Abstract
BACKGROUND
Case studies have suggested the efficacy of catheter-free, electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia (VT) using stereotactic body radiation therapy (SBRT), though prospective data is lacking.
METHODS
We conducted a prospective phase I/II trial of noninvasive cardiac radioablation in adults with treatment-refractory episodes of VT or cardiomyopathy related to premature ventricular contractions (PVCs). Arrhythmogenic scar regions were targeted by combining noninvasive anatomic and electrical cardiac imaging with a standard SBRT workflow followed by delivery of a single fraction of 25 Gray (Gy) to the target. The primary safety endpoint was treatment-related serious adverse events (SAE) in the first 90 days. The primary efficacy endpoint was any reduction in VT episodes (tracked by indwelling ICDs) or any reduction in PVC burden (as measured by 24-hour Holter monitor) comparing the 6 months before and after treatment (with a 6 week blanking window after treatment). Health-related quality of life was assessed using the Short Form (SF-36) questionnaire.
RESULTS
Nineteen patients were enrolled (17 for VT, 2 for PVC-cardiomyopathy). Median noninvasive ablation time was 15.3 minutes (range, 5.4–32.3). In the first 90 days, 2/19 patients (10.5%) developed a treatment-related SAE. Median number of VT episodes was reduced from 119 (range 4–292) to 3 (range 0–31, p < 0.001). Reduction was observed for both ICD shocks and anti-tachycardia pacing. VT episodes or PVC burden were reduced in 17/18 evaluable patients (94%). The frequency of VT episodes or PVC burden was reduced by 75% in 89% of patients. Overall survival was 89% at 6 months and 72% at 12 months. Use of dual antiarrhythmic medications decreased from 59% to 12% (p=0.008). Quality of life improved in 5 of 9 SF-36 domains at 6 months.
CONCLUSIONS
Noninvasive electrophysiology-guided cardiac radioablation is associated with markedly reduced ventricular arrhythmia burden with modest short-term risks, reduction in antiarrhythmic drug use, and improvement in quality of life.
CLINICAL TRIAL REGISTRATION
URL: https://clinicaltrials.gov. Unique identifiers: NCT02919618
Keywords: Ventricular tachycardia, noninvasive, stereotactic radiotherapy
INTRODUCTION
Ventricular tachycardia is a life-threatening heart rhythm disorder that is often caused by electrical reentry within and around patches of heterogeneous myocardial fibrosis.1 Invasive catheter procedures to treat VT first identify the circuits within the fibrosis then deliver thermal ablative energy to these regions.2 Stereotactic body radiotherapy (SBRT) is a technique that delivers precise, high doses of radiation to targets in the body with reduced exposure to adjacent normal tissue.3 When used to treat tumors, SBRT can achieve high rates of tumor control and minimal toxicity, including tumors adjacent to the heart.4, 5 SBRT has a potential to treat VT by delivering ablative energy noninvasively, minimizing procedural risk for the patient.6 Preclinical studies exploring SBRT using various forms of particle therapy (photons, protons, carbon) for cardiac ablation have demonstrated histological changes and electrophysiological effects without acute or sub-acute adverse effects over 6 month follow-up.7–9
Choosing the precise location to target for ablation, while avoiding healthy tissue and surrounding thoracic structures, requires a synthesis of anatomic and electrical information. A pilot case series used cardiac MRI, cardiac SPECT, 12-lead ECG during VT and electrocardiographic imaging (ECGI) to guide cardiac SBRT.10 The result demonstrated a strong antiarrhythmic effect that controlled VT storm and allowed reduction of antiarrhythmic medication. Other case reports have yielded generally positive short-term results without report of significant complications.11–13 While late toxic effects of radiotherapy to the heart have been reported in the treatment of thoracic tumors, late toxicity from high-dose SBRT applied to focal areas of previously injured heart is unknown.14, 15
Formal prospective evaluation of short-term risk, late toxicities, and antiarrhythmic effects of noninvasive cardiac radioablation has not been completed. Here we describe the results of a prospective trial of noninvasive cardiac radioablation for the control of treatment-refractory VT.
METHODS
The data, analytical methods, and study materials will be made available at Washington University Open Scholarship (https://openscholarship.wustl.edu/) 12 months after analysis of the primary endpoints.
Trial Design
The ENCORE-VT trial was a prospective single-arm phase I/II trial conducted at a single center. Local institutional review board (IRB) approved the study without Investigational Device Exemption (IDE) based on interpretation of the indications for use and risk of linear accelerators and electrocardiographic imaging technology. All participants provided informed consent, and an independent Data and Safety Monitoring Committee (DSMC) reviewed data semi-annually and provided guidance on study continuation. Subsequent to study enrollment, FDA reviewed the protocol and categorized the research as a significant risk study, which requires IDE approval. Although the FDA did not approve the protocol, analysis plan, or data, the investigators, IRB, and FDA worked together to ensure that the appropriate human subject protections were in place.
The trial was designed by CGR and PSC. The first draft of the manuscript was written by CGR and PSC, and decision to publish was made by all authors. Data were analyzed by CGR, PSC, and PPS. The authors vouch for the completeness and accuracy of the data and analyses.
Patients
Eligible patients were ≥ 18 years old and had a) ≥ 3 episodes of sustained monomorphic VT, or b) cardiomyopathy (left ventricular ejection fraction (LVEF) < 50%) related to monomorphic PVCs (PVC > 20%), and required failure of ≥ 1 antiarrhythmic medication and ≥ 1 catheter ablation (or have a contraindication to catheter ablation). Patients could not have received past radiotherapy to the anticipated treatment field. Patients were deemed ineligible if they had heart failure dependent on inotropes and/or a left-ventricular assist device, or were deemed unlikely to live 12 months in the absence of VT. Patients were also ineligible if they had polymorphic VT or ventricular fibrillation, more than 3 distinct clinical VT morphologies or more than 5 induced VT morphologies during noninvasive-programmed stimulation (NIPS) testing.
Intervention
Targeting
Protocol-specified baseline studies for targeting included a cardiac CT, cardiac MRI, PET/CT, 12-lead ECG, and acquisition of ECGI during induced VT during NIPS testing. NIPS and ECGI methods have been previously described. 10, 16–19 A synthesis of imaging studies and electrophysiologic mapping were used to guide SBRT in each patient with the principle being to target all areas of ventricular scar approximating the VT exit site that harbor related circuits. Full details are in the Online Data Supplement and as summarized in Figures S1-S3.
Treatment and Follow-up
Patients received a single dose of 25 Gy delivered with SBRT to the arrhythmogenic target as defined above. The general method for SBRT planning and delivery has been previously described.10 Full study-specific details regarding the SBRT procedure, including simulation, treatment planning, and delivery, are available in the Online Data Supplement. Following completion of treatment, a pre-specified ICD programming plan was implemented for all patients, which included a zone for detection at least 20 ms slower than the slowest clinical or induced VT. ICDs were remotely monitored as part of clinical care. If not contraindicated, oral anticoagulation was prescribed during the first month after treatment. Study visits occurred at day 3, at 2, 4, and 6 weeks, 6, and 12 months, and annually thereafter. Adverse events were continuously assessed, and ICD interrogation was performed at each study visit. A 12-lead ECG was obtained at day 3, at 6 weeks, and 3, 6, and 12 months. For patients with PVCs, 24 hour Holter monitor was performed at week 6, and months 3, 6, and 12. Chest CT was performed along with ECGI (without NIPS) at 3 and 12 months.
Outcome Measures and Statistical Analysis
The ENCORE-VT trial was designed with co-primary endpoints of (1) safety and (2) efficacy. The primary safety endpoint was defined as the rate of ≤ 90 day serious adverse events (SAEs) defined using Common Terminology Criteria for Adverse Events (CTCAE, v4.0) criteria that were treatment-related (possibly, probably, or definitely related to study treatment). SAEs were defined as any grade 3 toxicity requiring hospitalization, or any grade 4–5 toxicity. An early stopping rule was set to halt protocol enrollment if 5 or more of the first 10 patients developed a SAE.
The primary efficacy endpoint was defined as the number of subjects with any reduction in number of ICD treatments for VT or 24-hour PVC burden comparing the 6 month period before and after SBRT, with a 6 week blanking period after treatment to allow for a treatment effect. ICD treatments are composed of ICD shocks and anti-tachycardia pacing (ATP). Additional select pre-specified secondary objectives reported herein include stricter efficacy endpoints (50% reduction, 95% reduction), a patient-derived endpoint (reduction in shocks for VT patients, improvement in cardiac function for PVCs), overall survival, late (>90 day to 1 year) adverse events, and quality of life as measured by the SF-36 questionnaire.
The study was powered as a balance between assuring a high likelihood of safety with a preliminary assessment of efficacy. Considering all patients had failed previous treatments to halt VT, the population in this study was expected to be at higher risk, and an assumption was made that SAE rates up to 20% and efficacy as low as 40% would be clinically acceptable. Using a one-sided one sample test for proportions, 19 patients provided a 75.4% power to determine that the SAE rate was not truly higher than 20% (range, 5–20%, alpha = 0.0829) and a 81.5% power to determine that efficacy was not worse than 40% (range, 40–65%, alpha = 0.0885).
Continuous variables are reported as the median and range. The Wilcoxon signed-rank test was used to compare the number of VT events, ICD shocks, and ATP events between baseline and 6 month time points. McNemar’s paired testing was used to assess changes in the proportions of anti-arrhythmic use. For quality of life analysis, mean values in each of the Short Form-36 measures at baseline, 6 weeks, and 6 months were compared using a repeated measures ANOVA with Greenhouse-Geisser correction. Median follow-up was calculated from the date of treatment to the date of last scheduled follow-up or death. Kaplan-Meier analysis was used to estimate the survival function. All statistics were performed in IBM SPSS Statistics for Windows, Version 24.0, Armonk, NY, 2016.
RESULTS
Patients and Treatment
Table 1 outlines the demographic data for the cohort, that was characterized by median age 66 years, 89.5% male (n=17), ischemic cardiomyopathy (n=11, 57.9%), median LVEF 25% (range, 15–58), New York Heart Association (NYHA) class III/IV (73.7%). The median number of previous catheter ablations prior to enrollment was 1 (range, 0–4). Three patients did not have prior catheter ablation due to mechanical AVR and MVR (n=1), severely reduced LV ejection fraction with medical comorbidities precluding hemodynamic support (n=1), presence of mobile LV thrombus (n=1). Median follow up was 13 months, and no patients were lost follow-up
TABLE 1. Patient Demographics.
NYHA = New York Heart Association, VT = Ventricular tachycardia, ICD = Implantable Cardioverter Defibrillator, PVC = Premature ventricular contraction, ATP = Antitachycardia pacing, ACE = Angiotensin Converting Enzyme, ARB = Angiotensin Receptor Blocker, COPD = Chronic Obstructive Pulmonary Disease
| Variable | N = 19 |
|---|---|
| Median Age (year) (range) | 66 (49 – 81) |
| Sex (n) (%) Male Female |
17 (89.5) 2 (10.5) |
| Race (n) Caucasian Black/AA Asian |
17 (89.5) 1 (5.3) 1 (5.3) |
| Median Body Mass Index (kg/m2) (range) | 33.0 (24.3 – 48.6) |
| Median Age-Adjusted Charlson Score (range) | 4 (2 – 13) |
| Type of Cardiomyopathy (n) (%) Ischemic Non-ischemic Idiopathic Myocarditis (chronic) Valvular |
11 (57.9) 8 (42.1) 5 2 1 |
| NYHA Class (n) (%) I II III IV |
1 (5.3) 4 (21.1) 10 (52.6) 4 (21.1) |
| Median Left ventricular ejection fraction (%) (range) |
25 (15 – 58) |
| Median Number of Previous Catheter Ablations (range) |
1 (0–4) |
| Total Number of Prior Catheter Ablation Approaches (n) Endocardial Epicardial |
25 4 |
| Study Eligibility Criteria (n) (%) Incessant VT VT storm (>3 in 24 hours) ICD therapies (>3 shock or ATP in 6 months) PVC-related cardiomyopathy |
2 (10.5) 10 (52.6) 5 (26.3) 2 (10.5) |
| Device (n) (%) Single- or dual-chamber ICD Bi-ventricular ICD None |
8 (42.1) 10 (52.6) 1 (5.3) |
| More than one antiarrhythmic drug at baseline (n) (%) |
11 (57.9) |
| Current Antiarrhythmic drugs (n) (%) High dose amiodarone (≥300mg/day) Low dose amiodarone (<300mg/day) Class III (excluding amiodarone) Class I |
11 (57.9) 1 (5.3) 6 (31.6) 12 (63.2) |
| Other medications (n) (%) Beta-blocker Angiotensin Converting Enzyme (ACE)-Inhibitor Angiotensin Receptor Blocker (ARB) Oral Anticoagulation |
18 (94.7) 10 (52.6) 7 (36.8) 14 (73.7) |
| COPD/emphysema (n) (%) | 4 (21.1) |
| Diabetes Mellitus, Type 2 (n) (%) | 7 (36.8) |
| Hypertension (n) (%) | 10 (52.6) |
| Chronic Kidney Disease, Stage ≥3 (n) (%) | 9 (47.4) |
Patients were enrolled for either ICD-treated VT (n=17) or PVC-related cardiomyopathy (n=2). Of the 17 patients with ICD-treated VT, 10 were considered VT storm (three or more VT episodes in 24 hours) and two were in sustained VT at the time of treatment. More than half (n=11, 57.9%) the patients were on >1 antiarrhythmic drug and taking ≥ 300 mg of amiodarone daily at the time of treatment.
Targeting and treatment characteristics are reported in Table 2. Ten patients were excluded from cardiac MRI primarily due to abandoned ICD leads. All patients underwent CT, nuclear imaging, and induction of VT with subsequent 12-lead ECG and ECGI images. Patients had a median of 2 VTs induced (range, 1–5).
TABLE 2. Targeting and Treatment Details.
ECG = Electrocardiography, VT = Ventricular Tachycardia, LV = Left ventricle, SBRT = Stereotactic Body Radiotherapy
| Targeting data acquired at baseline (n) (%) Magnetic Resonance Imaging Nuclear imaging Electrocardiographic imaging 12-lead ECG |
9 (47.4) 19 (100) 19 (100) 19 (100) |
| Non-invasive Programmed Stimulation Median # of induced VT (range) Median Cycle length of induced VT (milliseconds) (range) |
2 (1–5) 360 (230–690) |
| Target location (n) * Segments in Left Ventricle Anterior Lateral Inferior Septal Apex LV Summit Segments in Right ventricle |
5 6 6 3 4 2 1 |
| Median Target Volume (cc’s) (range) † Gross target volume Internal target volume Planning target volume |
25.4 (6.4–88.6) 31.0 (17.7–128.9) 98.9 (60.9–298.8) |
| Immobilization system (n) (%) Vacuum immobilization (Elekta BodyFIX) Abdominal compression (CDR FreedomX) |
3 (15.8) 16 (84.2) |
| Stereotactic Body Radiotherapy technique Volumetric modulated arc therapy Fixed-field intensity modulated therapy |
17(89.5) 2 (10.5) |
| Linear accelerator Varian TrueBeam Varian Edge |
3 (15.8) 16 (84.2) |
| Median Stereotactic Body Radiotherapy beam-on time (min) (range) | 15.3 (5.4–32.3) |
Segments refers to inclusion of a location as part of targeting for SBRT. Multiple patients had targets that involved more than one segment, so total number adds up to greater than 19.
The target is referred to as the gross target volume (GTV). After outlining the GTV, an additional area around the GTV was added to account for internal motion of the GTV caused by breathing and cardiac motion, as assessed by review of the 4D‐CT. This is called the internal target volume (ITV). Finally, an additional safety margin of 5 mm was added to the ITV region for treatment planning to create a planning target volume (PTV), which accounts for any residual uncertainties in patient setup, motion, and delivery.
Median gross target volume was 25.4 cc (range, 6.4–88.6). The PVC patients had the smallest GTV (6.4 cc, 11.5 cc). Accounting for motion and conservative additional margins for setup and delivery, median planning target volume was 98.9 cc (range, 60.9–298.8). Most (n=17, 89.5%) were treated with a volumetric modulated arc therapy technique. Median beam-on time was 15.3 minutes (5.4–32.3).
Safety
No acute toxicity was observed during or immediately after SBRT. No adverse effects were observed with ICDs during or after SBRT. A list of all ≤ 90 day SAEs is shown in Table 3. Two patients (10.5%) experienced a grade 3 treatment-related (possibly, probably, or definitely related) SAE. One patient was hospitalized 65 days after treatment with a heart failure exacerbation (grade 3) and was conservatively scored as possible. Another patient was hospitalized at 80 days with pericarditis that improved with prednisone and was scored as probable. No grade 4 toxicity was recorded. An additional patient died 17 days after treatment in a nursing home resulting from an accident that was scored as unrelated and did not contribute to the 6 months primary efficacy endpoint. Early stopping rules were not met, and the DSMC recommended completion of the study.
TABLE 3.
Serious Adverse Events (SAEs) Within 90 Days of Treatment. The first column organizes CTCAE v4.0 adverse events by system and then further divides into specific adverse events. The first row indicates the grade as well as the attribution. No grade 4 events were recorded, and are thus not tabulated. Protocol-specific SAEs (SAEs that were possibly, probably, or definitely relative to study treatment) are bolded. CTCAE v4.0 = Common Terminology Criteria for Adverse Events version 4.0.
| CTCAE v4.0 System/Toxicity | Grade 3 | Grade 5 | |||
|---|---|---|---|---|---|
| Unrelated | Unlikely | Possible | Probable | Unlikely | |
| Cardiac disorders | |||||
| Heart Failure | 1 | ||||
| Pericarditis | 1 | ||||
| Gastrointestinal disorders | |||||
| Diarrhea | 1 | ||||
| Nausea | 1 | ||||
| General disorders and administration site conditions | |||||
| Other - Accident | 1 | ||||
| Immune system disorders | |||||
| Allergic Reaction | 1 | ||||
| Investigations | |||||
| Alanine Aminotransferase increased | 1 | ||||
| Aspartate Aminotransferase increased | 1 | ||||
| Respiratory, thoracic and mediastinal disorders | |||||
| Other – Influenza | 1 | ||||
| Vascular disorders | |||||
| Flushing | 1 | ||||
A complete list of adverse events inclusive of all attributions, grades, and time frames after treatment is shown in Table S1 in the Online Data Supplement. Adverse events probably or definitely related to treatment were generally grade 1–2 (8 events in 4 patients, (22%)). Transient grade 1 fatigue and hypotension were common. Three patients required adjustment of antihypertensive medication due to hypotension within two weeks of treatment. Other common grade 1–2 adverse events included dizziness, dyspnea, and nausea. Two patients (11.1%) developed grade 2 radiation pneumonitis that resolved with steroids. Pericardial effusions were documented 6 times in 5 patients (28%); 3 were asymptomatic, 1 resolved with medical management, and 2 were higher grade (1 possibly related grade 3, 1 unrelated grade 4 after epicardial access). Patients with symptomatic pericarditis or pneumonitis were treated with prednisone delivered at 1 mg/kg daily (max 60 mg), tapered by 10–20 mg per week based on symptoms. Six patients (33%) had at least one hospital admission for heart failure at any point during follow-up.
Efficacy
Of the eighteen patients who survived 6 months, the primary efficacy endpoint of reduction in VT episodes or PVC burden was achieved in 17/18 (94%) patients. Figure 1 shows the frequency of VT episodes and 24-hour PVC burden before and after noninvasive cardiac radioablation for all 18 patients.
FIGURE 1. Assessment of Treatment Efficacy.
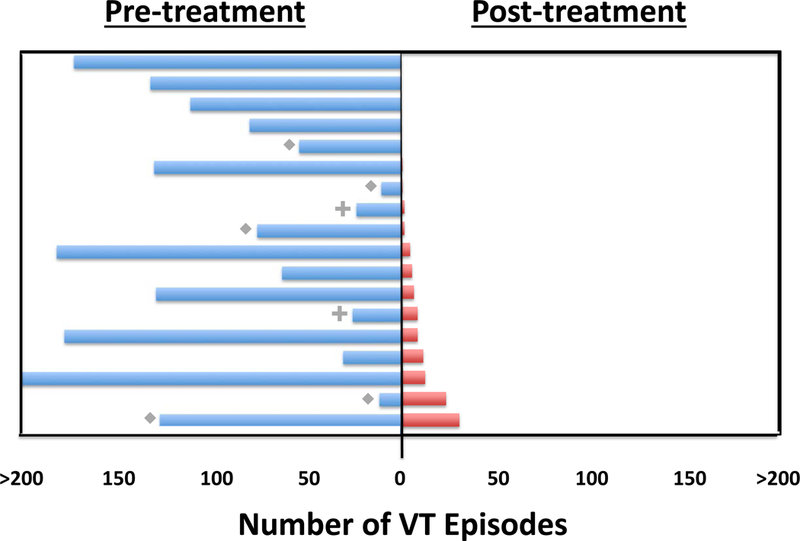
There were 18 patients who survived to 6 months. Patients with incessant VT or sustained slow VT below the ICD detection rate are noted with a diamond (n=5); these episodes were not included in the total. Patients with PVC-mediated cardiomyopathy are noted with a plus (n=2) and displayed as the PVC burden (percentage) captured on a 24 hour Holter monitor. Each line represents an individual patient; blue lines indicate pre-ablation and red lines post-ablation. Upper boundaries are artificially truncated at 200 episodes. Patients are arranged by recurrences during follow-up, ranging from greatest (bottom) to least (top). Frequency of VT was significantly reduced from a median of 119 episodes in the 6 months pre-ablation to a median of 3 episodes in the post-blanking period through 6 months (p < 0.001). For 2 patients with PVC-related cardiomyopathy, 24-hour PVC burden reduced from 24% to 2% and 26% to 9%. The frequency of VT episodes or PVC burden was reduced by 75% in 89% of patients. VT = Ventricular tachycardia, ICD = Implantable Cardioverter Defibrillator, PVC = Premature ventricular contraction
For 16 evaluable patients with ICD-treated VT, there were 1778 VT episodes in aggregate in the 6 months prior to treatment. During the 6-week blanking period, there were 149 episodes. For the next 4.5 months, there were 111 VT episodes (94% total VT episode reduction). The median number of VT episodes decreased from the 6 month pre-ablation period (119, range 4–292) to the 6 month post-ablation period (3, range 0–31, p<0.001). Significant reductions in the median number of ICD shocks (pre-ablation 4, range 0–30 versus post-ablation 0, range 0–7, p=0.002), and for ICD ATP (pre-ablation 81, range 0–292 versus post-ablation 3.5, range 0–29, p=0.001) were observed.
For 2 patients with PVC-related cardiomyopathy, 24-hour PVC burden reduced from 24% to 2% and 26% to 9%. LVEF improved by 13% and 8%, respectively (Figure S4 in the Online Data Supplement).
Pre-specified secondary endpoints included a 50% reduction and 95% reduction in VT episodes or 24-hour PVC burden. This endpoint was achieved in 94% and 61% of patients, respectively. The frequency of VT episodes or PVC burden was reduced by 75% in 89% of patients. A pre-specified secondary endpoint was elimination of ICD shocks and/or improvement in LVEF, which was reached in 72% of patients. Though VT burden was reduced in nearly all, many (11/16, 69%) had some recurrence of VT between the end of the six-week blanking period and six-months. Overall survival was 89% at 6 months and 72% at 12 months (Figure 2A). All-cause mortality is detailed in Table S2 in the Online Data Supplement.
FIGURE 2. Summary of Select Secondary Endpoints.
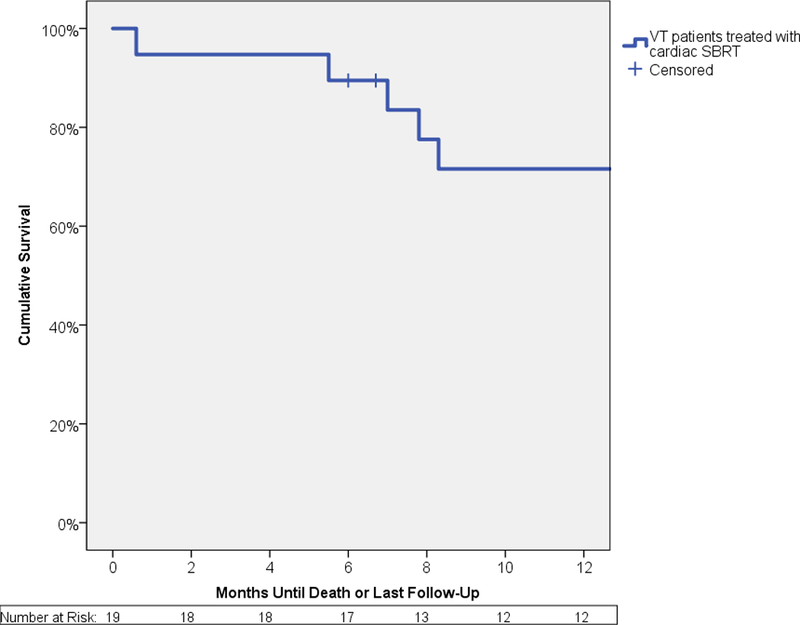
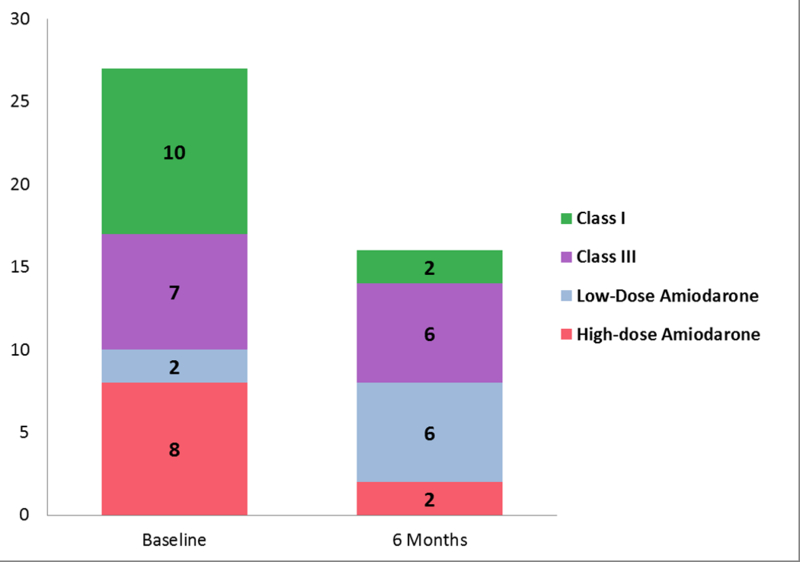
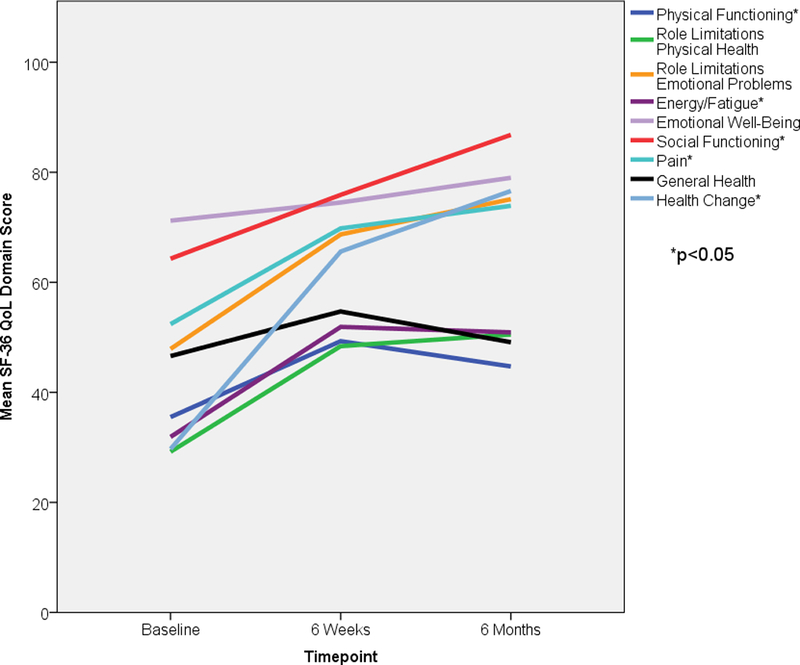
Panel A shows a Kaplan-Meier curve of overall survival for all patients. Actuarial overall survival at 6 months was 89% and 12 months was 72%. Panel B shows a stacked bar graph of anti-arrhythmic medication usage in patients, at baseline and at 6 months after treatment. The y-axis represents the total number of anti-arrhythmic medications used, with the sizes of each color being directly proportional to the number of agents used in that particular class of anti-arrhythmic medication. Amiodarone usage is split into high dose (≥300 mg/day) and low dose (<300 mg/day). Class I agents consisted of mexiletine and flecainide. Class III agents consisted of sotalol. Panel C graphically represents mean scores reported by the 16 patients who were alive at 6 months at baseline, 6 weeks, and 6 months after treatment in 3 selected domains of the Short Form-36 questionnaire – Social Functioning in blue, Health Change in green, General Health in purple. Asterisks denote a significant change (p<0.05) in mean scores over time. VT = Ventricular tachycardia, SBRT = Stereotactic Body Radiotherapy. Figure 2A – Overall Survival for All Patients Underdoing Noninvasive Cardiac Radioablation. Figure 2B - Antiarrhythmic Medication Use. Figure 2C – Select Short Form-36 Quality of Life Measures
Figure 2B shows the distribution of antiarrhythmic medication use before treatment and at six months. Use of dual antiarrhythmic medications decreased from 59% to 12% (p=0.008). Use of high-dose amiodarone (>300 mg per day) decreased from 47% to 12% (p=0.03). Use of class 1 agents decreased from 59% to 12% (0.008). Three patients stopped antiarrhythmic medication completely.
Selected patient-reported quality of life scores are shown in Figure 2C at baseline, 6 weeks, and 6 months after treatment. Significant improvements were observed in perceived health change and social functioning categories. No changes were observed in general health domain. Overall, significant improvements were observed in 5 of 9 domains, as shown in Figure S5 and Table S3 in the Online Data Supplement. Quality of life scores did not decline in any domain.
DISCUSSION
In this prospective phase I/II trial, noninvasive electrophysiology-guided cardiac radioablation was associated with a markedly reduced VT burden with modest short-term risks, reduction in antiarrhythmic drug use, and improvement in quality of life in patients with treatment-refractory VT.
The short-term safety of this technique is apparent: there were no acute procedural toxicities; there were no adverse effects to indwelling ICD systems; there was only one probable treatment-related SAE (pericarditis) within 90 days that improved with medical management. Patients were treated in the ambulatory setting, with a median treatment time of 15 minutes. This represents a major improvement from current acute procedural risk after invasive catheter ablation (6–7%).20, 21
Patients with VT that recurs after a catheter ablation procedure are at 4- to 6-fold increased risk for death.22 Recurrence of VT in patients with reduced heart function or advanced heart failure symptoms has been associated with a one-year transplant-free survival rate of 31–59%.23 Although the sample size in this study is small, the one-year survival of 72% observed with noninvasive cardiac radioablation compares favorably to survival of similar patients.
Specialized cardiac structures (coronary arteries, valves, papillary muscles, and conduction system) may be exposed to radiation based on anatomic location of scar. We did not require use of a fiducial or employ a tracking technique. However, the gain of such a technique for our patients would be expected to be limited given the modest additional volume of target treated to incorporate motion alone (Median 6 cc increase from GTV to ITV). We did, however, add a conservative additional 5 mm margin to create the final PTV based on the remaining uncertainties. The size of ablation volumes necessary to achieve arrhythmia control will likely decrease with improvements in a) measuring and controlling for complex cardiopulmonary motion during SBRT delivery coupled with b) refinement of cardiac imaging synthesis and c) improved understanding of arrhythmic circuits to define the minimum volume for targeting arrhythmias noninvasively.
For most patients, the antiarrhythmic effect of cardiac radioablation happened within the first 6 weeks after treatment. This effect occurred well before the expected time course of myocyte cell death mechanisms and replacement fibrosis.14 Improved understanding of the effect of radiation on diseased cardiac tissues will further improve the safety and efficacy of this technique.
Patient reported quality of life scores were lowest in categories related to physical functioning, fatigue, and general health. Expectedly, these did not improve over the 6 months of follow-up; advanced cardiomyopathies are not expected to recover with this treatment. Highest scores and significant improvement at six months were observed in categories associated with social functioning and health-related optimism. This may reflect a patient group regaining confidence after emotionally traumatic storms of VT, ICD shocks, and repeated hospitalizations.24
Limitations of this study include the non-randomized nature of the trial, limited long term follow-up, treatment at a single center, and limited number of patients and narrow patient selection which prohibits generalization to a larger population. As such, this technique remains investigational. Availability of ECGI remains limited outside of select institutions, so further evaluation of the necessity and/or optimal patient selection for use of this modality will be important. A critical next step in evaluation of this technique will be execution of a multi-center trial, to demonstrate scalability of this approach outside of a single institution, while refining best practices for safety and efficacy. This technique may be of particular global interest given the more ubiquitous availability of SBRT compared with catheter ablation labs and expertise. Close collaboration between radiation oncologists and cardiac electrophysiologists is paramount for this technique, to minimize patient risk and achieve highest levels of ablation accuracy. Similar collaborative workflow models currently exist between radiation oncology and neurosurgery for intracranial radiosurgery.
In conclusion, in patients with intractable VT, the use of EP-guided noninvasive stereotactic cardiac radiotherapy was associated with a marked reduction in the burden of VT with reduced antiarrhythmic medication use and improved quality of life.
Supplementary Material
Clinical Perspective
What Is New?
ENCORE-VT prospectively examined safety and efficacy of noninvasive targeting coupled with a single dose of stereotactic body radiotherapy for patients with refractory VT.
No acute toxicity was observed. Delayed pericarditis/effusion (28%) and pneumonitis (11.1%) occurred, which was generally responsive to medical therapy.
Of 18 evaluable patients, any reduction in VT or PVCs occurred in 17 (94%), with a concurrent reduction in anti-arrhythmic medication.
What Are the Clinical Implications?
Results support continued research into noninvasive cardiac radioablation, with a multi-institutional trial planned.
With limited numbers of patients and lack of long-term safety and efficacy data, this treatment remains investigational.
Acknowledgments
SOURCES OF FUNDING
All financial support was provided by Washington University (Departments of Radiation Oncology (CGR) and Medicine, Cardiovascular Division (PSC)) or from Barnes-Jewish Hospital Foundation competitive grants (PSC). Support for ECGI processing and analyses were provided by NIH R01 HL033343 (YR). No industry-related support was used.
Footnotes
DISCLOSURES
Dr. Robinson has received research grants with Varian Medical Systems, Inc. and Elekta AB (both Significant), and consulting and speaking honoraria from Varian Medical Systems, Inc. and ViewRay Technologies, Inc. (both Modest). Dr. Hugo has received research grants with Varian Medical Systems, Inc. and ViewRay Technologies, Inc. (both Significant). Dr. Knutson has received research grants with Varian Medical Systems, Inc. (Modest), and consulting and speaking honoraria from Varian Medical Systems, Inc. (Modest). Dr. Mutic has received grants, consulting honoraria, and non-financial support from Varian Medical Systems, Inc. (Significant), grants, honoraria, and non-financial support from ViewRay Technologies, Inc. (Modest), grants and non-financial support from Siemens Medical Solutions (Modest), and non-financial support from Philips (Modest). Dr. Cooper has received consulting and speaking honoraria from Medtronic and Abbott (both Modest). Dr. Smith has received research grant support from Medtronic and Boston Scientific (both Modest). Dr. Woodard has received non-financial support from Siemens Medical Solutions (Modest). Dr. Rudy has received royalties from CardioInsight Technologies (Significant). Dr. Cuculich has received consulting honoraria from Medtronic (Modest).The other authors have no relevant conflicts to report
REFERENCES
- 1.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ and Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 2.Dukkipati SR, Koruth JS, Choudry S, Miller MA, Whang W and Reddy VY. Catheter Ablation of Ventricular Tachycardia in Structural Heart Disease: Indications, Strategies, and Outcomes-Part II. J Am Coll Cardiol. 2017;70:2924–2941. [DOI] [PubMed] [Google Scholar]
- 3.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tome WA, Verellen D, Wang L and Yin FF. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. [DOI] [PubMed] [Google Scholar]
- 4.Reshko LB, Kalman NS, Hugo GD and Weiss E. Cardiac radiation dose distribution, cardiac events and mortality in early-stage lung cancer treated with stereotactic body radiation therapy (SBRT). J Thorac Dis. 2018;10:2346–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roach MC, Robinson CG, DeWees TA, Ganachaud J, Przybysz D, Drzymala R, Rehman S, Kashani R and Bradley JD. Stereotactic Body Radiation Therapy for Central Early-Stage NSCLC: Results of a Prospective Phase I/II Trial. J Thorac Oncol. 2018. doi: 10.1016/j.jtho.2018.07.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.John RM, Shinohara ET, Price M and Stevenson WG. Radiotherapy for ablation of ventricular tachycardia: Assessing collateral dosing. Comput Biol Med. 2018. doi: 10.1016/j.compbiomed.2018.08.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Lehmann HI, Graeff C, Simoniello P, Constantinescu A, Takami M, Lugenbiel P, Richter D, Eichhorn A, Prall M, Kaderka R, Fiedler F, Helmbrecht S, Fournier C, Erbeldinger N, Rahm AK, Rivinius R, Thomas D, Katus HA, Johnson SB, Parker KD, Debus J, Asirvatham SJ, Bert C, Durante M and Packer DL. Feasibility Study on Cardiac Arrhythmia Ablation Using High-Energy Heavy Ion Beams. Sci Rep. 2016;6:38895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Refaat MM, Ballout JA, Zakka P, Hotait M, Al Feghali KA, Gheida IA, Saade C, Hourani M, Geara F, Tabbal M, Sfeir P, Jalbout W, Al-Jaroudi W, Jurjus A and Youssef B. Swine Atrioventricular Node Ablation Using Stereotactic Radiosurgery: Methods and In Vivo Feasibility Investigation for Catheter-Free Ablation of Cardiac Arrhythmias. J Am Heart Assoc. 2017;6:e007193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Wong D, Weidlich G, Fogarty T, Jack A, Sumanaweera T and Maguire P. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm. 2010;7:802–810. [DOI] [PubMed] [Google Scholar]
- 10.Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, Faddis M, Gleva M, Noheria A, Smith TW, Hallahan D, Rudy Y and Robinson CG. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med. 2017;377:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cvek J, Neuwirth R, Knybel L, Molenda L, Otahal B, Pindor J, Murárová M, Kodaj M, Fiala F, Branny M and Feltl D. Cardiac Radiosurgery for Malignant Ventricular Tachycardia. Cureus. 2014:6;e190. [Google Scholar]
- 12.Jumeau R, Ozsahin M, Schwitter J, Vallet V, Duclos F, Zeverino M, Moeckli R, Pruvot E and Bourhis J. Rescue procedure for an electrical storm using robotic non-invasive cardiac radio-ablation. Radiother Oncol. 2018;128:189–191. [DOI] [PubMed] [Google Scholar]
- 13.Loo BW Jr., Soltys SG, Wang L, Lo A, Fahimian BP, Iagaru A, Norton L, Shan X, Gardner E, Fogarty T, Maguire P, Al-Ahmad A and Zei P. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8:748–750. [DOI] [PubMed] [Google Scholar]
- 14.Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D and Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–325. [DOI] [PubMed] [Google Scholar]
- 15.Menezes KM, Wang H, Hada M and Saganti PB. Radiation Matters of the Heart: A Mini Review. Front Cardiovasc Med. 2018;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuculich PS, Zhang J, Wang Y, Desouza KA, Vijayakumar R, Woodard PK and Rudy Y. The electrophysiological cardiac ventricular substrate in patients after myocardial infarction: noninvasive characterization with electrocardiographic imaging. J Am Coll Cardiol. 2011;58:1893–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanathan C, Ghanem RN, Jia P, Ryu K and Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004;10:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Cuculich PS, Zhang J, Desouza KA, Vijayakumar R, Chen J, Faddis MN, Lindsay BD, Smith TW and Rudy Y. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Sci Transl Med. 2011;3:98ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Cooper DH, Desouza KA, Cuculich PS, Woodard PK, Smith TW and Rudy Y. Electrophysiologic Scar Substrate in Relation to VT: Noninvasive High-resolution Mapping and Risk Assessment with ECGI. Pacing Clin Electrophysiol. 2016;39:781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santangeli P, Frankel DS, Tung R, Vaseghi M, Sauer WH, Tzou WS, Mathuria N, Nakahara S, Dickfeldt TM, Lakkireddy D, Bunch TJ, Di Biase L, Natale A, Tholakanahalli V, Tedrow UB, Kumar S, Stevenson WG, Della Bella P, Shivkumar K, Marchlinski FE and Callans DJ. Early Mortality After Catheter Ablation of Ventricular Tachycardia in Patients With Structural Heart Disease. J Am Coll Cardiol. 2017;69:2105–2115. [DOI] [PubMed] [Google Scholar]
- 21.Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, Rivard L, Gula L, Leong-Sit P, Essebag V, Nery PB, Tung SK, Raymond JM, Sterns LD, Veenhuyzen GD, Healey JS, Redfearn D, Roux JF and Tang AS. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med. 2016;375:111–121. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, Gonzalez MD, Worley SJ, Daoud EG, Hwang C, Schuger C, Bump TE, Jazayeri M, Tomassoni GF, Kopelman HA, Soejima K and Nakagawa H. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. [DOI] [PubMed] [Google Scholar]
- 23.Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K, Yu R, Vangala S, Tseng CH, Choi EK, Khurshid S, Patel M, Mathuria N, Nakahara S, Tzou WS, Sauer WH, Vakil K, Tedrow U, Burkhardt JD, Tholakanahalli VN, Saliaris A, Dickfeld T, Weiss JP, Bunch TJ, Reddy M, Kanmanthareddy A, Callans DJ, Lakkireddy D, Natale A, Marchlinski F, Stevenson WG, Della Bella P and Shivkumar K. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sears SF, Rosman L, Sasaki S, Kondo Y, Sterns LD, Schloss EJ, Kurita T, Meijer A, Raijmakers J, Gerritse B and Auricchio A. Defibrillator shocks and their effect on objective and subjective patient outcomes: Results of the PainFree SST clinical trial. Heart Rhythm. 2018;15:734–740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


