Abstract
Menopause is associated with declines in physical activity and skeletal muscle strength. Physical activity is also reduced in rodents after ovariectomy (OVX) and whole-body estrogen receptor α (ERα) knockout. However, it is unclear if the effects are estradiol (E2) specific. Thus, the overall purpose of this study was to investigate the effects of the ovarian hormones, E2 and progesterone (P4), and skeletal muscle ERα (skmERα) on physical activity and skeletal muscle contractility in female mice.
Methods:
Study 1: Forty female C57Bl/6J mice were given free access to running wheels for 2 weeks to assess baseline running and randomized into 4 treatment groups: OVX, OVX+E2, OVX+P4, OVX+E2+P4. All mice underwent OVX, returned to wheels for 2 weeks, received hormone pellet implants and returned to running wheels for 6 weeks, after which soleus muscle contractility testing was completed. Study 2: Thirty-two skeletal muscle specific ERα knock-out (skmERαKO) mice and wildtype (WT) littermates were randomized into 4 groups: skmERαKO-Run, skmERαWT-Run, skmERαKO-Sed, and skmERαWT-Sed. Run mice were given free access to wheels for 20 wk and sedentary (Sed) mice maintained normal cage activities. At the end point, muscle contractility was tested.
Results:
Study 1: OVX+E2+P4 group ran greater distances than both the OVX and OVX+P4 groups (p≤0.009). After fatiguing contractions, soleus muscles of the OVX+E2+P4 group maintained greater submaximal force than those of other groups (p=0.023). Immediately after the fatiguing contractions, OVX+E2+P4 muscles had greater maximal force production than the OVX+E2 group (p=0.027). Study 2: There were no differences in running distance between skmERαWT and skmERαKO mice (p=0.240). Soleus muscles of skmERαKO mice were more fatigable (p<0.001) and did not recover force as well as skmERαWT mice (p<0.001). In vivo isometric, concentric and eccentric torque was decreased in skmERαKO mice compared to skmERαWT mice (p≤0.029).
Conclusions:
Combined treatment of E2+P4 in OVX mice restored physical activity, predominantly driven by E2, and protected soleus muscles against fatigue. Muscle of skmERαKO mice was weak regardless of physical activity. Although 20 wk of wheel running partially prevented force loss during fatigue in skmERαKO mice, force production during recovery remained low, indicating that estradiol function through ERα in skeletal muscle.
Keywords: Progesterone, Estradiol, ERα, Fatigue, Skeletal muscle, Wheel running
1. Introduction
There has long been an association between aging and decreased physical activity (Caspersen 2000; Troiano 2008). In females, one possible contributor for a change in activity level is the decline in ovarian hormones, which occurs around the time of menopause. Postmenopausal women taking estrogen-based hormone therapy are more physically active and participate in greater levels of moderate to vigorous activity than those who did not (Andersen 2003; Choudhury 2011). Using a model of ovarian hormone suppression via gonadotropin releasing hormone antagonist treatment (Lupron™), Melanson (2018) reported women receiving estrogen add-back treatment tended to participate in greater moderate-to-vigorous physical activity per day than women receiving a placebo (Melanson 2018). Therefore, estrogen appears to play a role in modulating levels of physical activity after menopause.
Rodent models of surgical menopause further support a strong relationship between ovarian hormones, particularly estradiol, and physical activity. Multiple studies have reported substantial decreases in voluntary wheel running distance in mice (Morgan 2001; Gorzek 2007) and rats (Rodier 1971; Stern 1972; Kadi 2002; Hertrampf 2006) after ovariectomy (OVX), while treatment with 17-β estradiol (E2) after OVX restores voluntary wheel running activity to pre-surgical levels (Morgan 2001; Kadi 2002; Hertrampf 2006; Gorzek 2007). In addition to voluntary wheel running, measures of cage activity in mice have also been shown to decrease after OVX and be restored with E2 treatment (Ogawa 2003; Greising 2011; Greising 2011). Collectively, results support the premise that a positive relationship between E2 and levels of physical activity exist in both rodent models and humans.
While there is a putative relationship between E2 and levels of physical activity, the impact of the other major ovarian hormone, progesterone (P4), is not as well understood. To our knowledge, there have only been two investigations of effects of P4 alone on physical activity in rodent models (Rodier 1971; Hoffman-Goetz 2002). Rodier (1971) reported that injections of P4 given to rats with normal estrous cycles resulted in decreased wheel running activity, and P4 treatment after OVX had no effect on wheel running (Rodier 1971). Similarly, Hoffman-Goetz (2002) treated ovariectomized mice with P4 and reported no difference in voluntary wheel running distance compared with placebo treatment (Hoffman-Goetz 2002). We are not aware of any investigation which used simultaneous E2 and P4 treatment to assess physical activity in female rodents. Thus, the first objective of this study was to compare the effects of combined ovarian hormone treatment to single hormone treatments on running wheel distance in ovariectomized mice.
In addition to decreased physical activity levels, skeletal muscle contractile function also declines at the time of menopause, and there is evidence showing that the muscle dysfunction can be mitigated with E2 treatment (Phillips 1996; Skelton 1999; Greising 2009). The few studies to investigate skeletal muscle following P4 treatment alone report increased protein fractional synthesis, as well as mitochondrial hydrogen peroxide (H2O2) emissions in skeletal muscle from post-menopausal women (Kane 2011; Smith 2014), however effects of P4 on contractile function have not been fully elucidated. One study used a combined E2 and P4 treatment and showed increased strength of the back extensor muscles in postmenopausal women (Heikkinen 1997). Similarly, research using rodent models is supportive of E2 improving muscle contractile function after OVX (Moran 2007; Greising 2009; Greising 2011; Greising 2011) but minimal research has been conducted on P4 (Schneider 2004). Thus, a second objective of this study was to investigate the effects combined ovarian hormone treatment as well as single hormone treatments have on muscle contractile function with emphasis on muscle fatigue.
After determining that E2 treatment with or without P4 resulted in increased levels of physical activity and lessened muscle fatigue in ovariectomized mice compared to P4 treatment alone, we conducted a second study to determine if the α estrogen receptor (ERα) specifically in skeletal muscle mediated those effects. Rodent studies have reported estrogen and progesterone receptors are present in areas of the brain that are responsible for motivation for movement (Brinton 2008; Barth 2015), and that whole-body deletion of ERα decreases levels of cage activities in mice (Ribas 2010). However, when ERα is ablated only in skeletal muscle (skmERαKO), physical activity in terms of cage activity is unaffected (Collins 2018). Our third objective was therefore to determine if wheel running distance and skeletal muscle adaptations to exercise, namely fatigue resistance and recovery, was impaired in female skmERαKO mice compared to their wildtype littermates.
2. Methods
2.1. Animals and experimental design of study 1
To address objectives one and two we designed a study to determine if treatment with combined E2+P4 treatment as well as E2 or P4 alone 1) restored wheel running distance after OVX and 2) improved soleus muscle contractile function, specifically protection against fatigue, after OVX. Female C57BL/6 mice (n=40) were purchased from Jackson Labs at approximately4 months of age. Mice were group housed, 5 mice per cage, and allowed to acclimate for 1 week prior to study start. At the initiation of the study, mice were individually housed in cages with access to running wheels for 14 days to assess baseline running distance (Pre; Figure 1). Running distance over the last 7 Pre-days was used to rank order mice into 4 groups: OVX (n=6), OVX+E2 (n=7), OVX+P4 (n=7), OVX+E2+P4 (n=5). Rank ordering of baseline running wheel distances was used to ensure no difference among groups prior to intervention. The initial 7 days were not used because of high variability from mouse-to-mouse as novelty to the wheels and acclimation occurred. All mice then underwent OVX as previously described (Moran 2006). In brief, mice were anesthetized via isoflurane (1–2% at 0.2 L/min), dorsal incisions were made, ovaries removed, and incisions closed with 6–0 silk suture and 7-mm wound clips. Two hours prior to surgery all mice received subcutaneous injection of 2mg/kg slow release buprenorphine (ZooPharm, Windsor, CO). Fourteen mice were euthanized via CO2 after OVX due to development of dermatitis, and one mouse died during pellet implantation, leaving 25 mice completing the study.
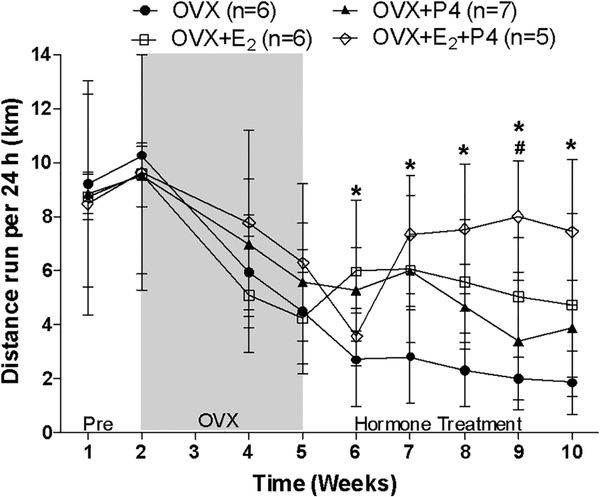
Fig. 1.
Study 1. Average distance run per 24 h plotted as weekly averages (means ± SD). Pre was prior to any intervention. Hormone treatment was initiated at week 5. Group data were analyzed by One-way ANOVA at each week. No differences among groups from wk. 1–5.★Significantly different from OVX at given week. #Significantly different from OVX + P4.
Mice recovered from surgery for 7 days and then were moved to individual cages with running wheels for another 14 days (OVX; Figure 1). At the completion of the OVX running period, hormone treatment was delivered using 60-day time-release hormone pellets (Innovative Research of America, Sarasota, FL) implanted at the base of the neck. Hormone pellets consisted of 0.18 mg E2 so that mice received approximately 3μg/day, 42 mg P4 so that mice received approximately 700 μg/day, or a pellet that contained combined E2 and P4 (42.18 mg total hormones). The doses were chosen based on previous studies which measured these hormone amounts to mimic physiologic hormone levels in mice (Nelson 1981; Farr 1995; Schneider 2004; Moran 2007).
After hormone pellet implantation, mice returned to the running wheels for ~ 42 days (Hormone Treatment; Figure 1). Body masses were measured weekly for the duration of the study. At the completion of the hormone treatment, mice were weighed and anesthetized via an intraperitoneal injection of sodium pentobarbital (100 mg/kg body mass) and left soleus muscles were tested ex vivo for contractile function to determine if exercise-induced adaptations were affected by the female sex hormones. Contralateral soleus muscles were dissected, snap frozen in liquid nitrogen and stored at −80o for enzymatic assay and protein content analysis. Whole blood was collected via exsanguination, allowed to clot, and spun at 10,000 g for 10 minutes at room temperature. Serum was collected and stored at −80oC with a subset of serum samples used for LC-MS/MS analysis of hormone concentrations.
2.2. Animals and experimental design of study 2
To address objective three, we designed a study to determine the impact of ablating ERα in skeletal muscle on wheel running and exercise. Female skeletal muscle specific estrogen receptor α knock-out mice (skmERαKO) and their littermates (skmERαWT), ages 8–16 weeks, were obtained from our colony at the University of Minnesota (n=32). Generation and characterization of these mice has previously been described (Collins 2018). SkmERαKO and skmERαWT mice were randomized into 4 groups: skmERαWT–Sed (n=6), skmERαKO–Sed (n=5), skmERαWT–Run (n=11), and skmERαKO–Run (n=10). Sedentary mice were group housed 3–4 mice per cage while mice in the run group were individually housed in cages with access to a running wheel for 16 weeks. Weekly body masses and daily food intake were measured over the duration of the study. At the end of week 16, all mice underwent Echo MRI for body composition analyses using Echo MRI 3-in-1 (Echo Medical System; Houston, TX). Mice were given continued access to wheels until the day of muscle testing. At week 17, mice were anesthetized via inhalation of isoflurane (1.5%) with O2 flow rate of 0.125 L/min, and underwent in vivo contractility testing of the plantar flexors (soleus, plantaris, and gastrocnemius muscles). While still anesthetized, mice received an intraperitoneal injection of sodium pentobarbital (100 mg/kg body mass) followed by ex vivo contractility testing of the right soleus muscle. Immediately following testing, the contralateral soleus was dissected snap frozen in liquid nitrogen and stored at −80o for enzymatic assays.
All animals for studies 1 and 2 were housed on a 14h:10h light:dark cycle, with free access to water and phytoestrogen free chow (Harland Teklad, 2919, Madison, WI). All protocols and animal care procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee.
2.3. Voluntary wheel running
Mice were individually housed in wheel running cages (Mouse Single Activity Wheel Chamber model 80820, Lafayette Instrument Company, Lafayette, IN). The wheel diameter measures 12.7 cm, width measures 5.72 cm, and revolutions are counted via optical sensor. The run surface of the wheels consisted of 38 rods spaced 6.14 mm apart. Data were recorded via Activity Wheel Monitor Software Model 86065. Each wheel was calibrated weekly with 1-g resistance using resistance brakes (Model 86070-B1, Lafayette Instruments) to prevent the wheel from over-rotating as the mouse moved on/off the wheel and to assure that each mouse ran against an equivalent resistance for the duration of the study.
2.4. Ex vivo soleus muscle contractile function
The isolated muscle preparation for ex vivo muscle testing was conducted as previously described (Moran 2005) using a 1.2 ml bath filled with Krebs-Ringer buffer, maintained at 25oC. Following the peak isometric tetanic contraction (Po) measurements, peak concentric force was measured by stimulating the muscle during a 10% Lo shortening contraction. Next, soleus muscles underwent a protocol of submaximal fatiguing contractions. Soleus muscles were stimulated for 1000 ms at 40 Hz every 5 s for 5 min, for a total of 60 contractions (Greising 2011). Recovery from fatigue was assessed by re-measuring Po immediately following the fatigue protocol and every 5 min for 20–25 min.
2.5. In vivo plantar flexor muscle function
As previously described for in vivo testing of the plantar flexor muscles, the peroneal branch of the sciatic nerve was first severed to avoid recruitment of the dorsi flexor muscles (Baltgalvis 2012). The ankle joint was then positioned such that the foot was perpendicular to the tibia and the knee was stabilized by a clamp to inhibit movement of the lower limb. The foot was secured to a footplate that was attached to the shaft of a servomotor (300B-LR; Aurora Scientific, Aurora, Ontario, Canada). Contraction of the plantar flexors was elicited via stimulation of the sciatic nerve through platinum percutaneous electrodes attached to a stimulator (E2–12 and S48; Grass Telefactor, Warwick, RI). Stimulation voltage and needle electrode placement were optimized during 5–15 isometric tetanic contractions (400-ms train of 0.1-ms pulses at 200 Hz). Following optimization, contractile function of the plantar flexors was assessed by measuring isometric torque as a function of stimulation frequency (10–300 Hz), with highest recorded torque defined as peak isometric torque. Next, for the torque-velocity relationship, the foot was passively moved to 20° dorsiflexion, and a concentric contraction was performed at 300 Hz with the foot simultaneously moving to 20° plantarflexion at varying rates (1200–100°/s). Power was calculated by multiplying the torque generated at a particular velocity by that velocity.
2.6. LC-MS/MS method for serum hormone concentration
Serum E2 was precipitated, eluted and measured using tandem LC-MS/MS methods as previously described (Le 2018) and here with the addition of serum P4 quantification. A micro LC column (2.7 μm, C18 90 Å, Halo Fused core, 100 × 0.3 mm, Eksigen, SCIEX; Framingham, MA, USA) was used in series with an AB SCIEX QTRAP 5500 mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The MS was operated in negative (for E2) and positive (for P4) ionization modes using MRM scans with a collision energy of −55V (for E2) and +15V (for P4), source temperature of 295o C, with nitrogen as the collision and ion source gas. The E2 molecule was identified by a m/z of 271.2 Da and P4 identified by a m/z of 314.4 Da. LCMS/MS results were analyzed by peak area of the chromatograph using MultiQuant™(SCIEX).
2.7. Total protein content
Frozen muscles were homogenized in RIPA lysis buffer containing protease inhibitor tablet (11836170001, Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitor II and III cocktails (P5726 and P0044, Sigma Aldrich, St. Louis, MO) using bullet blender (Midwest Scientific, St. Louis, MO). Homogenates were centrifuged at 664 g for 10 min at 4o C and the supernatant was collected. Protein lysates were assayed in duplicate for total protein concentration using BCA protein assay with albumin standards (Pierce Biotechnology, Rockford, IL).
2.8. Mitochondrial enzyme assays
Muscles were homogenized in 33 mM potassium phosphate buffer (pH 7.4) at a muscle-buffer ratio of 1:20 using teflon- on- glass tissue grinder on ice. Protein concentration of each homogenate was measured in triplicate using BCA protein assay kit. Citrate synthase (CS) activity was measured in duplicate, and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity was measured in triplicate for lateral gastrocnemius muscles and in duplicate for soleus muscles as previously described (Landisch 2008). Enzyme activities were normalized to total protein content.
2.9. Statistical analyses
Data are presented as means ± SD. One-way ANOVAs were used for determining differences among the four groups in study 1 with use of Holm-Sidak post hoc tests when indicated. Two-way Repeated Measures ANOVA was used to determine weekly running wheel differences in study 2 with Holm-Sidak post hoc tests when indicated, and independent t-tests were used to determine differences in total running wheel distance between skmERαWT and skmERαKO groups. Two-way ANOVAs with Holm-Sidak post hoc tests were used to determine differences among groups for all other parameters of study 2. Significance was set at p<0.05.
3. Results
3.1. Study 1: E2 and P4 hormone treatments
Body masses were not different among the OVX, OVX+E2, OVX+P4, OVX+E2+P4 groups at the beginning of the study or 10 weeks later, although there was a trend for mice receiving a single hormone treatment, E2 or P4, to have relatively low body mass at the end of the study (Table 1). Mice treated with E2 had uterine masses 10–15 times greater than OVX and OVX+P4 mice (Table 1). Uterine masses of the OVX+E2+P4 mice fell in between those of the single hormone treated mice, indicative of progesterone antagonizing the E2 effect on uterine tissue.
Table 1:
Body and uterine masses of mice in study 1.
| OVX (n=6) | OVX+E2 (n=5) | OVX+P4 (n=6) | OVX+E2+P4 (n=3) | One-Way ANOVA p-value | |
|---|---|---|---|---|---|
| Body Mass – Pre OVX (g) | 20.9 ± 0.96 | 21.4 ± 0.95 | 20.7 ± 1.30 | 20.3 ± 0.92 | 0.469 |
| Body Mass – Harvest (g) | 28.4 ± 3.09 | 25.5 ± 1.57 | 25.7 ± 3.30 | 27.8 ± 1.72 | 0.052 |
| Uterine Mass (mg) | 15.0 ± 4.06 | 151.7 ± 33.0* | 11.0 ± 3.94# | 90.1 ± 12.9 | 0.001 |
Data are mean ± SD.
Significantly different from OVX (p<0.005);
Significantly different from OVX+E2 (p<0.005).
3.1.1. Serum hormone concentrations
Serum E2 levels were 22.4 ± 10.8 pg/ml in mice treated with E2, whereas OVX mice without E2 treatment had levels of 4.9 ± 5.5 pg/ml. For comparison, serum E2 from intact, normal estrous-cycling mice was 16.0 ± 12.3 pg/ml. P4 levels were 243 ± 90.4 pg/ml in OVX mice,70.9 ± 38.9 pg/ml in OVX+E2 mice, 1488 ± 339 pg/ml in OVX+P4 mice, and 933 ± 596 pg/ml in OVX+E2+P4 mice. For comparison, serum P4 from intact, normal estrous-cycling mice was 1901 ± 297 pg/ml.
3.1.2. Running wheel distance
Twenty-four hour running distances among the groups prior to OVX were not different (p=0.873). Following OVX at week 3, mice decreased daily distances from 9.8± 0.6 km/24h at week 2 to 5.7 ± 0.6 km/24h at week 5 (Figure 1). Treatment groups did not differ among each other at weeks 2–5 (p≥0.326). At weeks 6 and 8, OVX+E2 mice ran greater distances than OVX mice (p≤0.019). From weeks 7–10, the OVX+E2+P4 group ran greater distances than the OVX group (p≤0.009) and at week 9 ran greater distance than the OVX+P4 group (p=0.004).
Running distance at week 10 was also analyzed for average distances run during light hours and dark hours. During the 10 h dark cycle, OVX+E2+P4 mice ran 53% greater distance than OVX mice (p=0.003), similar to the total 24-h results. There was no difference in distance run among the four groups during the 14 h of light (0.52 ± 0.16 km/14h; p=0.320).
3.1.3. Soleus muscle contractility
There was no difference in soleus muscle size (mass, length, or physiological cross-sectional area) or total protein content among groups (Table 2). There were also no differences among groups in peak twitch, tetanic, or concentric force generation or active and passive stiffness (Table 2).
Table 2:
In vitro soleus muscle contractile properties in study 1.
| OVX (n=6) | OVX+E2 (n=5) | OVX+P4 (n=6) | OVX+E2+P4 (n=3) | One-Way ANOVA p-value | |
|---|---|---|---|---|---|
| Muscle mass (mg) | 14.4 ± 1.59 | 12.2 ± 2.19 | 11.7 ± 1.56 | 13.2 ± 0.54 | 0.060 |
| Muscle length (mm) | 9.65 ± 0.15 | 9.48 ± 0.40 | 9.76 ± 0.09 | 9.65 ± 0.30 | 0.632 |
| Physiological CSA (mm2) | 1.98 ± 0.21 | 1.71 ± 0.27 | 1.60 ± 0.21 | 1.83 ± 0.12 | 0.063 |
| Total protein (mg) | 1.36 ± 0.22 | 1.38 ± 0.45 | 1.26 ± 0.26 | 1.34 ± 0.25 | 0.980 |
| Peak twitch force (mN) | 22.7 ± 5.62 | 20.6 ± 7.70 | 21.9 ± 7.26 | 22.1 ± 9.49 | 0.973 |
| Peak isometric tetanic force (mN) | 211 ± 28.2 | 164 ± 48.4 | 178 ± 26.8 | 155 ± 37.7 | 0.167 |
| Peak concentric force (mN) | 105 ± 21.2 | 71.0 ± 27.3 | 80.6 ± 20.4 | 68.3 ± 31.7 | 0.095 |
| Active stiffness (N/m) | 398 ± 47.5 | 345 ± 82.8 | 366 ± 70.9 | 385±107 | 0.682 |
| Passive stiffness (N/m) | 17.2 ± 1.80 | 23.5 ± 10.9 | 18.9 ± 1.47 | 20.0 ± 4.27 | 0.215 |
Data are mean ± SD
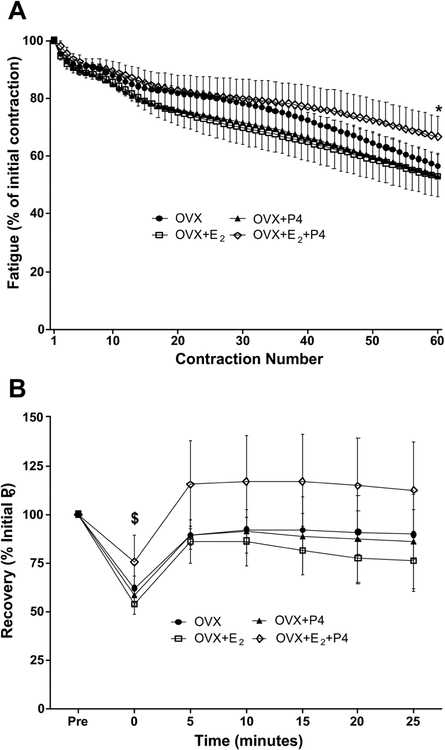
During repeated submaximal isometric contractions designed to fatigue isolated soleus muscle, there was a difference among groups at contractions 40, 50, and 60 with the OVX+E2+P4 mice losing less force than the OVX+E2 and OVX+P4 mice at contraction 60 (p=0.023; Figure 2A). In order to determine recovery from fatigue, force from a single maximal isometric tetanic contraction was measured immediately following fatigue (time 0; Figure 2B) and then again, every 5 min for 25 min. At time 0, mice treated with OVX+E2+P4 produced greater force than OVX+E2 mice (p=0.027; Figure 2B). At 5–25 min after the fatiguing protocol there were no differences among groups in force production (p≥0.055).
Fig. 2.
Study 1. A) Force loss over 60 submaximal isometric contractions presented as a percent of the initial force (means ± SD).★OVX + E2 + P4 significantly different from OVX + P4 and OVX + E2. B) Recovery of maximal isometric force after 60 submaximal fatiguing contractions presented as a percent of initial maximal isometric force (means ± SD). $ OVX + E2 + P4 significantly different from OVX + E2.
To determine if greater oxidative metabolism in soleus muscles contributed to reduced fatigability in OVX+E2+P4 mice, β-HAD activity was measured but did not differ among groups (p=0.349). However, citrate synthase enzyme activity was 50% greater in soleus muscles from OVX+E2+P4 mice compared to those from OVX and OVX+E2 mice (p=0.015).
3.2. Study 2: Skeletal muscle ERα ablation
3.2.1. Body composition
There were significant effects of wheel running on all measures of body composition (Table 3). Mice in the run groups, regardless of genotype, weighed ~8 g less and had ~8 g less fat mass than mice in the Sed groups (Table 3). Run mice also had ~1 g more lean mass than Sed mice. Subcutaneous and visceral fat pad masses of Run mice weighed 3–4 times less compared to the Sedentary groups. There was no difference in any measure of body composition between female mice with and without skeletal muscle ERα (Table 3).
Table 3:
Body and fat masses for skmERαWT and skmERαKO female mice in study 2.
| Groups | 2-Way ANOVA p-values | ||||||
|---|---|---|---|---|---|---|---|
| skmERαWT–Sed (n=6) | skmERαKO–Sed (n=5) | skmERαWT–Run (n=11) | skmERαKO–Run (n=10) | Genotype | Activity | Interaction | |
| Total body mass (g) | 31.8 ± 7.39 | 34.1 ± 5.45 | 26.6 ± 2.22 | 26.1 ± 3.02 | 0.566 | <0.001 | 0.384 |
| Fat mass (g) | 11.8 ± 5.96 | 14.4 ± 4.09 | 4.90 ± 1.26 | 4.67 ± 2.41 | 0.360 | <0.001 | 0.274 |
| Lean mass (g) | 19.2 ± 1.76 | 19.0 ± 1.23 | 20.4 ± 1.38 | 20.5 ± 1.09 | 0.852 | 0.014 | 0.836 |
| % Fat mass | 35.4 ± 9.72 | 41.6 ± 4.84 | 18.3 ± 3.63 | 17.3 ± 6.64 | 0.278 | <0.001 | 0.137 |
| % Lean mass | 62.2 ± 8.65 | 56.4 ± 5.10 | 77.2 ± 4.22 | 79.1 ± 6.75 | 0.406 | <0.001 | 0.105 |
| Subcutaneous fat pad mass (mg) | 1627 ± 1177 | 1986 ± 649 | 365 ±131 | 401 ± 198 | 0.361 | <0.001 | 0.457 |
| Visceral fat pad mass (mg) | 2036 ± 1111 | 2693 ± 766 | 608 ± 322 | 681 ± 456 | 0.138 | <0.001 | 0.231 |
Data are mean ± SD.
3.2.2. Running wheel distance
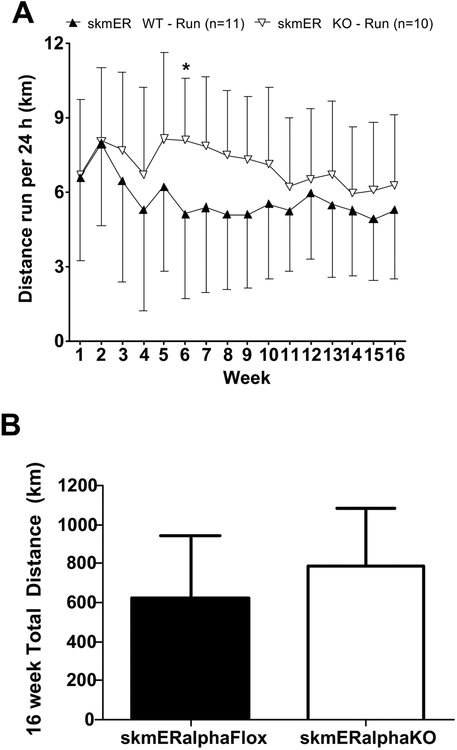
A significant interaction between genotype and week was found for running wheel distance (p=0.002). Post-hoc analysis revealed skmERαKO mice ran a greater distance than skmERαWT mice only at week 6 (p=0.030; Figure 3A). However, the total distance run over 16 weeks was not different between mice with and without skeletal muscle ERα (p=0.236; Figure 3B). Running distance at week 6 was analyzed for average distances run during light hours and dark hours. During 10 h dark cycle, skmERαKO mice ran 45% greater distance than skmERαWT mice (p=0.024), similar to the total 24-h results. There was no difference in distance run among the four groups during the 14 h of light (0.37 ± 0.11 km/14h; p=0.062).
Fig. 3.
Study 2. A) Average distance run per 24 h plotted as weekly averages (means ± SD). Data analyzed by repeated measures 2-way ANOVA.★skmERαKO significantly different from skmERαWT mice. B) Total distance run over 16 weeks, analyzed by paired t-test.
3.2.3. Soleus muscle contractility
Soleus muscle mass and physiological CSA were larger in skmERαKO mice compared to skmERαWT mice (Table 4). Soleus muscles from Run mice weighed more and had larger physiological CSA than those from Sed mice (Table 4). Soleus muscles from Sed mice produced 21% greater twitch force and were more resistant to passive stretch than those from Run mice, regardless of genotype (Table 4). Muscle length, peak isometric tetanic force, and active stiffness were not affected by genotype or activity (Table 4).
Table 4:
In vitro soleus muscle contractile properties of skmERαWT and skmERαKO mice in study 2.
| Groups | Two-way ANOVA p-values | ||||||
|---|---|---|---|---|---|---|---|
| skmERαWT–Sed (n=6) | skmERαKO–Sed (n=5) | skmERαWT–Run (n=11) | skmERαKO–Run (n=10) | Genotype | Activity | Interaction | |
| Muscle mass (mg) | 8.22 ± 1.21 | 10.4 ± 0.95 | 10.7 ± 0.71 | 11.8 ± 1.53 | 0.003 | <0.001 | 0.283 |
| Muscle length (mm) | 10.5 ± 0.54 | 10.7 ± 0.69 | 10.3 ± 0.49 | 10.2 ± 0.35 | 0.638 | 0.114 | 0.433 |
| Physiological CSA (mm2) | 1.07 ± 0.13 | 1.29 ± 0.10 | 1.38 ± 0.11 | 1.54 ± 0.22 | 0.006 | <0.001 | 0.835 |
| Peak twitch force (mN) | 28.4 ± 6.22 | 29.4 ± 6.18 | 22.1 ± 5.09 | 23.1 ± 7.03 | 0.691 | 0.019 | 0.991 |
| Peak isometric tetanic force (mN) | 220 ± 40.1 | 221 ± 17.1 | 202 ± 38.3 | 192 ± 46.9 | 0.641 | 0.172 | 0.763 |
| Active stiffness (N/m) | 421 ± 91.3 | 448±103 | 391 ±108 | 370 ±102 | 0.942 | 0.197 | 0.563 |
| Passive stiffness (N/m) | 13.0 ± 1.77 | 13.3 ± 0.97 | 15.3 ± 2.43 | 15.4 ± 1.73 | 0.841 | 0.008 | 0.907 |
Data are mean ± SD.
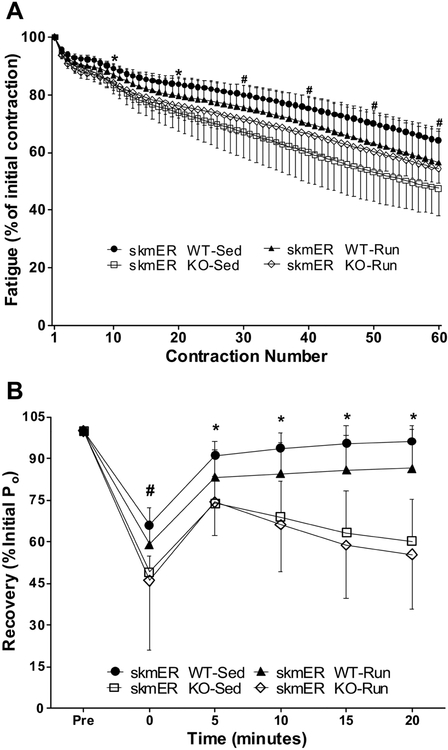
Fatigability of soleus muscles over the course of 60 submaximal isometric contractions was affected by genotype with skmERαKO mice losing a greater percentage of force than skmERαWT mice at contraction numbers 10 and 20 (p<0.001; Figure 4A). At contractions 30, 40, 50, and 60 there was a significant interaction between genotype and activity, with the skmERαWT-Sed mice losing less force than the skmERαKO-Sed mice (p≤0.045; Figure 4A). At time point 0 during recovery skmERαKO-Run mice and skmERαWT-Sed mice produced greater force than skmERαKO-Sed mice, (p=0.01; Figure 4B). At time points 5, 10, 15, and 20 min after fatigue, skmERαWT mice produced greater force than the skmERαKO mice (p≤0.005; Figure 4B).
Fig. 4.
Study 2. A) Force loss over 60 submaximal isometric contractions presented as a percent of the initial force (means ± SD). Data analyzed by 2-way ANOVA at every 10th contraction.★skmERαWT mice significantly different from skmERαKO mice (main effect of genotype). #skmERαWT-Sed mice significantly different from skmERαKO-Sed mice (post-hoc result following significant interaction). B) Recovery of maximal force after 60 submaximal fatiguing contractions presented as a percent of initial maximal isometric force (means ± SD). #skmERαWT-Sed and skmERαKO-Run mice significantly different from skmERαKO-Sed mice (post-hoc result following significant interaction).★skmERαWT significantly different from skmERαKO mice (main effect of genotype).
3.2.4. Plantar flexor muscle contractility
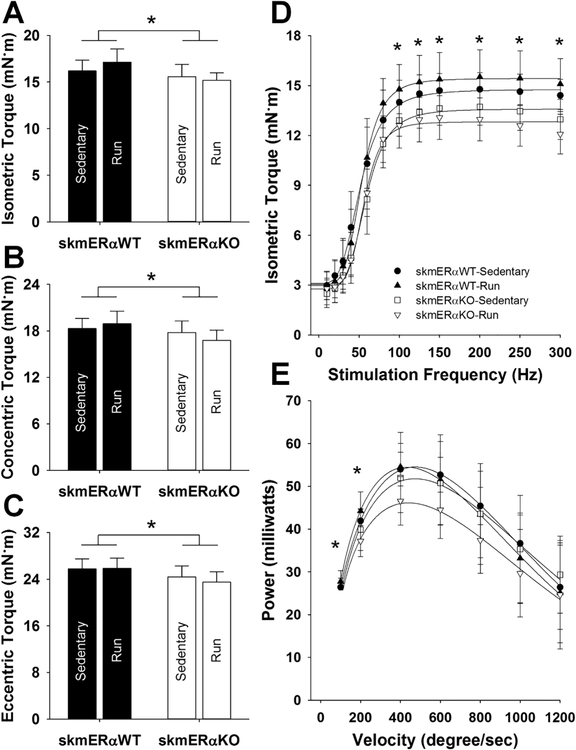
In order to assess the larger group of posterior lower leg muscle recruited during wheel running we assessed in vivo plantar flexor muscle contractility. There was a significant effect of genotype across all peak contraction types, both in absolute peak torque and when peak torque is normalized to muscle mass, with skmERαKO mice producing 7–8% less torque regardless of running activity (isometric, p=0.014; concentric, p=0.029; and eccentric, p=0.013; Figures 5AC). Rates of contraction and relaxation were not different among groups (p≥0.12; data not shown). When activated across submaximal to maximal stimulation frequencies, there were significant effects of genotype with skmERαKO plantar flexors producing less isometric torque at ≥100 Hz (p<0.01; Figure 5D). SkmERαKO mice also produced 11–12% less power at 100 and 200o/s relative to skmERαWT mice (Figure 5E). β-HAD activity in the lateral head of the gastrocnemius muscle was significantly greater in the Run groups (p=0.033), however citrate synthase activity among the groups did not differ with genotype or activity (p≥0.311).
Fig. 5.
Study 2. A–C) Plantar flexor in vivo peak torques (means ± SD). D) Isometric torque as a function of stimulation frequency. E) Power produced at increasing velocities.★skmERαWT mice significantly different from skmERαKO mice.
4. Discussion
Physical activity levels are detrimentally impacted by loss of ovarian hormones in female rodents and treatment with E2 can reverse or prevent the decline (Stern 1972; Morgan 2001; Kadi 2002; Hertrampf 2006; Gorzek 2007). However, when ovarian function declines or when ovaries are surgically removed levels of P4 are affected in addition to E2. Clinically, P4 treatment is indicated for treatment of postmenopausal hot flashes (Prior 2010) as well as used in combination with a form of estrogen in hormone therapy for postmenopausal women with an intact uterus. Therefore, our first objective in study 1 was to investigate the effects of a combined hormone treatment as well single hormone treatments on voluntary wheel running as a form of physical activity in female mice. First, we confirmed with LC-MS/MS that serum concentrations of E2 and P4 were low in OVX mice and in hormone-treated mice were within physiological ranges reported for C57Bl/6J mice with normal estrous cycles (Nelson 1981). The latter was important to ensure mice were not being treated with supraphysiological doses of hormones.
As expected wheel running distance decreased following OVX by ~58%. A novel result of this work is that the combined E2+P4 treatment in study 1 restored wheel running to within 20% of pre-surgical levels. Treatment with E2 alone increased wheel running distance however treatment with P4 alone did not (Figure 1). These P4 results are consistent with previous studies showing that P4 treatment following OVX did not improve wheel running distance in rodents (Young 1945; Rodier 1971; Hoffman-Goetz 2002). As with voluntary wheel running, OVX results in reduced cage activity and is attenuated by E2 treatment (Greising 2011; Greising 2011). Together these results suggest that the primary ovarian hormone affecting physical activity is E2.
In addition to physical activity, ovarian hormone status also has been reported to influence skeletal muscle contractility. Our lab and others have reported that E2 treatment after OVX is protective against fatigue (Hou 2010; Greising 2011). To better understand the role of both ovarian hormones on fatigue and recovery, we investigated the effects of combined E2+P4 and single hormone treatments on soleus muscle contractile function in study 1. Our results suggest that combined E2+P4 hormone treatment provides the greatest protection against fatigue (Figure 2). To our knowledge there is only one other investigation into the effects of a combined hormone treatment on skeletal muscle fatigue (Schneider 2004). Schneider (2004) reported that single hormone treatment with P4 best protected the plantar flexor muscles against fatigue compared to treatments with E2, E2+P4, and placebo. There are a few key differences between that study and ours. In our protocol, we used submaximal isometric contractions to induce fatigue of the isolated soleus muscle; their protocol used maximal eccentric contractions of the plantar flexor muscles in vivo (i.e., soleus, gastrocnemius, and plantaris muscles). Additionally, we extended our recovery period to 25 minutes vs. 3 minutes. By extending the recovery period we are able to show that all treatments aided recovery to at least 80% of the initial force, indicating fatigue rather than injury. Our fatigue protocol resulted in 25–45% decreases in force immediately after the protocol, while theirs caused 73–86% decreases in force. Thus, it is difficult to truly compare the results of our study to those of Schneider (2004) due to these key differences, though in both studies P4 appeared to be beneficial. Because of the paucity of research utilizing a combine hormone treatment or P4 alone, more research is needed to fully elucidate muscle fatigue mechanisms.
Because mice that received the combined hormone treatment also ran the greatest distance, it is plausible that hind limb muscles from that group of mice also would have the highest oxidative capacity. Our results showed citrate synthase activity, indicative of mitochondrial content, was greater in soleus muscles from mice given the combined E2+P4 treatment thus representing a possible mechanism for their increased fatigue resistance.
E2 functions by binding to ERs and ERα in particular is present in a wide range of tissues including skeletal muscle (Kuiper 1997; Pavao 2001; Lemoine 2003; Wiik 2009; Baltgalvis 2010). Our lab has previously demonstrated that E2 utilizes ERα to elicits its effects on skeletal muscle function (Collins 2018). Therefore, in study 2 our objective was to determine if deletion of skmERα affects voluntary wheel running, and if such exercise training would rescue previously reported deficits in muscle function. One limitation of this study is the difference in housing among our groups, with the mice in the run groups housed individually, while the mice in the sedentary groups were group housed.
The results that wheel running distance did not differ between skmERαWT and skmERαKO mice (Figure 3) are consistent with our previous finding of no genotype difference between these mice in 24-h cage activities (Collins 2018). In contrast, studies investigating mice with whole-body deletion of ERα showed decreased levels of activity (Ogawa 2003; Ribas 2010). Together these results suggest that physical activity is regulated via central (i.e., brain), not peripheral (i.e., muscle) mechanisms, likely via E2 acting through ERα (Correa 2015; Su 2015).
We would anticipate long-term wheel running to have an impact on muscle fatigue, thus we isolated soleus muscle to assess fatigability and recovery from fatigue. Our results from study 2 showed that wheel running partially protected skmERαKO mice against fatigue (Figure 4A). However, skmERαKO mice only partially recovered maximal force generation after fatiguing contractions, and did not sustain this ability throughout the recovery (Figure 4B). Collectively these results are consistent with the results previously published in our lab (Collins 2018) as well as work from Ribas (2016) who reported decreased force production in soleus muscle of a similar mouse model of skeletal muscle deleted ERα (Ribas 2016). Changes in muscle oxidative capacity could help to explain the differences in fatigability, however our results indicated modest increases only in β-HAD activity in the run groups. Similarly, work by Ribas (2016) showed no differences in citrate synthase activity, expression of transcriptional regulators of mitochondrial biogenesis, or protein abundance of select components of the mitochondrial electron transport chain between their skeletal muscle specific ERαKO mice and control littermates (Ribas 2016). However, the authors did report reductions in calcium handling, ATP production, and elevated ROS production in mice lacking ERα in skeletal muscle, indicating dysfunctional mitochondria. A limitation of the present study is that we did not investigate measures of mitochondrial function in addition to the enzyme assays. It is possible that the exercise program used in this study was not intense enough or of a long enough duration to induce changes in the mitochondrial function to impact muscle fatigue. Future studies should further investigate the effects of varied training programs on mitochondrial function in mice lacking skeletal muscle ERα.
Although we would expect all these markers of oxidative capacity to change with exercise training, we did focus our analyses on the soleus muscle, which is already highly oxidative. We observed a significant weight gain in our sedentary groups, and we know that the soleus muscle works as a postural stabilizer when mice are performing normal activities. Thus, we speculated that perhaps the soleus muscles in the sedentary mice were required to work sufficiently hard against the gain in body mass that there were enough adaptations to equal the oxidative changes elicited via wheel running.
Wheel running in study 2 prevented the body and fat mass gain observed in the sedentary mice (Table 3). We did not find a difference in body mass between skmERαWT and skmERαKO mice which is in contrast to previous reports (Ribas 2016; Collins 2018). We also did not measure differences between genotypes in fat mass or % fat gain, similar to our previous report (Collins 2018).
In study 2 we also sought to determine if exercise training would rescue the previously reported decrements in muscle function of skmERαKO mice (Collins 2018). Our in vivo assessment of the plantar flexors indicated that long-term voluntary wheel running had no impact on skeletal muscle function in skmERαKO mice. Consistent with our characterization of the skmERα mice (Collins 2018), the present study found a genotype effect with the skmERαKO mice producing less in vivo plantar flexor muscle force, decreased force at higher stimulation frequencies and decreased power at slower velocities (Figure 4).
In summary, key findings from studies reported here demonstrate that: 1) although combined hormone treatment (E2+P4) restores wheel running distances, this is likely driven by E2 working through ERα in tissue outside of skeletal muscle, 2) combined hormone treatment (E2+P4) protects against skeletal muscle fatigue, 3) skeletal muscle ERα is necessary for optimal muscle contractile function, and 4) exercise training partially protects muscle lacking ERα against skeletal muscle fatigue. Thus, physical activity and skeletal muscle function are both affected by ovarian hormone status. The clinical relevance of this work is based on the premise that maintenance of physical activity and skeletal muscle function are critical for women to maintain independence throughout the lifespan. As such, elucidating the mechanisms through which ovarian hormones elicit their effects is essential to determining potential therapeutic targets for menopausal-related dysfunctions.
Highlights:
Combined E2+P4 treatment best restores wheel running activity in OVX female mice
ERα is necessary for optimal skeletal muscle force production
Physical activity did not improve overall skeletal muscle contractility in mice lacking muscle ERα
Acknowledgements:
The authors would like to thank Bruce A. Witthuhn with the College of Biological Sciences for his technical assistance establishing the LC-MS/MS serum analyses.
Funding: This work was supported by National Institutes of Health Grants RO1-AG031743, T32-AR007612, T32-AR050938, T32-AG029796 as well as a grant from the American Diabetes Association BS-1–15–170.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RE, Crespo CJ, Franckowiak SC, Walston JD (2003). “Leisure-time activity among older U.S. women in relation to hormone-replacement-therapy initiation.” J Aging Phys Act 11(1): 82–89. [Google Scholar]
- Baltgalvis KA, Call JA, Cochrane GD, Laker RC, Yan Z, Lowe DA (2012). “Exercise training improves plantarflexor muscle function in mdx mice.” Med Sci Sports Exerc 44: 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltgalvis KA, Greising SM,Warren GL,Lowe DA (2010). “Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle.” PLoS One 5(4): e10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J (2015). “Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods.” Front Neurosci 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J (2008). “Progesterone receptors: Form and function in brain.” Front Neuroendocrinol 29(2): 313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen CJ, Pereira MA, Curran KM (2000). “Changes in physical activity patterns in the United States, by sex and cross-sectional age.” Med Sci Sports Exerc 32(9): 1601–1609. [DOI] [PubMed] [Google Scholar]
- Choudhury F, Bernstein L, Hodis HN, Frank Z, Mack WJ (2011). “Physical activity and sex hormone levels in estradiol and placebo treated postmenopausal women.” Menopause 18(10): 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BC, Mader TL, Cabelka CA, Inigo MR, Spangenburg EE, Lowe DA (2018). “Deletion of estrogen receptor α in skeletal muscle results in impaired contractility in female mice.” J Appl Physiol (1985) 124(4): 980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA (2015). “An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females.” Cell Rep 10(1): 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE (1995). “Effect of ovarian steroids on footshock avoidance learning and retention in female mice.” Physiol Behav 58(4): 715–723. [DOI] [PubMed] [Google Scholar]
- Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA (2007). “Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice.” Med Sci Sports Exerc 39(2): 248–256. [DOI] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA (2011). “Estradiol’s beneficial effect on murine muscle function is independent of muscle activity.” J Appl Physiol (1985) 110(1): 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Lowe DA, Warren GL (2009). “Hormone therapy and skeletal muscle strength: a meta-analysis.” J Gerontol A Biol Sci Med Sci 64(10): 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA (2011). “Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice.” Exp Gerontol 46(8): 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen J, Kyllonen E, KurttilaMatero E, WilenRosenqvist G,Lankinen KS,Rita H, Vaananen HK (1997). “HRT and exercise: Effects on bone density, muscle strength and lipid metabolism. A placebo controlled 2-year prospective trial on two estrogenprogestin regimens in healthy postmenopausal women.” Maturitas 26(2): 139–149. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Degen GH, Kaid AA, Laudenbach-Leschowsky U, Siebel J, Di Virgilio AL, Diel P (2006). “Combined effects of physical activity, dietary isoflavones and 17- beta estradiol on movement drive, body weight, and bone mineral density in ovariectomized female rats.” Planta Med 72(6): 484–487. [DOI] [PubMed] [Google Scholar]
- Hoffman-Goetz L, Fietsch CL (2002). “Lymphocyte apoptosis in ovariectomized mice given progesterone and voluntary exercise.” J Sports Med Phys Fitness 42(4): 481–487. [PubMed] [Google Scholar]
- Hou YX, Jia SS, Liu YH (2010). “17-beta estradiol accentuates contractility in rat genioglossal muscle via regulation of estrogen receptor alpha.” Arch Oral Biol 55(4): 309–317. [DOI] [PubMed] [Google Scholar]
- Kadi F, Karlsson C, Larsson B, Eriksson J, Larval M, Billig H, Jonsdottir IH (2002). “The effects of physical activity and estrogen treatment on rat fast and slow skeletal muscles following ovariectomy.” J Muscle Res Cell Motil 23(4): 335–339. [DOI] [PubMed] [Google Scholar]
- Kane DA, Lin CT, Anderson EJ, Kwak HB, Cox JH, Brophy PM, Hickner RC,Neufer PD, Cortright RN (2011). “Progesterone increases skeletal muscle mitochondrial H 2O 2 emission in nonmenopausal women.” Am J Physiol Endocrinol Metab 300(3): E528–E535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B,Grandien K,Enmark E,Häggblad J,Nilsson S,Gustafsson JA (1997). “Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta.” Endocrinology 138(3): 863–870. [DOI] [PubMed] [Google Scholar]
- Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA (2008). “Adaptive and nonadaptive responses to voluntary wheel running by mdx mice.” Muscle Nerve 38(4): 1290–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le G, Novotny SA,Mader TL,Greising SM,Chan SSK,Kyba M,Lowe DA,Warren GL (2018). “A moderate estradiol level enhances neutrophil number and activity in muscle after traumatic injury but strength recovery is accelerated.” J Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine S, Granier Pascale,Tiffoche Christophe,Rannou-Bekono Francoise,,Thieulant Marie-Lise,Delamarche Paul (2003). “Estrogen receptor alpha mRNA in human skeletal muscles.” Med Sci Sports Exerc 35(3): 439–443. [DOI] [PubMed] [Google Scholar]
- Melanson EL, Lyden K, Gibbons E, Gavin KM, Wolfe P, Wierman ME, Schwartz RS, Kohrt WM (2018). “Influence of estradiol status on physical activity in premenopausal women.” Med Sci Sports Exerc 50(8): 1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Warren GL,Lowe DA (2005). “Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice.” Exp Gerontol 40(12): 966–975. [DOI] [PubMed] [Google Scholar]
- Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA (2007). “Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice.” J Appl Physiol (1985) 102(4): 1387–1393. [DOI] [PubMed] [Google Scholar]
- Moran AL, Warren GL, Lowe DA (2006). “Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution.” J Appl Physiol (1985) 100(2): 548–559. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW (2001). “Effects of estrogen on activity and fear-related behaviors in mice.” Horm Behav 40(4): 472–482. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE (1981). “Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice.” Biol Reprod 24(4): 784–794. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW (2003). “Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity.” Endocrinology 144(1): 230–239. [DOI] [PubMed] [Google Scholar]
- Pavao M, Traish AM (2001). “Estrogen receptor antibodies: specificity and utility in detection, localization and analyses of estrogen receptor alpha and beta.” Steroids 66(1): 1–16. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Sanderson AG, Birch K, Bruce SA, Woledge RC (1996). “Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle.” The Journal of Physiology 496 (2): 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior JC, Hitchcock CL (2010). “Progesterone for vasomotor symptoms: a 12-week randomized, masked placebo-controlled trial in healthy, normal-weight women 1–20 years since final menstrual flow.” Endocr Rev 31(Suppl 1): S51–S51. [Google Scholar]
- Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ,Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS, Hevener AL (2016). “Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females.” Sci Transl Med 8(334): 334ra354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL (2010). “Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice.” Am J Physiol Endocrinol Metab 298(2): E304–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier WI (1971). “Progesterone-Estrogen Interactions in the Control of Activity-Wheel Running in the Female Rat.” J Comp Physiol Psychol 74(3): 365–373. [DOI] [PubMed] [Google Scholar]
- Schneider BS, Fine JP, Nadolski T, Tiidus PM (2004). “The effects of estradiol and progesterone on plantarflexor muscle fatigue in ovariectomized mice.” Biol Res Nurs 5(4): 265–275. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Phillips SK, Bruce SA, Naylor CH, Woledge RC (1999). “Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women.” Clin Sci (Lond) 96(4): 357–364. [PubMed] [Google Scholar]
- Smith G, Yoshino J, Reeds D, Bradley D, Burrows R, Heisey J, Moseley A, Mittendorfer B (2014). “Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women.” J Clin Endocrinol Metab 99: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J, Murphy M (1972). “The effects of thyroxine and estradiol benzoate on wheel running activity in female rats.” Physiol Behav 9: 79–82. [DOI] [PubMed] [Google Scholar]
- Su P, Cao X, He Y, Zhu L, Yang Y, Saito K, Wang C, Yan X, Hinton AO Jr., Zou F, Ding H, Xia Y, Yan C, Shu G, Wu SP, Yang B, Feng Y, Clegg DJ, DeMarchi R, Khan SA, Tsai SY, DeMayo FJ, Wu Q, Tong Q, Xu Y (2015). “Estrogen receptor-α in medial amygdala neurons regulates body weight.” J Clin Invest 125(7): 2861–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigani D, Dodd KW, Masse LC, Tilert T, McDowell M (2008). “Physical activity in the United States measured by accelerometer.” Med Sci Sports Exerc 40(1): 181–188. [DOI] [PubMed] [Google Scholar]
- Wiik A, Ekman M,Johansson O,Jansson E,Esbjornsson M (2009). “Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue.” Histochem Cell Biol 131(2): 181–189. [DOI] [PubMed] [Google Scholar]
- Young WC, Fish WR (1945). “The ovarian hormones and spontaneous running activity in the female rat.” Endocrinology 36(3): 181–189. [Google Scholar]