Summary
Ancillary factors, not directly related to treatment, often play a significant role by affecting therapeutic outcome. A search of the literature was conducted including words related to the placebo phenomenon and orofacial diseases. Therefore, critical factors have been grouped into three major categories: (a) the natural course of the diseases; (b) the regression of the symptoms to their mean intensity; and (c) placebo response. This topical narrative review describes the elements mentioned above, provides an up-to-date overview of the hot topics and gaps in the field and indicates developing and future research direction of the orofacial pain field. Such a knowledge might be positively used during daily clinical practice to optimise the management of orofacial pain diseases, as well as in conducting future clinical trials for validating new interventions.
Keywords: bias, chronic pain, orofacial pain, placebo effect, placebo response, regression to the mean
1 |. INTRODUCTION
Orofacial pain has been described to affect nearly 16%-22% of the American population, with the most common type of orofacial pain being dentoalveolar, reported by 9%-12% of the population, followed by musculoligamentous pain, reported by 5%-6% of the population.1–3 Evidence-based treatment of orofacial pain patients, as stated by the guidelines of the American Academy of Orofacial Pain, is based on several procedures including patient education and self-management, behavioural therapy, pharmacologic management, physical therapy, orthopaedic appliance therapy, dental and occlusal therapy, and surgery.1
The outcome of such therapies is highly dependent on the specific diagnosis. Outcomes are usually fair in patients suffering from odontogenic pain and temporomandibular disorders (TMD), with significant reduction in pain symptoms and related disability and increase in mandibular function;4–6 they are hardly predictable in patients with neuropathic pain.7–9
Ancillary factors, in addition to pain treatment, often play a significant role in the healing processes and affect response to treatments and consequently therapeutic outcomes. Such factors can be grouped into three major categories: (a) the natural course of the diseases; (b) the regression of the symptoms to their mean intensity; and (c) placebo response. The term “ancillary,” from the word “ancilla,” indicates “an aid to achieving or mastering something difficult.” While these factors become problematic in the context of clinical trials, they become a helpful resource in daily clinical practice.
This review describes the elements mentioned above, discussing how they can be used positively during clinical management of orofacial pains, but also how they should be controlled in the context of clinical trials for research purpose.
2 |. MATERIALS AND METHODS
A literature search was carried out to identify relevant articles explicating the role of ancillary factors in the treatment of orofacial pain. A PubMed search was performed using key words such as “natural history,” “natural course,” “spontaneous remission” and “clinical evolution,” associated with the name of the specific orofacial pain diseases as listed in the guidelines of the American Academy of Orofacial Pain,1 including “neuropathic pain,” “trigeminal neuralgia,” “pretrigeminal neuralgia,” “glossopharyngeal neuralgia,” “burning mouth syndrome,” “oral mucosal pain,” “stomatitis,” “aphthous stomatitis,” “traumatic stomatitis,” “herpetic gingivostomatitis,” “geographic tongue,” “temporomandibular disorders,” “TMD,” “temporomandibular joint disorders,” “TMJ,” “TMJ arthralgia,” “TMJ arthritis,” “disc displacement,” “disc displacement with reduction,” “disc displacement without reduction,” “closed lock,” “myofascial pain,” “masticatory myalgia,” “masticatory muscle pain,” “masticatory myositis,” “face pain,” “TMJ arthritis,” “TMJ arthrosis,” “temporal tendonitis,” “dental pain,” “tooth pain,” “dental abscess,” “pulpitis” and “odontogenic pain.” A similar approach has been used with the term “regression to the mean,” and orofacial pain diseases, as well as with “placebo,” “placebo effect” and “placebo response.”
Titles and abstracts were reviewed by one author (MM) and selected to describe potential factors that might influence orofacial pain management. This article does not mean to be a systematic review, rather a topical narrative review that synthesises knowledge in the field of orofacial pain and the placebo phenomenon, providing an up-to-date overview of the hot topics and gaps in the field that are still developing, and indicates the future research direction of the orofacial pain field. The results of those papers are displayed and discussed in the following sections.
2.1 |. Natural course of the disease
Many diseases are self-limiting. Consequently, it is of paramount importance for the clinician to be familiar with such illness behaviour in order to recommend and interpret the results of a therapy. For example, the common cold has a mean duration of about 10 days.10 As a result, any therapy started during this period is going to be successful in a few days. This is also true for several orofacial pain conditions.
2.1.1 |. Neuropathic pain
Usually, neuropathic conditions causing pain do not resolve spontaneously without treatment. However, some cases of spontaneous remission have been reported.11 A retrospective study on patients with burning mouth syndrome (a burning pain in the mouth that may occur every day for months or longer) examined the clinical charts of 53 patients and showed that, even though the majority of the subjects reported either no change in oral symptoms (49%), moderate improvement (28.3%) or worsening of the oral complaints (18.9%), 3.7% of them reported complete spontaneous remission of oral symptoms without any treatment.11 A similar percentage of spontaneous remission (3.2%) has also been observed in a more recent study.12
Also, as reported in a prospective survey of 1052 patients with persistent facial pain, patients with typical trigeminal neuralgia reported periods of spontaneous remission. In about 36% of the subjects, the pain-free intervals could last for months.13
2.1.2 |. Oral mucosal pain
Many painful lesions of the oral mucosa frequently spontaneously resolve without treatment. Among the various conditions affecting the oral mucosa, the most common disease affecting about 10%-20% of the general population is aphthous stomatitis.14,15 It is a typically self-limiting pathology, because the lesions usually heal within 7-14 days without treatment.14,16
Also, traumatic stomatitis, with lesions caused by physical, chemical or thermal insults to the mucosal tissues, is a self-limiting condition. Since the offending cause is generally immediately discontinued, healing is normally quick.17
Recurrent herpetic gingivostomatitis is a highly prevalent viral infection of the oral mucosa. The herpes simplex virus is latent in the trigeminal ganglion and can be periodically reactivated causing unilateral erythematous vesicular eruptions, followed by crusting and spontaneous healing after about 14 days.18,19
Another disease affecting the oral mucosa is geographic tongue, a benign inflammatory disorder characterised by delineated areas of erythema on the dorsum and the borders of the tongue. The lesions vary in size and shape with periods of exacerbations and remissions.20
2.1.3 |. Temporomandibular disorders
Temporomandibular disorders are often remitting, self-limiting or fluctuating over time.1 This characteristic was confirmed by Kamisaka etal, who examined 367 randomly selected citizens of Okayama City twice by the use of a mailed questionnaire.21 The second time, the questionnaire was mailed to the subjects 4 years after they filled out the first one. Prevalence of TMD symptoms was similar in the two surveys; however, significant fluctuation of the symptoms was observed. More than half of the subjects who reported temporomandibular joint (TMJ) pain in the first questionnaire did not report TMJ pain 4 years later in the second questionnaire. Also, 41% of the subjects who reported limited mouth opening in the first questionnaire did not report such limitation 4 years later. Moreover, 22% of the subjects who reported TMJ sounds in the first questionnaire did not report the same symptom in the second questionnaire.21 Similar results were obtained by Manfredini et al, who evaluated the natural course of TMD symptoms in a population of patients with low levels of pain-related impairment according to the Research Diagnostic Criteria for TMD.22,23 Sixty-nine subjects were clinically assessed during the first evaluation and at 2- to 3-year follow-up. The percentage of patients with muscle disorders decreased from 68.1% to 23.1%, about 6% of the patients with disc displacement without reduction with limited mouth opening showed no limitation at follow-up, patients with TMJ arthralgia decreased from 30.4% to 14.4%, and subjects with TMJ osteoarthritis/osteoarthrosis decreased from 27.5% to 24.6%.22
In addition, Kononen demonstrated a fluctuation of TMJ sounds during the 9 years of observation of 128 Finnish adolescents, and only 2% of the subjects reported a TMJ sound at each examination.24
In a recent longitudinal study using computed tomography (CT) and magnetic resonance imaging (MRI), at baseline and after 8 years, Schiffman et al demonstrated that, for the vast majority of the subjects, disc displacement and degenerative joint diseases do not progress. However, an interesting finding is that about 8% of subjects showed improvement of their status.25
Masticatory myofascial pain was evaluated by Rammelsberg et al in a 5-year epidemiologic study. Among the 165 subjects presenting with myofascial pain at baseline, 31% had their pain persisting over a period of 5 years, nevertheless, 33% had complete remission of the symptoms.26 In a different study, among 391 TMD patients, 84 were classified as having only muscle disorders in the first evaluation (37 men, 47 women). At one-year follow-up, the prevalence decreased to 71 subjects (27 men, 44 women), and at two-year follow-up, the prevalence further decreased to 53 subjects (22 men, 31 women). Moreover, subjects with muscle disorders who did not need treatment increased from 27% at the first evaluation to 55% at the second evaluation and slightly decreased to 42% at the third evaluation.27
When specifically examining the condition of disc displacement without reduction, many studies demonstrated that this disorder spontaneously improves with time with a reduction in patients’ pain and dysfunction. In four studies by Sato et al, approximately half of the subjects with disc displacement without reduction improved after about 1 year without treatment, and the success rate was similar to the patients who were treated with a stabilisation splint.28–31 Improvement included mandibular range of motion, limitation of mouth opening, condylar mobility and electromyographic activity during chewing movement.28–31
Yura examined 40 patients with unilateral acute “closed lock” of the TMJ for a period of 12 weeks and found that allowing the disorder to take its natural course without any treatment, only 15 joints had not resolved successfully after 2 weeks, and only 2 of them had been unsuccessful after 12 weeks.32
Kurita et al confirmed such outcome extending the follow-up to 2.5 years and found that about 40% of the patients completely resolved the symptoms, over 30% of them improved, and 25% of the subjects did not improve and needed some treatment.33
Examining the condition of disc displacement with reduction, two relevant studies show that many cases stay stable over time, while others can develop a disc displacement without reduction. However, such progression is rarely accompanied by symptoms of permanent locking.34,35
2.2 |. Regression to the mean
The natural course of diseases can vary remarkably; in the previous section, we described studies reporting examples of self-limiting disorders. Here, we decided to dedicate one section to a particular kind of symptom behaviour in the natural course of the diseases: the regression to the mean. “Regression to the mean” is an expression used to describe a phenomenon occurring when a measurement taken when its value is extreme tends to be closer to the mean value in a successive measurement.36 The concept of regression to the mean is a serious concern in clinical studies when biological values varying above or below a physiological level are assessed.36
Chronic pain is habitually oscillating and fluctuating in time, with alternated high and low levels of pain intensity. Such pain behaviour may influence when a person seeks treatment, because when the level of pain flares up is usually when patients search for evaluation and therapy. Consequently, the improvement of the symptoms following the start of the treatment may also be due to factors not directly related to the treatment itself, such as a regression of the symptoms to their mean intensity (Figure 1).37
FIGURE 1.
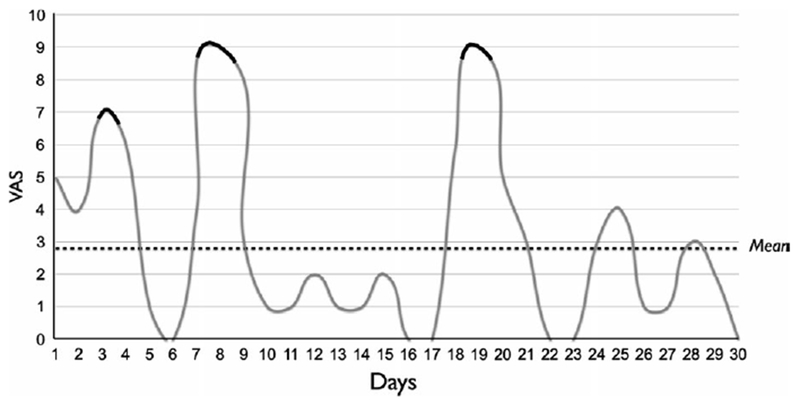
Variation of pain intensity in a 1-month period. The highest peaks of pain intensity are highlighted in black, those are the moments during which the patient sought treatment. VAS, visual analogue scale
In a survey by Whitney et al, the authors tried to uncover the effect of the regression to the mean by comparing the decrease in the symptoms in 2 groups of TMD patients. In the first group, only subjects seeking care for their pain were included, in the second group (control group), only patients not seeking care were enrolled. Such selection was based on the assumption that, as shown in Figure 1, the patients in the first group would be probably in a period of higher pain intensity, and a period of improvement would probably follow due to regression to the mean. Pain level was measured on a 100 mm visual analogue scale. After 1-year follow-up, in the subjects in the first group, a significant 14.7-point reduction in pain intensity was observed, while no mean reduction was noticed in the patients in the control group, regardless of the treatment received.37
2.3 |. Placebo response
The “placebo effect” is a phenomenon occurring during therapy, but it is not directly related to the specific effect of the treatment. It is rather related to what is “around” the treatment, such as the doctor-patient relationship, the environment of the medical office, nature of the treatment, patients’ expectancy and prior experience, repetition of the procedures and the type and route of administration of the treatment.38–43 Placebo effects can be very powerful and lead to significant improvement of the symptoms.44
It is important to specify the difference between the true placebo effect and the perceived placebo effect or placebo response. In fact, the latter is referred to the improvement of the symptoms in the subjects included in the control group of a trial and is the summation of the true placebo effect with other factors such as the natural course of the disease, the regression to the mean and other non-specific time-related factors such as changes in the skill of the investigators, modifications in the familiarity of the patients with the medical environment, seasonal variations of the symptoms and patients adjustments in lifestyle.45,46 Except for the trials where a nointervention group is present, the improvement of the symptoms in the placebo-controlled group of the studies reported in this section is not ascribed to true placebo effect, but to a placebo response.
Most of the information on placebo response can be obtained examining the placebo arm of randomised controlled trials (RCTs) evaluating the efficacy of different types of treatments for orofacial pain conditions.
2.3.1 |. Temporomandibular disorders
The following studies can be used to show the placebo response in the treatment of TMD.
A number of trials were carried out to test the effect of oral appliances by comparing real appliances with placebo appliances. Splint therapy was carried out by the use of conventional and palatal non-occluding appliances. The results were compared and showed similar outcome in terms of palpation score, pain diary score and improvement of maximum mouth opening.47,48
In a study by Dao et al,48 63 patients with myofascial pain of the masticatory muscles were divided into 3 groups: (a) a passive control group, where the subjects worn an oral appliance for only 30 minutes during their appointments at the clinic; (b) an active control group, where the subjects worn a placebo appliance, with only palatal coverage and without any occlusal contact, for 24 hours a day; (c) a treatment group, where the subjects wore an oral appliance for 24 hours a day. Surprisingly, all the patients reported improvement of their pain and quality of life without any significant difference between the groups.48,49
Placebo response was also evident while comparing occlusal adjustment, performed to remove significant slides and non-working side interferences, with mock occlusal adjustment. The effect of such treatment showed no significant difference in the improvement of signs and symptoms of TMD and in the kinesiographic and electromyographic measurements between the treatment and control group.50,51
The placebo response of sham dry needling of active myofascial trigger points in the masseter muscle was evident in TMD patients, but low. Pain pressure threshold in the masseter muscle and the lateral pole of the condyle increased by 8% and 7.4%, respectively.52
Also, sham acupuncture was shown to decrease pain in patients with muscular TMD. Sham acupuncture was performed by inserting acupuncture needles to a depth of only 2-4 mm (compared to a depth of 10-30 mm of real acupuncture treatment) into non-acupuncture points. Mean pain reduction was equal to 26%, with two patients reporting over 70% decrease in their pain.53
Another treatment suggested for the management of myofascial TMD pain is the injection of botulinum toxin type A into the masseter muscle. In a randomised, controlled, double-blind multicentre study, the placebo response of isotonic saline solution resulted in 40% pain reduction after 1 month and 33% pain reduction after 3 months.54
Several pharmacologic therapies have been compared to placebo in TMD patients: analgesic/anti-inflammatory drugs, muscle relaxants and anticonvulsants. The placebo response ranged from 14% to 40% pain relief.55–63 Specifically, when comparing two groups of TMD patients complaining of joint pain and myofascial pain not responsive to occlusal splint, behavioural and physical therapy, administration of low-dose clonazepam in the experimental group induced 22.5% and 20.7% decrease in left and right TMJ pain, respectively, in the control group.55 In addition, a second study comparing clonazepam with cyclobenzaprine and placebo in subjects with myofascial pain showed a 40% placebo response in the control group after 3 weeks.56 A similar placebo response was achieved in a trial comparing the use of diazepam and ibuprofen in chronic myogenous orofacial pain: the group treated with placebo reached 40% pain relief.57 Significant decrease in TMJ pain was reported by the subjects enrolled in the control group of a randomised controlled study assessing the effect of chondroitin sulphate and glucosamine hydrochloride for TMJ arthralgia and osteoarthritis. Such decrease was equal to 34.7%.58 Conversely, the use of placebo compared to Mersyndol, a combination of anti-inflammatory, analgesic and antihistamine medications, in patients with TMD pain, elicited a placebo response of 26.9% pain relief, compared to naproxen and celecoxib elicited a placebo response of 33.7% pain relief, and compared to gabapentin elicited a placebo response of 24.3% pain relief.59–61
Two studies were carried out to evaluate the use of topical medication on the skin overlying the TMJ. Lobo Lobo etal used Theraflex-TMJ, an analgesic and anti-inflammatory cream containing methyl-salicylate, copper- and zinc-pyrocarboxylate, while Winocur et al used capsaicin cream.62,63
The placebo response obtained varied from 13.9% in the former study to 39.8% in the latter, when evaluating TMJ and masseter pain.62,63
For a review of the supposed mechanisms of the placebo effect, see Greene et al38
2.3.2 |. Neuropathic pain
The amount of placebo response in patients with neuropathic pain can be inferred from the evaluation of the placebo arm of randomised controlled studies on the treatment of different types of orofacial pain.
The placebo response on the management of trigeminal neuralgia has been described in a crossover study alternating the use of car-bamazepine and placebo every 2 weeks of treatment, for 8 weeks.64 The group starting with the placebo reported 26% reduction in pain in the first 2-week period on placebo and 17% improvement in the third 2-week period on placebo. The group starting with the actual treatment reported 5% reduction in the pain in the second 2-week period on placebo and 15% improvement in the fourth 2-week period on placebo.64
When BOTOX injections were used, the response rate of the placebo group was equal to 32.1% of the patients that reported that their pain was “very much improved” after 8 weeks of treatment.65
Another chronic orofacial pain condition characterised by neuropathic pain, such as burning mouth syndrome, has been shown to elicit inconsistent placebo response. In a placebo-controlled clinical study, among the 33 patients included in the control group, only two reported significant improvement of the symptoms after 6 months and only one reported complete resolution of the pain.66 However, when laser therapy was used instead of tablet medications, the placebo response increases leading to almost half of the cases reaching total relief of the symptoms, confirming the more powerful placebo effect of technologic procedures.67
Nevertheless, a recent review examining the placebo arm of 12 RCTs investigating burning mouth syndrome showed a positive placebo response in 6 of them. The mean placebo response, indicated as a percentage of actual therapy response, was 72%, demonstrating a strong perceived placebo effect.68
2.3.3 |. Dental pain
The role of placebo can also be observed in patients with tooth pain.
One hundred emergency patients with a diagnosis of irreversible pulpitis and with severe tooth pain received either a real medication or a placebo medication and were then evaluated every 10 minutes by cold testing for a 1-hour period. The difference between the two groups was not statistically significant; however, both groups showed a reduction in pain intensity from severe to moderate. Only 20%-34% of the patients still had severe pain after 60 minutes.69 Another study evaluating the effect of a topical medication on toothache due to caries, fracture and lost restorations displayed a significant placebo response. Despite the fact that a higher number of responders were achieved by administering the topical real medication (benzocaine), 46.7% of the subjects in the placebo group reported decrease in pain.70
A different type of dental pain, the post-surgical pain following the surgical extraction of impacted third molars, was treated with different formulations of aspirin tablets compared to placebo. Although aspirin resulted in higher levels of pain reduction, the use of placebo produced perceptible pain relief in 62%-70.5% of the subjects and meaningful relief in 30%-37.1% of the subjects. Over 40% of such patients reported fair-to-excellent relief after treatment.71
In a similar study, placebo elicited 5.05 total pain relief, as determined by summation of assessments of pain relief scores as measured on a visual analogue scale (VAS) over a 0- to 6-hour period, compared to 14.98 of oxycodone 5 mg/ibuprofen 400 mg, which was the medication that achieved the greatest analgesia.72 Similar results were reported in a trial comparing different formulations of intravenous diclofenac and ketorolac and placebo. Diclofenac and ketorolac were shown to be superior to placebo, nevertheless, administration of placebo produced 62.8 decrease in total pain relief, as determined by adding VAS scores recorded over a 0- to 6-hour period.73
3 |. DISCUSSION
In the present review, ancillary factors playing a significant role in the treatment of different types of orofacial pain have been examined. Full awareness and understanding of such aspects are essential for the clinicians for a correct planning and evaluation of orofacial pain therapies. The effect of the natural course of the diseases, regression to the mean and placebo response always occur during treatments, regardless of the efficacy of the cure itself; therefore, they can introduce significant bias in the outcome of the therapy. When the natural course of the disease is self-limiting and the patient comes for evaluation when the symptoms are severe, we already know that the pain is going to have a remission regardless of the treatment we can start. In addition, placebo effects and placebo responses elicited by the therapy can further magnify the effect of the cure. We may also try to enhance the placebo effect by exploiting different features that are associated with more advantageous outcome, such as improving the doctor-patient relationship, ameliorating the environment of the medical office, choosing the most appropriate nature, type and route of administration of the treatment. All these characteristics can be positively used to improve the outcome of treatments and the resolution of the patients’ symptoms.
For these reasons, the ancillary factors mentioned in this review are always important components of any therapeutic strategy and should be frequently sought in clinical practice. It is remarkably different when the goal is to run a clinical trial to evaluate the effect of a treatment. In this case, all these aspects need to be controlled because they can introduce a significant bias to the results of the trial.
To minimise the effect of the natural course of the disease, RCTs should include a non-treatment patient group, in addition to the placebo-controlled group, to which the placebo group could be compared.39,45,46
To minimise the effect of the regression to the mean, it has been proposed to use the average of a number of measurements as baseline value instead of a single measurement. This method reduces the variability of the quantifications and therefore should reduce the effect of regression in succeeding measurements.36
A second way to control the effect of fluctuation of the symptoms over time is the use of pattern analysis while evaluating the results. Such analysis has been suggested for the treatment of psychiatric conditions, but the concept can be extended to other chronic disorders. It consists in giving a 1 score to the patients that were “improved” or “much improved” for each week of treatment and a 0 score to all the other subjects. In a six-week period, the pattern can vary from 000000 to 111111, but a stable and non-fluctuating improvement (for example 000111 or 001111) is suggestive of a true therapy effect.74,75
The inclusion of a placebo-controlled group in RCTs is perpetrated to contain the effect of placebo and reduce the consequent bias to the results. Effort should be made to blind the subjects and the clinicians, when possible, according to the type of treatment. When it is not possible to blind the clinicians (for example in case of surgical procedures), different investigators blind to the therapy should evaluate the results.
It has also been suggested to exclude placebo responders during wash-in periods of the trial. However, this is considered unethical because it precludes the possibility of receiving active treatments to the entire category of patients. Such exclusion also compromises the generalisability of the results, because placebo responders might also respond differently to active treatments.39
In addition, significant effort should be made by the investigators while carrying out the trials in order to minimise the placebo effect. Any direct and indirect influence to the patients, and comments on the treatments carried out, must be avoided.39
A different approach was suggested and named the “open-hidden paradigm.” Instead of eliminating the specific effect of the treatment, like in the placebo group of RCTs, the context of the treatment is eliminated by using a preprogrammed computer-controlled infusion pump to deliver the medication intravenously. The pump is concealed, and no clinicians or nurses are present in the room; therefore, the patients do not know when they are receiving the treatment. If a drug is really effective, pain reduction will correlate with the time that the drug is administered. However, this can be carried out in limited cases.76 Moreover, future studies including a no-intervention arm can help disentangle the contribution of each of these factors to the overall outcome and separate them from potential true placebo effects.
A major limitation of this study is the lack of a systematic search and analysis of the literature, which would have reduced the bias of the results associated with differently selected studies.
4 |. CONCLUSIONS
This review highlighted the effect of ancillary factors not directly related to active treatment on the outcome of orofacial pain therapies. The natural course of the diseases, regression to the mean and placebo response can significantly improve orofacial pain symptoms. Clinicians need to be familiar with such elements, to be able to use them during clinical management of orofacial pains, but also to control them during clinical trials for research purpose. It is important to take it into account when judging clinical results or designing clinical research. In addition, since the effect of these ancillary factors can be remarkable, it must be emphasised the use of non-invasive and reversible strategies for the management of orofacial pains; irreversible treatments must be limited to specific cases.
ACKNOWLEDGEMENTS
No conflict of interest declared. This study was carried out without funding.
REFERENCES
- 1.de Leeuw R, Klasser GD, eds. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management, 6th edn. Chicago, IL: Quintessence; 2018. [Google Scholar]
- 2.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. [DOI] [PubMed] [Google Scholar]
- 3.Horst OV, Cunha-Cruz J, Zhou L, Manning W, Manci L, DeRouen TA. Prevalence of pain in the orofacial regions in patients visiting general dentists in the Northwest Practice-based Research Collaborative in Evidence-based DENTistry research network. J Am Dent Assoc. 2015;146:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roldan-Barraza C, Lanko S, Villanueva J, Araya I, Lauer HC. A systematic review and meta-analysis of usual treatment versus psychosocial interventions in the treatment of myofascial temporomandibular disorder pain. Journal of Oral & Facial Pain and Headache. 2014;28:205–222. [DOI] [PubMed] [Google Scholar]
- 5.Vena DA, Collie D, Wu H, et al. Prevalence of persistent pain 3 to 5 years post primary root canal therapy and its impact on oral health-related quality of life: PEARL Network findings. Journal of Endodontics. 2014;40:1917–1921. [DOI] [PubMed] [Google Scholar]
- 6.Nixdorf DR, Moana-Filho EJ, Law AS, McGuire LA, Hodges JS, John MT. Frequency of persistent tooth pain after root canal therapy: a systematic review and meta-analysis. Journal of Endodontics. 2010;36:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun A, Wu KM, Wang YP, Lin HP, Chen HM, Chiang CP. Burning mouth syndrome: a review and update. J Oral Pathol Med. 2013;42:649–655. [DOI] [PubMed] [Google Scholar]
- 8.Haviv Y, Zadik Y, Sharav Y, Benoliel R. Painful traumatic trigeminal neuropathy: an open study on the pharmacotherapeutic response to stepped treatment. Journal of Oral & Facial Pain and Headache. 2014;28:52–60. [DOI] [PubMed] [Google Scholar]
- 9.Salah S, Thomas L, Ram S, Clark GT, Enciso R. Systematic review and meta-analysis of the efficacy of oral medications compared with placebo treatment in the management of postherpetic neuralgia. Journal of Oral & Facial Pain and Headache. 2016;30:255–266. [DOI] [PubMed] [Google Scholar]
- 10.Arruda E, Pitkäranta A, Witek TJ Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sardella A, Lodi G, Demarosi F, Bez C, Cassano S, Carrassi A. Burning mouth syndrome: a retrospective study investigating spontaneous remission and response to treatments. Oral Dis. 2006;12:152–155. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-de Rivera-Campillo E, López-López J. Evaluation of the response to treatment and clinical evolution in patients with burning mouth syndrome. Medicina Oral, Patologia Oral y Cirugia Bucal. 2013;18:e403–e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen P Facial pain. II. A prospective survey of 1052 patients with a view of: character of the attacks, onset, course, and character of pain. Acta Neurochir 1990;107:121–128. [DOI] [PubMed] [Google Scholar]
- 14.Vucicevic Boras V, Savage NW. Recurrent aphthous ulcerative disease: presentation and management. Aust Dent J 2007;52:10–15. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay A, Shetty KV. Recurrent aphthous stomatitis. Otolaryngol Clin North Am. 2011;44:79–88. [DOI] [PubMed] [Google Scholar]
- 16.Woo SB, Sonis ST. Recurrent aphthous ulcers: a review of diagnosis and treatment. J Am Dent Assoc. 1996;127:1202–1213. [DOI] [PubMed] [Google Scholar]
- 17.Tosti A, Piraccini BM, Peluso AM. Contact and irritant stomatitis. Semin Cutan Med Surg. 1997;16:314–319. [DOI] [PubMed] [Google Scholar]
- 18.Westley S, Seymour R, Staines K. Recurrent intra-oral herpes simplex 1 infection. Dental Update 2011;38:368–370. [DOI] [PubMed] [Google Scholar]
- 19.Scott DA, Coulter WA, Lamey PJ. Oral shedding of herpes simplex virus type 1: a review. J Oral Pathol Med. 1997;26:441–447. [DOI] [PubMed] [Google Scholar]
- 20.Assimakopoulos D, Patrikakos G, Fotika C, Elisaf M. Benign migratory glossitis or geographic tongue: an enigmatic oral lesion. Am J Med. 2002;113:751–755. [DOI] [PubMed] [Google Scholar]
- 21.Kamisaka M, Yatani H, Kuboki T, Matsuka Y, Minakuchi H. Four-year longitudinal course of TMD symptoms in an adult population and the estimation of risk factors in relation to symptoms. Journal of Orofacial Pain. 2000;14:224–232. [PubMed] [Google Scholar]
- 22.Manfredini D, Favero L, Gregorini G, Cocilovo F, Guarda-Nardini L. Natural course of temporomandibular disorders with low pain-related impairment: a 2-to-3-year follow-up study. J Oral Rehabil. 2013;40:436–442. [DOI] [PubMed] [Google Scholar]
- 23.Dworkin S, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examination and specifications, critique. Journal of Craniomandibular Disorders: Facial & Oral Pain. 1992;6:301–355. [PubMed] [Google Scholar]
- 24.Kononen M, Waltimo A, Nystrom M. Does clicking in adolescence lead to painful temporomandibular joint locking? Lancet. 1996;347:1080–1081. [DOI] [PubMed] [Google Scholar]
- 25.Schiffman EL, Ahmad M, Hollender L, et al. Longitudinal stability of common TMJ structural disorders. J Dent Res. 2017;96:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rammelsberg P, LeResche L, Dworkin S, Mancl L. Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by Research Diagnostic Criteria for temporomandibular disorders. Journal of Orofacial Pain. 2003;17:9–20. [PubMed] [Google Scholar]
- 27.Kuttila M, Kuttila S, Niemi PM, Alanen P, Le Bell Y. Fluctuation of treatment need for temporomandibular disorders and age, gender, stress, and diagnostic subgroup. Acta Odontol Scand. 1997;55:350–355. [DOI] [PubMed] [Google Scholar]
- 28.Sato S, Kawamura H, Motegi K. Management of nonreducing temporomandibular joint disk displacement. Evaluation of three treatments. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80:384–388. [DOI] [PubMed] [Google Scholar]
- 29.Sato S, Goto S, Kawamura H, Motegi K. The natural course of nonreducing disc displacement of the TMJ: relationship of clinical findings at initial visit to outcome after 12 months without treatment. Journal of Orofacial Pain. 1997;11:315–320. [PubMed] [Google Scholar]
- 30.Sato S, Takahashi K, Kawamura H, Motegi K. The natural course of nonreducing disk displacement of the temporomandibular joint: changes in condylar mobility and radiographic alterations at one-year follow up. Int J Oral Maxillofac Surg. 1998;27:173–177. [DOI] [PubMed] [Google Scholar]
- 31.Sato S, Kawamura H. Natural course of non-reducing disc displacement of the temporomandibular joint: changes in electromyographic activity during chewing movement. J Oral Rehabil. 2005;32:159–165. [DOI] [PubMed] [Google Scholar]
- 32.Yura S Natural course of acute closed lock of the temporomandibular joint. Br J Oral Maxillofac Surg. 2012;50:646–649. [DOI] [PubMed] [Google Scholar]
- 33.Kurita K, Westesson PL, Yuasa H, Toyama M, Machida J, Ogi N. Natural course of untreated symptomatic temporomandibular joint disc displacement without reduction. J Dent Res. 1998;77:361–365. [DOI] [PubMed] [Google Scholar]
- 34.Kalaykova S, Lobbezoo F, Naeije M. Two-year natural course of anterior disc displacement with reduction. Journal of Orofacial Pain. 2010;24:373–378. [PubMed] [Google Scholar]
- 35.Naeije M, Te Veldhuis AH, Te Veldhuis EC, Visscher CM, Lobbezoo F. Disc displacement within the human temporomandibular joint: a systematic review of a ‘noisy annoyance’. J Oral Rehabil. 2013;40:139–158. [DOI] [PubMed] [Google Scholar]
- 36.Davis CE. The effect of regression to the mean in epidemiologic and clinical studies. Am J Epidemiol. 1976;104:493–498. [DOI] [PubMed] [Google Scholar]
- 37.Whitney CW, Von Korff M. Regression to the mean in treated versus untreated chronic pain. Pain. 1992;50:281–285. [DOI] [PubMed] [Google Scholar]
- 38.Greene CS, Goddard G, Macaluso GM, Mauro G. Topical review: placebo responses and therapeutic responses. How are they related?. Journal of Orofacial Pain 2009;23:93–107. [PubMed] [Google Scholar]
- 39.Dumitriu A, Popescu BO. Placebo effects in neurological diseases. Journal of Medicine and Life. 2010;3:114–121. [PMC free article] [PubMed] [Google Scholar]
- 40.Autret A, Valade D, Debiais S. Placebo and other psychological interactions in headache treatment. The Journal of Headache and Pain. 2012;13:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meissner K, Fässler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941–1951. [DOI] [PubMed] [Google Scholar]
- 42.de Craen AJ, Tijssen JG, de Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol. 2000;247:183–188. [DOI] [PubMed] [Google Scholar]
- 43.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–133. [DOI] [PubMed] [Google Scholar]
- 44.Pramod GV, Shambulingappa P, Shashikanth MC, Lele S. Analgesic efficacy of diazepam and placebo in patients with temporomandibular disorders: a double blind randomized clinical trial. Indian Journal of Dental Research. 2011;22:404–409. [DOI] [PubMed] [Google Scholar]
- 45.Ernst E, Resch KL. Concept of true and perceived placebo effects. BMJ. 1995;311:551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colloca L Treatment of pediatric migraine (Letter to the editor). N Engl J Med. 2017;376:1387–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Ani Z, Gray RJ, Davies SJ, Sloan P, Glenny AM. Stabilization splint therapy for the treatment of temporomandibular myofascial pain: a systematic review. J Dent Educ. 2005;69:1242–1250. [PubMed] [Google Scholar]
- 48.Dao TT, Lavigne GJ, Charbonneau A, Feine JS, Lund JP. The efficacy of oral splints in the treatment of myofascial pain of the jaw muscles: a controlled clinical trial. Pain. 1994;56:85–94. [DOI] [PubMed] [Google Scholar]
- 49.Rubinoff MS, Gross A, McCall WD. Conventional and non-occluding splint therapy compared for patients with myofascial pain dysfunction syndrome. General Dentistry. 1987;35:502–506. [PubMed] [Google Scholar]
- 50.Tsolka P, Morris RW, Preiskel HW. Occlusal adjustment therapy for craniomandibular disorders: a clinical assessment by a double-blind method. J Prosthet Dent 1992;68:957–964. [DOI] [PubMed] [Google Scholar]
- 51.Tsolka P, Preiskel HW. Kinesiographic and electromyographic assessment of the effects of occlusal adjustment therapy on craniomandibular disorders by a double-blind method. J Prosthet Dent. 1993;69:85–92. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Carnero J, La Touche R, Ortega-Santiago R, et al. Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. Journal of Orofacial Pain. 2010;24:106–112. [PubMed] [Google Scholar]
- 53.Goddard G, Karibe H, McNeill C, Villafuerte E. Acupuncture and sham acupuncture reduce muscle pain in myofascial pain patients. Journal of Orofacial Pain 2002;16:71–76. [PubMed] [Google Scholar]
- 54.Emberg M, Hedenberg-Magnusson B, List T, Svensson P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blind multicenter study. Pain. 2011;152:1988–1996. [DOI] [PubMed] [Google Scholar]
- 55.Harkins S, Linford J, Cohen J, Kramer T, Cueva L. Administration of clonazepam in the treatment of TMD and associated myofascial pain: a double-blind pilot study. Journal of Craniomandibular Disorders. 1991;5:179–186. [PubMed] [Google Scholar]
- 56.Herman CR, Schiffman EL, Look JO. The effectiveness of adding pharmachologic treatment with Clonazepam or Cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: a randomized clinical trial. Journal of Orofacial Pain. 2002;16:64–70. [PubMed] [Google Scholar]
- 57.Singer E, Dionne R. A controlled evaluation of ibuprofen and diazepam for chronic orofacial muscle pain. Journal of Orofacial Pain. 1997;11:139–146. [PubMed] [Google Scholar]
- 58.Nguyen P, Mohamed SE, Gardiner D, Salinas T. A randomized double-blind clinical trial of the effect of chondroitin sulfate and glucosamine hydrochloride on temporomandibular joint disorders. Journal of Craniomandibular Practice. 2001;19:130–139. [DOI] [PubMed] [Google Scholar]
- 59.Gerschman JA, Reade PD, Burrows GD. Evaluation of a proprietary analgesic/antihistamine in the management of pain associated with temporomandibular joint pain dysfunction syndrome. Aust Dent J. 1984;29:300–304. [DOI] [PubMed] [Google Scholar]
- 60.Ta LE, Dionne RA. Treatment of painful temporomandibular joints with cyclooxygenase-2 inhibitor: a randomized placebo-controlled comparison of celecoxib to naproxen. Pain. 2004;111:13–21. [DOI] [PubMed] [Google Scholar]
- 61.Kimos P, Biggs C, Mah J, et al. Analgesic action of Gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial. Pain. 2007;127:151–160. [DOI] [PubMed] [Google Scholar]
- 62.Lobo Lobo S, Mehta N, Forgione AG, et al. Journal of Craniomandibular Practice. 2004;22:137–144. [DOI] [PubMed] [Google Scholar]
- 63.Winocur E, Gavish A, Halachmi M, Eli I, Gazit E. Topical application of capsaicin for the treatment of localized pain in the temporomandibular joint area. Journal of Orofacial Pain 2000;14:31–36. [PubMed] [Google Scholar]
- 64.Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29:265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Lian Y, Ma Y, et al. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. The Journal of Headache and Pain. 2014;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodríguez de Rivera Campillo E, López-López J, Chimenos-Küstner E. Response to topical clonazepam in patients with burning mouth syndrome: a clinical study. Bull Group Int Rech Sci Stomatol Odontol 2010;49:19–29. [PubMed] [Google Scholar]
- 67.Sugaya NN, Silva ÉF, Kato IT, Prates R, Gallo CB, Pellegrini VD. Low intensity laser therapy in patients with burning mouth syndrome: a randomized, placebo-controlled study. Brazilian Oral Research. 2016;30:e108. [DOI] [PubMed] [Google Scholar]
- 68.Kuten-Shorrer M, Kelley JM, Sonis ST, Treister NS. Placebo effect in burning mouth syndrome: a systematic review. Oral Dis. 2014;20:e1–e6. [DOI] [PubMed] [Google Scholar]
- 69.Fowler S, Fullmer S, Drum M, Reader A. Does acetaminophen/hydrocodone affect cold pulpal testing in patients with symptomatic irreversible pulpitis? A prospective, randomized, double-blind, placebo-controlled study. Journal of Endodontics. 2014;40:1958–1960. [DOI] [PubMed] [Google Scholar]
- 70.Hersh EV, Stoopler ET, Secreto SA, DeRossi SS. A study of benzocaine gel dosing for toothache. Journal of Clinical Dentistry. 2005;16:103–108. [PubMed] [Google Scholar]
- 71.Cooper SA, Voelker M. Evaluation of onset of pain relief from micronized aspirin in a dental pain model. Inflammopharmacology. 2012;20:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Litkowski LJ, Christensen SE, Adamson DN, Van Dyke T, Han SH, Newman KB. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model. Clin Ther 2005;27:418–429. [DOI] [PubMed] [Google Scholar]
- 73.Christensen K, Daniels S, Bandy D, et al. A double-blind placebo-controlled comparison of a novel formulation of intravenous diclofenac and ketorolac for postoperative third molar extraction pain. Anesth Prog. 2011;58:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quitkin FM, Rabkin JD, Markowitz JM, Stewart JW, McGrath PJ, Harrison W. Use of pattern analysis to identify true drug response. A replication. Arch Gen Psychiatry. 1987;44:259–264. [DOI] [PubMed] [Google Scholar]
- 75.Howland RH. Understanding the placebo effect. Part 1: placebo use in clinical trials. J Psychosoc Nurs Ment Health Serv 2008;46:17–20. [DOI] [PubMed] [Google Scholar]
- 76.Koshi EB, Short CA. Placebo theory and its implications for research and clinical practice: a review of the recent literature. Pain Practice. 2007;7:4–20. [DOI] [PubMed] [Google Scholar]


