Abstract
TREM2 was suggested to be an important regulator of microglia during neurodegeneration, but previous studies report conflicting results in relation to soluble TREM2 (sTREM2) in CSF when using clinical criteria to classify Alzheimer’s disease (AD). The present study explores sTREM2 CSF levels and their associations with other biomarkers and cognitive measures in a prospective AD cohort. Based on the available CSF biomarker information, 497 subjects were classified according to the 2018 National-Institute on Aging-Alzheimer’s Association (NIA-AA) research framework guidelines, which group biomarkers into those of amyloid-β deposition, tau pathology and neurodegeneration. CSF sTREM2 concentrations were associated with markers of neurodegeneration and fibrillar tau pathology, but not amyloidosis; sTREM2 concentrations were increased in tTau positive vs negative individuals; sTREM2 was not related to cognitive and other biomarker changes over time; and sTREM2 concentrations increased over time in tTau positive vs negative individuals with AD pathophysiology. The present study provides evidence in support of sTREM2 in CSF as a marker of neuroinflammation across the spectrum of early clinical AD. sTREM2 is linked to neuronal injury and may therefore offer complementary information relevant for diagnostic purposes and novel treatment approaches targeting the immune system in AD.
Keywords: Alzheimer’s disease, dementia, mild cognitive impairment, biomarker, neuroinflammation, neurodegeneration, imaging, prediction
1. Introduction
Microglia activation and other innate immune responses seem to be associated with most neurodegenerative conditions, but the underlying mechanisms are still poorly understood. Single nucleotide polymorphisms (SNPs) related to microglia function were shown to be linked to neurodegeneration. For example, SNPs in the Triggering receptor expressed on myeloid cells 2 (TREM2) gene have been associated with Alzheimer’s disease (AD) and other neurodegenerative disorders, including Parkinson’s disease, dementia with Lewy bodies, amyotrophic lateral sclerosis and frontotemporal dementia (FTD) (Borroni et al., 2014; Cady et al., 2014; Cuyvers et al., 2014; Guerreiro, R. et al., 2013; Guerreiro, R.J. et al., 2013; Jonsson et al., 2013; Rayaprolu et al., 2013; Walton et al., 2016).
TREM2 was suggested to be an important regulator of microglia during neurodegeneration (Kleinberger et al., 2017; Ulland et al., 2017). Functional imaging studies on mice using positron-emission-tomography (PET) showed that TREM2 plays a role in microglial activation during normal aging and is needed to maintain physiological cerebral energy metabolism (Kleinberger et al., 2017). The soluble form of TREM2 (sTREM2) is accessible as a biomarker in cerebrospinal fluid (CSF) since its ectodomain is shed by ADAM10 in a process similar to the intramembrane proteolysis of APP (Kleinberger et al., 2014). However, several previous studies report conflicting results with CSF sTREM2 concentrations shown to be increased (Heslegrave et al., 2016; Piccio et al., 2016; Suarez-Calvet et al., 2016b), decreased (Kleinberger et al., 2014) or normal in AD (Gispert et al., 2017), suggesting that sTREM2 levels vary with disease progression. Findings in autosomal dominant AD suggest that CSF sTREM2 increases several years before the expected symptom onset in mutation carriers, but after Amyloid β (Aβ) deposition and neuronal injury have already occurred (Suarez-Calvet et al., 2016a). Moreover, previous work indicates that sTREM2 is associated with biomarkers of tau fibrillar pathology, neurodegeneration and glial proteins in AD, but not with Aβ pathophysiology (Gispert et al., 2016; Heslegrave et al., 2016; Piccio et al., 2016; Suarez-Calvet et al., 2016b). It was also reported in former National Football League (NFL) players that cumulative exposure to repetitive head impact and microglial activation estimated by CSF sTREM2 were linked to higher CSF tTau levels, but not to CSF Aβ or pTau (Alosco et al., 2018); it was suggested that chronic microglial activation may subsequently lead to tau hyperphosphorylation associated with chronic traumatic encephalopathy (Cherry et al., 2016).
The available evidence suggests that sTREM2 levels increase in association with neuronal damage rather than with the appearance of Aβ plaques per se and that sTREM2 may be linked to neurodegeneration in the absence of fibrillar Tau pathology. It remains, however, to be clarified how sTREM2 is related to the core pathological changes along the AD continuum. Therefore, in the present study we utilized the biomarker-based classification system proposed in the recently published National Institute of Aging-Alzheimer’ Association (NIA-AA) research framework (Jack et al., 2018). This approach allowed us to contrast groups of individuals with different biomarker profiles. We tested the main hypotheses that sTREM2 concentrations differ between the NIA-AA groups; and that cross-sectional and longitudinal associations between sTREM2 and other relevant AD markers are also related to the NIA-AA groups.
2. Methods
Data used in the preparation of this article were obtained from the AD Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) on 16th March 2018. The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET and additional biological markers and clinical and neuropsychological assessments can be combined to measure the progression of mild cognitive impairment (MCI) and early clinical AD. Participants were aged between 55–90 (inclusive), considered cognitively normal (CN), MCI or AD dementia diagnosed individuals, and underwent serial evaluations of functional, biomedical, neuropsychological and clinical status at various intervals.
2.1. Study population
AD could only be diagnosed in the dementia stage until recently different sets of criteria were presented with a stronger focus on biomarkers, including neuroimaging and CSF proteins. The guidelines proposed by the NIA-AA conceptualized AD as a progressive disorder including all possible stages from asymptomatic to severely impaired (Jack et al., 2011; McKhann, 2011), allowing AD to be diagnosed in the MCI stage if the presence of AD pathophysiology is supported by the typical biomarker findings (Giaccone et al., 2011). In the recently updated guidelines (i.e. the NIA-AA research framework (Jack et al., 2018), AD is defined by its pathological processes, which can be measured in vivo using biomarkers, and not by its clinical consequences. The relevant biomarker measures are grouped into those of Aβ deposition (A), tau pathology (T) and neurodegeneration (N), which in the present study were assessed by decreased Aβ42, increased phosphorylated Tau181 (pTau181) and increased total-Tau (tTau) in the CSF, respectively. CSF biomarker values were binarized into normal vs abnormal for the purposes of ATN classification. References for the cut-off values used to define CSF biomarker abnormality are provided in the “Established biomarkers” section below.
The current study utilised data collected at baseline and over 48-months in 12-month increments from individuals participating in ADNI 1, ADNI 2 and ADNI GO, for whom complete CSF biomarker information was available (Aβ42, pTau181 and tTau). The study cohort included subjects with a baseline clinical classification of CN (n=138), MCI (n=302) or AD dementia (n=57). CN was defined as MMSE score between 25 and 30, inclusive; CDR score of 0; no evidence of depression; and no memory complaints. MCI was defined as MMSE score between 24 and 30, inclusive; CDR score of 0.5; report of memory complaints; and no significant functional impairment; all individuals with MCI also met Petersen criteria (Petersen, 2004). Finally, subjects with AD dementia met NIA-AA criteria and the diagnostic guidelines of the National Institute of Neurological and Communicative Disorders and Stroke-AD and Related Disorders Association (NINCDS–ADRDA) (McKhann et al., 2011) for AD dementia and probable AD (McKhann et al., 1984), respectively.
Based on the NIA-AA research framework guidelines (Jack et al., 2018), each ADNI participant was assigned to a group defined by their respective biomarker profile according to the ATN classification system, irrespective of clinical status as suggested before (Baldacci et al., 2017). Only CN individuals with an A−T−N− profile were considered as healthy controls to exclude preclinical/prodromal AD cases from this group; in addition, MCI patients with an A−T−N− profile who had reverted to CDR 0 at their last available ADNI follow-up visit were also considered healthy controls for the purposes of the present study, resulting in a final set of 79 controls. To study the effects of fibrillar tau pathology and neuronal injury separately in individuals along the AD continuum, the following groups were defined (irrespective of clinical status): A+T−N− (N = 54), A+T+N− (N = 64), A+T+N+(N=129). Furthermore, individuals with suspected non-AD pathology (SNAP) were defined as A−T+N− (N = 32) and A−T+N+ (N = 20). All other biomarker profiles were not considered for the present study to exclude individuals with non-AD (co)pathologies. The final groups are presented in Table 1.
Table 1.
Study groups based on the ATN classification system
| ATN profile (diagnosis) | Study group | N |
|---|---|---|
| A−T−N− (CN)* | Healthy controls | 79 |
| A+T−N− (CN, MCI, AD) | 54 | |
| A+T+N− (CN, MCI, AD) | AD continuum | 64 |
| A+T+N+ (CN, MCI, AD) | 129 | |
| A−T+N− (CN, MCI, AD) | SNAP | 32 |
| A−T+N+ (CN, MCI, AD) | 20 | |
| A−T−N+ (AD) | Non-AD change | 1 |
| A+T− N+ (AD) | Concomitant non-AD change | 1 |
| A−T−N− (MCI, AD) | Normal AD biomarkers | 98 |
Grey fields indicate ADNI participants classified as either healthy controls or patients along the Alzheimer’s continuum, who were included in the study; uncoloured fields indicate ADNI participants who were excluded from the study because of non-Alzheimer’s changes or normal biomarker results despite a diagnosis of MCI or dementia.
Includes MCI individuals classified as CDR = 0 at their last available ADNI visit (N = 11) Abbreviations: CN, cognitively normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease (dementia); SNAP, suspected non-AD pathology; ATN: amyloid/tau/neurodegeneration classification
Included subjects had available structural MRI scans; PET scans to measure glucose metabolisms (F18-fluorodeoxyglucose, FDG) and fibrillar Aβ load (F18-Florbetapir or C11-PiB; note, PiB PET and Florbetapir PET yield comparable Aβ binding patterns, utilising different Aβ imaging tracer compounds (Jagust, W. et al., 2009; Landau et al., 2013)); CSF proteins (Aβ1–42, tTau and pTau181); and apolipoprotein E (APOE) ε4 allele carrier status (dichotomised into carriers vs non-carriers). Additionally included were validated summary metrics for the memory (ADNI-mem; derived from: Rey Auditory Verbal Learning test, AD Assessment Scale-cognitive subscale, MMSE and Wechsler Memory Test-logical memory I) (Crane et al., 2012) and executive cognitive domains (ADNI-ef; derived from the Wechsler Adult Intelligence Scale-Revised Digit Symbol Substitution and Digit Span backwards, Trail Making Test parts A and B, animal and vegetable Category Fluency and Clock Drawing Test) (Gibbons et al., 2012). Summary cognitive scores were chosen over individual cognitive tests to use more comprehensive and robust measures of domain-specific cognitive performance. ADNI was reviewed and approved by all host study site review boards and participants completed informed consent after receiving a comprehensive description of ADNI.
2.2. Established Biomarkers
A detailed description of biomarker acquisition and performance measures in ADNI can be obtained by registered users at http://adni.loni.usc.edu/, with image and CSF collection protocols available elsewhere (Jack et al., 2008; Jagust, W. et al., 2009; Landau et al., 2010; Landau et al., 2012). Briefly, TaqMan quantitative polymerase chain reaction assays were used for genotyping APOE nucleotides 334 TC and 472 CT with an ABI 7900 real-time thermocycler (Applied Biosystems, Foster City, CA) using DNA freshly prepared from whole blood samples (Bonner-Jackson et al., 2012). Mean FDG count was obtained per subject based on a composite region of interest in an AD typical hypometabolic pattern (Jagust, W. et al., 2009; Landau et al., 2010). For Aβ PET analysis, standardized update value ratios (SUVRs) were calculated with a standardized cortical anatomical automatic labelling volume-of-interest template placed on spatially normalized image volumes using a whole-cerebellum reference region, as previously described (Barthel et al., 2011). Composite SUVRs were calculated as the unweighted mean of the left and right lateral temporal, frontal, posterior cingulate/precuneus and parietal cortices. FreeSurfer software (http://surfer.nmr.mgh.harvard.edu) was utilised to extract MRI (1.5 T) measured hippocampal volume where an atlas-based approach was implemented and has been validated for use in subjects with a great deal of morphologic variability. Uncorrected hippocampal volume for head size was used as a previous study showed that the association between hippocampal volumes and cognition was not altered by intracranial volume normalization (Voevodskaya et al., 2014). Routine peptide CSF measures were generated from aliquot samples collected at the same time (Kim et al., 2011) using commercially available enzyme-linked immunosorbent assays (ELISAs). Validated cut-offs were applied to a differential between normal and pathological findings for CSF pTau181 and tTau (Jagust, W.J. et al., 2009; Shaw et al., 2009a; Steenland et al., 2014), CSF Aβ (Landau et al., 2010; Landau et al., 2013; Shaw et al., 2009a; Steenland et al., 2014), FDG PET (Jagust, W. et al., 2009; Landau et al., 2010; Landau et al., 2012), MRI hippocampal volume (Jagust, W. et al., 2009; Landau et al., 2010; Landau et al., 2012; Landau et al., 2013; Shaw et al., 2009a; Steenland et al., 2014) and Aβ PET (Jagust, W. et al., 2009; Landau et al., 2010; Landau et al., 2012; Landau et al., 2013; Shaw et al., 2009b; Steenland et al., 2014). Neurofilament light chain (NF-L) was measured in CSF and plasma, using the same antibodies in a commercially available sandwich ELISA and transferred to a SIMOA assay, respectively (Mattsson et al., 2017).
2.3. sTREM2 measurements
CSF sTREM2 concentrations were measured in ADNI using two different validated approaches in parallel at two different laboratories: (i) at German Center for Neurodegenerative Disorders Munich an ELISA using the Mesoscale Discovery (MSD) electrochemiluminescence platform was used (Kleinberger et al., 2014; Suarez-Calvet et al., 2016a; Suarez-Calvet et al., 2016b); the assay consists of a biotinylated polyclonal goat IgG anti-human TREM2 antibody as capture antibody, which is raised against aminoacids 19–174 of human TREM2; a monoclonal mouse IgG anti-human TREM2 antibody as a detection antibody, which is raised against aminoacids 1–160 of human TREM2; and a SULFOTAG– labeled goat polyclonal IgG anti-mouse secondary antibody. Recombinant human TREM2 protein, corresponding to the extracellular domain of human TREM2 (aminoacids 1–174) is used as a standard. The electrochemical signal is measured using the SECTOR Imager 2400 (MSD). All measurements are performed in duplicate and the average is subsequently used for the statistical analyses.
(ii) at Washington University, Department of Neurology, sTREM2 levels were measured using ELISA method (Piccio et al., 2008; Piccio et al., 2016), which uses an antihuman TREM2 monoclonal antibody (clone 20G2) as capture antibody, a biotinylated mouse anti-human TREM-2 mAb (clone 29E3) as detection antibody and recombinant human sTREM2 (Sino Biological Inc.) to generate the standard curve. Again, all measurements are performed in duplicate and the average is subsequently used for the statistical analyses. More information about the two different sTREM2 assays, including quality assurance measures, is available on the ADNI website (http://adni.loni.usc.edu/).
2.4. Statistical analysis
Statistical analysis was performed in R (Version 3.5.1, The R Foundation for Statistical Computing) and IBM SPSS Statistics (Version 20, IBM, New York, USA). Statistical significance level for all tests was α = 0.05. Analysis of group differences in relation to demographic data, fluid biomarkers, neuroimaging and cognitive test results was performed with Kruskal-Wallis test or chi square test, as appropriate, with post-hoc pairwise comparisons where relevant. Differences in sTREM2 concentration between the ATN groups were tested with one-way analysis of variance (ANOVA). sTREM2 was not normally distributed, therefore a cube root transformation was performed prior to subsequent analysis. Associations between CSF sTREM2 and other fluid biomarkers, imaging and cognitive test data at baseline were analysed using linear regression models corrected for age, sex, educational level and APOE genotype. Longitudinal associations between sTREM2 concentrations at baseline, fluid biomarkers, imaging and cognitive test data were assessed using linear mixed-effects model (LME), in which sTREM2 baseline CSF concentration was dichotomized into high vs low by median split method. The LME was calculated with autoregressive covariance matrices, random intercept and slopes for time and sTREM2 concentration as fixed factor. The interaction between sTREM2 concentration and time (baseline, month 12, month 24, month 36 and month 48) and the fixed effects of time and group (sTREM2 baseline high vs low) were tested for each ATN group and the entire study cohort. T-tests using Satterthwaite’s method were performed to reveal significance for the fixed effects. Changes in sTREM2 concentration over the course of the disease (i.e. between baseline and month 24) where assessed using a repeated ANOVA.
3. Results
Characteristics of the study population are presented in Table 2. APOE genotype, ADNI_mem and ADNI_ef, CSF sTREM2, Aβ1–42, tTau and pTau181, and CSF/plasma NFL differed between the ATN groups at baseline. CSF sTREM2 concentrations measured using the two different technologies were highly correlated (ρ = 0.83, p < 0.001); therefore, only the MSD measurements were used for all subsequent statistical analyses because of the higher sensitivity of the electrochemiluminescence platform compared to standard ELISA technology.
Table 2.
Characteristics of the study population
| Variable | A−T−(N−) (CN)* (N=79) | A+T−N− (CN, MCI, AD) (N = 54) | A+T+N− (CN, MCI, AD) (N = 64) | A+T+N+ (CN, MCI, AD) (N = 129) | A−T+N− (CN, MCI, AD) (N=32) | A−T+N+ (CN, MCI, AD) (N=20) | p Value |
|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 72.4 (6.3) | 74.2 (6.6) | 74.9 (5.8) | 73.8 (7.8) | 72.3 (7.4) | 76.1 (7.5) | 0.105a |
| Female, N (%) | 43 (49.8) | 33 (47.6) | 33 (47.3) | 48 (50.2) | 31 (47.1) | 55 (51.0) | 0.120b |
| Education, mean (SD) years | 16.0 (2.9) | 15.1 (2.9) | 16.3 (2.9) | 15.8 (2.7) | 15.8 (2.8) | 15.9 (3.2) | 0.313a |
| APOE ε4 carriers, N (%) | 16 (37) | 56 (50.2) | 67 (47.3) | 71 (45.4) | 16 (36.9) | 25 (44.4) | < 0.001b |
| ADNI_ef, mean (SD) | 0.78 (0.66) | 0.11 (0.83) | −0.67 (0.95) | −0.15 (0.90) | 0.58 (0.83) | 0.34 (0.66) | < 0.001a |
| ADNI_mem, mean (SD) | 0.92 (0.55) | 0.26 (0.65) | 0.06 (0.78) | −0.19 (0.72) | 0.72 (0.64) | 0.64 (0.69) | < 0.001a |
| NF-L plasma* mean (SD), pg/ml | 27.38 (10.65) | 46.75 (39.19) | 46.94 (27.34) | 39.46 (12.96) | 23.43 (6.96) | 33.92 (17.44) | 0.01a |
| NF-L CSF* mean (SD), pg/mL | 995.56 (258.74) | 1261.22 (635.52) | 1247.16 (483.92) | 1629.62 (513.34) | 1347.34 (394.49) | 1320.60 (204.12) | < 0.001a |
| CSF sTREM2 mean (SD), pg/mL | 2572.10 (1596.09) | 3110.76 (1425.49) | 3617.73 (1651.78) | 4577.71 (2151.30) | 3716.45 (2031.35) | 5317.08 (2619.21) | < 0.001a |
| CSF Aβ42, mean (SD), ng/l | 261.10 (39.79) | 144.83 (29.08) | 139.57 (32.64) | 140.26 (27.02) | 287.21 (55.43) | 255.04 (45.87) | < 0.001a |
| CSF tTau, mean, (SD), ng/l | 53.43 (15.90) | 59.62 (14.71) | 74.71 (14.49) | 158.87 (60.27) | 68.65 (16.00) | 122.79 (33.20) | < 0.001a |
| CSF pTau181, mean, (SD), ng/l | 16.00 (3.44) | 18.09 (3.28) | 34.51 (10.36) | 42.48 (16.69) | 28.46 (4.20) | 31.77 (9.33) | < 0.001a |
Kruskal Wallis
Chi-square test
only ADNI 1 data available
Abbreviations: CN, cognitively normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease (dementia); SNAP; APOE, apolipoprotein E; ADNI, Alzheimer’s Disease Neuroimaging Initiative; ADNI_ef, executive domain summary score; ADNI_mem, memory domain summary score; CSF: cerebrospinal fluid; NF-L, neurofilament light chain; sTREM2, soluble Triggering receptor expressed on myeloid cells 2; A, amyloid-; tTau, total tau; pTau, phosphorylated tau; ATN:amyloid/tau/neurodegeneration classification
3.1. Cross-sectional associations between CSF sTREM2, other biomarkers and cognition
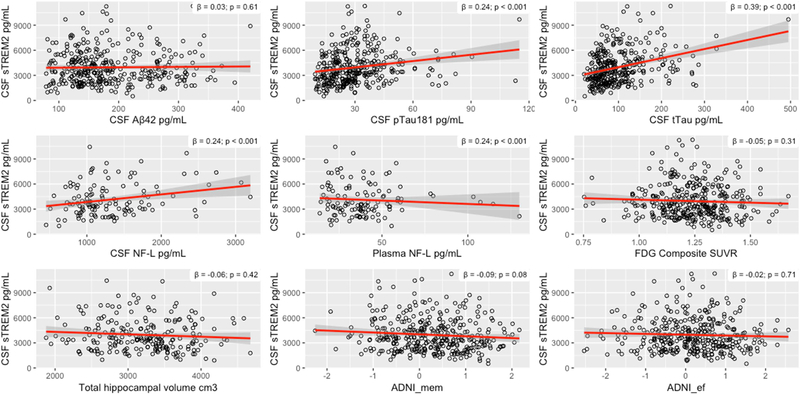
A multiple linear regression analysis at baseline revealed positive associations between CSF concentrations of sTREM2 and tTau (β = 0.06, p < 0.001), pTau181 (β = 0.24, p < 0.001) and NF-L (β = 0.21, p = 0.03). No significant associations were found for CSF Aβ42 (β = 0.03, p = 0.61) and plasma NF-L (β = -0.12, p = 0.21). FDG-PET (β = -0.06, p = 0.31) and MRI total hippocampal volume (β = -0.06, p = 0.42) showed no significant associations with sTREM concentration. There was an association on trend level between baseline CSF sTREM2 with ADNI_mem (β = -0.1, p = 0.08) and no association with ADNI_ef scores (β = -0.02, p = 0.71). Correlations are shown in Figure 1.
Figure 1. Associations between CSF sTREM2, other biomarkers and cognitive performance in the entire study cohort.
Abbreviations: ADNI, Alzheimer’s Disease Neuroimaging Initiative; ADNI_ef, executive domain summary score; ADNI_mem, memory domain summary score; CSF: cerebrospinal fluid; NF-L, neurofilament light chain; sTREM2, soluble Triggering receptor expressed on myeloid cells 2; Aβ, amyloid-β; total tau; pTau, phosphorylated tau; ATN: amyloid/tau/neurodegeneration classification
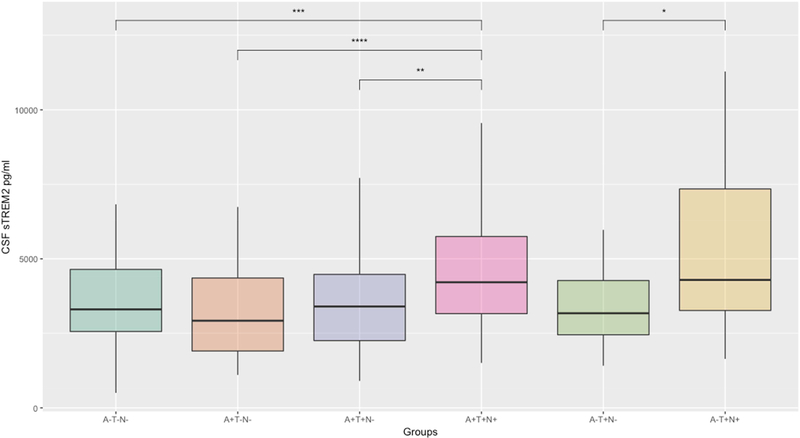
3.2. CSF sTREM2 concentration differences between ATN groups
Mean sTREM2 concentrations per group are shown in Table 2 and Figure 2. tTau positive (A+T+N+) participants showed significantly higher concentrations of sTREM2 compared to healthy controls (A−T−N−, p < 0.01) as well as other tTau negative stages of AD (A+T−N−, p < 0.001; A+T−N−, p < 0.01). This strong dependency of sTREM2 on tTau was also present in the SNAP group (suspected non-AD pathology, i.e. elevated pTau/tTau but normal Aβ); AT+N- showed significantly lower sTREM2 concentrations compared to A−T+N+ (p = 0.02).
Figure 2: CSF sTREM2 concentration differences between the ATN sub-groups.
* p = 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
Abbreviations: CSF, cerebrospinal fluid; sTREM2, soluble Triggering receptor expressed on myeloid cells 2;
3.3. Longitudinal associations between CSF sTREM2, cognitive performance and neuroimaging markers
Longitudinal changes in cognitive performance (ADNI_mem and ADNI_ef) and imaging outcomes (FDG-PET composite SUVR, MRI hippocampal volume) were analysed in relation to baseline sTREM2 concentrations. LME analysis showed no time by sTREM2 group interaction (low vs high) for the entire study cohort as well as for the ATN sub-groups individually. With advancing disease stage, a more pronounced decline in all tested variables was observed. There was no significant difference in ADNI_mem, ADNI_ef, FDG SUVR or MRI hippocampal volume between the sTREM2 groups. Estimates of the fixed effects and significances for the factors time and group as well as the time by group interactions are shown in Table 3.
Table 3.
Results of the linear mixed effects model analysis for ADNI_mem, ADNI_ef, FDG PET SUVR and hippocampal volume. Estimates of the fixed effects (ß values) are shown for the fixed factors time and group and time by group interaction for the whole study population and the ATN sub-groups (p values obtained from t-tests using Satterthwaite’s method)
| Variables | All participants | A−T−N− | A+T−N− | A+T+N− | A+T+N+ | A−T+N− | A−T+N+ |
|---|---|---|---|---|---|---|---|
|
ADNI_mem |
β, (p) | β, (p) | β, (p) | β, (p) | β, (p) | β, (p) | β, (p) |
| Time | −0.111 (<0.001) | 0.026 (0.17) | −0.066 (0.02) | 0.030 (<0.001) | 0.024 (<0.001) | −0.029 (0.42) | −0.099 (0.06) |
| Group (sTREM2) | −0.151 (0.08) | −0.174 (0.08) | 0.353 (0.07) | 0.099 (0.68) | −0.088 (0.52) | 0.275 (0.28) | −0.017 (0.96) |
| Interaction (time × group) | 0.013 (0.54) | 0.028 (0.29) | 0.078 (0.16) | −0.004 (0.94) | 0.030 (0.40) | 0.040 (0.54) | 0.095 (0.17) |
|
ADNI_ef |
|||||||
| Time | −0.109 (<0.001) | 0.003 (0.90) | −0.115 (0.01) | −0.168 (<0.001) | −0.212 (<0.001) | 0.016 (0.70) | −0.038 (0.54) |
| Group (sTREM2) | −0.049 (0.63) | 0.080 (0.66) | −0.051 (0.85) | 0.163 (0.55) | −0.142 (0.41) | 0.362 (0.30) | −0.067 (0.85) |
| Interaction (time × group) | −0.012 (0.65) | −0.045 (0.24) | 0.057 (0.50) | 0.006 (0.93) | 0.014 (0.77) | 0.063 (0.40) | 0.037 (0.66) |
|
FDG PET comp. SUVR |
|||||||
| Time | −0.020 (<0.001) | −0.005 (0.28) | −0.014 (0.01) | −0.027 (<0.001) | −0.040 (<0.001) | NA | −0.014 (<0.001) |
| Group (sTREM2) | 0.002 (0.91) | −0.036 (0.26) | 0.003 (0.96) | 0.056 (0.16) | 0.015 (0.56) | NA | −0.015 (0.91) |
| Interaction (time × group) | −0.001 (0.88) | 0.001 (0.83) | 0.007 (0.46) | −0.007 (0.58) | 0.008 (0.32) | NA | −0.0002 (0.88) |
|
Hippocampal volume |
|||||||
| Time | −393.33 (<0.001) | −465.04 (<0.001) | −310.04 (<0.001) | −281.79 (<0.001) | −474.31 (<0.001) | −332.51 (0.001) | −393.33 (<0.001) |
| Group (sTREM2) | −61.99 (0.80) | −551.85 (0.36) | −13.95 (0.98) | 773.10 (0.12) | −597.71 (0.11) | 1931.71 (0.08) | −61.99 (0.80) |
| Interaction (time × group) | 16.42 (0.72) | 209.47 (0.07) | −99.42 (0.48) | 128.66 (0.27) | 105.67 (0.12) | −362.72 (0.07) | 16.42 (0.72) |
Abbreviations: ADNI, Alzheimer’s Disease Neuroimaging Initiative; ADNI_ef, executive domain summary score; ADNI_mem, memory domain summary score; sTREM2, soluble Triggering receptor expressed on myeloid cells 2; ATN: amyloid/tau/neurodegeneration classification; FDG PET, F18-fluorodeoxyglucose positron-emissiontomography; SUVR, standardised uptake value ratio.
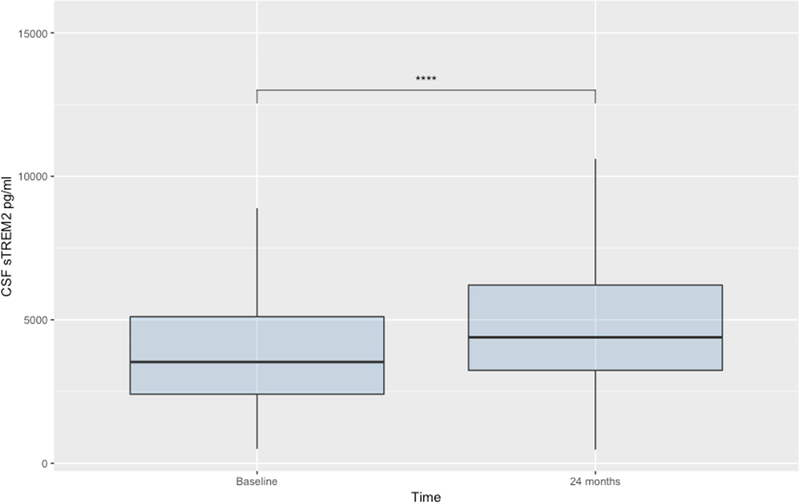
3.4. Changes in sTREM2 concentration over the course of the disease
Changes of sTREM2 concentrations measured with the MSD assay over time were analysed using a repeated measure ANOVA, comparing baseline vs 24-month measurements. No significant time by ATN group interaction was found (P = 0.44), but a significant increase in sTREM2 concentration after 48 months was demonstrated in the entire cohort (p < 0.001) (Figure 3). Pairwise comparisons revealed significant differences between A+T+N+ vs A−T−N− (p < 0.01), A+T−N− (p < 0.01) and A+T+N− (p < 0.01).
Figure 3: Changes in CSF sTREM2 concentration in the whole study cohort of the AD spectrum.
**** p < 0.0001
Abbreviations: CSF, cerebrospinal fluid; sTREM2, soluble Triggering receptor expressed on myeloid cells 2
4. Discussion
An improved mechanistic understanding of biomarker changes related to different types of pathology is a key prerequisite of improving the diagnosis, prevention and clinical management of AD. Neuroinflammation has become a key target for biomarker research and TREM2 has recently been identified as an interesting new biomarker candidate, reflecting microglial activation and innate immune response in different neurodegenerative diseases including AD. Previous studies indicate that sTREM2 concentrations in CSF are increased in the early symptomatic stages of AD (Suarez-Calvet et al., 2016b), but the association of CSF sTREM2 with other more established fluid and imaging biomarkers is not well understood, in particular when it comes to biomarker changes and associations over time.
In a bid to determine how CSF sTREM2 concentrations are related to the core pathological changes along the AD continuum, we utilized a subset of the ADNI cohort with available CSF and other relevant biomarker results. We decided to apply a biomarker-based staging system based on the recently published NIA-AA research framework, which is a strength of our approach that allowed us to explore associations in relation to the underlying presence or absence of key aspects of AD pathophysiology (i.e. Aβ, pTau and Tau) rather than the clinical phenotype.
The main findings of our study are that (i) different established immunologic methods result in comparable CSF sTREM2 measurements; (ii) CSF sTREM2 concentrations are correlated with CSF markers of fibrillar tau (pTau181) and neuronal injury (tTau and NF-L) but not amyloidosis (CSF Aβ42); (iii) tTau positive individuals have higher CSF concentrations of sTREM2 compared to both healthy controls and tTau negative patients along the AD continuum; (iv) CSF sTREM2 is not associated with cognitive and other biomarker changes over time; and (v) CSF sTREM2 concentrations increase over time in tTau positive AD patients compared to tTau negative patients with AD pathophysiology.
The findings of the present study are in line with previous results indicating that higher sTREM2 concentrations in CSF are linked to neuronal injury (i.e. CSF tTau and NFL), even in the absence of Aβ pathology as indicated by differences between healthy controls and SNAP individuals (Suarez-Calvet et al., 2016b). Our study extends previous evidence by showing that fibrillar tau pathology in the absence of neurodegeneration is not associated with increased sTREM2 CSF levels. The findings also suggest that tau and Aβ may have opposite associations with sTREM2 in CSF, with Aβ being associated with lower sTREM2 concentrations in the absence of tau pathology (non-significant finding, but conclusion supported by Figure 1). These findings may explain why some previous studies report conflicting results in relation to sTREM2 changes when individuals are classified according to their clinical status rather than their AD biomarker profile, which results in biologically heterogenous cohorts (Heslegrave et al., 2016; Piccio et al., 2016; Suarez-Calvet et al., 2016b).
The association between TREM2 and tTau seems to follow a consistent pattern, both for genetic associations with the TREM2 gene and for the soluble form in CSF. For example, carriers of the TREM2 rs75932628 risk allele showed increased levels of CSF tTau but not Aβ42. These consistent findings suggest that sTREM2 increases if neuronal injury occurs, even if Aβ pathology is absent. Increased sTREM2 seems to be a consequence rather than a cause of neurodegeneration, in line with reports that the TREM2 receptor mediates the phagocytosis of apoptotic neurons by microglia (Wang et al., 2015). At the same time, sTREM2 as a biomarker of AD is likely to offer complementary, rather than redundant, information to tTau and pTau, which are passively released to CSF from decaying neurons whereas the production and shedding of TREM2 by microglia is a physiological process, making sTREM2 a biomarker candidate of microglia activation in the context of AD. The association between microglial activation and increased sTREM2 concentrations is in line with elevated TREM2 expression (Matarin et al., 2015) and higher translocator protein (TSPO) tracer uptake in PET studies (Brendel et al., 2017). There are conflicting hypotheses if microglial activation during early AD stages is neuroprotective, detrimental or merely reactive to advancing Aβ pathology. The increase of sTREM2 during physiological aging and the association between misfolded mutant TREM2 and impaired microglial phagocytosis may point to a protective effect, at least as neurodegeneration starts to develop (Kleinberger et al., 2014; Piccio et al., 2016). However, distinct disease associated TREM2 mutations may have opposite mechanisms on the cellular level because, for example, R47H mutations associated with AD do not show decreased TREM2 shedding (Kleinberger et al., 2014).
A few limitations of the study must be acknowledged. Firstly, Standardisation of biomarker cut-offs is currently limited and results often vary among laboratories; however, current cut-offs have been used in numerous ADNI studies, and appear to show reasonable validity for the purposes of this paper (Alexopoulos et al., 2014; Jagust, W. et al., 2009; Landau et al., 2010; Landau et al., 2012; Landau et al., 2013; Shaw et al., 2009b). Secondly, the ADNI cohort is mainly white, middle class, educated and without any major comorbidity, thus, it would be important to repeat such a study with a larger and more widely represented demographic which also considers relevant comorbid conditions such as cerebrovascular disease. Thirdly, our analyses only consider biomarker and clinical changes over the period of 48 months and longer follow-up periods may reveal additional informative changes and associations. Fourthly, there was no histopathological verification of the clinical diagnoses, but the ADNI cohort is on purpose enriched with probable pre-dementia AD cases, evidenced by the first autopsy studies (Toledo et al., 2013). Finally, there is some missing data, especially in later visit time-points as study participants drop off, which is a typical pattern observed in prospective clinical research.
To conclude, our work provides intriguing evidence in support of sTREM2 in CSF as a marker of neuroinflammation across the spectrum of early clinical AD; sTREM2 is linked statistically and biologically to neuronal injury and may therefore offer complementary information relevant for diagnostic purposes and novel treatment approaches targeting the immune system. Moving forward, future studies should use biomarker information to further categorise clinical AD stages (e.g. MCI) and to explore the biological mechanisms underlying the dynamic sTREM2 changes.
Highlights.
Biomarkers useful in diagnosing early Alzheimer, i.e. before dementia onset
Established biomarkers related to core pathophysiology, including amyloid-β and tau
Alzheimer also characterized by activation of innate immune system
TREM2 related to immune response, soluble form detectable in cerebrospinal fluid
Soluble TREM2 differs between Alzheimer stages, related to tau and other biomarkers
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514. The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- Alexopoulos P, Grimmer T, Perneczky R, Kurz A, Kriett L, Klupp E, Förster S, Yakushev I, Haller B, Gray K, Laskaris N, Drzezga A, Fellgiebel A, 2014. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer's disease. Alzheimer's and Dementia. [DOI] [PubMed] [Google Scholar]
- Alexopoulos P, Tsolakidou A, Roselli F, Arnold A, Grimmer T, Westerteicher C, Leante MR, Forstl H, Livrea P, Kurz A, Perneczky R, 2012. Clinical and neurobiological correlates of soluble amyloid precursor proteins in the cerebrospinal fluid. Alzheimers Dement 8(4), 304–311. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Tripodis Y, Fritts NG, Heslegrave A, Baugh CM, Conneely S, Mariani M, Martin BM, Frank S, Mez J, Stein TD, Cantu RC, McKee AC, Shaw LM, Trojanowski JQ, Blennow K, Zetterberg H, Stern RA, 2018. Cerebrospinal fluid tau, Abeta, and sTREM2 in Former National Football League Players: Modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacci F, Toschi N, Lista S, Zetterberg H, Blennow K, Kilimann I, Teipel S, Cavedo E, Dos Santos AM, Epelbaum S, Lamari F, Dubois B, Floris R, Garaci F, Bonuccelli U, Hampel H, 2017. Two-level diagnostic classification using cerebrospinal fluid YKL-40 in Alzheimer’s disease. Alzheimers Dement 13(9), 993–1003. [DOI] [PubMed] [Google Scholar]
- Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, Hiemeyer F, Wittemer-Rump SM, Seibyl J, Reininger C, Sabri O, Florbetaben Study G, 2011. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 10(5), 424–435. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Okonkwo O, Tremont G, Alzheimer’s Disease Neuroimaging I, 2012. Apolipoprotein E epsilon2 and functional decline in amnestic mild cognitive impairment and Alzheimer disease. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry 20(7), 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Ferrari F, Galimberti D, Nacmias B, Barone C, Bagnoli S, Fenoglio C, Piaceri I, Archetti S, Bonvicini C, Gennarelli M, Turla M, Scarpini E, Sorbi S, Padovani A, 2014. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol Aging 35(4), 934 e937–910. [DOI] [PubMed] [Google Scholar]
- Brendel M, Kleinberger G, Probst F, Jaworska A, Overhoff F, Blume T, Albert NL, Carlsen J, Lindner S, Gildehaus FJ, Ozmen L, Suarez-Calvet M, Bartenstein P, Baumann K, Ewers M, Herms J, Haass C, Rominger A, 2017. Increase of TREM2 during Aging of an Alzheimer’s Disease Mouse Model Is Paralleled by Microglial Activation and Amyloidosis. Front Aging Neurosci 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady J, Koval ED, Benitez BA, Zaidman C, Jockel-Balsarotti J, Allred P, Baloh RH, Ravits J, Simpson E, Appel SH, Pestronk A, Goate AM, Miller TM, Cruchaga C, Harms MB, 2014. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol 71(4), 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Tripodis Y, Alvarez VE, Huber B, Kiernan PT, Daneshvar DH, Mez J, Montenigro PH, Solomon TM, Alosco ML, Stern RA, McKee AC, Stein TD, 2016. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun 4(1), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D, Alzheimer’s Disease Neuroimaging I, 2012. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6(4), 502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuyvers E, Bettens K, Philtjens S, Van Langenhove T, Gijselinck I, van der Zee J, Engelborghs S, Vandenbulcke M, Van Dongen J, Geerts N, Maes G, Mattheijssens M, Peeters K, Cras P, Vandenberghe R, De Deyn PP, Van Broeckhoven C, Cruts M, Sleegers K, consortium B, 2014. Investigating the role of rare heterozygous TREM2 variants in Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging 35(3), 726 e711–729. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Arzberger T, Alafuzoff I, Al-Sarraj S, Budka H, Duyckaerts C, Falkai P, Ferrer I, Ironside JW, Kovacs GG, Meyronet D, Parchi P, Patsouris E, Revesz T, Riederer P, Rozemuller A, Schmitt A, Winblad B, Kretzschmar H, 2011. New lexicon and criteria for the diagnosis of Alzheimer’s disease. Lancet neurology 10(4), 298–299; author reply 300–291. [DOI] [PubMed] [Google Scholar]
- Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK, Alzheimer’s Disease Neuroimaging I, 2012. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6(4), 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispert JD, Monte GC, Suarez-Calvet M, Falcon C, Tucholka A, Rojas S, Rami L, Sanchez-Valle R, Llado A, Kleinberger G, Haass C, Molinuevo JL, 2017. The APOE epsilon4 genotype modulates CSF YKL-40 levels and their structural brain correlates in the continuum of Alzheimer’s disease but not those of sTREM2. Alzheimers Dement (Amst) 6, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispert JD, Suarez-Calvet M, Monte GC, Tucholka A, Falcon C, Rojas S, Rami L, Sanchez-Valle R, Llado A, Kleinberger G, Haass C, Molinuevo JL, 2016. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimers Dement 12(12), 1259–1272. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J, Alzheimer Genetic Analysis G, 2013. TREM2 variants in Alzheimer’s disease. N Engl J Med 368(2), 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Lohmann E, Bras JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, Emre M, Singleton A, Hardy J, 2013. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol 70(1), 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, Ohrfelt A, Blennow K, Hardy J, Schott J, Mills K, Zetterberg H, 2016. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH, 2011. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3), 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14(4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, J LW, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW., 2008. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of magnetic resonance imaging: JMRI 27(4), 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, Landau SM, Shaw L, Trojanowski J, Koeppe R, Reiman E, Foster N, Petersen RC, Weiner M, Price J, Mathis C, 2009. Relationships between biomarkers in aging and dementia. Neurology 73(15), 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, Mathis CA, Alzheimer’s Disease Neuroimaging I, 2009. Relationships between biomarkers in aging and dementia. Neurology 73(15), 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K, 2013. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368(2), 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, Shaw LM, Trojanowski JQ, Potkin SG, Huentelman MJ, Craig DW, DeChairo BM, Aisen PS, Petersen RC, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging I, 2011. Genome-wide association study of CSF biomarkers Abeta1–42, t-tau, and p-tau181p in the ADNI cohort. Neurology 76(1), 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, Brendel M, Mracsko E, Wefers B, Groeneweg L, Xiang X, Focke C, Deußing M, Suárez-Calvet M, Mazaheri F, Parhizkar S, Pettkus N, Wurs W, Feederle R, Bartenstein P, Mueggler T, Arzberger T, Knuesel I, Rominger A, Haass C, 2017. The FTD-like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion and glucose metabolism. EMBO J, DOI: 10.15252/embj.201796516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, Tahirovic S, Lleo A, Alcolea D, Fortea J, Willem M, Lammich S, Molinuevo JL, Sanchez-Valle R, Antonell A, Ramirez A, Heneka MT, Sleegers K, van der Zee J, Martin JJ, Engelborghs S, Demirtas-Tatlidede A, Zetterberg H, Van Broeckhoven C, Gurvit H, Wyss-Coray T, Hardy J, Colonna M, Haass C, 2014. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med 6(243), 243ra286. [DOI] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman E, Foster N, Aisen PS, Petersen RC, Shaw L, Trojanowski J, Jack CR, Weiner M, Jagust W, 2010. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75(3), 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Jagust WJ, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Weiner MW, 2012. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Annals of Neurology 72(4), 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, Shaw LM, Jagust WJ, 2013. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann. Neurol. 74(6), 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarin M, Salih DA, Yasvoina M, Cummings DM, Guelfi S, Liu W, Nahaboo Solim MA, Moens TG, Paublete RM, Ali SS, Perona M, Desai R, Smith KJ, Latcham J, Fulleylove M, Richardson JC, Hardy J, Edwards FA, 2015. A genomewide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology. Cell Rep 10(4), 633–644. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging I, 2017. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 74(5), 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM, 1984. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7), 939–944. [DOI] [PubMed] [Google Scholar]
- McKhann GM, 2011. Changing concepts of Alzheimer disease. JAMA 305(23), 2458–2459. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, 2004. Mild cognitive impairment as a diagnostic entity. J Intern Med 256(3), 183–194. [DOI] [PubMed] [Google Scholar]
- Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt RE, Fenoglio C, Rinker J 2nd, Naismith RT, Panina-Bordignon P, Passini N, Galimberti D, Scarpini E, Colonna M, Cross AH., 2008. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 131(Pt 11), 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Deming Y, Del-Aguila JL, Ghezzi L, Holtzman DM, Fagan AM, Fenoglio C, Galimberti D, Borroni B, Cruchaga C, 2016. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol 131(6), 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, Lippa C, Josephs KA, Knopman DS, White CL 3rd, Caselli R, Mackenzie IR, Miller BL, Boczarska-Jedynak M, Opala G, Krygowska-Wajs A, Barcikowska M, Younkin SG, Petersen RC, Ertekin-Taner N, Uitti RJ, Meschia JF, Boylan KB, Boeve BF, Graff-Radford NR, Wszolek ZK, Dickson DW, Rademakers R, Ross OA, 2013. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee V, Trojanowski J, 2009a. Cerebrospinal Fluid Biomarker Signature in Alzheimer’s Disease Neuroimaging Initiative Subjects. Ann. Neurol 65(4), 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee V, Trojanowski J, 2009b. Cerebrospinal Fluid Biomarker Signature in lzheimer's Disease Neuroimaging Initiative Subjects. Ann. Neurol. 65(4), 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Zhao L, Goldstein F, Cellar J, Lah J, 2014. Biomarkers for predicting cognitive decline in those with normal cognition. J Alzheimers Dis 40(3), 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Calvet M, Araque Caballero MA, Kleinberger G, Bateman RJ, Fagan AM, Morris JC, Levin J, Danek A, Ewers M, Haass C, Dominantly Inherited Alzheimer N, 2016a. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci Transl Med 8(369), 369ra178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Calvet M, Kleinberger G, Araque Caballero MA, Brendel M, Rominger A, Alcolea D, Fortea J, Lleo A, Blesa R, Gispert JD, Sanchez-Valle R, Antonell A, Rami L, Molinuevo JL, Brosseron F, Traschutz A, Heneka MT, Struyfs H, Engelborghs S, Sleegers K, Van Broeckhoven C, Zetterberg H, Nellgard B, Blennow K, Crispin A, Ewers M, Haass C, 2016b. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med 8(5), 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, Householder E, Ayutyanont N, Roontiva A, Bauer RJ, Eisen P, Shaw LM, Davatzikos C, Weiner MW, Reiman EM, Morris JC, Trojanowski JQ, Alzheimer’s Disease Neuroimaging I, 2013. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun 1(1), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM, Colonna M, 2017. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 170(4), 649–663 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voevodskaya O, Simmons A, Nordenskjold R, Kullberg J, Ahlstrom H, Lind L, Wahlund LO, Larsson EM, Westman E, Alzheimer’s Disease Neuroimaging I, 2014. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Frontiers in aging neuroscience 6, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton RL, Soto-Ortolaza AI, Murray ME, Lorenzo-Betancor O, Ogaki K, Heckman MG, Rayaprolu S, Rademakers R, Ertekin-Taner N, Uitti RJ, van Gerpen A, Wszolek ZK, Smith GE, Kantarci K, Lowe VJ, Parisi JE, Jones DT, Savica R, Graff-Radford J, Knopman DS, Petersen RC, Graff-Radford NR, Ferman TJ, Dickson DW, Boeve BF, Ross OA, Labbe C, 2016. TREM2 p.R47H substitution is not associated with dementia with Lewy bodies. Neurol Genet 2(4), e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M, 2015. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160(6), 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Ewers M, Teipel S, Burger K, Wallin A, Blennow K, He P, McAllister C, Hampel H, Shen Y, 2007. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry 64(6), 718–726. [DOI] [PubMed] [Google Scholar]