23 (21.3) | 31 (28.7) | 0.472 (0.209, 1.064) | 0.070 | |
| Shared injection equipment d | n = 58 | ||||||
| No | 25 | 43.1 | 12 (20.7) | 13 (22.4) | - | - | |
| Yes | 33 | 56.9 | 11 (19.0) | 22 (37.9) | 1.846 (0.635, 5.369) | 0.260 | |
| Multiple sex partner d | |||||||
| No | 95 | 78.5 | 26 (27.7) | 42 (44.7) | - | - | |
| Yes | 26 | 21.5 | 7 (7.4) | 19 (20.2) | 1.680 (0.621, 4.545) | 0.307 | |
| Consistent condom use d | |||||||
| No | 105 | 86.8 | 30 (31.9) | 57 (60.6) | - | - | |
| Yes | 16 | 13.2 | 3 (3.2) | 4 (4.3) | 0.702 (0.147, 3.342) | 0.657 | |
Note:
SD: Standard deviation;
Virally suppressed: Viral load < 200 copies/mL;
Health CD4 count: CD4 count ≥ 500 cells/mm3;
In the past 30 days;
Optimal ART adherence: Adherence ≥95%;
Odds ratio;
Confidence interval
Correlates of optimal ART adherence
Table 1 shows the bivariate correlates of optimal ART adherence, and Table 2 shows the independent correlates associated with this outcome in multivariate modeling. Three factors were independently correlated with optimal ART adherence: being virally suppressed (aOR=6.470, p=0.038) and having higher motivation to adhere to ART (aOR=1.171, p=0.011) were positively correlated, while having anticipated HIV-related stigma (aOR=0.384, p=0.015) was negatively correlated with optimal ART adherence. Furthermore, we also found a significant interaction effect that involved motivation to adherence to ART combined with drug injection to be correlated with optimal ART adherence (aOR=1.086, p=0.049). The results of the post hoc analyses showed that drug injection had significant influence on ART adherence at lower levels of motivation to adhere to ART (Effect=−0.3764, p=0.033). This effect was, however, nonsignificant at greater levels of motivation to adhere to ART.
Table 2:
Multivariate logistic regression models of factors associated with optimal ART adherence (N=121)
| Variables | Optimal ART Adherence b |
||
|---|---|---|---|
| aOR c | 95% CI d | p | |
| Ethnicity | |||
| Non-White | - | - | - |
| White | 0.574 | 0.170, 1.940 | 0.372 |
| High school graduate | |||
| No | - | - | - |
| Yes | 0.632 | 0.208, 1.923 | 0.419 |
| HIV diagnosis duration (Years) | 1.064 | 0.998, 1.134 | 0.058 |
| Virally suppressed a | |||
| No | - | - | - |
| Yes | 6.470 | 1.106, 37.846 | 0.038 |
| Motivation: HIV risk reduction | 0.985 | 0.849, 1.142 | 0.839 |
| Motivation to adhere to ART | 1.171 | 1.037, 1.324 | 0.011 |
| Anticipated HIV-related stigma | 0.384 | 0.178, 0.831 | 0.015 |
| Active drug injection (past 30 days) | |||
| No | - | - | - |
| Yes | 0.270 | 0.001, 12.895 | 0.676 |
| Motivation to adhere to ART * drug injection | 1.086 | 1.003, 1.141 | 0.049 |
| R2 = 0.446 | |||
| Hosmer and Lemeshow Test: Chi-square = 8.776; p = 0.362 | |||
Note:
Virally suppressed: Viral load < 200 copies/mL;
Optimal ART adherence: Adherence ≥ 95%;
aOR: Adjusted odds ratio;
CI: Confidence interval
Participants in the current study were recruited over the course of five years thus presenting history/maturation as a potential threat to internal validity. We, therefore, redid the analysis for Table 2 adjusting for the year of recruitment in the multivariable model but there were no significant differences observed in the results (see Table 1, Supplemental Digital Content).
Test of mediation
Next, we examined the role of motivation to adhere to ART on the relationship between HIV-related stigma and ART adherence. Participants who anticipated HIV-related stigma were significantly less likely to have higher motivation to adhere to ART (B=−0.583, p=0.025; path a). Higher motivation to adhere to ART was, in turn, positively associated with optimal ART adherence (B=0.209, p=0.037; path b). The relationship between HIV-related stigma and ART adherence also emerged as significant (B=−0.248, p=0.042; path c). This relationship was, however, non-significant after controlling for motivation to adhere to ART (B=−0.127, p=0.059; path c’), thus supporting the hypothesized mediation effect. The formal two-tailed significance test demonstrated that the indirect effect was significant (Sobel z = −0.121, p = 0.043). Bootstrap results confirmed the Sobel test (Table 3), with a bootstrapped 95% confidence interval around the indirect effect not containing zero (−0.203, −0.064). All of these analyses support our hypothesis of an indirect effect (i.e., mediation) of HIV-related stigma on ART adherence via motivation to adhere to ART (Figure 1).
Table 3:
Regression results for simple mediation
| Variable | Path | Coeff. | SE | t/z | p |
|---|---|---|---|---|---|
| ART adherence regressed on stigma | c | −0.248 | 0.239 | −1.037 | 0.042 |
| Motivation to adhere to ART regressed on stigma | a | −0.583 | 0.320 | −1.821 | 0.025 |
| ART adherence regressed on Motivation to adhere to ART | b | 0.209 | 0.090 | 2.322 | 0.037 |
| ART adherence regression on stigma controlling for motivation to adhere to ART | c’ | −0.127 | 0.038 | −3.342 | 0.059 |
| Motivation to adhere to ART |
Indirect Effect of Stigma on ART adherence |
||||
| Effect | Boot SE | Boot LLCI | Boot ULCI | ||
| −0.121 | 0.034 | −0.203 | −0.064 | ||
| Sobel test |
Tests for Indirect Effect |
||||
| Effect | SE | z | p | ||
| −0.121 | 0.032 | −3.781 | 0.043 | ||
Note: Controlled for age, gender, sexual orientation, ethnicity, marital status, education, and income; ART = Antiretroviral therapy; Stigma refers to anticipated HIV-related stigma
Discussion
This study, to our knowledge, is the only study that examines factors correlated with optimal ART adherence in PLWH on methadone – a group of PWUD whose adherence is already increased by virtue of being on opioid agonist therapy.16 Several key findings emerged that provide guidance for tailoring HIV prevention and treatment in PWUD. First, despite the known ART adherence benefits of being prescribed methadone,16 optimal adherence levels remained relatively low but similar to other studies,35,36 suggesting the need for further intervention.
PLWH on MMT often encounter numerous barriers that contribute to sub-optimal ART adherence, including ongoing drug use, stigma and discrimination, chaotic lifestyle, and complex array of social, medical, and psychological issues.15,37–41 The finding that viral suppression is associated with optimal adherence has been demonstrated in multiple studies, and similar to these, it was the single most important correlate. Unlike other studies,42,43 ongoing drug use like cocaine, did not adversely influence medication adherence. Clinicians and researchers alike often find viral suppression to be an excellent marker for adherence yet identifying suboptimal adherence before virological failure occurs would be an important target for intervention.
The finding that higher levels of motivation to adhere to ART is an important and new finding in PLWH on MMT. Patients with substance use disorders, generally, have lower motivation levels, which includes individuals’ beliefs and attitudes about the consequences of adherence, perhaps attributed to depressive symptoms (not measured here). As part of future interventions, factors that increase motivation, either by screening and treating underlying depression or better informing patients about the benefits of ART adherence, as stipulated in the IMB feedback model,44,45 could potentially increase motivation levels generally and most importantly focus on ART.
Concerning is the finding that increasing levels of anticipated stigma (i.e., fear that stigma will be experienced) are correlated with suboptimal adherence. This finding has been demonstrated elsewhere among PLWH,29,46–48 but less well-described in patients with substance use disorders. It is possible that anticipated stigma associated with both HIV infection and drug use may discourage these individuals to disclose their HIV status. This, in turn, may lead to underutilization of available HIV treatment services and sub-optimal adherence because of fear of rejection and discrimination. New strategies that specifically address anticipated stigma may enhance ART adherence among HIV-positive methadone-maintained patients as well as their overall health status. One highly effective strategy, HIV TasP3–6 has the potential to curb HIV incidence at the population level, and they may reduce stigma related to HIV and its effects.48 The scientific evidence behind TasP led to the Undetectable = Untransmittable (U=U) campaign,49 which has been rapidly gathering momentum, having been endorsed by more than 400 organizations from 60 different countries including the US Centers for Disease Control and Prevention.50 This U=U strategy has not, however, transcended drug-using populations with regard to uptake. Such strategies remove the absolute need to disclose their HIV status and markedly reduces the negative consequences (e.g., HIV-related stigma) to PLWH through the disclosure process and thus may improve adherence to ART.
Our findings further demonstrated that there is a complex interplay between motivation to adhere to ART, injection-related practices, and optimal ART adherence. Although non-significant in the current study, similar studies have found that people who inject drugs have been less likely to achieve optimal adherence.10–12 As an extension of prior findings, our results point toward an interactive effect of motivation to adhere to ART and drug injection on individuals’ ART adherence. That is, those who injected drugs were likely to report optimal ART adherence only if they had higher motivation to adhere to ART. This highlights the importance of precisely targeting the impact of drug-related risk behaviors, while enhancing motivation to adhere to ART.
The results of this study also provide preliminary evidence of how an individual’s anticipated HIV-related stigma may influence their ART adherence, taking into consideration their motivation to adhere to ART. Our data demonstrated a significant mediating effect of motivation for ART adherence in the relationship between HIV-related stigma and ART adherence. That is, HIV-related stigma was negatively associated with motivation to adhere to ART. Higher motivation, in turn, was associated with optimal ART adherence. This mediation effect demonstrates that motivation to adhere to ART may be an important path through which HIV-related stigma influences individuals’ adherence to ART. This finding reinforces our prior finding that efforts to improve ART adherence should consider ways to harness motivation so that individuals better adhere to their treatment regimen.
Findings from this study are not without limitations. First, participants were recruited from MMT sites within one county, potentially limiting generalizability of findings to HIV-positive patients on MM nationwide. Second, we relied on self-reported measures of ART adherence as well as several correlates of adherence, which may have been subject to reporting bias, particularly over-estimating adherence and underreporting risk behaviors. Third, the data were cross-sectional in nature, thus limiting our ability to infer direct causation from the associations we found. Fourth, the study sample was relatively small, which may have limited our ability to detect significant associations of other relevant variables. Fifth, the inclusion of participants meeting specific eligibility criteria (e.g., able to understand, speak, and read English, not actively suicidal or psychotic, met DSM-V criteria for opioid use disorder, stable on methadone) may limit our ability to generalize the findings to other risk populations. Despite these limitations, our findings significantly contribute to the literature to date and have important implications for interventions targeting medication adherence among high-risk populations.
There have been substantial advances in the use of ART for the treatment and prevention of HIV infection. Optimal adherence to ART is, however, vital to sustained HIV suppression, reduced risk of HIV transmission, and improved overall health and quality of life.1,2 Findings from this study underscore the complexities surrounding ART adherence among high-risk HIV-positive methadone-maintained patients. Our findings are unique given the relative dearth of research on ART adherence practices relative to the factors we were able to examine in our analyses. Further, the results make a significant contribution to our understanding of the explanatory pathways through which various factors influence ART adherence. As HIV prevention efforts rely upon the TasP approaches51,52 future interventions approaches will need to carefully address population-specific needs (e.g., harm reduction, overcoming stigma, improving motivation for medication adherence) that may not be evident, but may strongly influence HIV prevention outcomes.
Supplementary Material
Acknowledgments
The authors thank Brian Sibilio and Pramila Karki for the contributions to this trial
Source of Funding: This work was supported by grants from the National Institute on Drug Abuse for research (R01 DA032290 to MMC) and for career development (K24 DA017072 to FLA; K02 DA033139 to MMC).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. Aids. 2013;27(6):973–979. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–29. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. New England Journal of Medicine. 2016;375(9):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavinton B, Grinsztejn B, Phanuphak N, et al. HIV treatment prevents HIV transmission in male serodiscordant couples in Australia, Thailand and Brazil. Paper presented at: JOURNAL OF THE INTERNATIONAL AIDS SOCIETY2017. [Google Scholar]
- 6.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. Jama. 2016;316(2):171–181. [DOI] [PubMed] [Google Scholar]
- 7.Zwahlen M, Harris R, May M, et al. Mortality of HIV-infected patients starting potent antiretroviral therapy: comparison with the general population in nine industrialized countries. Int J Epidemiol. 2009;38(6):1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross R, Yip B, Lo Re V 3rd, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194(8):1108–1114. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 10.Azar P, Wood E, Nguyen P, et al. Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC Infectious Diseases. 2015;15(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Chunqing L, Sung-Jae L, Le Anh T, Nan F, Nguyen Anh T. Antiretroviral therapy adherence and self-efficacy among people living with HIV and a history of drug use in Vietnam. International Journal of STD & AIDS. 2017;28(12):1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Wilson TE, Adedimeji A, et al. The Impact of Substance Use on Adherence to Antiretroviral Therapy Among HIV-Infected Women in the United States. AIDS and Behavior. 2018;22(3):896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer JP, Althoff AL, Altice FL. Optimizing Care for HIV-Infected People Who Use Drugs: Evidence-Based Approaches to Overcoming Healthcare Disparities. Clinical Infectious Diseases. 2013;57(9):1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha R, Copenhaver MM. Viral suppression among HIV-infected methadone-maintained patients: The role of ongoing injection drug use and adherence to antiretroviral therapy (ART). Addictive behaviors. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrestha R, Karki P, Huedo-Medina TB, Copenhaver M. Treatment engagement moderates the effect of neurocognitive impairment on antiretroviral therapy adherence in HIV-infected drug users in treatment. Journal of the Association of Nurses in AIDS Care. 2017;28(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low AJ, Mburu G, Welton NJ, et al. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Medicine. 2014;12(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevelius JM, Saberi P, Johnson MO. Correlates of antiretroviral adherence and viral load among transgender women living with HIV. AIDS care. 2014;26(8):976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seghatol-Eslami VC, Dark H, Raper JL, Mugavero MJ, Turan JM, Turan B. Interpersonal and intrapersonal factors as parallel independent mediators in the association between internalized HIV stigma and ART adherence. Journal of acquired immune deficiency syndromes (1999). 2017;74(1):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahana SY, Fernandez MI, Wilson PA, et al. Rates and correlates of antiretroviral therapy use and virologic suppression among perinatally and behaviorally infected HIV+ youth linked to care in the United States. Journal of acquired immune deficiency syndromes (1999). 2015;68(2):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalichman SC, Eaton L, Kalichman MO, Cherry C. Medication beliefs mediate the association between medical mistrust and antiretroviral adherence among African Americans living with HIV/AIDS. Journal of health psychology. 2017;22(3):269–279. [DOI] [PubMed] [Google Scholar]
- 22.Ekstrand ML, Heylen E, Mazur A, et al. The Role of HIV Stigma in ART Adherence and Quality of Life Among Rural Women Living with HIV in India. AIDS and Behavior. 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferro EG, Weikum D, Vagenas P, et al. Alcohol use disorders negatively influence antiretroviral medication adherence among men who have sex with men in Peru. AIDS care. 2015;27(1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha R, Krishnan A, Altice FL, Copenhaver M. A non-inferiority trial of an evidence-based secondary HIV prevention behavioral intervention compared to an adapted, abbreviated version: Rationale and intervention description. Contemp Clin Trials. 2015;44:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5. [DOI] [PubMed] [Google Scholar]
- 26.Bowen EA, Canfield J, Moore S, Hines M, Hartke B, Rademacher C. Predictors of CD4 health and viral suppression outcomes for formerly homeless people living with HIV/AIDS in scattered site supportive housing. AIDS Care. 2017;29(11):1458–1462. [DOI] [PubMed] [Google Scholar]
- 27.Crepaz N, Tang T, Marks G, Hall HI. Viral Suppression Patterns Among Persons in the United States With Diagnosed HIV Infection in 2014. Ann Intern Med. 2017;167(6):446–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huedo-Medina TB, Shrestha R, Copenhaver M. Modeling a Theory-Based Approach to Examine the Influence of Neurocognitive Impairment on HIV Risk Reduction Behaviors Among Drug Users in Treatment. AIDS and Behavior. 2016;20(8):1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV Stigma Mechanisms and Well-Being Among PLWH: A Test of the HIV Stigma Framework. AIDS and Behavior. 2013;17(5):1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowling-Guyer S, Johnson ME, Fisher DG, et al. Reliability of Drug Users’ Self-Reported HIV Risk Behaviors and Validity of Self-Reported Recent Drug Use. Assessment. 1994;1(4):383–392. [Google Scholar]
- 31.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Statistics in medicine. 1997;16(9):965–980. [DOI] [PubMed] [Google Scholar]
- 32.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological methodology. 1982;13(1982):290–312. [Google Scholar]
- 33.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36(4):717–731. [DOI] [PubMed] [Google Scholar]
- 34.IBM SPSS Statistics for Windows, Version 23 [computer program]. Armonk, NY: IBM Corp.; 2015. [Google Scholar]
- 35.Malta M, Magnanini MMF, Strathdee SA, Bastos FI. Adherence to Antiretroviral Therapy Among HIV-Infected Drug Users: A Meta-Analysis. AIDS and Behavior. 2010;14(4):731–747. [DOI] [PubMed] [Google Scholar]
- 36.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to Antiretroviral Therapy and Virologic Failure: A Meta-Analysis. Medicine. 2016;95(15):e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruce RD, Altice FL. Clinical care of the HIV-infected drug user. Infect Dis Clin North Am. 2007;21(1):149–179, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhlmann S, Milloy MJ, Kerr T, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105(5):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palepu A, Milloy MJ, Kerr T, Zhang R, Wood E. Homelessness and Adherence to Antiretroviral Therapy among a Cohort of HIV-Infected Injection Drug Users. Journal of Urban Health. 2011;88(3):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cioe PA, Gamarel KE, Pantalone DW, Monti PM, Mayer KH, Kahler CW. Cigarette Smoking and Antiretroviral Therapy (ART) Adherence in a Sample of Heavy Drinking HIV-Infected Men Who Have Sex with Men (MSM). AIDS and Behavior. 2017;21(7):1956–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrestha R, Altice FL, Sibilio B, Copenhaver MM. HIV Sero-Status Non-disclosure Among HIV-Infected Opioid-Dependent Individuals: The Roles of HIV-Related Stigma, Risk Behavior, and Social Support. J Community Health. 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Socías ME, Wood E, Small W, et al. Methadone maintenance therapy and viral suppression among HIV-infected opioid users: The impacts of crack and injection cocaine use. Drug & Alcohol Dependence. 2016;168:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azar P, Wood E, Nguyen P, et al. Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC infectious diseases. 2015;15(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horvath KJ, Smolenski D, Amico KR. An empirical test of the information-motivation-behavioral skills model of ART adherence in a sample of HIV-positive persons primarily in out-of-HIV-care settings. AIDS Care. 2014;26(2):142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amico KR, Barta W, Konkle-Parker DJ, et al. The Information–Motivation–Behavioral Skills Model of ART Adherence in a Deep South HIV+ Clinic Sample. AIDS and Behavior. 2009;13(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logie CH, Lacombe-Duncan A, Wang Y, et al. Pathways From HIV-Related Stigma to Antiretroviral Therapy Measures in the HIV Care Cascade for Women Living With HIV in Canada. Journal of Acquired Immune Deficiency Syndromes (1999). 2018;77(2):144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. Journal of the International AIDS Society. 2013;16(3Suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing Mechanisms Linking HIV-Related Stigma, Adherence to Treatment, and Health Outcomes. American Journal of Public Health. 2017;107(6):863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prevention Access Campaign. Risk of sexual transmission of HIV from a person living with HIV who has undetectable viral load. 2018; https://www.preventionaccess.org/consensus. Accessed March 24, 2018.
- 50.The Lancet HIV. U=U taking off in 2017. The Lancet HIV. 2017;4(11):e475. [DOI] [PubMed] [Google Scholar]
- 51.Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of hiv infection in adults: 2016 recommendations of the international antiviral society–usa panel. JAMA. 2016;316(2):191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volberding PA. HIV Treatment and Prevention: An Overview of Recommendations From the 2016 IAS–USA Antiretroviral Guidelines Panel. Topics in Antiviral Medicine. 2017;25(1):17–24. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


