Abstract
This pilot work examined associations of brain grey matter volumes (GMV) with perceived fatigability in older adults to elucidate disablement mechanisms. A subsample (n=29; age=77.2±5.5; 86% female) of participants from the Lifestyle Interventions and Independence for Elders (LIFE) Study was utilized to quantify GMV for regions of interest in the basal ganglia and limbic system normalized to intracranial volume. The Pittsburgh Fatigability Scale measured physical and mental fatigability (score 0–50; higher physical fatigability ≥15; higher mental fatigability ≥13). We used an exploratory alpha level of p<0.1. Nineteen (66%) participants had higher physical fatigability, 19 (66%) had higher mental fatigability, of these, 17 (57%) had both. Right hippocampal volumes/ICV were smaller in participants with higher verses lower physical fatigability (0.261±0.039 vs. 0.273± 0.022, p=0.07); associations were similar for right putamen and bilateral thalamus. Higher mental fatigability was associated with smaller right hippocampus, thalamus, and posterior cingulum and bilateral amygdala. Higher fatigability in older adults may be associated with smaller volumes of the basal ganglia and limbic system, indicating mechanisms for further exploration.
Keywords: Fatigue, grey matter volumes, magnetic resonance imaging, aging
1.0. Introduction
Among older adults, fatigue may precede decline in physical performance and subsequent impaired or reduced mobility (Ekmann, Petersen, Manty, Christensen, & sAvlund, 2012; Simonsick et al., 2016; Simonsick et al., 2018). Fatigue is an independent predictor of both mortality and incident disability; therefore, managing fatigue levels may be important for maintaining functional independence (Ekmann et al., 2012; Eldadah, 2010; Simonsick et al., 2016). Fatigability is a whole-body measure of an individual’s fatigue anchored to a standardized task or activity of a specific duration (Eldadah, 2010). Compared to global fatigue, fatigability provides better insight into the degree to which an individual is limited either physically or mentally by fatigue because it accounts for self-pacing bias (Eldadah, 2010; Glynn et al., 2015; Simonsick et al., 2016).
Past research on fatigue and the brain suggests a neural component to fatigability, which may contribute to our knowledge in the underlying pathophysiology of age-related fatigability. Strong evidence exists that the neurotransmitter dopamine may have an important role in fatigue (Dobryakova, Genova, DeLuca, & Wylie, 2015; Lin et al., 2016; Karshikoff, Sundelin & Lasselin, 2017). Neuroimaging studies have identified neural correlates of fatigue in disease states such as Parkinson’s disease, traumatic brain injury, stroke and multiple sclerosis (Delcua, Genova, Capili & Wylie, 2009; Harrington, 2012; Kluger, Krupp, & Enoka, 2013; Rocca et al., 2014); however, neural correlates of fatigability have not been identified in older adults free of neurologic disease (Nakagawa et al., 2016). Based on this previous fatigue work we will examine regions of interest including the limbic cortex (amygdala, hippocampus, orbitofrontal cortex, medial superior frontal gyrus), basal ganglia (caudate, putamen, thalamus), and cingulate cortex (Harrington, 2012; Kluger et al., 2013; Nakagawa et al., 2016; Rocca et al., 2014).
Therefore, this cross-sectional pilot study aims to identify brain regions that may be responsible for, or related to, perceived physical fatigability levels in older adults. We hypothesize that there will be an inverse relationship between lower cortical grey matter volumes and higher perceived physical fatigability scores. An exploratory aim is to examine the relationship between brain regions and mental fatigability, as validation of this construct is ongoing.
2.0. Materials and methods
2.1. Study population
The Lifestyle Interventions and Independence for Elders (LIFE) Study was a phase three, single-masked randomized controlled clinical trial evaluating the effects of long-term moderate-intensity physical activity compared to a health education intervention on physical function in sedentary older adults (N=1635) age 70–89 with compromised physical function at baseline (Pahor et al., 2014). After randomization and prior to starting the assigned intervention program, participants from the University of Pittsburgh field center were given the opportunity to enroll, if interested and eligible, in brain magnetic resonance imaging (LIFE-MRI) and/or muscle mitochondria and fatigability (LIFE-Mito) ancillary studies (Rosano et al., 2016; Santanasto et al., 2016). Published elsewhere are the details on screening and eligibility for both ancillary studies (Rosano et al., 2016; Santanasto et al., 2016). In brief, all participants had to meet MRI inclusion criteria (e.g., no metal implants or claustrophobia). Since these ancillary studies recruited late in LIFE enrollment, lower functioning participants and African Americans were oversampled to meet overall study target criteria. Thirty-two participants enrolled in both ancillary studies; however, we excluded three participants from our analyses due to flagged MRI findings or missing PFS data. Thus, the final analytic sample was 29 participants that had complete data for both the 7T MRI scan and PFS.
2.2. Assessment of perceived physical and mental fatigability
At baseline, before intervention activities began, and within approximately 2–3 weeks of the brain MRI, participants from LIFE-Mito completed the Pittsburgh Fatigability Scale (PFS), a 10-item self-administered questionnaire that assessed perceived physical and mental fatigability (Glynn et al., 2015). Participants were asked to rate on a scale from 0 (no fatigue) to 5 (extreme fatigue) the level of physical and mental fatigue they expected or imagined they would feel after completing ten different activities ranging in type and intensity. Responses were summed to create separate total physical and mental fatigability scores ranging from 0 (no physical/mental fatigue) to 50 (extreme physical/mental fatigue).
A cutpoint for higher physical fatigability, (PFS score ≥15) versus lower fatigability (PFS score <15), was established during the initial validation of the scale. Midpoint values (mean adjusted higher PFS fatigability score – half of the adjusted mean difference) were calculated by comparing higher versus lower fatigability for several non-PFS fatigability and performance measures used in the validation study and then averaged to obtain the cutpoint (Glynn et al., 2015; Cooper et al. 2018; Simonsick et al. 2018). We used a similar approach to assign a higher mental fatigability cutpoint of ≥13 versus lower mental fatigability <13 (Simonsick et al. 2018).
2.3. Assessment of brain regions
Magnetic Resonance images were acquired at the MR Research center at the University of Pittsburgh on a 7-Tesla human scanner (Magnetom, Siemens Medical Solutions, Erlangen Germany) using an eight-channel head coil (Rapid Biomedical GmbH, Rimpar, Germany). High-resolution T1-weighted 3D MPRAGE sequences were used for volumetric analyses and were acquired in the axial orientation (TR/TE = 3,430/3.54, voxel size: 0.7 × 0.7 × 0.7 mm, 256 slices) (16). As previously described, a semi-automated skull stripping of each MPRAGE image was conducted and a linear, hierarchical, demon-based registration was used to segment images (Wu, Carmichael, Lopez-Garcia, Carter, & Aizenstein, 2006).
We assessed neuroimaging variables for normality and transformed non-normal variables as appropriate. Regions of interest (ROIs) were identified using the Automated Anatomical Labeling (AAL) atlas, selected based on studies of fatigue and included the limbic cortex (amygdala, hippocampus, orbitofrontal cortex, medial superior frontal gyrus), basal ganglia (caudate, putamen, and thalamus), cingulate cortex, and the middle frontal gyrus (Harrington, 2012; Kluger et al., 2013; Nakagawa et al., 2016; Rocca et al., 2014; Tzourio-Mazoyer et al., 2002). We used a specialized atlas to identify ROIs in order to account for the contrast specific to our 7T acquisition. Gray matter volume (GMV) was obtained using Brain Extraction Tool as the volume contained within the “inner skull”(Smith, 2002). The ratio of GMV to intracranial volume (ICV) was used in all analyses.
2.4. Assessment of covariates and performance measures
Age, sex, race, education level, body mass index (BMI), medical conditions including cardiovascular disease and diabetes/high blood sugar (both self-report of physician diagnosis), and depression measured by the Center for Epidemiologic Study Depression Scale (depression: ≥16) were collected as part of the main LIFE baseline visit (Fielding et al., 2011; Pahor et al., 2014). Further, physical and cognitive performance measures were obtained from the main LIFE study baseline examination and were used for descriptive characteristic purposes only. Measures included: usual gait speed (m/s), usual paced 400-m walk time (seconds), Short Physical Performance Battery score (SPPB, 0–12), Modified Mini-Mental State Examination (3MS) score (range 0–100) and the Digit Symbol Substitution Test (DSST) score (Guralnik et al., 2000; Guralnik et al., 1994; Radloff, 1997; Teng & Chui, 1987).
2.5. Statistical analyses
Descriptive statistics for the analytic sample by fatigability status were calculated. We examined average brain regions by fatigability status, and T-tests and Wilcoxon Rank Sum Tests were performed. As this was an exploratory, hypothesis generating initial study to identify potential associations, the significance level was set at p<0.1. T-tests and Wilcoxon Rank Sum tests assessed continuous covariates of fatigability (higher versus lower for both physical and mental). To assess categorical covariate associations with higher versus lower fatigability, chi-squared tests were performed, including Fisher Exact tests when expected cell counts were less than 5.
Separate bivariate and multivariable logistic regression models examined the odds of having higher, compared with lower, fatigability associated with the GMV/ICV of each brain ROI. Physical and mental fatigability were assessed separately and covariates calculated were entered into models one at a time to determine whether each covariate explained any observed associations between brain region volumes and fatigability. Covariates were included in final multivariable models if they were significant at the p≤0.1 level. Due to the small sample size and exploratory nature of these analyses, we did not correct for multiple comparisons. The ratios obtained for GMV to ICV were adjusted by a factor of 100 before they were added into the model for meaningful interpretation of parameters estimates.
3.0. Results
The mean age (±standard deviation [SD]) of the study population was 77.2 ± 5.5 years, range 70 to 88 years, predominately female (86.2%, n=25), 37.9% white (n=11) and were lower functioning with a mean SPPB score of 7.6±1.4 (Table 1). Physical and mental fatigability scores were 20.2±9.1 and 15.8±9.7, respectively, and 65.5% of participants were categorized as having higher fatigability for both the physical and mental subscales. There was overlap between participants in higher physical and mental fatigability categories; of the participants who had higher physical fatigability, 89.9% (n=17) reported higher mental fatigability. Of the participants who had higher mental fatigability, 89.5% (n=17) had higher physical fatigability. There were no differences by physical fatigability status in any demographic, lifestyle, medical history or cognitive function measures, except for SPPB, gait speed and 400m walk time (Table 1). Higher mental fatigability was associated with older age, smoking status, SPPB score, gait speed and 400m walk time.
Table 1.
Baseline Characteristics of the Analytic Sample from the LIFE Study by Perceived Fatigability Status (N=29). Mean (standard deviations) or numbers (%) are reported.
| Perceived Fatigability Status | |||||
|---|---|---|---|---|---|
| Physical | Mental | ||||
| Higher | Lower | Higher | Lower | ||
| Characteristic | Total | (≥15) | (<15) | (≥13) | (<13) |
| N=29 | (n=19) | (n=10) | (n=19) | (n=10) | |
| Age, years | 77.2±5.5 | 77.5±5.9 | 76.6±4.9 | 78.5±5.6 | 74.9±4.6* |
| Race, Caucasian/White | 11(37.9) | 9(47.4) | 2(20.0) | 8(42.1) | 3(30.0) |
| Sex, Female | 25(86.2) | 17(89.5) | 8(80.0) | 17(89.5) | 8(80.0) |
| Education≥ High School, years | 9(31.0) | 6(31.6) | 3(30.0) | 5(26.32) | 4(40.0) |
| Smoking Status, Former or Current | 10(34.5) | 5(26.3) | 5(50.0) | 4(21.1) | 6(60.0)* |
| Body Mass Index, m/kg2 | 31.4±4.9 | 31.6±5.2 | 30.8±4.4 | 31.3±5.0 | 31.5±5.0 |
| Cardiovascular Disease, Yes, self-report | 6(20.7) | 5(26.3) | 1(10.0) | 4(21.1) | 2(20.0) |
| Diabetes/high blood sugar, Yes, self-report | 10(34.5) | 7(36.8) | 3(30.0) | 7(36.8) | 3(30.0) |
| Depression Score, ≥16 | 4(13.8) | 4(21.1) | 0(0.0) | 4(21.1) | 0(0.0) |
| Usual gait speed, m/s | 0.81±0.20 | 0.73±0.15 | 0.96±0.20* | 0.76±0.20 | 0.90±0.16* |
| Usual-paced 400-m walk time, seconds | 491.8±119.6 | 523.2±131.0 | 432.2±64.1* | 519.5±127.2 | 439.3±86.1* |
| Short Physical Performance Battery Score, 0–12 points | 7.6±1.4 | 7.2±1.5 | 8.5±0.7* | 7.3±1.5 | 8.2±1.3* |
| Mini-Mental State Examination score, Points | 90.9±5.7 | 91.1±6.0 | 90.3±5.2 | 90.5±5.7 | 91.7±5.8 |
| Digit Symbol Substitution Score, points | 44.8±13.0 | 47.0±13.0 | 40.8±12.8 | 46.4±12.5 | 41.9±14.2 |
P<0.1 for the test of mean differences between higher and lower fatigability within physical or mental fatigability status
In bivariate analyses, mean (SD) grey matter volume (GMV) of the right hippocampus was lower for those with higher compared to lower physical fatigability, p=0.07 (Table 2). Similar associations were found for physical fatigability in the right putamen (p=0.05) and left and right thalamus, p=0.04 and p=0.08, respectively. For mental fatigability, the associations were similar for the right hippocampus and thalamus with addition of the right posterior cingulum (p=0.05) and left (p=0.02) and right amygdala (p=0.05) (Table 2). Figure 1 highlights significant GMV ROIs with p<0.1.
Table 2.
Mean (Standard Deviation) Grey Matter Volume as a Percentage of Intracranial Volume (nm3) by Perceived Fatigability Status (The LIFE Study, N=29)
| Perceived Fatigability Status | ||||
|---|---|---|---|---|
| Physical | Mental | |||
| Region of Interesta | Higher | Lower | Higher | Lower |
| (≥15) | (<15) | (≥13) | (<13) | |
| (n=19) | (n=10) | (n=19) | (n=10) | |
| Whole brain | 27.21(1.44) | 27.54(1.05) | 27.20(1.49) | 27.56(0.91) |
| Right Subcortical Structures | ||||
| Amygdala | 0.08(0.02) | 0.09(0.01) | 0.08(0.01) | 0.09(0.01)* |
| Hippocampusb | 0.26(0.04) | 0.27(0.02)* | 0.26(0.04) | 0.28(0.02)** |
| Frontal Middle Orbital gyrusb | 0.14(0.02) | 0.13(0.02) | 0.14(0.02) | 0.13(0.02) |
| Frontal Superior Medial gyrus | 0.33(0.05) | 0.33(0.04) | 0.32(0.06) | 0.33(0.04) |
| Anterior Caudate | 0.29(0.04) | 0.29(0.06) | 0.29(0.04) | 0.28(0.04) |
| Putamenb | 0.27(0.05) | 0.29(0.03)** | 0.27(0.04) | 0.30(0.06) |
| Thalamus | 0.28(0.03) | 0.31(0.02)* | 0.28(0.03) | 0.31(0.02)*** |
| Anterior Cingulate cortex | 0.23(0.04) | 0.22(0.03) | 0.22(0.03) | 0.23(0.04) |
| Middle Cingulate cortex | 0.31(0.06) | 0.30(0.04) | 0.31(0.07) | 0.30(0.04) |
| Posterior Cingulate cortexb | 0.05(0.01) | 0.05(0.01) | 0.04(0.01) | 0.05(0.01)** |
| Frontal Middle gyrus | 0.67(0.07) | 0.67(0.05) | 0.68(0.07) | 0.64(0.02) |
| Left Subcortical Structures | ||||
| Amygdala | 0.07(0.01) | 0.08(0.01) | 0.07(0.01) | 0.09(0.01)** |
| Hippocampusb | 0.28(0.04) | 0.28(0.02) | 0.27(0.04) | 0.28(0.02) |
| Frontal Middle Orbital gyrusb | 0.12(0.03) | 0.12(0.02) | 0.12(0.03) | 0.11(0.02) |
| Frontal Superior Medial gyrus | 0.41(0.06) | 0.42(0.04) | 0.41(0.06) | 0.41(0.04) |
| Anterior Caudate | 0.26(0.03) | 0.26(0.03) | 0.26(0.03) | 0.26(0.04) |
| Putamenb | 0.25(0.04) | 0.27(0.04) | 0.25(0.03) | 0.28(0.06) |
| Thalamus | 0.29(0.03) | 0.31(0.02)** | 0.29(0.03) | 0.31(0.02)** |
| Anterior Cingulate cortex | 0.24(0.05) | 0.23(0.04) | 0.24(0.03) | 0.24(0.04) |
| Middle Cingulate cortex | 0.28(0.06) | 0.25(0.04) | 0.27(0.06) | 0.26(0.04) |
| Posterior Cingulate cortexb | 0.07(0.02) | 0.06(0.01) | 0.07(0.02) | 0.07(0.01) |
| Frontal Middle gyrus | 0.69(0.06) | 0.69(0.05) | 0.69(0.06) | 0.68(0.05) |
Gray Matter Volume divided by Intracranial Volume multiplied by a factor of 100
Non-parametric test
P<0.1 for test of mean differences between higher and lower fatigability within physical or mental fatigability status
P<0.05 for test of mean differences between higher and lower fatigability within physical or mental fatigability status
P<0.01 for test of mean differences between higher and lower fatigability within physical or mental fatigability status
Figure 1.
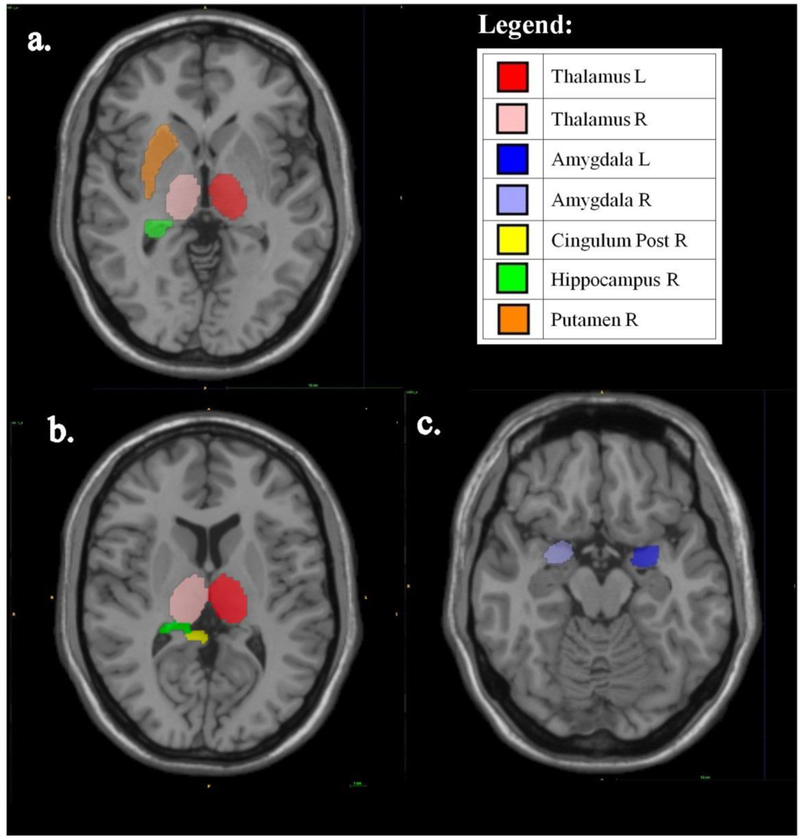
Axial Images of Grey Matter Volumes for Significant Regions of Interest by a) Physical Fatigability, b) and c) Mental Fatigability
Table 3 includes the logistic regression models for each brain region that was significantly associated with physical and mental fatigability identified by bivariate t-tests from Table 2. For the left thalamus, every one unit increase in normalized GMV/ICV was associated with 28.6% lower odds of having higher physical fatigability after adjusting for smoking (OR: 0.714, 95%CI: 0.517, 0.985, p=0.04). We found similar results for the left thalamus and mental fatigability after adjusting for smoking (OR: 0.646, 95% CI: 0.444, 0.939, p=0.02) (Table 3). For every one unit increase in GMV/ICV of the right thalamus, the odds of having higher mental fatigability was reduced by 39% (OR: 0.610, 95% CI: 0.407, 0.912, p=0.02). This relationship remained significant after adjusting for age and smoking (Table 3). No other regions of interest remained significant after adjustment.
Table 3.
Logistic Regression Models for the Odds of Higher Perceived Fatigability being associated with GMV/ICV Anatomical Brain Regions (The LIFE Study, N=29)
| Perceived Physical Fatigability | |||
|---|---|---|---|
| Model 1. | Model 2. | Model 3. | |
| Unadjusted | Adjusted for age | Adjusted for smoking status | |
| Region of Interesta | OR (95% CI) | OR(95% CI) | OR(95% CI) |
| Amygdala (L) | 0.60(0.26, 1.37) | 0.60(0.25, 1.44) | 0.62(0.27, 1.45) |
| Amygdala(R) | 0.72(0.39, 1.33) | 0.73(0.38, 1.38) | 0.82(0.41, 1.65) |
| Posterior Cingulate (R) | 1.02(0.48, 2.17) | 1.04(0.48, 2.24) | 0.92(0.41, 2.04) |
| Hippocampus(R) | 0.90(0.71, 1.13) | 0.90(0.71, 1.14) | 0.87(0.68, 1.12) |
| Putamen(R) | 0.91(0.76, 1.09) | 0.91(0.76, 1.09) | 0.94(0.77, 1.14) |
| Tdalamus(L) | 0.75(0.55, 1.01) | 0.75(0.55, 1.01) | 0.71(0.52, 0.99) |
| Tdalamus(R) | 0.75(0.55, 1.02) | 0.73(0.52, 1.03) | 0.72(0.52, 1.02) |
| Perceived Mental Fatigability | |||
| Model 1. | Model 2. | Model 3. | |
| Unadjusted | Adjusted for age | Adjusted for smoking status | |
| Region of Interesta | OR (95% CI) | OR(95% CI) | OR(95% CI) |
| Amygdala (L) | 0.41(0.16, 1.07) | 0.49(0.18, 1.30) | 0.39(0.13, 1.15) |
| Amygdala(R) | 0.50(0.24, 1.04) | 0.56(0.26, 1.17) | 0.61(0.27 1.36) |
| Posterior Cingulate (R) | 0.66(0.31, 1.42) | 0.71(0.31, 1.59) | 0.49(0.20, 1.19) |
| Hippocampus(R) | 0.87(0.68, 1.10) | 0.88(0.68, 1.12) | 0.81(0.62, 1.05) |
| Putamen(R) | 0.87(0.71, 1.06) | 0.85(0.66, 1.08) | 0.91(0.73, 1.14) |
| Tdalamus(L) | 0.72(0.53, 0.99) | 0.72(0.51, 1.01) | 0.65(0.44, 0.94) |
| Tdalamus(R) | 0.61(0.41, 0.91) | 0.64(0.42, 0.96) | 0.46(0.24, 0.87) |
Note. (OR) = Odds Ratio; (CI) = Confidence Interval; (L)=Left subcortical; (R)=Right subcortical; Bolded values denote p<0.05
Gray Matter Volume divided by Intracranial Volume multiplied by a factor of 100
4.0. Discussion
Lower GMV/ICV of the hippocampus, putamen, and thalamus were associated with greater odds of having higher physical fatigability in a sample of lower functioning older adults. The direction of these findings is consistent with our hypothesized association between brain volumes and fatigability status. The strongest relationship for physical fatigability was with the putamen, a component of the basal ganglia that has previously been identified as a neural correlate of fatigue in healthy younger adults (Nakagawa et al., 2016).
To our knowledge, this is the first study of neural correlates of fatigability in a sample of older adults without neurologic disorders, but our results can be informed by studies in younger adults and those with neurological diseases to elucidate plausible mechanisms from these preliminary findings. The putamen, a primary component of the basal ganglia, has previously been implicated in motor control and learning habits and skills and has been identified as a neural correlate of fatigue in healthy younger adults (Durieux, Schiffmann, & de Kerchove d’Exaerde, 2011; Nakagawa et al., 2016; O’Doherty & John, 2009). Motivation and reward theories related to fatigue are also associated with dopamine, a key neurotransmitter in the basal ganglia (John.Salamone & Mercè, 2012). Relationships between dopamine functioning and basal ganglia were not explored in the present study. However, we speculate that the relationship between physical fatigability and the putamen may be related to dopamine functioning that plays a role in motivation and reward. Direct measures of dopamine functioning may be useful in future work to better understand if the association observed between the putamen and physical fatigability was attributed to changes or interruptions in dopaminergic function.
The hippocampus, which is deeply involved in the stress response, has previously been identified as a region of the brain that interacts with dopaminergic systems related to motivational behavior (Calabresi, 2013). A recent study of younger adults identified the basal ganglia as a critical region involved in fatigue, but not the hippocampus (Nakagawa et al., 2016). To our knowledge, we are the first group to identify the hippocampus as a potential brain region involved in the perception of physical fatigability. Plausible mechanisms for the hippocampus’ role in fatigability may be related to neuronal loss in dopaminergic pathways seen in the hippocampus, which has previously been explored in the context of fatigue (Calabresi, 2013). Fatigue may result from the disruption of communication of the associative network between the striatum and prefrontal cortex (Dobryakova et al., 2015). Although our work could not explore these mechanisms, previous evidence of hippocampal involvement in the dopaminergic pathway that has been related to fatigue outcomes supports our finding that the hippocampal grey matter volume may be related to fatigability in older adults (Nakagawa et al., 2016).
The thalamus, an important relay center in the brain, may be related to fatigability as all pathways that project to the cerebral cortex do so after synapsing in the thalamus (Blumenfeld, 2012). A change in thalamic activity has also been proposed as a reason for perceived higher fatigability (Chaudhuri & Behan, 2000). An increase in thalamic inhibition or a shift in reciprocal state of activation between the thalamus and the subthalamic nucleus may result in a modified cortical response to the basal ganglia (Chaudhuri & Behan, 2000). In the event the dopaminergic drive to the pallidothalamic cortical loop is reduced, frontal activation will be suppressed. As such, motivational influences or emotion may contribute to goal-oriented cognition and behavior and perceived fatigability. It is important to note that alterations in the thalamo-cortical loop have been shown to be associated with Parkinson’s Disease and depression, which commonly include symptoms of fatigue (McCormick, 1999).
We also identified associations for lower GMV/ICV of the amygdala, posterior cingulate and thalamus with greater odds of having higher mental fatigability. These results suggest that there is overlap in the neurobiology of physical and mental fatigability, but with some key differences. Notably, the thalamus is an area associated with both physical and mental fatigability and, as stated above, plays a key role as a central relay center in the brain involved in regulation of many different functions.
Lower GMV of the amygdala was associated with higher mental fatigability but not with physical fatigability. Lower activity in the amygdala has previously been associated with fatigability in patients with multiple sclerosis (Spiteri et al., 2017). The amygdala may play a partial role in the relationship between mental fatigability and motivation, as mental fatigue and motivation are closely related concepts with shared neuro-biological mechanisms (Karshikoff, Sundelin & Lasselin 2017). The feeling of fatigue has long been thought to be a conscious manifestation of the body maintaining homeostatic control (Gibson et al., 2003; Stephan et al., 2016). The conscious sensation of fatigue or perception of fatigability may also be related to other emotions such as anger, fear and memory of a prior activity (Gibson et al., 2003).
In the present study, we defined mental fatigability as a measure of the body’s susceptibility to fatigue related to physical and mental activities that engage cognitive function. In our results, the posterior cingulate cortex was associated with mental fatigability outcomes but not physical fatigability. A study in MS patients also identified associations of the posterior cingulate with fatigue outcomes (Pardini et al., 2015). Based on our definition, it is plausible that mechanisms related to mental fatigability through the posterior cingulate cortex may be related to inefficient cognitive function, which is implicated in perceptions of mental fatigability related to demanding tasks that specifically involve cognitive control (Leech & Sharp, 2014). The posterior cingulate cortex has notably been identified as a highly connected region of the brain that has a high baseline metabolic rate (Hagmann et al., 2008; Raichle et al., 2001). The high metabolic state of the posterior cingulate is responsive to an individual’s cognitive state, where a demanding task such as a perceptual decision or a motor response is required. Additionally, the activity of the posterior cingulate cortex in a healthy brain is related to cognitive load where failure to appropriately deactivate the brain region is associated with inefficient cognitive function in both the healthy and damaged brain (Bonnelle et al., 2011; Crone et al., 2011; Singh & Fawcett, 2008; Sonuga-Barke & Castellanos, 2007; Weissman, Roberts, Visscher, & Woldorff, 2006).
Due to the novel nature of this work, we are limited in our ability to compare findings to the work of others. An oversampling of African Americans in the second half of the main LIFE study recruitment period when these ancillary studies were conducted resulted in >60% of African Americans in our analytic sample. Consequently, the racial imbalance coupled with lower physical function of our participants may result in limited generalizability. The cross-sectional design prevented investigation of causality between brain regional volume and fatigability. In addition, we interpret these results with caution due to the small sample size, multiple comparisons, and our limited ability to assess confounders. However, this work serves as an important first step for development of future studies that aim to explore the pathophysiology of physical and mental fatigability in older adults without neurologic disease.
We have identified a number of strengths in this pilot work. This is the first study to examine brain volumes in relation to both perceived physical and mental fatigability. Measuring fatigability instead of fatigue accounts for the inherent issue of self-pacing bias and thus provides greater capacity to assess fatigue’s role in the disablement pathway. Also, 7T MRI is a novel neuroimaging modality that allows accurate volumetric measurement of regions of interest at high resolution.
5.0. Conclusions
Lower brain volumes of the hippocampus, putamen, and thalamus were associated with higher physical fatigability in a small sample of older adults. Further, we assessed neural correlates of mental fatigability that contribute to evidence for mental and physical fatigability as separate constructs. A smaller thalamus was associated with higher mental fatigability, but the posterior cingulate and amygdala were related with mental fatigability but not physical fatigability. Plausible mechanisms for higher fatigability include alterations in dopaminergic function, regulation of sleep and sensory interpretation, emotional formation and processing, learning and memory, and/or motivational behavior, which are supported by our findings. Future studies at the intersection of epidemiology, neurobiology and population neuroscience research will help us better understand the pathophysiology and brain regions related to perceived physical and mental fatigability in healthy older adults and may illuminate vulnerable structural neuronal networks related to the disablement pathway.
Highlights.
Provides initial evidence that fatigability in aging has a neurobiological component
Specific regions in the basal ganglia and limbic system may be related to fatigability
Regions were similar for physical and mental, but more regions associated with mental fatigability
Acknowledgements
The LIFE Main Study
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement (grant number UO1 AG22376) and a supplement from the National Heart, Lung and Blood Institute (grant number 3U01AG022376–05A2S), and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
Administrative Coordinating Center, University of Florida, Gainesville, FL
Marco Pahor, MD – Principal Investigator of the LIFE Study
Jack M. Guralnik, MD, PhD – Co- Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD)
Stephen D. Anton, PhD
Thomas W. Buford, PhD
Christiaan Leeuwenburgh, PhD
Susan G. Nayfield, MD, MSc
Todd M. Manini, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ching-ju Lu, MPH
This research is partially supported by the University of Florida Claude D. Pepper Older Americans Independence Center (grant number 1 P30 AG028740).
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston Salem, NC
Michael E. Miller, Ph.D. – DMAQC Principal Investigator
Mark A. Espeland, Ph.D. – DMAQC Co- Investigator
Walter T. Ambrosius, PhD
William Applegate, MD
Daniel P. Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
Delilah Cook, CCRP
Curt D. Furberg, MD, PhD
Lea N. Harvin, BS
Leora Henkin, MPH, Med
John Hepler, MA
Fang-Chi Hsu, PhD
Kathy Joyce
Laura Lovato, MS
Juan Pierce, AB
Wesley Roberson, BSBA
Julia Robertson, BS
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
W. Jack Rejeski, PhD
Jeffrey A. Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon L. Mihalko, PhD
Janine M. Jennings, PhD
National Institutes of Health, Bethesda, MD
Evan C. Hadley, MD (National Institute on Aging)
Sergei Romashkan, MD, PhD (National Institute on Aging)
Kushang V. Patel, PhD (National Institute on Aging)
National Heart, Lung and Blood Institute, Bethesda, MD
Denise Bonds, MD, MPH
Field Centers
Northwestern University, Chicago, IL
Mary M. McDermott, MD – Field Center Principal Investigator
Bonnie Spring, PhD – Field Center Co-Investigator
Joshua Hauser, MD – Field Center Co-Investigator
Diana Kerwin, MD – Field Center Co-Investigator
Kathryn Domanchuk, BS
Rex Graff, MS
Alvito Rego, MA
Pennington Biomedical Research Center, Baton Rouge, LA
Timothy S. Church, MD, PhD, MPH – Field Center Principal Investigator
Steven N. Blair, PED (University of South Carolina)
Valerie H. Myers, PhD
Ron Monce, PA-C
Nathan E. Britt, NP
Melissa Nauta Harris, BS
Ami Parks McGucken, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi K. Millet, MPA, BS
Catrine Tudor-Locke, PhD, FACSM
Ben P. Butitta, BS
Sheletta G. Donatto, MS, RD, LDN, CDE
Shannon H. Cocreham, BS
Stanford University, Palo Alto, CA
Abby C. King, Ph.D. – Field Center Principal Investigator
Cynthia M. Castro, PhD William L. Haskell, PhD
Randall S. Stafford, MD, PhD Leslie A. Pruitt, PhD
Veronica Yank, MD
Kathy Berra, MSN, NP-C, FAAN
Carol Bell, NP Rosita M. Thiessen Kate P. Youngman, MA Selene B. Virgen, BAS
Eric Maldonado, BA Kristina N. Tarin, MS, CSCS Heather Klaftenegger, BS Carolyn A. Prosak, RD Ines Campero, BA
Dulce M. Garcia, BS José Soto, BA Linda Chio, BA David Hoskins, MS
Tufts University, Boston, MA
Roger A. Fielding, PhD – Field Center Principal Investigator
Miriam E. Nelson, PhD
Sara C. Folta, PhD
Edward M. Phillips, MD
Christine K. Liu, MD
Erica C. McDavitt, MS
Kieran F. Reid, PhD, MPH
Dylan R. Kirn, BS
Evan P. Pasha, BS
Won S. Kim, BS
Julie M. Krol, MS
Vince E. Beard, BS
Eleni X. Tsiroyannis, BS
Cynthia Hau, BS, MPH
Dr. Fielding’s contribution is partially supported by the U.S. Department of Agriculture, under agreement No. 58–1950-0–014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
This research is also supported by the Boston Claude D. Pepper Older Americans Independence Center (grant number 1P30AG031679) and the Boston Rehabilitation Outcomes Center (grant number 1R24HD065688–01A1).
University of Florida, Gainesville, FL
Todd M. Manini, PhD – Field Center Principal Investigator Marco Pahor, MD – Field Center Co- Investigator
Stephen D. Anton, PhD
Thomas W. Buford, PhD
Michael Marsiske, PhD
Susan G. Nayfield, MD, MSc
Bhanuprasad D. Sandesara, MD
Mieniecia L. Black, MS
William L. Burk, MS
Brian M. Hoover, BS
Jeffrey D. Knaggs, BS
William C. Marena, MT, CCRC
Irina Korytov, MD
Stephanie D. Curtis, BS
Megan S. Lorow, BS
Chaitalee S. Goswami
Melissa A. Lewis
Michelle Kamen BS
Jill N. Bitz
Brian K. Stanton, BS
Tamika T. Hicks, BS
Charles W. Gay, DC
Chonglun Xie, MD (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Holly L. Morris, MSN, RN, CCRC (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Floris F. Singletary, MS, CCC-SLP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jackie Causer, BSH, RN (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Susan Yonce, ARNP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Katie A. Radcliff, M.A. (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Mallorey Picone Smith, B.S. (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jennifer S. Scott, B.S. (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Melissa M. Rodriguez B.S. (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Margo S. Fitch, P.T. (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Mendy C. Dunn, BSN (Assessment) (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jessica Q. Schllesinger, B.S. Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
This research is partially supported by the University of Florida Claude D. Pepper Older Americans Independence Center (1 P30 AG028740).
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH – Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH – Field Center Co-Investigator
Bret H. Goodpaster, PhD
Oscar Lopez, MD
Nancy W. Glynn, PhD
Neelesh K. Nadkarni, MD, PhD
Diane G. Ives, MPH
Mark A. Newman, PhD
George Grove, MS
Kathy Williams, RN, BSEd, MHSA
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA (deceased)
Pamela Vincent, CMA
Allison Gerger, BS
Jamie R. Romeo, BS
Lauren C. Monheim, BS
The Pittsburgh Field Center is partially supported by the Pittsburgh Claude D. Pepper Older Americans Independence Center (grant number P30 AG024827).
Wake Forest University, Winston Salem, NC
Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator
Anthony P. Marsh, PhD – Field Center Co-Principal Investigator
Tina E. Brinkley, PhD
Jamehl S. Demons, MD
Kaycee M. Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
The Wake Forest University Field Center is, in part, supported by the Claude D. Pepper Older Americans Independence Center (grant number 1 P30 AG21332).
Yale University, New Haven, CT
Thomas M. Gill, M.D. – Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM – Field Center Co-Principal Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, M.P.H. (VA Connecticut Healthcare System)
Nathalie de Rekeneire, MD, MS
Joanne M. McGloin, MDiv, MS, MBA
Raeleen Mautner, PhD
Sharon M. Huie-White, MPH
Luann Bianco, BA
Janice Zocher
Karen C. Wu, RN
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Rina Castro, LPN
Sean Halpin, MA
Matthew Brennan, MA
Theresa Barnett, MS, APRN
Lynne P. Iannone, MS, CCRP
Maria A. Zenoni, MS
Julie A. Bugaj, MS
Christine Bailey, MA
Peter Charpentier, MPH
Geraldine Hawthorne-Jones
Bridget Mignosa
Lynn Lewis
Dr. Gill is the recipient of an Academic Leadership Award (grant number K07AG3587) from the National Institute on Aging.
The Yale Field Center is partially supported by the Claude D. Pepper Older Americans Independence Center (grant number P30AG021342).
Cognition Coordinating Center, Wake Forest University, Winston Salem, NC
Jeff Williamson, MD, MHS – Center Principal Investigator
Kaycee M Sink, MD, MAS – Center Co-Principal Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Mark Espeland, PhD
Janine Jennings, PhD
Valerie K. Wilson, MD
Electrocardiogram Reading Center, University of Florida, Gainesville, FL
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
Anita Szady, MD
Spirometry Reading Center, Yale University, New Haven, CT
Geoffrey L. Chupp, MD
Gail M. Flynn, RCP, CRFT
Thomas M. Gill, MD
John L. Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
Dr. Fragoso is the recipient of a Career Development Award from the Department of Veterans Affairs.
Cost Effectiveness Analysis Center
Erik J. Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System)
Robert M. Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health)
Funding
This work was supported by “Ultra-high-field neuroimaging in elderly after a two-year exercise intervention” and Claude D. Pepper Older Americans Independence Centers at the University of Florida (grant numbers 1R01AG044474–02, P30 AG024827); University of Pittsburgh, Center for Aging and Population Health Pilot Grant Program, and the Department of Epidemiology Student Small Grant Program (LIFE-MITO). Adam Santanasto was supported by a Ruth L. Kirschstein, National Research Service Award from the National Institute on Aging (grant number T32-AG-000181). Development of the Pittsburgh Fatigability Scale was supported by a Pittsburgh Claude D. Pepper Older Americans Independence Center, Developmental Pilot Grant from the National Institutes of Health (grant number P30 AG024826 to NWG). The Intramural Research Program, National Institute on Aging (NWG) also supported this work; and analyses were supported in part by the National Institute on Aging (grant number K01AG053431 to ALR).
Footnotes
Conflict of Interest/ Disclosure Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blumenfeld H (2012). Neuroanatomy Through Clinical Cases (pp. 1006).
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, …Sharp DJ (2011). Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(38), 13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Castrioto A, Di Filippo M, & Picconi B (2013). New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson’s disease - The Lancet Neurology. Lancet Neurol, 12(9), 811–821. doi:doi: 10.1016/S1474-4422(13)70118-2 [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, & Behan PO (2000). Fatigue and basal ganglia. Journal of the Neurological Sciences, 179(1), 34–42. doi: 10.1016/S0022-510X(00)00411-1 [DOI] [PubMed] [Google Scholar]
- Crone JS, Ladurner G, Höller Y, Golaszewski S, Trinka E, & Kronbichler M (2011). Deactivation of the default mode network as a marker of impaired consciousness: an fMRI study. PloS One, 6(10), e26373. doi: 10.1371/journal.pone.0026373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova E, Genova HM, DeLuca J, & Wylie GR (2015). The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol, 6, 52. doi: 10.3389/fneur.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca J, Genova HM, Capili EJ, Wylie GR (2009). Functional Neuroimaging of Fatigue. Physical Medicine and Rehabilitation clinics of North America, 20(2), 325–37. Doi: 10.1016/j.pmr.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Durieux PF, Schiffmann SN, & de Kerchove d’Exaerde A (2011). Targeting neuronal populations of the striatum. Front Neuroanat, 5, 40. doi: 10.3389/fnana.2011.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekmann A, Petersen I, Manty M, Christensen K, & Avlund K (2012). Fatigue, general health, and ischemic heart disease in older adults. J Gerontol A Biol Sci Med Sci, 68(3), 279–285. [DOI] [PubMed] [Google Scholar]
- Eldadah BA (2010). Fatigue and fatigability in older adults. Pm r, 2(5), 406–413. doi: 10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- Fielding RA, Rejeski WJ, Blair S, Church T, Espeland MA, Gill TM, … Pahor M (2011). The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci, 66(11), 1226–1237. doi: 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson ASC, Baden DA, Lambert MI, Lambert EV, Harley YXR, Hampson D, … Noakes TD (2003). The Conscious Perception of the Sensation of Fatigue. Sports Medicine, 33(3), 167–176. doi: 10.2165/00007256-200333030-00001 [DOI] [PubMed] [Google Scholar]
- Glynn NW, Santanasto AJ, Simonsick EM, Boudreau RM, Beach SR, Schulz R, & Newman AB (2015). The Pittsburgh Fatigability scale for older adults: development and validation. J Am Geriatr Soc, 63(1), 130–135. doi: 10.1111/jgs.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, … Wallace RB (2000). Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci, 55(4), M221–231. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, … Wallace RB (1994). A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol, 49(2), M85–94. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, & Sporns O (2008). Mapping the structural core of human cerebral cortex. PLoS biology, 6(7), e159. doi: 10.1371/journal.pbio.0060159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME (2012). Neurobiological studies of fatigue. Prog Neurobiol, 99(2), 93–105. doi: 10.1016/j.pneurobio.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Salamone, & Mercè C (2012). The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron, 76(3), 470–485. doi: 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karshikoff B, Sundelin T, & Lasselin J (2017). Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Frontiers in Immunology, 8, 21 10.3389/fimmu.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger BM, Krupp LB, & Enoka RM (2013). Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology, 80(4), 409–416. doi: 10.1212/WNL.0b013e31827f07be [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, & Sharp DJ (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(1), 12–32. doi: 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ren P, Cotton K, Porsteinsson A, Mapstone M, & Heffner KL (2016). Mental fatigability and heart rate variability in mild cognitive impairment. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry, 24(5), 374–378. 10.1016/j.jagp.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA (1999). Are thalamocortical rhythms the rosetta stone of a subset of neurological disorders? Nature Medicine, 5(12), 1349–1351. doi: 10.1038/70911 [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeuchi H, Taki Y, Nouchi R, Kotozaki Y, Shinada T, … Kawashima R (2016). Basal ganglia correlates of fatigue in young adults. Sci Rep, 6, 21386. doi: 10.1038/srep21386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty BWB, & John P (2009). Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action. Neuropsychopharmacology, 35(1), 48. doi:doi: 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, … investigators L. s. (2014). Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA, 311(23), 2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini M, Bonzano L, Bergamino M, Bommarito G, Feraco P, Murugavel A, … Roccatagliata L (2015). Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England), 21(4), 442–447. doi: 10.1177/1352458514546791 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1997). The CES-D Scale 10.1177/014662167700100306 10.1177/014662167700100306. doi: 10.1177/014662167700100306 [DOI]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. doi: 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, Parisi L, Pagani E, Copetti M, Rodegher M, Colombo B, … Filippi M (2014). Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology, 273(2), 511–520. doi: 10.1148/radiol.14140417 [DOI] [PubMed] [Google Scholar]
- Rosano C, Guralnik J, Pahor M, Glynn NW, Newman AB, Ibrahim TS, … Aizenstein HJ (2016). Hippocampal Response to a 24-Month Physical Activity Intervention in Sedentary Older Adults. Am J Geriatr Psychiatry, 25(3), 209–217. doi: 10.1016/j.jagp.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanasto AJ, Coen PM, Glynn NW, Conley KE, Jubrias SA, Amati F, … Newman AB (2016). The relationship between mitochondrial function and walking performance in older adults with a wide range of physical function. Exp Gerontol, 81, 1–7. doi: 10.1016/j.exger.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, & Ferrucci L (2016). Fatigued, but not frail: Perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc, 64(6), 1287–1292. doi: 10.1111/jgs.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Schrack JA, Santanasto AJ, Studenski SA, Ferrucci L and Glynn NW (2018), Pittsburgh Fatigability Scale: One‐page predictor of mobility decline in mobility‐intact older adults. J Am Geriatr Soc doi: 10.1111/jgs.15531 [DOI] [PMC free article] [PubMed]
- Singh KD, & Fawcett IP (2008). Transient and linearly graded deactivation of the human default-mode network by a visual detection task. NeuroImage, 41(1), 100–112. doi: 10.1016/j.neuroimage.2008.01.051 [DOI] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Hum Brain Mapp, 17(3), 143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, & Castellanos FX (2007). Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and Biobehavioral Reviews, 31(7), 977–986. doi: 10.1016/j.neubiorev.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Spiteri S, Hassa T, Claros-Salinas S, Dettmers C, Schoenfeld MA (2017). Neural correlates of of effort-dependent and effort-ndependent cognitive fatigue components in patients with multiple sclerosis. Multiple sclerosis journal, 1–11. doi. 10.1177/1352458517743090 [DOI] [PubMed]
- Stephan KE, Manjaly ZM, Mathys CD, Weber LAE, Paliwal S, Gard T,…Petzschner FH (2016). Allostatis self-efficacy: A metacognitive theory of dyshomeosatsis-induced faigue and depression. Front Hum Neurosci doi.org/10.3389/fnhum.2016.00550. [DOI] [PMC free article] [PubMed]
- Teng EL, & Chui HC (1987). The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry, 48(8), 314–318. [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, & Woldorff MG (2006). The neural bases of momentary lapses in attention. Nature Neuroscience, 9(7), 971–978. doi: 10.1038/nn1727 [DOI] [PubMed] [Google Scholar]
- Wu M, Carmichael O, Lopez-Garcia P, Carter CS, & Aizenstein HJ (2006). Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp, 27(9), 747–754. doi: 10.1002/hbm.20216 [DOI] [PMC free article] [PubMed] [Google Scholar]


