Abstract
Objective
To evaluate the quality of enhancement and solid organ lesion depiction using weight-based IV contrast dosing calculated by injector software versus fixed IV contrast dose in oncologic abdominal CT examinations.
Methods
This Institutional Review Board-exempt retrospective cohort study included 134 patients who underwent single-phase abdominal CT before and after implementation of weight-based IV contrast injector software. Patient weight, height, body mass index and body surface area were determined. Two radiologists qualitatively assessed examinations (4 =markedly superior to −4 for markedly inferior), and Hounsfield unit measurements were performed.
Results
Enhancement (estimated mean −0.05, 95% CI [−0.19 to 0.09], p = 0.46) and lesion depiction (estimated mean −0.01, 95% CI [−0.10 to 0.07], p = 0.79) scores did not differ between CT examinations using weight-based IV contrast versus fixed IV contrast dosing when a minimum of 38.5 g of iodine was used. However, the scores using weight-based IV contrast dosing were lower when the injector software calculated and delivered less than 38.5 g of iodine (estimated mean −0.81, 95% CI [−1.06 to −0.56], p < 0.0001). There were no significant differences in measured Hounsfield units between the CT examinations using weight-based IV contrast dosing versus fixed IV contrast dosing.
Conclusions
Oncologic CT image quality was maintained or improved with weight-based IV contrast dosing using injector software when using a minimum amount of 38.5 g of iodine.
Keywords: iodine contrast, abdomen, IV, weight-based dosing, computed tomography
Introduction
The optimal CT scanning of patients requires a tailored approach that takes into account multiple factors inherent to the patient, scanner, contrast media and clinical scenario. With an increased focus on personalized medicine, precise and highly reproducible scanning is required, beyond what has already been achieved. Particularly in oncology, variations in image quality and contrast phase can limit the detection of disease or subtle interval changes in a tumor.1–3 Reducing this variability is becoming increasingly important as earlier predictive and accurate tumor response assessment to treatment is desired.
Contrast injection protocols have been an area of intense research in an effort to improve diagnostic accuracy.4–13 Specifically, the timing and degree of organ enhancement play critical roles in oncologic lesion detection.14 This has historically been optimized by altering the iodine contrast concentration, volume, and injection rate, as well as through the use of a saline chaser.15 While optimized contrast injection parameters are critical for robust imaging, further attention must be given to patient-related factors that affect imaging and can be partially mitigated. These include conditions such as congestive heart failure, which alters cardiovascular circulation and thus contrast enhancement, as well as body habitus.16–21
Studies have shown that maximum hepatic enhancement is inversely related to body weight.21, 22 Furthermore, work such as that by Kondo et al. has shown other parameters such as lean body weight to better correlate with contrast enhancement compared to total body weight (TBW).11, 12 Despite long-standing published literature, our experience has been that highly personalized injection protocols are not typically used, likely because this would require an operational change that would include calculations or additional software and because the results of a more simplified approach are typically adequate for clinical purposes.
Weight-based contrast injection can provide multiple benefits during imaging. First, larger patients are often underdosed with respect to intravenous (IV) contrast, and thus weight-based dosing (WBD) can improve contrast enhancement.23 Second, smaller patients typically receive more contrast than needed, which can potentially increase the risk of contrast-induced neuropathy in at-risk patient populations.24–28 Third, WBD can allow more precise tailoring of the IV contrast load based on clinical necessity such as allowing more contrast for the presurgical staging of the liver versus less contrast for the clinical evaluation of possible appendicitis. In smaller patients, there are also potential cost-savings, especially when lower kVp protocols are used since similar enhancement can be obtained at lower IV contrast doses.29–33
The purpose of our study was to evaluate abdominal CT for quality of contrast enhancement, solid organ lesion depiction and contrast volume distribution across patient sizes between a commercially-available injector software that uses weight-based IV contrast injection and our fixed contrast dose oncologic protocols. We also correlated the calculated contrast volumes from the scanner software to TBW, body mass index (BMI), and body surface area (BSA).
Materials and Methods
This Health Insurance Portability and Accountability Act compliant retrospective cohort study was approved by our quality improvement board (Institutional Review Board–exempt) and the need for informed consent was waived.
PATIENT POPULATION
After instituting the use of weight-based contrast IV volume adjustment on a single scanner, patients were selected (66 men, 68 women; mean age, 59 years; age range, 23–80 years). Power analysis assumed a 0.5 difference in the mean quality scores and a standard deviation of 1; 128 patients would yield 80% power to detect this difference with a 2-sided type I error rate of 5%. The radiology information system at our institution, a tertiary oncologic center, was searched for patients who had undergone contrast-enhanced computed tomography (CT) scanning of the abdomen from 12/1/2016 – 1/15/2017 with our single-phase WBD protocol using WBD software and who also had an otherwise equivalent fixed contrast dose prior examination for comparison. This search yielded matched protocol specifications and injection parameters without the need of exclusion criteria.
The age, sex, weight, height, primary cancer type, and BMI were recorded for each patient. BSA was calculated using two equations, BSA1 ((kg × cm)1/2)/60)34 and BSA2 (0.1173 × kg0.6466). BSA1 is the commonly used Mosteller method, which is also used at our institution for the calculation of certain chemotherapeutic dosing regimens. BSA2 is another commonly used calculation elsewhere, which has been reported to be more accurate in obese patients than a third BSA calculation using the Du Bois equation.35 The number of days between the WBD and fixed contrast dose examinations was recorded.
IMAGING PARAMETERS
All patients underwent CT scanning of the abdomen utilizing an identical imaging protocol performed on a Revolution CT system (GE Healthcare): gantry speed of 0.7 seconds, pitch of 0.5:1, table speed of 40 mm/rotation, beam collimation of 80 mm with detector configuration of 128 × 0.6 mm, and 120 kVp using tube current modulation.
Standard routine protocols at our institution vary injections parameters based on axial digital field of view (DFOV) selected by the technologist: patients with a prescribed DFOV of 40 and below receive 125 mL of IV contrast injected at 3 mL/s and those with a DFOV of 42 and above receive 150 mL of IV contrast injected at 4 mL/s. The type of IV contrast is selected based on an estimated glomerular filtration rate cutoff of 45 mL/min/1.73 m2. In our study, 129 patients received 350 mg of iodine per mL as iohexol (Omnipaque 350; General Electric, Waukesha, WI) and 5 patients received 320 mg of iodine per mL as iodixanol (Visipaque 320; General Electric). The WBD studies were matched to fixed dose examinations for identical IV contrast injection rate and contrast type.
Certegra® P3T® software (Bayer HealthCare, LLC; Whippany, NJ) was used to prescribe contrast volume based on input values of weight and iodine concentration using a weight factor of 0.6 gI/kg and maximum volume of 150 mL. This was the highest weight factor allowed, which empirically provided the best match to our standard examinations.
WBD and fixed dose examinations were performed with bolus tracking using a 100–Hounsfield unit (HU) trigger value in the abdominal aorta at the level of the celiac artery and a scan delay of 46 s to obtain a portovenous phase of scanning followed by a 120 s delay phase of the kidneys and urinary bladder.
Patients were categorized as large and small based on DFOV size into Group L (DFOV of 42 and above) and Group S (DFOV of 40 and below), respectively. Groups S was further divided by the amount of IV contrast suggested by the injection software. During the initial assessment of this software at our institution, we empirically noted that the enhancement quality was noticeably inferior below an approximate software suggested level of 110 mL of IV contrast (38.5 g of iodine). Therefore, we subdivided Group S into Slow and Shigh for patients receiving less than or more than 38.5 g of IV iodine, respectively.
QUALITATIVE ANALYSIS
Two radiologists (S.G. and N.W.-B., with 5 and 7 years of post-fellowship experience in abdominal imaging) qualitatively assessed the quality of contrast enhancement in each WBD examination and the relevant comparison examination using 2.5 mm reconstructions.
The examinations were reviewed independently over 3 sessions. The WBD examinations were rated for overall contrast enhancement quality against our standard high-quality oncologic comparison examinations using a previously published comparative scale.36 A score of 4 was to be given if the WBD examination was markedly superior (likely improving diagnosis), 3 for moderately superior (probable influence on diagnosis), 2 for mildly superior (possible influence on diagnosis), 1 for slightly superior (no influence on diagnosis), 0 for no clear difference between exams, −1 for slightly inferior (no influence on diagnosis), −2 for mildly inferior (possible influence on diagnosis), −3 for moderately inferior (probable influence on diagnosis), and −4 for markedly inferior (likely impairing diagnosis).
Using the same rating scale, images were also reviewed for focal, non-calcified lesions within the solid organs. The number, type, and organ location were recorded for each lesion and a single, overall score for lesion depiction was provided for each patient. The first reader for each case marked lesions so that the same lesions were reviewed by both readers; this study did not aim to assess lesion detection accuracy.
There was no time limit for image review.
QUANTITATIVE ANALYSIS
Authors not involved in the qualitative interpretation of images (K.B., radiology resident; L.V., abdominal radiology fellow) measured numerous two-dimensional regions of interest (ROI) on the WBD and comparison examinations: three regions within the liver at the level of the portal vein bifurcation, main portal vein (MPV), each hepatic vein, each psoas muscle, suprarenal and infrarenal inferior vena cava supra- and infra-renal (IVC), aorta at the celiac artery origin, spleen, and right and left subcutaneous fat. No ROIs were obtained on images with artifacts and any focal abnormality such as calcification was carefully avoided.
The contrast-to-noise ratio (CNR) relative to psoas muscle was calculated for the liver using the equation (ROIi − ROIm)/SD, where ROIi is the mean Hounsfield unit value for the anatomy of interest, ROIm is the mean Hounsfield unit value of psoas muscles, and SD is the mean image noise based on subcutaneous fat using the average standard deviation in Hounsfield units.37, 38 Liver-to-spleen ratios were calculated for the WBD and comparison examinations on both the portovenous and delayed contrast phases.
A value of ‘total vascular enhancement’ (TVE) was calculated, which represents the summation of HUs in the MPV, average of the three hepatic veins, aorta, infrarenal IVC and suprarenal IVC. TVE was also calculated per gram of injected iodine in each patient (TVE/gI).
A difference in HUs of 10 was considered clinically significant since previous studies have shown that reviewers are not consistently able to identify differences in HUs below this level.39, 40
STATISTICAL ANALYSES
Summaries of reader scores and of HU ROIs were provided in frequencies, mean, standard deviation, and range. Weighted Kappa statistics (quadratic weights) was used to assess agreement between two readers. A linear mixed model was used to estimate and compare mean reader scores based on DFOV status. Patient level was included in the mixed model as a random effect. HU ROIs were analyzed and compared by DFOV using analysis of variance. Comparisons between WBD and fixed dose scan were performed using the paired t-test. Linear regression was used to evaluate the relationship between weight, BMI, BSA1, and BSA2 compared with TVE/gI and contrast volume. All tests were two-sided and p-values of 0.05 or less were considered statistically significant. Statistical analyses were carried out using SAS version 9.4, and regression lines were plotted using JMP 12 (SAS Institute, Cary, NC).
Results
PATIENT CHARACTERISTICS
The 134 patients who met study criteria were assessed together and between subsets. The entire group mean BMI was 27.79 ± 7.26 kg/m2 with a mean WBD amount of injected IV iodine of 44.60 ± 6.25 g and mean amount of WBD injected iodine per weight of 0.61 ± 0.12 g/kg. Mean iodine injected per weight in WBD Groups Slow, Shigh, and L was 0.62 ± 0.03 g/kg (Range, 0.60–0.69 g/kg), 0.60 ± 0.06 g/kg (Range, 0.47–0.91 g/kg), and 0.54 ± 0.08 g/kg (Range, 0.18–0.61 g/kg), respectively. On fixed dose examinations, the respective iodine injected per weight was 0.73 ± 0.06 g/kg (Range, 0.65–0.86 g/kg), 0.57 ± 0.10 g/kg (Range, 0.38–0.91 g/kg), and 0.58 ± 0.12 g/kg (Range, 0.18–0.87 g/kg) (Table 1).
Table 1.
Patient characteristics with enhancement and contrast calculations.
| Weight (kg) |
BMI (kg/m2) |
BSA1 | BSA2 | Iodine Injected [WBD] (g) |
Iodine Injected [fixed] (g)* |
TVE/gI [WBD] |
TVE [WBD] |
TVE/gI [fixed]* |
TVE [fixed]* |
gI/kg [WBD] |
gI/kg [fixed]* |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (N=134) | ||||||||||||
| Min | 46 | 17.82 | 1.42 | 1.39 | 32.20 | 38.89 | 12.21 | 623.99 | 9.47 | 490.41 | 0.18 | 0.18 |
| Max | 285 | 86.04 | 3.80 | 4.54 | 52.15 | 52.54 | 30.13 | 1211.18 | 29.53 | 1261.74 | 0.91 | 0.91 |
| Mean | 79.36 | 27.79 | 1.86 | 1.91 | 44.60 | 45.73 | 19.96 | 872.48 | 19.56 | 882.64 | 0.58 | 0.61 |
| SD | 25.02 | 7.26 | 0.19 | 0.22 | 6.25 | 4.78 | 3.86 | 116.56 | 4.06 | 149.94 | 0.07 | 0.12 |
| Group L (DFOV 42–48) (N=56) | ||||||||||||
| Min | 59 | 22.04 | 1.58 | 1.64 | 32.90 | 43.61 | 12.21 | 623.99 | 9.47 | 490.41 | 0.18 | 0.18 |
| Max | 285 | 86.04 | 3.80 | 4.54 | 52.15 | 52.54 | 24.83 | 1093.94 | 25.86 | 1201.54 | 0.61 | 0.87 |
| Mean | 93.52 | 31.79 | 2.09 | 2.19 | 48.59 | 51.12 | 17.64 | 849.44 | 16.73 | 853.38 | 0.54 | 0.58 |
| SD | 30.38 | 8.64 | 0.31 | 0.40 | 4.36 | 1.69 | 2.89 | 114.06 | 3.50 | 167.55 | 0.08 | 0.12 |
| Group Shigh(DFOV 40 & Below >110 mL IV contrast) (N=49) | ||||||||||||
| Min | 46 | 18.66 | 1.42 | 1.39 | 37.66 | 40.00 | 14.93 | 669.99 | 14.56 | 601.50 | 0.47 | 0.38 |
| Max | 109 | 39.08 | 2.25 | 2.44 | 52.08 | 43.79 | 28.84 | 1211.18 | 25.53 | 1089.88 | 0.91 | 0.91 |
| Mean | 75.94 | 26.42 | 1.89 | 1.92 | 45.20 | 41.88 | 20.18 | 905.11 | 20.19 | 845.54 | 0.60 | 0.57 |
| SD | 11.83 | 4.53 | 0.17 | 0.19 | 4.45 | 1.06 | 3.18 | 122.56 | 2.40 | 103.52 | 0.06 | 0.10 |
| Group Slow(DFOV 40 & Below <110 mL IV contrast) (N=29) | ||||||||||||
| Min | 51 | 17.82 | 1.47 | 1.49 | 32.20 | 38.89 | 20.07 | 725.45 | 18.40 | 793.89 | 0.60 | 0.65 |
| Max | 64 | 25.15 | 1.77 | 1.73 | 38.43 | 43.75 | 30.13 | 1054.57 | 29.53 | 1261.74 | 0.69 | 0.86 |
| Mean | 57.79 | 22.37 | 1.61 | 1.62 | 35.87 | 41.82 | 24.06 | 861.83 | 23.96 | 1001.80 | 0.62 | 0.73 |
| SD | 4.07 | 1.72 | 0.09 | 0.07 | 1.67 | 1.02 | 2.91 | 100.97 | 2.80 | 120.21 | 0.03 | 0.06 |
TVE = total vascular enhancement, BSA = body surface area, WBD = weight-based dosing, BMI = body mass index,
refers to data from comparison fixed contrast dose examinations
The frequency of primary neoplasms in descending order were melanoma (N=23), breast (N=19), sarcoma (N=19), colon (N=15), prostate (N=9), renal cell carcinoma (N=7), lung (N=6), squamous cell (N=6), endometrial (N=5), gastric (N=4), peritoneal (N=3), pancreas (N=3), biliary (N=3), small intestine (N=2), appendiceal (N=2), lymphoma (N=2), testicular (N=2) and single cases of epithelioid angiomyolipoma, desmoplastic small round cell tumor, ovarian, cervical and esophageal malignancies.
The mean number of days between WBD and comparison examinations was 113 ±77 days.
QUALITATIVE IMAGE ANALYSIS
Enhancement scores and lesion depiction scores of WBD versus fixed dose examinations were significantly lower in the Slow group, whereas Groups L and Shigh scores were not significantly different. (Table 2). With a volume threshold difference of 15 mL between IV contrast usage of WBD and comparison examinations, a higher volume was used in 17 WBD cases, a lower volume was used in 32 cases, and similar volumes were used in 85 cases. This threshold was chosen because it is a conservative estimate of IV contrast volume necessary to increase HUs by more than 10 in the liver.22, 39, 40
Table 2.
Estimated mean reader scores comparing DFOV groups when assessing WBD to fixed-contrast dose examinations.
| Score Type | DFOV Group |
Compared two levels | Estimated Difference | Standard Error |
LCL | UCL | P-value | |
|---|---|---|---|---|---|---|---|---|
| Overall contrast enhancement | L | Slow | 0.67 | 0.16 | 0.35 | 0.98 | 0.0001 | |
| L | Shigh | −0.19 | 0.14 | −0.46 | 0.07 | 0.33 | ||
| Slow | Shigh | −0.86 | 0.16 | −1.18 | −0.54 | <.0001 | ||
| Lesion depiction | L | Slow | 0.25 | 0.09 | 0.07 | 0.44 | 0.022 | |
| L | Shigh | −0.14 | 0.08 | −0.30 | 0.03 | 0.23 | ||
| Slow | Shigh | −0.39 | 0.10 | −0.59 | −0.20 | 0.0004 | ||
Significant statistical differences are in bold, DFOV = digital field of view, WBD = weight-based dosing LCL = 95% lower confidence limit, UCL = 95% upper confidence limit
The frequencies of reader scores are listed in Table 3. The readers identified 477 lesions: 238 within the kidneys (218 cysts, 20 malignant lesions), 195 within the liver (80 cysts, 43 malignant lesions, 15 hemangiomas, 57 indeterminate lesions), 20 within the adrenal glands (12 adenomas, 7 malignant lesions, 1 indeterminate lesion), 17 within the spleen (8 cysts, 6 hemangiomas, 2 malignant lesions, 1 indeterminate lesion), and 7 within the pancreas (5 malignant lesions, 1 cyst, 1 indeterminate lesion).
Table 3.
Frequencies of reader scores by reader and DFOV group.
| Score type | Reader | Score* | DFOV group | All | ||
|---|---|---|---|---|---|---|
| Overall contrast enhancement | Reader 1 | L | Slow | Shigh | ||
| −2 | 3 | 1 | 1 | 5 | ||
| −1 | 15 | 19 | 13 | 47 | ||
| 0 | 22 | 8 | 22 | 52 | ||
| 1 | 15 | 1 | 13 | 29 | ||
| 2 | 1 | 0 | 0 | 1 | ||
| Reader 2 | L | Slow | Shigh | |||
| −2 | 1 | 6 | 2 | 9 | ||
| −1 | 22 | 15 | 7 | 44 | ||
| 0 | 24 | 8 | 24 | 56 | ||
| 1 | 6 | 0 | 14 | 20 | ||
| 2 | 3 | 0 | 2 | 5 | ||
| Lesion depiction | Reader 1 | L | Slow | Shigh | ||
| −2 | 2 | 0 | 0 | 2 | ||
| −1 | 1 | 3 | 1 | 5 | ||
| 0 | 42 | 23 | 36 | 101 | ||
| 1 | 3 | 0 | 1 | 4 | ||
| 2 | 0 | 0 | 1 | 1 | ||
| Reader 2 | L | Slow | Shigh | |||
| −2 | 1 | 2 | 0 | 3 | ||
| −1 | 6 | 10 | 3 | 19 | ||
| 0 | 38 | 14 | 31 | 83 | ||
| 1 | 3 | 0 | 4 | 7 | ||
| 2 | 0 | 0 | 1 | 1 | ||
Readers did not ascribe any ratings of −4, −3, 3 or 4
QUANTITATIVE IMAGE ANALYSIS
There were no clinically significant differences in the measured HUs for the entire group of patients (each size group combined) comparing WBD to the standard fixed dose exams; the liver measured 5 HUs lower in the WBD group, which was statistically significant (p=0.001). However, when assessing by DFOV subgroups, HUs for the Slow group were significantly lower with WBD in the MPV, aorta, liver, and TVE. The Slow group also did not demonstrate the improved CNR with WBD that was seen in the L and Shigh groups. Conversely, the Shigh group demonstrated higher HUs in the MPV, aorta and TVE; however, the liver HUs were not significantly different for WBD versus fixed dose examinations (Table 4).
Table 4.
Comparisons of HU ROIs between WBD and fixed dose examinations by DFOV group level.
| ROI | DFOV Group | WBD exam | Fixed dose exam | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% LCL | 95% UCL | Mean | 95% LCL | 95% UCL | |||
| MPV | L | 202.66 | 195.00 | 210.32 | 200.33 | 191.35 | 209.31 | 0.64 |
| Slow | 199.59 | 188.95 | 210.23 | 234.57 | 222.10 | 247.05 | <0.0001 | |
| Shigh | 224.83 | 216.64 | 233.01 | 201.04 | 191.45 | 210.64 | <0.0001 | |
| Aorta | L | 186.96 | 178.93 | 194.99 | 180.55 | 172.26 | 188.84 | 0.13 |
| Slow | 183.74 | 172.59 | 194.90 | 213.51 | 201.99 | 225.03 | <0.0001 | |
| Shigh | 196.09 | 187.51 | 204.67 | 180.69 | 171.83 | 189.55 | 0.0049 | |
| Liver | L | 121.25 | 116.47 | 126.03 | 122.47 | 116.60 | 128.34 | 0.60 |
| Slow | 125.11 | 118.46 | 131.75 | 148.80 | 140.64 | 156.95 | <0.0001 | |
| Shigh | 124.05 | 118.94 | 129.17 | 123.31 | 117.03 | 129.58 | 0.77 | |
| Liver-Spleen Ratio | L | 0.91 | 0.87 | 0.94 | 0.90 | 0.86 | 0.93 | 0.56 |
| Slow | 0.95 | 0.90 | 0.99 | 0.94 | 0.89 | 0.99 | 0.60 | |
| Shigh | 0.86 | 0.82 | 0.89 | 0.88 | 0.84 | 0.92 | 0.12 | |
| TVE | L | 845.99 | 815.74 | 876.23 | 847.68 | 812.18 | 883.18 | 0.92 |
| Slow | 868.49 | 826.46 | 910.53 | 1012.82 | 963.49 | 1062.16 | <0.0001 | |
| Shigh | 905.11 | 872.77 | 937.44 | 845.54 | 807.58 | 883.49 | 0.0013 | |
| CNRliver | L | 6.37 | 5.71 | 7.04 | 5.74 | 5.05 | 6.43 | 0.028 |
| Slow | 6.86 | 5.93 | 7.79 | 7.85 | 6.89 | 8.80 | 0.083 | |
| Shigh | 6.59 | 5.87 | 7.30 | 5.75 | 5.01 | 6.48 | 0.033 | |
| Liver (Delayed phase) | L | 91.71 | 88.12 | 95.31 | 89.63 | 85.08 | 94.19 | 0.40 |
| Slow | 95.90 | 90.86 | 100.94 | 108.78 | 102.50 | 115.05 | <0.0001 | |
| Shigh | 92.62 | 88.81 | 96.42 | 91.49 | 86.66 | 96.31 | 0.50 | |
| Liver Spleen Ratio (Delayed phase) | L | 0.98 | 0.94 | 1.02 | 0.95 | 0.91 | 1.00 | 0.17 |
| Slow | 0.99 | 0.93 | 1.04 | 0.98 | 0.92 | 1.04 | 0.46 | |
| Shigh | 0.96 | 0.93 | 1.00 | 0.97 | 0.93 | 1.02 | 0.52 | |
Significant statistical differences are in bold, TVE = total vascular enhancement, CNR = contrast-to-noise ratio, MPV = main portal vein, LCL = lower confidence limit, UCL = upper confidence limit, WBD = weight-based dosing, ROI = region of interest, DFOV = digital field of view
When comparing HUs between DFOV groups within just the WBD examinations, HUs were significantly lower in Group L than in Group Shigh for MPV, with a mean difference of −22.17 ± 5.67 [95% CI −33.38 to −10.96, p = 0.0001] and for TVE with a mean difference of −59.12 ± 22.38 [95% CI −103.40 to −14.84, p = 0.009]; the only other significant difference was lower HUs for the MPV in Group Slow compared with Shigh with a mean difference of −25.24 ± 6.79 [95% CI −38.66 to −11.81, p = 0.0003].
The mean image noise on WBD examinations for Groups Slow, Shigh and L were 9.5 ± 2.5 (Range, 4.6–15.4), 9.9 ± 2.3 (Range, 5.2–15.6), and 9.8 ± 2.2 (Range, 6–15.3), respectively; on the comparison examinations, the respective noise was 10.3 ± 2.5 (Range, 5.7–15.8), 10.6 ± 2.6 (Range, 4.4–18.2), and 10.6 ± 1.9 (Range, 6.6–15.5).
Regression analyses were performed on contrast volume and TVE/gI. Weight, BSA1, and BSA2 correlated well and similarly to one another versus contrast volume and TVE/gI across groups Slow, Shigh, and L; respective R2 values for the entire group (each size group combined) were 0.78, 0.79, and 0.81 and 0.58, 0.65, and 0.59. Conversely, R2 values for BMI with contrast volume and TVE/gI were only 0.56 and 0.26, respectively.
Discussion
Our study revealed that, when selecting an appropriate minimum IV iodine contrast dose, the quality of contrast enhancement and solid organ lesion depiction can be preserved and a better distribution of contrast usage can be obtained when using weight-based injector software. Weight-based IV contrast injection using injector software has not been specifically evaluated in the abdominal literature even though the commercially available software is available to many practices. There was no overall significant contrast-related difference between WBD examinations and our standard fixed contrast dosing of examinations; however, importantly, a portion of patients in each size group were better optimized to receive an increased or decreased contrast dose through the use of WBD.
Qualitative evaluation of overall contrast enhancement and lesion depiction found no significant difference between WBD and fixed-dose exams in Group L or Group Shigh. Quantitatively, the only statistically significant difference between the entire combined groups of WBD versus comparison examinations was in the liver; however, this difference of 5 HU is not considered clinically significant. Previous studies have shown that reviewers are not consistently able to identify differences in HUs of less than 10.39, 40 When assessing by group level, Group Shigh had statistically and clinically significant improvement in quantitative enhancement in the MPV, aorta, and TVE. Interestingly, when assessing between group levels, Group L was significantly lower than Group Shigh for HUs in the MPV and TVE; this is attributable to the setting of maximum contrast volume at 150 mL. Depending on the intended clinical use, increasing this maximum level could be considered to maintain an even distribution of contrast usage and thus image quality. Improvements in CNRliver noted on WBD examinations for Groups L and Shigh were related to mild differences in noise between examinations.
Our results with a larger sample size, specifically in an oncologic population with lesion evaluation, support those of George et al., which also showed an improved distribution of contrast enhancement and image quality across a spectrum of patient sizes using WBD.41 George et al. also demonstrated the use of TBW to be an acceptable measure for the choice of contrast volume, as did Svensson et al..42 This finding was reproduced in the current study, which showed TBW and BSA performed similarly when compared with our vendor-specific injection software that uses a proprietary calculation. Of note, BMI correlated very poorly with these measures and thus would not be well-suited to define contrast volume groups in clinical practice.
Our WBD examinations allow an IV contrast range of 110–150 mL when using the injector software. The choice of a 110 mL minimum volume (38.5 g of iodine) was made during implementation when an empiric loss of contrast was noted near this level, despite the fact that the software suggested a lower volume based on patient weight. One subset, Group Slow, of smaller patients was identified in our study to assess this empiric observation. In addition, this subset was used to determine whether image quality could be maintained during reduced contrast volume, which could potentially be employed such as in the setting of renal impairment. Although contrast-induced acute kidney injury has been shown to be rare in patients with a stable estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73 m2 or greater, contrast risk in patients with a stable eGFR of 30–44 mL/min/1.73 m2 is controversial, according to recent conflicting evidence, and those with a stable eGFR of less than 30 mL/min/1.73 m2 are still considered to be at increased risk43. It remains reasonable to use the least amount of IV contrast as possible and to consider more aggressive reduction of contrast volume in patients with impaired renal function. There was a noticeable reduction in image quality in Group Slow for which the minimum contrast volume of 110 mL (38.5 g of iodine) was removed, allowing the software a full lower range of volume selection (Fig. 1). Readers rated overall contrast enhancement and lesion depiction to be inferior in Group Slow and HUs were significantly lower for the MPV, aorta, liver and TVE (Tables 2 and 4). This insufficient enhancement in small patients even while using the same gI/kg level has been previously recognized and the use of lean body weight has been proposed to mitigate this limitation.44 Unfortunately, this limitation was still apparent with the use of our vendor-specific injector WBD software.
Fig. 1.
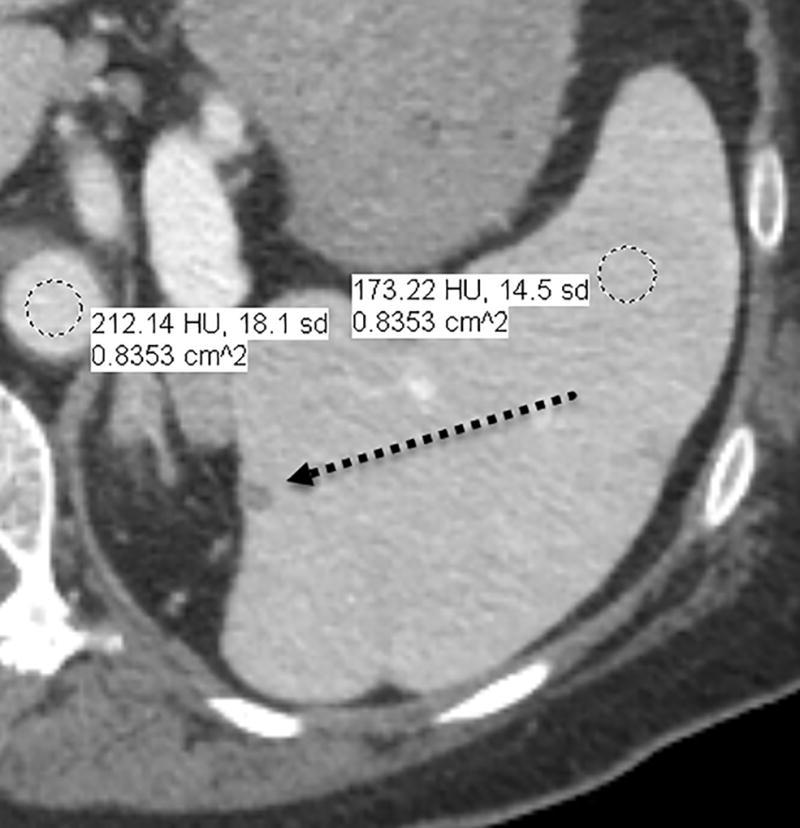
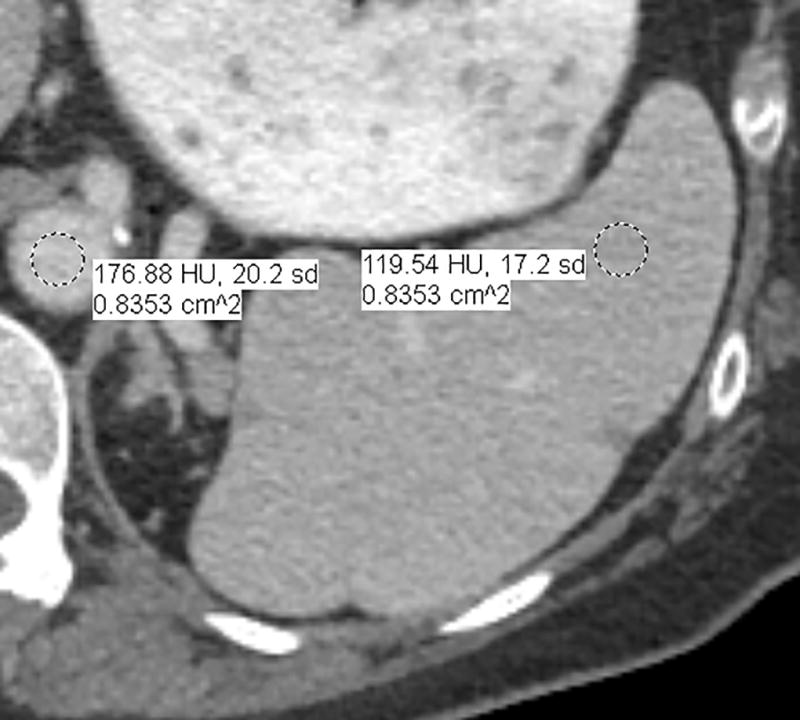
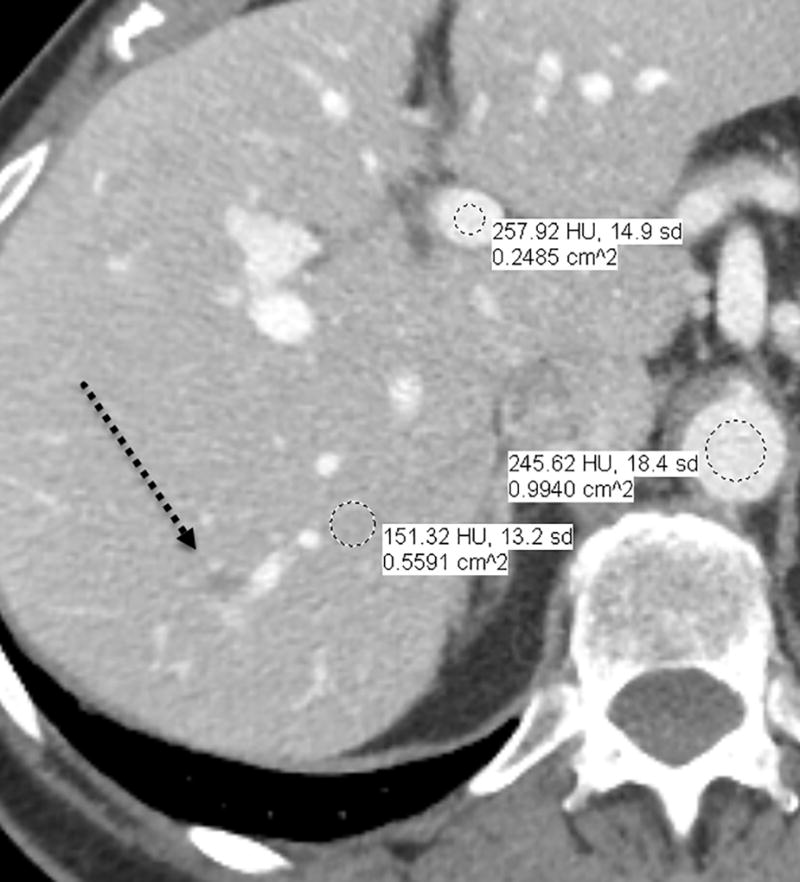
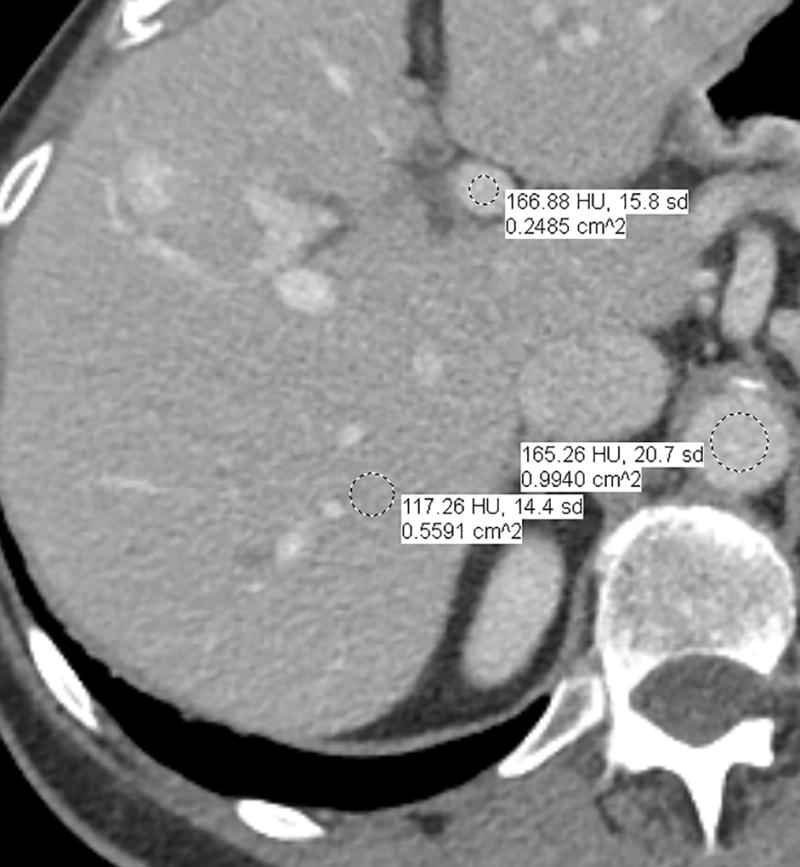
a–d—Two case examples from different patients, each demonstrating a small benign finding (a lesion that is unchanged between examinations) for comparison between fixed-dose (a,c) and weight-based dosing (WBD) (b,d) examinations when IV contrast load was allowed below 110 mL (38.5 g of iodine) on the WBD examination. Inferior overall enhancement and lesion depiction was noted qualitatively by readers and upon quantitative measurements for the WBD cases. Readers noticed that a subtle splenic lesion (a, arrow) and the nodular enhancement of a hemangioma (c, arrow) were only barely seen on the WBD examinations.
When assessing the choice of fixed-dose versus WBD, image quality and patient safety are most important. However, there are also implications of cost to be considered. With the historical use of a single division of patients into two fixed-dose IV contrast volume groups at our institution, we suspected that there would be three categories of effect by using WBD: one in which there was no significant difference in contrast volume, a group of slightly above average patient sizes that would receive less contrast than our standard, and a group of slightly below average patient sizes that would receive an increased contrast volume relative to our standard. This was confirmed in our study; our group of large patients (Group L) received a mean dose of 0.54 gI/kg with WBD versus 0.58 gI/kg on comparison examinations and our group of smaller patients (Group Shigh) received a mean dose of 0.60 gI/kg versus 0.57 gI/kg on comparison examinations. Specifically, 32 WBD cases used a lower volume in Group L and 17 WBD cases used a higher volume in group Shigh than for the comparison exams. In our practice, the degree of cost savings would likely be muted by our need to maintain robust oncologic imaging quality, particularly of the liver. A range of 0.489 – 0.75 gI/kg has been reported as the necessary contrast dose for proper hepatic enhancement. Kondo et al. demonstrated that 0.6 gI/kg TBW was an important level above which resultant images were deemed to be good quality in more than 90% of patients.12, 23, 44 Kondo et al. also showed that patients receiving 22 gI/m2 based on BSA had an even better correlation with enhancement than did the TBW group; the mean values from our study were 23.97 gI/m2 and 23.35 gI/m2 using BSA1 and BSA2 methods of calculation, respectively. Using the calculation from Heiken et al. and our target gI/kg of 0.6, our detailed oncologic evaluations appear to require a mean hepatic enhancement (mean Δ HU) of 57.6 HU.22 Using data from Davenport et al. (2017), and comparing to their fixed dose protocol of 125 mL, there is a projected additional cost of $102,384 for a sample of 6737 patients if a TBW factor of 0.625gI/kg was used with a 150 mL maximum contrast load. Although products and pricing contrast vary between institutions, we suspect that this projection would apply to our Group S and that a cost savings, similar in degree, would apply to our Group L.45
There were some limitations in our study. First, inherent in studies between two time points, the clinical status of the patient may change. In our study, the most commonly expected change would be variation in hepatic steatosis between examinations related to chemotherapeutic regimens. To account for this, we calculated the HU liver-spleen ratio on every examination and there was no significant change between time points. In addition, a change in cardiovascular status was a potential concern between examinations; we address this in our standard clinical practice and we addressed this in the study through the detailed use of contrast bolus-tracking. Second, our study was retrospective and not intended to directly assess potential clinical impact, which requires further investigation. Furthermore, the type and degree of effects related to WBD software implementation will vary depending on the initial protocols used within each practice.
In conclusion, weight-based contrast dosing using injector software maintained or improved IV contrast enhancement and lesion depiction across patient sizes when using a minimum contrast volume of 110 mL (38.5 g of iodine). Total body weight and body surface area correlated well with the software selected contrast volumes, whereas BMI was a poor predictor of IV contrast volume needed to maintain contrast enhancement across patient sizes. Our study, which used WBD relative to our standard fixed-dosing approach, suggests that patients of above-average size present opportunities for lowering contrast usage, whereas certain patients of below-average size may benefit from an increased amount of contrast with WBD.
Acknowledgments
Funding: Supported by institutional CCSG (cancer center support grant) from the NIH/National Cancer Institute under award number P30CA016672.
A special thank you to David Martinez, RT for his assistance during implementation.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
IRB Statement: HIPAA-compliant, IRB-exempt quality improvement project
References
- 1.Solomon J, Marin D, Roy Choudhury K, et al. Effect of Radiation Dose Reduction and Reconstruction Algorithm on Image Noise, Contrast, Resolution, and Detectability of Subtle Hypoattenuating Liver Lesions at Multidetector CT: Filtered Back Projection versus a Commercial Model-based Iterative Reconstruction Algorithm. Radiology. 2017;284(3):777–787. doi: 10.1148/radiol.2017161736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paushter DM, Zeman RK, Scheibler ML, et al. CT evaluation of suspected hepatic metastases: comparison of techniques for i.v. contrast enhancement. AJR Am J Roentgenol. 1989;152(2):267–71. doi: 10.2214/ajr.152.2.267. [DOI] [PubMed] [Google Scholar]
- 3.Platt JF, Glazer GM. IV contrast material for abdominal CT: comparison of three methods of administration. AJR Am J Roentgenol. 1988;151(2):275–7. doi: 10.2214/ajr.151.2.275. [DOI] [PubMed] [Google Scholar]
- 4.Bae KT, Heiken JP, Brink JA. Aortic and hepatic contrast medium enhancement at CT. Part II. Effect of reduced cardiac output in a porcine model. Radiology. 1998;207(3):657–62. doi: 10.1148/radiology.207.3.9609887. [DOI] [PubMed] [Google Scholar]
- 5.Bae KT, Heiken JP, Brink JA. Aortic and hepatic contrast medium enhancement at CT. Part I. Prediction with a computer model. Radiology. 1998;207(3):647–55. doi: 10.1148/radiology.207.3.9609886. [DOI] [PubMed] [Google Scholar]
- 6.Bae KT, Heiken JP, Brink JA. Aortic and hepatic peak enhancement at CT: effect of contrast medium injection rate--pharmacokinetic analysis and experimental porcine model. Radiology. 1998;206(2):455–64. doi: 10.1148/radiology.206.2.9457200. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita Y, Komohara Y, Takahashi M, et al. Abdominal helical CT: evaluation of optimal doses of intravenous contrast material--a prospective randomized study. Radiology. 2000;216(3):718–23. doi: 10.1148/radiology.216.3.r00se26718. [DOI] [PubMed] [Google Scholar]
- 8.Awai K, Inoue M, Yagyu Y, et al. Moderate versus high concentration of contrast material for aortic and hepatic enhancement and tumor-to-liver contrast at multi-detector row CT. Radiology. 2004;233(3):682–8. doi: 10.1148/radiol.2333031617. [DOI] [PubMed] [Google Scholar]
- 9.Awai K, Hiraishi K, Hori S. Effect of contrast material injection duration and rate on aortic peak time and peak enhancement at dynamic CT involving injection protocol with dose tailored to patient weight. Radiology. 2004;230(1):142–50. doi: 10.1148/radiol.2301021008. [DOI] [PubMed] [Google Scholar]
- 10.Awai K, Hori S. Effect of contrast injection protocol with dose tailored to patient weight and fixed injection duration on aortic and hepatic enhancement at multidetector-row helical CT. Eur Radiol. 2003;13(9):2155–60. doi: 10.1007/s00330-003-1904-x. [DOI] [PubMed] [Google Scholar]
- 11.Ho LM, Nelson RC, Delong DM. Determining contrast medium dose and rate on basis of lean body weight: does this strategy improve patient-to-patient uniformity of hepatic enhancement during multi-detector row CT? Radiology. 2007;243(2):431–7. doi: 10.1148/radiol.2432060390. [DOI] [PubMed] [Google Scholar]
- 12.Kondo H, Kanematsu M, Goshima S, et al. Abdominal multidetector CT in patients with varying body fat percentages: estimation of optimal contrast material dose. Radiology. 2008;249(3):872–7. doi: 10.1148/radiol.2492080033. [DOI] [PubMed] [Google Scholar]
- 13.Dean PB, Kivisaari L, Kormano M. Contrast enhancement pharmacokinetics of six ionic and nonionic contrast media. Invest Radiol. 1983;18(4):368–74. doi: 10.1097/00004424-198307000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Haider MA, Amitai MM, Rappaport DC, et al. Multi-detector row helical CT in preoperative assessment of small (< or = 1.5 cm) liver metastases: is thinner collimation better? Radiology. 2002;225(1):137–42. doi: 10.1148/radiol.2251011225. [DOI] [PubMed] [Google Scholar]
- 15.Dorio PJ, Lee FT, Jr, Henseler KP, et al. Using a saline chaser to decrease contrast media in abdominal CT. AJR Am J Roentgenol. 2003;180(4):929–34. doi: 10.2214/ajr.180.4.1800929. [DOI] [PubMed] [Google Scholar]
- 16.Dean PB, Violante MR, Mahoney JA. Hepatic CT contrast enhancement: effect of dose, duration of infusion, and time elapsed following infusion. Invest Radiol. 1980;15(2):158–61. doi: 10.1097/00004424-198003000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Berland LL, Lee JY. Comparison of contrast media injection rates and volumes for hepatic dynamic incremented computed tomography. Invest Radiol. 1988;23(12):918–22. doi: 10.1097/00004424-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Claussen CD, Banzer D, Pfretzschner C, et al. Bolus geometry and dynamics after intravenous contrast medium injection. Radiology. 1984;153(2):365–8. doi: 10.1148/radiology.153.2.6484168. [DOI] [PubMed] [Google Scholar]
- 19.Heiken JP, Brink JA, McClennan BL, et al. Dynamic contrast-enhanced CT of the liver: comparison of contrast medium injection rates and uniphasic and biphasic injection protocols. Radiology. 1993;187(2):327–31. doi: 10.1148/radiology.187.2.8475268. [DOI] [PubMed] [Google Scholar]
- 20.Harmon BH, Berland LL, Lee JY. Effect of varying rates of low-osmolarity contrast media injection for hepatic CT: correlation with indocyanine green transit time. Radiology. 1992;184(2):379–82. doi: 10.1148/radiology.184.2.1620831. [DOI] [PubMed] [Google Scholar]
- 21.Kormano M, Partanen K, Soimakallio S, et al. Dynamic contrast enhancement of the upper abdomen: effect of contrast medium and body weight. Invest Radiol. 1983;18(4):364–7. doi: 10.1097/00004424-198307000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Heiken JP, Brink JA, McClennan BL, et al. Dynamic incremental CT: effect of volume and concentration of contrast material and patient weight on hepatic enhancement. Radiology. 1995;195(2):353–7. doi: 10.1148/radiology.195.2.7724752. [DOI] [PubMed] [Google Scholar]
- 23.Kondo H, Kanematsu M, Goshima S, et al. Body size indexes for optimizing iodine dose for aortic and hepatic enhancement at multidetector CT: comparison of total body weight, lean body weight, and blood volume. Radiology. 2010;254(1):163–9. doi: 10.1148/radiol.09090369. [DOI] [PubMed] [Google Scholar]
- 24.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 25.Katzberg RW, Haller C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Suppl. 2006;(100):S3–7. doi: 10.1038/sj.ki.5000366. [DOI] [PubMed] [Google Scholar]
- 26.McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368–75. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 27.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33(6):1756–824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 28.Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36(5):1542–8. doi: 10.1016/s0735-1097(00)00917-7. [DOI] [PubMed] [Google Scholar]
- 29.Wintermark M, Maeder P, Verdun FR, et al. Using 80 kVp versus 120 kVp in perfusion CT measurement of regional cerebral blood flow. AJNR Am J Neuroradiol. 2000;21(10):1881–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Sigal-Cinqualbre AB, Hennequin R, Abada HT, et al. Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology. 2004;231(1):169–74. doi: 10.1148/radiol.2311030191. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama Y, Awai K, Funama Y, et al. Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology. 2005;237(3):945–51. doi: 10.1148/radiol.2373041655. [DOI] [PubMed] [Google Scholar]
- 32.Ertl-Wagner BB, Hoffmann RT, Bruning R, et al. Multi-detector row CT angiography of the brain at various kilovoltage settings. Radiology. 2004;231(2):528–35. doi: 10.1148/radiol.2312030543. [DOI] [PubMed] [Google Scholar]
- 33.Waaijer A, Prokop M, Velthuis BK, et al. Circle of Willis at CT angiography: dose reduction and image quality--reducing tube voltage and increasing tube current settings. Radiology. 2007;242(3):832–9. doi: 10.1148/radiol.2423051191. [DOI] [PubMed] [Google Scholar]
- 34.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 35.Livingston EH, Lee S. Body surface area prediction in normal-weight and obese patients. Am J Physiol Endocrinol Metab. 2001;281(3):E586–91. doi: 10.1152/ajpendo.2001.281.3.E586. [DOI] [PubMed] [Google Scholar]
- 36.Goodenberger MH, Wagner-Bartak NA, Gupta S, et al. Computed Tomography Image Quality Evaluation of a New Iterative Reconstruction Algorithm in the Abdomen (Adaptive Statistical Iterative Reconstruction-V) a Comparison With Model-Based Iterative Reconstruction, Adaptive Statistical Iterative Reconstruction, and Filtered Back Projection Reconstructions. J Comput Assist Tomogr. 2018;42(2):184–190. doi: 10.1097/RCT.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin D, Nelson RC, Schindera ST, et al. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm--initial clinical experience. Radiology. 2010;254(1):145–53. doi: 10.1148/radiol.09090094. [DOI] [PubMed] [Google Scholar]
- 38.Telesmanich ME, Jensen CT, Enriquez JL, et al. Third version of vendor-specific model-based iterativereconstruction (Veo 3.0): evaluation of CT image quality in the abdomen using new noise reduction presets and varied slice optimization. Br J Radiol. 2017;90(1077):20170188. doi: 10.1259/bjr.20170188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foley WD, Berland LL, Lawson TL, et al. Contrast enhancement technique for dynamic hepatic computed tomographic scanning. Radiology. 1983;147(3):797–803. doi: 10.1148/radiology.147.3.6844616. [DOI] [PubMed] [Google Scholar]
- 40.Bressler EL, Alpern MB, Glazer GM, et al. Hypervascular hepatic metastases: CT evaluation. Radiology. 1987;162(1 Pt 1):49–51. doi: 10.1148/radiology.162.1.3024210. [DOI] [PubMed] [Google Scholar]
- 41.George AJ, Manghat NE, Hamilton MC. Comparison between a fixed-dose contrast protocol and a weight-based contrast dosing protocol in abdominal CT. Clin Radiol. 2016;71(12):1314 e1–1314 e9. doi: 10.1016/j.crad.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Svensson A, Nouhad J, Cederlund K, et al. Hepatic contrast medium enhancement at computed tomography and its correlation with various body size measures. Acta Radiol. 2012;53(6):601–6. doi: 10.1258/ar.2012.120268. [DOI] [PubMed] [Google Scholar]
- 43.Davenport MS, Cohan RH, Ellis JH. Contrast media controversies in 2015: imaging patients with renal impairment or risk of contrast reaction. AJR Am J Roentgenol. 2015;204(6):1174–81. doi: 10.2214/AJR.14.14259. [DOI] [PubMed] [Google Scholar]
- 44.Onishi H, Murakami T, Kim T, et al. Abdominal multi-detector row CT: effectiveness of determining contrast medium dose on basis of body surface area. Eur J Radiol. 2011;80(3):643–7. doi: 10.1016/j.ejrad.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 45.Davenport MS, Parikh KR, Mayo-Smith WW, et al. Effect of Fixed-Volume and Weight-Based Dosing Regimens on the Cost and Volume of Administered Iodinated Contrast Material at Abdominal CT. J Am Coll Radiol. 2017;14(3):359–370. doi: 10.1016/j.jacr.2016.09.001. [DOI] [PubMed] [Google Scholar]


