Abstract
Zinc is the second most abundant metal in human and serves as an essential trace element in the body. During the past decades, zinc has been found to play important roles in central nervous system, such as the development of neurons and synaptic activities. An imbalance of zinc is associated with brain diseases. The blood-brain barrier (BBB) maintains the homeostasis of the microenvironment, regulating the balance of zinc in the brain. A compromised BBB is the main cause of severe complications in cerebral ischemic patients, such as hemorrhage transformation, inflammation and edema. Recent studies reported that zinc in the brain may be a potential target for integrative protection against ischemic brain injury. Although zinc has long been regarded as important transmitters in central nervous system, the critical role of zinc dyshomeostasis in damage to the BBB has not been fully recognized. In this review, we summarize the role of the BBB in regulating homeostasis of zinc in physiological conditions and the effects of changes in zinc levels on the permeability of the BBB in cerebral ischemia. The integrity of BBB maintains the homeostasis of zinc in pathological conditions, while the balance of zinc in the brain and the circulation maintains the normal function of the BBB. Interrupting the zinc/BBB system will disturb the microenvironment in the brain, leading to pathological diseases. In stroke patients, zinc may serve as a potential target for protecting the BBB and reducing hemorrhage transformation, inflammation and edema.
Keywords: zinc, blood-brain barrier, endothelial cells, ischemia, zinc transporter
1. Introduction
Zinc is the second most abundant metal and serves as an essential trace element in the human body. A large amount of zinc is stored in neurons, especially in synaptic vesicles (Andreini et al. 2006; Frederickson et al. 2000). During the past decades, zinc has been found to play important roles in the central nervous system, especially in neuronal and synaptic activities, because zinc is released into the synaptic cleft during active neurotransmission. Under normal conditions, zinc released from the synaptic vesicles modulates both ionotropic and metabotropic post-synaptic receptors (Marger et al. 2014;Szewczyk 2013). Imbalance of zinc is related to mental retardation, epilepsy and neurodegenerative diseases (Berger and O’Leary 1975; Khan 2016; Morris and Levenson 2012; Ordak et al. 2018; Pochwat et al. 2015).
However, the critical role of zinc dyshomeostasis in the blood-brain barrier (BBB) damage has not been fully recognized. The BBB, a special structure in the brain, keeps the homeostasis of microenvironment, regulating the balance of zinc in the brain. The BBB is mainly composed of endothelium of brain microvessels. Tight junction proteins seal the space between endothelial cells and maintain the integrity of the BBB. The surroundings, such as extracellular matrix, astrocytes and pericytes, regulate the opening of the BBB. A compromised BBB is the main cause of severe complications in cerebral ischemic patients, including hemorrhage transformation, inflammation and edema (Dahl et al. 2018; Keep et al. 2018; Lu et al. 2018; Manaenko et al. 2018; Smith et al. 2018; Yuen et al. 2018). Recent studies reported that zinc in the brain may be a potential target for integrative protection against ischemic brain injury (Pan et al. 2015; Qi et al. 2016; Zhao et al. 2014).
In this review, we summarize the role of the BBB in regulating homeostasis of zinc in physiological conditions and the effects of zinc alteration on BBB permeability in cerebral ischemia.
2. The Homeostasis of Zinc in Physiological Conditions
Most of the zinc in the human body is found in zinc containing proteins. A bioinformatics study in human reported that over 2,800 proteins are potentially zinc-binding, which is approximately 10 percent of all proteins in human proteome, serving signaling, catalytic, and structural roles (Andreini et al. 2006). Most of the zinc-binding proteins have zinc-finger motifs, in which a zinc ion binds four invariant cysteine and/or histidine residues (Cys2/His2 being the most common types) to form a stable structure (Andreini et al. 2006; Zhou et al. 2015; Zhou et al. 2011), regulating protein-DNA, protein-RNA, and protein-protein interactions (Blackshear and Perera 2014; Ding et al. 2017; Huestis et al. 2016; Khmeleva et al. 2013; Nyborg and Peersen 2004; Zhou et al. 2016).
Beside that firmly bound in proteins, free zinc ions (Zn2+) or loosely bound, which are histochemically detectable and removable by chelator agents, are widely present in all organs, tissues, fluids, and secretions. More than 95 percent of zinc is located in the fat-free mass (testes, muscle, liver, and brain tissue). The distribution of zinc is well summarized previously (Hess et al. 2007).
Therefore, free zinc and zinc-binding proteins constitute zinc pools in human tissue, keeping the homeostasis of zinc in physiological conditions.
2.1. Zinc Pool in Plasma
A study with 134 healthy adults analyzed the concentration of free zinc in sera by electrothermal atomic absorption spectrophotometry, showing that free zinc in plasma is maintained at concentrations of 12–15 μmol/L. Most plasma zinc ions are bonded with albumin (12.4 +/− 2.2 μmol/l) and α2-macroglobulin (2.4 +/− 0.6 μmol/l), which serve as a zinc pool in circulation (Blindauer et al. 2009; Foote and Delves 1984). Another study measured the exchange of zinc in plasma zinc pool in seven healthy men using inductively coupled plasma mass spectrometry. Plasma zinc mass turned over at an average of 5.3 times per hour, demonstrating the active exchange in exchangeable zinc pool in plasma and active functions of zinc under physiological conditions (Pinna et al. 2001). The rest of plasma zinc (less than 0.01%) is ultrafilterable and complexed with amino acids, primarily cysteine and histidine (Hernell and Lonnerdal 2003; Hess et al. 2007; Samman et al. 2014). These studies demonstrate the important role of the zinc pool to keep the dynamic balance in plasma.
2.2. Distribution of Zinc in the Brain
Zinc ranks second in the trace metal elements in brain tissue after iron. Frederickson et al utilized the co-staining of zinc specific Timm-Danscher staining and neuronal specific Nissl staining to detect the distribution of zinc in the brain of rats. They found that zinc is highly concentrated in the neuronal-rich area, such as hippocampus, amygdala, cerebral cortex, and olfactory cortex (Frederickson et al. 2000). Recent studies suggest that the free zinc plays crucial roles in neuronal modulation, synaptic plasticity, learning, and memory (Blakemore and Trombley 2017; Corceiro et al. 2018; Kennedy et al. 2017; Nam et al. 2017).
2.3. Plasma Zinc Across the BBB
Studies reported that the BBB separates zinc between plasma and brain under normal physiological conditions. A study in sheep reported that the BBB kept the balance of zinc in brain tissue in physiological conditions. The authors infused zinc acetate solution (1 mg Zn/ml) intravenously at a rate of 1.0 ml/min for 30 min and then continuously at 0.125 ml/min for 7 h. Data showed that the infusion increased plasma zinc (pZn) concentration approximately 10-fold without altering the zinc concentration in cerebrospinal fluid (CSF Zn), suggesting that the BBB prevented pZn from penetrating into the brain (Blair-West et al. 1990). Another study in rats revealed that the replacement ratios of 65Zn to zinc in tissues were 40% in plasma, 16–20% in liver, kidney, blood, and prostate, while the replacement ratios in testis and cerebrum were very low (2–3%), suggesting that zinc uptakes in testis and brain may be regulated by the blood-testis barrier and BBB (Furuta et al. 1999).
Another study showed that increased cranial blood osmolality (4 M NaCl) or electroconvulsive shock (140 V, 2 s), which temporarily opens the BBB, increased CSF Zn up to the level of pZn and then fell back to the baseline of CSF Zn in normal condition (Blair-West et al. 1990), demonstrating that opening the BBB increases zinc translocation from blood to brain. These studies indicate that the BBB separates zinc between plasma and brain under normal physiological conditions.
2.4. Zinc Uptake in Endothelial Cells
However, a slight zinc exchange could be observed between the blood and endothelial cells under normal conditions. An interesting study using 65Zn isotope in normal rats measured the zinc uptake by brain from blood and the BBB permeability to zinc (Pullen et al. 1991). 65ZnCl2 in a bolus of normal saline was injected intravenously and 125I-albumin was also administrated as a vascular marker. At the first 30min of circulation, 65Zn fluxes between blood and brain were bidirectional to attain a steady state. After 30 min of circulation, 65Zn uptake became unidirectional from blood to brain, with a K value of 5×10−4 ml/min/g. The abilities of 65Zn uptake in different regions were ranked: cerebellum > midbrain > cortex > hippocampus> medulla > hypothalamus (Pullen et al. 1991). It is worth noting that there is a difference in value between the exchanging compartment in the brain and the brain’s plasma space. Therefore, there would be another compartment(s) where 65Zn rapidly exchanges. As they found very slight change of cisternal CSF during 5h of circulation, they speculated that the endothelial cell luminal might be the potential compartment to uptake 65Zn. This speculation is very reasonable. Recent studies have verified that endothelial cells are able to uptake zinc indeed by zinc transporters (see Section 2.5).
2.5. Zinc Transporters in Endothelial Cells
Several families of zinc transporter proteins have been identified to regulate zinc concentration and maintain zinc homeostasis. The Zrt- and Irt-like protein (ZIP) family increases cytoplasmic zinc by increasing zinc uptake, thereby decreasing plasma zinc and prompting zinc release from cellular vesicles. On the contrary, the zinc transporter family (ZnTs) reduces cytoplasmic zinc by exporting cellular zinc, which increases plasma zinc concentrations and helps the movement of cytoplasmic zinc into intracellular organelles (Hess et al. 2007). Metallothionein (MT), an inducible protein with a low molecular mass, has high affinity for metals, such as zinc. A high concentration of zinc induces the MT protein expression and forces formation of a zinc-MT binding complex, keeping the homeostasis of zinc in cells. The homeostasis of zinc in neurons has been well summarized (Colvin et al. 2000; Colvin et al. 2003). Here, we mainly focus on the regulation of zinc level in endothelial cells.
As zinc is especially rich in brain tissue and regulates BBB function, do brain-derived endothelial cells have any particular response to zinc alteration? An interesting study reported differential responses of brain and non-brain endothelial cells to zinc depletion (Di et al. 2005). The authors demonstrated that zinc depletion by zinc chelator, N,N,N’,N’-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) reduces transendothelial electrical resistance (TEER) in non-brain endothelial cells, such as human umbilical vein endothelial cells, human aortic endothelial cells, and human iliac vein endothelial cells. On the contrary, zinc depletion enhanced barrier function in human brain microvessel endothelial cells, suggesting the presence of specific mechanisms to counteract zinc deficiency in brain endothelial cells. However, the authors did not further investigate the specific mechanism in this unique response.
Recent studies have demonstrated that zinc transporters play critical roles in keeping zinc homeostasis. Lehmann et al reported that moderate zinc deficient treatment (1.5 micromol/L) increased the rate of isotope 65zinc uptake into endothelial cells and then transport 65zinc across the BBB into the albuminal chamber. On the contrary, moderate high zinc (50 micromol/L) decreased the rate of zinc uptake into endothelial cells but increased the rate of zinc release into the brain (Lehmann et al. 2002). Further in vivo studies are warranted to verify the capacity and mechanism of endothelial cells to uptake zinc.
3. Effects of Zinc Deficiency or Overload on the Damages of the BBB in Pathological Conditions
3.1. Increasing the Permeability of the BBB
As zinc is stored highly in synaptic vesicles and released from the terminals of neurons during neuronal diseases, most of literatures focus on the role of zinc in neurons. Recent studies revealed that the break of zinc homeostasis contributes to BBB disruption in pathological conditions.
On the one hand, zinc deficiency results in the BBB damages when exposed to cerebral ischemia. Using dynamic Magnetic Resonance Imaging (MRI), the BBB permeability was evaluated in zinc-deficient male weanling rats following 3 days exposure to hyperoxia (85% O2). Three-day exposure of hyperoxia caused a marginal loss of BBB integrity. However, zinc deficiency resulted in a significant increase in BBB permeability. In addition, MRI-visible free water was elevated in zinc deficient brains following hyperoxia treatment, indicating that the loss of BBB integrity may be associated with neuronal edema, which was mediated by oxidative stresses as measured by the ratio of oxidized to reduced glutathione (GSSG:GSH) (Noseworthy and Bray 2000).
On the other hand, zinc overload deteriorates BBB disruption following cerebral ischemia. Our recent study reported that zinc overload exacerbated BBB permeability in cultured endothelial monolayer under oxygen glucose deprivation conditions (cellular ischemic BBB model), with more leakage of FITC-labeled albumin from up-chamber to bottom-chamber of endothelial monolayer. Moreover, increased zinc accumulation was observed in isolated microvessels and in situ in the brain of cerebral ischemic rats. Treatment with a specific zinc chelator, TPEN, greatly attenuated BBB permeability as measured by Evan’s Blue leakage and magnetic resonance imaging. Our findings revealed that zinc accumulation in microvessels leads to loss of tight junction proteins (Occludin and Claudin-5) and cerebral ischemia-induced BBB damage, which was mediated through upregulating superoxide and matrix metalloproteinases (Qi et al. 2016).
However, the BBB permeability does not seem to respond to chronic zinc overload. Yorulmaz et al evaluated the effect of chronic zinc treatment on BBB permeability in pentylenetetrazole-induced epileptic seizures model of rats. Chronic oral ZnCl2 treatment (2 months) did not show significantly difference in BBB permeability (Yorulmaz et al. 2013).
It seems that chronic zinc overload may have time to develop compensatory pathways to counteract zinc overload, such as increasing zinc secretion from endothelial cells. Further studies are needed to investigate the regulation of compensatory pathways to overcome the zinc overload, which may have been induced to overcome rapid zinc accumulation such as cerebral ischemia.
These studies suggested that zinc homeostasis is critical to BBB integrity. Both zinc deficiency and zinc overload lead to BBB disruption in pathological conditions.
3.2. Increasing tPA Toxicity
Tissue plasminogen activator (tPA) is abundantly secreted in endothelial cells and the tPA-plasminogen-plasmin cascade system participates in thrombolysis, wound healing, and cancer progression (Hacke et al. 2008; Ibrahim et al. 2016; Lund et al. 2006; Montalvan, V et al. 2018; Story et al. 2017). Recent studies reported that alteration in zinc concentration regulates the levels of tPA in endothelial cells. Cho et al reported that 40 microM ZnCl2 incubation for 4 h significantly increased mRNA and protein levels of tPA in brain endothelial cells (Cho et al. 2013). Another study showed that free zinc enhanced tPA-induced cell death, which was substantially attenuated by zinc chelator TPEN, suggesting that zinc dyshomeostasis deteriorated tPA toxicity (Kim et al. 2015). These studies indicated that zinc might serve as a novel target to regulate tPA level, providing potential treatment for stroke, trauma, and cancer.
3.3. Altering the Levels of Tight Junctions and F-Actin
Tight junctions and the cytoskeleton maintain the morphology and integrity of endothelial cells. Recent studies showed that the level of zinc regulates the expression of tight junctions and F-action in endothelial cells. A study in a Madin-Darby canine kidney (MDCK) cell monolayer reported the effects of zinc on tight junctions. It was demonstrated that pathological levels of Zn2+ (>200 μM) significantly damaged the integrity of tight junctions (especially affecting efflux tight junctions opening), leading to the collapse of basolateral claudin-1, ZO-1, and F-actin via the GSK3beta/snail pathway (Xiao et al. 2018). Our recent study also demonstrated that zinc overload (150 μM) plus oxygen glucose deprivation (mimicking ischemic condition in vitro) lead to more severe degradation of occludin in brain endothelial cells than high concentration of zinc in normoxic condition, suggesting the pathological role of zinc in ischemic condition (Qi et al. 2016).
However, one study on aluminum toxicity obtained different results. It showed that zinc suppresses BBB injury in juvenile rats, which were exposed to aluminum. Zinc protected the integrity of the BBB and inhibited the decline of occludin and F-actin in endothelial cells (Song et al. 2008).
These different conclusions may be derived from the different level of base line of zinc. In ischemic condition, a lot of zinc has already accumulated in endothelial cells, leading to a high zinc level in microenvironment. Continuously increasing zinc will deteriorate the zinc dyshomeostasis and result in BBB disruption. On the contrary, aluminum exposure, which competitively inhibited zinc level, cause zinc deficiency in endothelial cells. Zinc treatment will help to recovery or return to the baseline of zinc, keeping the normal level of occludin and F-actin in endothelial cells. Therefore, the function of zinc may be different, depending on the status of zinc homeostasis.
3.4. Regulating Zinc Transporters in Endothelial Cells
Among the ZnTs family members, ZnT-1, −3, −4, −5, −6 are reported to express in the brain. It was reported that the expression of ZnT-1 and −2 were directly related to the fluctuation of Zn2+ in cells. On the contrary, ZnT-4 expression was little affected by cellular Zn2+ levels. ZnT-3 expression was generally found in synaptic vesicles, where locates a high concentration of histochemically reactive Zn2+. The role of ZnTs in neurons has been well summarized in several reviews (Colvin et al. 2000; Colvin et al. 2003).
Beside in the neurons, zinc transporters were also found to regulate zinc homeostasis in endothelial cells. Zinc transporters in endothelial cells have different responses when the zinc level fluctuates. Recent studies demonstrated that ZnT1 might be responsible for rapid fluctuations in zinc levels, but ZnT2 are more likely to be involved in maintaining zinc homeostasis in a long-term mechanism.
The longitudinal changes in zinc transport kinetics, metallothionein and zinc transporter expression in a porcine blood-brain barrier model were measured in response to a moderately excessive zinc environment. Zinc release from cells significantly increased after 12–24 hours of exposure, but fell back to baseline after 48–96 hours, as indicated by transport across the BBB. Expression of ZnT-1 increased by 169% within 12 h, but was no longer different from controls after 24 h. However, ZnT-2 and MT were elevated within 12 h and remained in a high level throughout the study (Bobilya et al. 2008). Moreover, ZnT-2 was found to sequester zinc into intracellular vesicles to maintain brain zinc homeostasis in longer-term zinc overload (Bobilya et al. 2008).
This study also showed that, unlike ZnTs, Zip1 kept constant during the entire experiments, suggesting that ZnTs are more sensitive and inducible to changing concentration of zinc than ZIPs. Pan et al reported that hypoxia/reoxygenation significantly decreased ZnT-1 protein level, whereas it did not change ZIP-1 protein levels, leading to intercellular free zinc accumulation and interrupting zinc homeostasis (Pan and Liu 2016). These studies suggested that zinc efflux might be a potential pathway to regulate intercellular zinc homeostasis during ischemic stroke.
4. Zinc Alteration in Circulation and Brain in Stroke Patients
Several clinical studies help us understand the breakdown of zinc homeostasis under ischemic conditions in human bodies. A clinical study with serum from 256 ischemic stroke patients and 180 healthy people demonstrated that the concentration of zinc was significantly lower in stroke patients than in healthy controls, suggesting that zinc represents an independent risk factor for stroke (Munshi et al. 2010).
A retrospective study in a single center compared the concentrations of serum zinc in 152 ischemic stroke and 72 transient ischemic attack (TIA) patients. All blood samples were obtained within 24 hours of admission and free zinc levels were analyzed by atomic absorption spectroscopy. The study showed that 35.7% of stroke patients had low zinc levels (65 mcg/dL). Stroke patients were more likely to have low zinc levels (odds ratio: 2.62, confidence interval: 1.92–3.57, P <0.003) compared to the TIA patients. The authors also reported that lower zinc levels (≤65 mcg/dL) are associated with severe strokes (National Institute of Health stroke scale >8) and independently associated with poor functional status at discharge (Modified Rankin Scale >3) (Bhatt et al. 2011).
The above two clinical studies reported the reduction of serum zinc after ischemic stroke, but the data need to be interpreted carefully. First, the reduction of zinc needs to be calibrated with the level of albumin and α2-macroglobulin in serum, as most of plasma zinc is associated with them. Second, atomic absorption spectroscopy measures the zinc atoms in the entire serum sample, being unable to distinguish between free zinc and combined zinc. It is unclear how free and combined zinc change respectively and which type of zinc is more associated with stroke. In addition, if serum zinc level is indeed reduced, it is not clear where the serum zinc goes in the ischemic condition. Unfortunately, it is not possible to obtain brain tissue of ischemic stroke patients to answer the questions.
Zinc level in the CSF from patients with other brain diseases than stroke may be used as references. A clinical study investigated zinc concentrations in CSF and serum in 28 healthy people and 74 patients with various neurological diseases, including multiple sclerosis, peripheral nervous system (poly-neuropathy, polyradiculitis) amyotrophic lateral sclerosis, Alzheimer’s disease, and other different neurological diseases with increased CSF protein (over 50 mg/ml) (Kapaki et al. 1989). Increased CSF Zn was found in the group of peripheral nervous system diseases and in the cases that had increased CSF protein concentrations. Neither group has abnormal level of serum zinc. This clinical research suggested that the compromised BBB may permit the passage of zinc to the subarachnoid space. However, this study did not mention the correlation between zinc and protein in the CSF, which need further investigations.
Conclusions and Perspectives
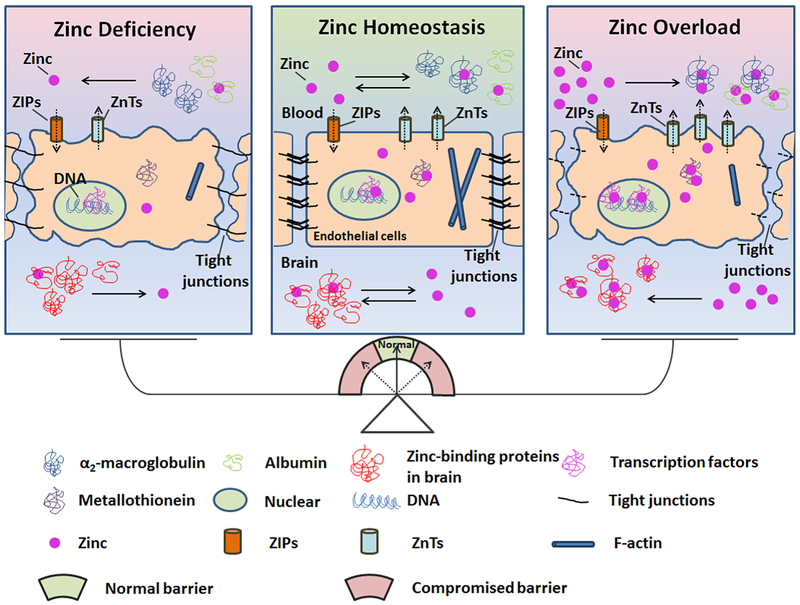
Although zinc has long been regarded as an important transmitter in central nervous system, the critical role of zinc dyshomeostasis in damage to the BBB has not been fully realized and understood. The integrity of the BBB maintains the homeostasis of zinc in pathological conditions, while the balance of zinc in the brain and in circulation maintains the normal function of the BBB. Interrupting the zinc/BBB system will damage the microenvironment in the brain, leading to pathological diseases, as illustrated in Figure 1. Zinc may serve as a potential target for protecting the BBB in stroke patients, and for reducing hemorrhage transformation, inflammation, and edema.
Figure 1.
Schematic diagram of the role of zinc homeostasis in the normal function of the BBB. Most zinc is found in zinc containing proteins, regulating protein-DNA, protein-RNA, and protein-protein interactions. Most plasma zinc is in binding with albumin and α2-macroglobulin, which serve as a zinc pool in circulation. The BBB separates the plasma zinc from the brain under physiological conditions. The ZIP and ZnTs families have been identified to regulate zinc concentration and keep zinc homeostasis in endothelial cells. The ZIP family increases cytoplasmic zinc by increasing zinc uptake, thereby decreasing plasma zinc and prompting zinc release. The ZnTs family reduces cytoplasmic zinc by exporting cellular zinc, which increases plasma zinc concentrations and helps the movement of cytoplasmic zinc into intracellular organelles. ZnTs are more easily inducible and sensitive to changes in concentration of zinc than ZIPs. Pathological insults, such as cerebral ischemia, break the zinc homeostasis and result in BBB disruption. The compromised BBB, which could not block plasma zinc into brain tissue, deteriorates the dyshomeostasis of zinc.
Acknowledgements:
This work was supported, in part, by grants from the National Natural Science Foundation of China (81571175, 81620108011) and U.S. National Institutes of Health (P30GM103400).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors have no conflict to declare.
References
- Andreini C, Banci L, Bertini I, Rosato A (2006) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5:196–201 [DOI] [PubMed] [Google Scholar]
- Berger ML, O’Leary JL (1975) Zinc distribution in mouse brain subsequent to hippocampal lesions. Arch Neurol 32:295–297 [DOI] [PubMed] [Google Scholar]
- Bhatt A, Farooq MU, Enduri S, Pillainayagam C, Naravetla B, Razak A, Safdar A, Hussain S, Kassab M, Majid A (2011) Clinical significance of serum zinc levels in cerebral ischemia. Stroke Res Treat 2010:245715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear PJ, Perera L (2014) Phylogenetic distribution and evolution of the linked RNA-binding and NOT1-binding domains in the tristetraprolin family of tandem CCCH zinc finger proteins. J Interferon Cytokine Res 34:297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair-West JR, Denton DA, Gibson AP, McKinley MJ (1990) Opening the blood-brain barrier to zinc. Brain Res 507:6–10 [DOI] [PubMed] [Google Scholar]
- Blakemore LJ, Trombley PQ (2017) Zinc as a Neuromodulator in the Central Nervous System with a Focus on the Olfactory Bulb. Front Cell Neurosci 11:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blindauer CA, Harvey I, Bunyan KE, Stewart AJ, Sleep D, Harrison DJ, Berezenko S, Sadler PJ (2009) Structure, properties, and engineering of the major zinc binding site on human albumin. J Biol Chem 284:23116–23124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobilya DJ, Gauthier NA, Karki S, Olley BJ, Thomas WK (2008) Longitudinal changes in zinc transport kinetics, metallothionein and zinc transporter expression in a blood-brain barrier model in response to a moderately excessive zinc environment. J Nutr Biochem 19:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MK, Sun ES, Kim YH (2013) Zinc-triggered induction of tissue plasminogen activator and plasminogen in endothelial cells and pericytes. Exp Neurobiol 22:315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Davis N, Nipper RW, Carter PA (2000) Zinc transport in the brain: routes of zinc influx and efflux in neurons. J Nutr 130:1484S–1487S [DOI] [PubMed] [Google Scholar]
- Colvin RA, Fontaine CP, Laskowski M, Thomas D (2003) Zn2+ transporters and Zn2+ homeostasis in neurons. Eur J Pharmacol 479:171–185 [DOI] [PubMed] [Google Scholar]
- Corceiro VN, Bastos FC, Matias CM, Dionisio JC, Santos RM, Rosario LM, Quinta-Ferreira RM, Quinta-Ferreira MEC (2018) Sulfamethoxazole induces zinc changes at hippocampal mossy fiber synapses from pregnant rats. Gen Physiol Biophys 37:213–221 [DOI] [PubMed] [Google Scholar]
- Dahl RH, Berg RMG, Taudorf S, Bailey DM, Lundby C, Larsen FS, Moller K (2018) A reassessment of the blood-brain barrier transport of large neutral amino acids during acute systemic inflammation in humans. Clin Physiol Funct Imaging 38:656–662 [DOI] [PubMed] [Google Scholar]
- Di CF, Siddharthan V, Paul-Satyaseela M, Kim KS (2005) Divergent effects of zinc depletion in brain vs non-brain endothelial cells. Biochem Biophys Res Commun 335:373–376 [DOI] [PubMed] [Google Scholar]
- Ding X, Zhou X, Cooper KL, Huestis J, Hudson LG, Liu KJ (2017) Differential sensitivities of cellular XPA and PARP-1 to arsenite inhibition and zinc rescue. Toxicol Appl Pharmacol 331:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote JW, Delves HT (1984) Albumin bound and alpha 2-macroglobulin bound zinc concentrations in the sera of healthy adults. J Clin Pathol 37:1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB (2000) Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr 130:1471S–1483S [DOI] [PubMed] [Google Scholar]
- Furuta S, Suzuki M, Toyama S, Miwa M, Sano H (1999) Tissue distribution of polaprezinc in rats determined by the double tracer method. J Pharm Biomed Anal 19:453–461 [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von KR, Wahlgren N, Toni D (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359:1317–1329 [DOI] [PubMed] [Google Scholar]
- Hernell O, Lonnerdal B (2003) Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace elements. Am J Clin Nutr 78:296–301 [DOI] [PubMed] [Google Scholar]
- Hess SY, Peerson JM, King JC, Brown KH (2007) Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull 28:S403–S429 [DOI] [PubMed] [Google Scholar]
- Huestis J, Zhou X, Chen L, Feng C, Hudson LG, Liu KJ (2016) Kinetics and thermodynamics of zinc(II) and arsenic(III) binding to XPA and PARP-1 zinc finger peptides. J Inorg Biochem 163:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SF, Issak M, Bayoumy AA, Abd El-Fatah DS (2016) Cutaneous (tPA) and Skeletal (TnI) mRNA as Markers of Aging in Contused Wound. J Forensic Sci 61:1007–1010 [DOI] [PubMed] [Google Scholar]
- Kapaki E, Segditsa J, Papageorgiou C (1989) Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acta Neurol Scand 79:373–378 [DOI] [PubMed] [Google Scholar]
- Keep RF, Andjelkovic AV, Xiang J, Stamatovic SM, Antonetti DA, Hua Y, Xi G (2018) Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab271678X18774666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RH, Wiqas A, Curley JP (2017) Evidence for mast cell-mediated zinc homeostasis: Increased labile zinc in the hippocampus of mast-cell deficient mice. Neurosci Lett 650:139–145 [DOI] [PubMed] [Google Scholar]
- Khan MZ (2016) A possible significant role of zinc and GPR39 zinc sensing receptor in Alzheimer disease and epilepsy. Biomed Pharmacother 79:263–272 [DOI] [PubMed] [Google Scholar]
- Khmeleva SA, Mezentsev YV, Kozin SA, Tsvetkov PO, Ivanov AS, Bodoev NV, Makarov AA, Radko SP (2013) Zinc-induced interaction of the metal-binding domain of amyloid-beta peptide with DNA. J Alzheimers Dis 36:633–636 [DOI] [PubMed] [Google Scholar]
- Kim HN, Kim TY, Yoon YH, Koh JY (2015) Pyruvate and cilostazol protect cultured rat cortical pericytes against tissue plasminogen activator (tPA)-induced cell death. Brain Res 1628:317–326 [DOI] [PubMed] [Google Scholar]
- Lehmann HM, Brothwell BB, Volak LP, Bobilya DJ (2002) Zinc status influences zinc transport by porcine brain capillary endothelial cells. J Nutr 132:2763–2768 [DOI] [PubMed] [Google Scholar]
- Lu L, Wang M, Yuan F, Wei X, Li W (2018) Roles of elevated 20HETE in the breakdown of blood brain barrier and the severity of brain edema in experimental traumatic brain injury. Mol Med Rep 17:7339–7345 [DOI] [PubMed] [Google Scholar]
- Lund LR, Green KA, Stoop AA, Ploug M, Almholt K, Lilla J, Nielsen BS, Christensen IJ, Craik CS, Werb Z, Dano K, Romer J (2006) Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J 25:2686–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaenko A, Yang P, Nowrangi D, Budbazar E, Hartman RE, Obenaus A, Pearce WJ, Zhang JH, Tang J (2018) Inhibition of stress fiber formation preserves blood-brain barrier after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab 38:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marger L, Schubert CR, Bertrand D (2014) Zinc: an underappreciated modulatory factor of brain function. Biochem Pharmacol 91:426–435 [DOI] [PubMed] [Google Scholar]
- Montalvan A,V, Rojas CZ, Aldave SR (2018) Controversies in cerebrovascular disease: High or low doses of recombinant tissue plasminogen activator to treat acute stroke? A literature review. Neurologia [DOI] [PubMed] [Google Scholar]
- Morris DR, Levenson CW (2012) Ion channels and zinc: mechanisms of neurotoxicity and neurodegeneration. J Toxicol 2012:785647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi A, Babu S, Kaul S, Shafi G, Rajeshwar K, Alladi S, Jyothy A (2010) Depletion of serum zinc in ischemic stroke patients. Methods Find Exp Clin Pharmacol 32:433–436 [DOI] [PubMed] [Google Scholar]
- Nam SM, Kim JW, Kwon HJ, Yoo DY, Jung HY, Kim DW, Hwang IK, Seong JK, Yoon YS (2017) Differential Effects of Low- and High-dose Zinc Supplementation on Synaptic Plasticity and Neurogenesis in the Hippocampus of Control and High-fat Diet-fed Mice. Neurochem Res 42:3149–3159 [DOI] [PubMed] [Google Scholar]
- Noseworthy MD, Bray TM (2000) Zinc deficiency exacerbates loss in blood-brain barrier integrity induced by hyperoxia measured by dynamic MRI. Proc Soc Exp Biol Med 223:175–182 [DOI] [PubMed] [Google Scholar]
- Nyborg JK, Peersen OB (2004) That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem J 381:e3–e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordak M, Bulska E, Jablonka-Salach K, Luciuk A, Maj-Zurawska M, Matsumoto H, Nasierowski T, Wojnar M, Matras J, Muszynska E, Bujalska-Zadrozny M (2018) Effect of Disturbances of Zinc and Copper on the Physical and Mental Health Status of Patients with Alcohol Dependence. Biol Trace Elem Res 183:9–15 [DOI] [PubMed] [Google Scholar]
- Pan R, Liu KJ (2016) ZNT-1 Expression Reduction Enhances Free Zinc Accumulation in Astrocytes After Ischemic Stroke. Acta Neurochir Suppl 121:257–261 [DOI] [PubMed] [Google Scholar]
- Pan R, Timmins GS, Liu W, Liu KJ (2015) Autophagy Mediates Astrocyte Death During Zinc-Potentiated Ischemia--Reperfusion Injury. Biol Trace Elem Res 166:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna K, Woodhouse LR, Sutherland B, Shames DM, King JC (2001) Exchangeable zinc pool masses and turnover are maintained in healthy men with low zinc intakes. J Nutr 131:2288–2294 [DOI] [PubMed] [Google Scholar]
- Pochwat B, Nowak G, Szewczyk B (2015) Relationship between Zinc (Zn (2+) ) and Glutamate Receptors in the Processes Underlying Neurodegeneration. Neural Plast 2015:591563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen RG, Franklin PA, Hall GH (1991) 65Zn uptake from blood into brain in the rat. J Neurochem 56:485–489 [DOI] [PubMed] [Google Scholar]
- Qi Z, Liang J, Pan R, Dong W, Shen J, Yang Y, Zhao Y, Shi W, Luo Y, Ji X, Liu KJ (2016) Zinc contributes to acute cerebral ischemia-induced blood-brain barrier disruption. Neurobiol Dis 95:12–21 [DOI] [PubMed] [Google Scholar]
- Samman S, Crossett B, Somers M, Bell KJ, Lai NT, Sullivan DR, Petocz P (2014) Metabolic profiling of plasma amino acids shows that histidine increases following the consumption of pork. Diabetes Metab Syndr Obes 7:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NM, Giacci MK, Gough A, Bailey C, McGonigle T, Black AMB, Clarke TO, Bartlett CA, Swaminathan IK, Dunlop SA, Fitzgerald M (2018) Inflammation and blood-brain barrier breach remote from the primary injury following neurotrauma. J Neuroinflammation 15:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Xue Y, Liu X, Wang P, Liu L (2008) Effects of acute exposure to aluminum on blood-brain barrier and the protection of zinc. Neurosci Lett 445:42–46 [DOI] [PubMed] [Google Scholar]
- Story M, Kwon SK, Robinson R, Fortis S (2017) Acute cor pulmonale due to pulmonary tumour thrombotic microangiopathy from renal cell carcinoma. BMJ Case Rep 2017: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B (2013) Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Yuan L, He W, Yang X (2018) Zinc ions regulate opening of tight junction favouring efflux of macromolecules via the GSK3beta/snail-mediated pathway. Metallomics 10:169–179 [DOI] [PubMed] [Google Scholar]
- Yorulmaz H, Seker FB, Demir G, Yalcin IE, Oztas B (2013) The effects of zinc treatment on the blood-brain barrier permeability and brain element levels during convulsions. Biol Trace Elem Res 151:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen NY, Chechneva OV, Chen YJ, Tsai YC, Little LK, Dang J, Tancredi DJ, Conston J, Anderson SE, O’Donnell ME (2018) Exacerbated brain edema in a rat streptozotocin model of hyperglycemic ischemic stroke: Evidence for involvement of blood-brain barrier Na-K-Cl cotransport and Na/H exchange. J Cereb Blood Flow Metab271678X18770844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Pan R, Li S, Luo Y, Yan F, Yin J, Qi Z, Yan Y, Ji X, Liu KJ (2014) Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke 45:1139–1147 [DOI] [PubMed] [Google Scholar]
- Zhou X, Cooper KL, Huestis J, Xu H, Burchiel SW, Hudson LG, Liu KJ (2016) S-nitrosation on zinc finger motif of PARP-1 as a mechanism of DNA repair inhibition by arsenite. Oncotarget 7:80482–80492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Cooper KL, Sun X, Liu KJ, Hudson LG (2015) Selective Sensitization of Zinc Finger Protein Oxidation by Reactive Oxygen Species through Arsenic Binding. J Biol Chem 290:18361–18369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG (2011) Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J Biol Chem 286:22855–22863 [DOI] [PMC free article] [PubMed] [Google Scholar]