Abstract
Objective
Features of cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy ( CADASIL) caused by NOTCH3 mutations vary between ethnicities and regions. In Taiwan, more than 70% of CADASIL patients carry the mutation hot spot of p.R544C. We investigated the prevalence of NOTCH3 p.R544C mutation in stroke patients in Taiwan.
Methods
This prospective, multicenter study recruited acute stroke patients within 10 days of symptom onset. The p.R544C mutation was identified by polymerase chain reaction with confronting two‐pair primers and sequencing. Clinical parameters, vascular risk factors, stroke subtypes, and stroke outcomes were analyzed.
Results
Of the 1970 stroke patients (mean age 61.1 ± 13.6 years, male 69.5%) included, 1705 (86.5%) had ischemic stroke and 265 (13.5%) had intracerebral hemorrhage. The prevalence of p.R544C in the study population was 2.8% (95% confidence interval [CI] = 2.1–3.5%). The prevalence was highest in patients with small vessel occlusion type of ischemic stroke (5.6%), followed by intracerebral hemorrhage (5.3%), and infarct of undetermined etiology (2.7%), and was low in patients with cardioembolism (0.8%) and large artery atherosclerosis (0.7%). All p.R544C patients with intracerebral hemorrhage were nonlobar hemorrhage. Sibling history of stroke (odds ratio [OR] = 4.50, 95% CI = 1.67–12.14 in ischemic stroke; OR = 6.03, 95% CI = 1.03–35.47 in intracerebral hemorrhage, respectively) and small vessel occlusion (OR, 4.03, 95% CI, 1.26–12.92) were significantly associated with p.R544C.
Interpretation
p.R544C NOTCH3 mutation is underdiagnosed in stroke patients in Taiwan, especially in those with small vessel occlusion and sibling history of stroke.
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy (CADASIL) is an autosomal dominant inherited vasculopathy and the most common single‐gene disorder causing stroke.1, 2, 3 The defective, 33‐exon NOTCH3 gene is located on chromosome 19.4 NOTCH3 mutations typically affect the number of highly conserved cysteine residues within the epidermal growth factor‐like repeat domain.1 Reports of more than 200 different NOTCH3 mutations in patients all over the world suggest that CADASIL has considerable genetic heterogeneity.5, 6, 7
The first case of CADASIL in Taiwan was reported in 2004.8 Over the past few years, the number of cases in Taiwan has increased owing to raised clinical awareness of the disease and advancements in molecular genetic testing.9 In 2015, Liao et al. reported that p.R544C in exon 11 was the most common mutation, accounting for 70.5% of the pedigrees of 112 patients with CADASIL belonging to 95 families of Chinese descent in Taiwan.10 This finding may indicate a specific genetic hot spot for mutations related to CADASIL in Taiwan.
It has been suggested that the prevalence of CADASIL is underestimated, especially in Asia.1, 7 However, the results of recent epidemiological studies investigating the prevalence of NOTCH3 mutations in stroke patients have varied.11, 12, 13, 14 Since p.R544C in exon 11 accounts for more than 70% of NOTCH3 mutations in Taiwan, we aim to investigate the prevalence of p.R544C in a cohort of patients with ischemic stroke (IS) or intracerebral hemorrhage (ICH) from a multicenter registry in Taiwan, and compare their epidemiological features with those without the mutation.
Materials and Methods
Study design and population
The study was conducted by the Formosa Stroke Genetics Consortium (FSGC), a group of six medical centers (National Taiwan University Hospital, Shin Kong Wu Ho‐Su Memorial Hospital, National Cheng Kung University Hospital, Taipei Veteran General Hospital, Chi‐Mei Medical Center, and Tri‐Service General Hospital), and three local hospitals (Taipei Medical University Hospital, Shuang Ho Hospital, and National Taiwan University Hospital, Yun‐Lin Branch) dedicated to studying the molecular biology of cerebrovascular diseases.15 An expert panel of stroke neurologists and epidemiologists developed a standard FSGC operation manual and protocol for biological sample collection.15 The study was approved by the ethics committees of the participating hospitals. Informed consent was obtained from all participants and/or their relatives.
Stroke patients were recruited if the subject presented within 10 days of symptom onset and received examination including computed tomography and/or magnetic resonance imaging for this index event. The criteria used for stroke diagnosis have been previously detailed.16 IS was further classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.17 Hypertension‐related ICH was defined as hemorrhage occurring in deep structures such as basal ganglia, thalamus, brainstem and cerebellum, with hypertension as indicated by a history of hypertension or sustained systolic blood pressure readings averaging >140 mmHg or of diastolic blood pressure averaging >90 mmHg after hospital admission.16 Patients with traumatic ICH, ICH colocalized with tumor, and primary subdural/epidural/subarachnoid hemorrhage were excluded.18
Each patient's vascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, heart disease, smoking habit), previous history of stroke or transient ischemic attack (TIA), and family history of first‐degree relatives (biological parents and siblings) ever had a stroke and vascular risk factors were collected. Modified Rankin Scale (mRS) was used to determine outcome at 1 month and 3 months after the stroke. Favorable outcome was defined as mRS score of ≦1.16
Data collection
Data were collected from participating hospitals.15 Each patient's information including the background information, vascular risk factors, related past histories, family history, and post stroke outcome was collected by study nurses, research assistants, and stroke neurologists on preadmission, admission, discharge, and at 1 and 3 months follow‐up. Investigators were certified to perform National Institutes of Health Stroke Scale (NIHSS) and mRS scoring. The study nurses received a standard training for the mRS scoring, data collection, and data entry through a web‐based database system.15 The data were abstracted from medical charts or self‐reported during interviews conducted by trained research assistants and study nurses. For patients with head MRI examination, white matter was divided into periventricular white matter (PVWM) and deep white matter (DWM), and the abnormally high signals on T2 or fluid‐attenuated inversion recovery (FLAIR) images were semiquantified and scored by using the Fazekas scale.
Genotyping
Genomic DNA was extracted using a nonorganic method and then stored at −80°C until use.15 The NOTCH3 mutation p.R544C was genotyped by PCR‐CTPP (polymerase chain reaction with confronting two‐pair primers). DNA was amplified with the primers: 5′ GTGGGGTGGAGTGGAAGTAAGTGG (F1) and 5′ GAGCAGTCGTCCACGTTGCA (R1) for the C allele, and 5′ TTGAGGGCACGCTGTGTGATC (F2) and 5′ ACTAGATGCACCATTCCCAAACCC (R2) for the T allele. The PCR amplification was performed for 35 cycles (denaturation at 95°C for 30 sec, annealing at 62°C for 30 sec, extension at 72°C for 30 sec, and final extension at 72°C for 10 min). PCR products of 479 and 216 bp for the TT genotype, 479, 303, and 216 bp for the TC genotype, 479 and 303 bp for the CC genotype were visualized on 2% agarose gel stained with ethidium bromide. The genotype identified by PCR‐CTPP analysis was confirmed by DNA sequencing analysis, and these analyses were completely concordant. To assure data quality, we sequenced 5% of samples using the ABI 3100 DNA sequencer (Applied Biosystems). The genotype concordance rate between duplicate samples was 100%.
Statistical analyses
Student's t‐test was used to compare continuous variables between p.R544C (+) patients and p.R544C (−) patients. The chi‐square test was used for analysis of categorical variables. Multivariate logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) after adjustment for potential confounders. All statistical analyses were performed with IBM SPSS statistical software (version 23.0, IBM SPSS Inc., USA). A two‐tailed P value <0.05 was considered statistically significant.
Results
By the end of September 2016, 1970 patients were included for this study. 1705 presented as IS (86.5%) and 265 as ICH (13.5%). The mean age was 61.1 ± 13.6 years, and 1370 (69.5%) were men. According to TOAST criteria, there were 580 (34.0%) large artery atherosclerosis (LAA), 496 (29.1%) small vessel occlusion (SVO), 247 (14.5%) cardioembolism, 121 (7.1%) other specific etiologies, and 261 (15.3%) undetermined etiologies in IS patients.
In our cohort, the prevalence of p.R544C mutation [(p.R544C (+)] was 2.8% (95% confidence intervals [CI] = 2.1–3.5%), being identified in 55 patients (mean age of 59.2 ± 11.1 years, male 63.6%). Of them, 41 patients (74.5%) were diagnosed with IS and 14 (25.5%) with ICH. Figure 1A shows the age distributions of patients with p.R544C (−) and p.R544C (+) respectively. Figure 1B shows the distribution of p.R544C (+) among patients with different stroke subtypes. Specifically, p.R544C (+) was most prevalent in patients with SVO (n = 28, 5.6%. 95% CI = 3.6–7.7%) followed by ICH (n = 14, 5.3%, 95% CI = 2.6–8.0%), infarct of undetermined etiology n = 7, 2.7%, 95% CI = 0.7–4.6%), cardioembolism (n = 2, 0.8%, 95% CI = 0–1.9%), and LAA (n = 4, 0.7%, 95% CI = 0–1.4%). Figure 2 provides the two demographic cases of LAA and ICH subtype stroke with p.R544C (+).
Figure 1.
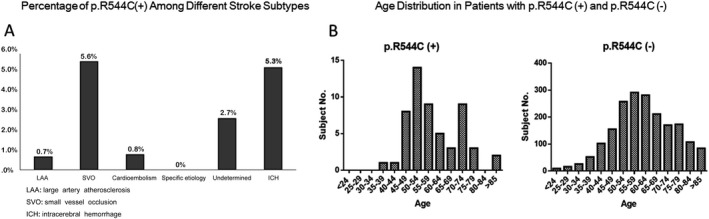
The age distribution in patients with and without p.R544C mutation (A). The distribution of p.R544C mutation in different stroke types. Small vessel occlusion and intracerebral hemorrhage were the two most common presentations in stroke patients with p.R544C mutation (B).
Figure 2.
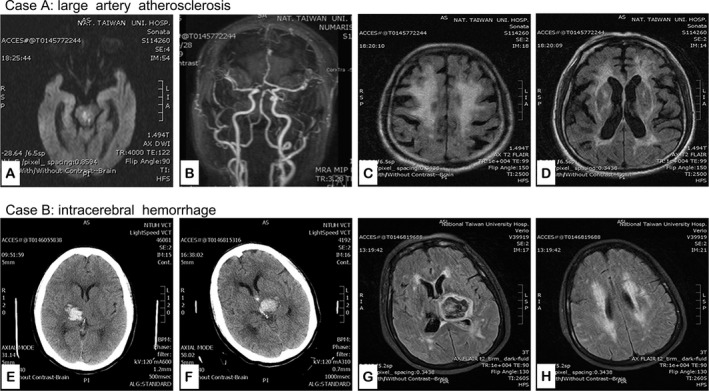
Demographic cases with p.R544C mutation for stroke patients with large artery atherosclerosis and ICH. Case A is a 67 year‐old man with hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, multiple myeloma, and old stroke. He had acute diplopia and left limb weakness. Head MRI showed acute bilateral paramedian pontine infarcts, more on the right side on diffusion weighted imaging (arrow) (A) and focal stenosis in middle segment of basilar artery (arrow) (B). FLAIR showed severe diffuse white matter change (C) and high signal intensity in bilateral external capsules (D). Case B is a 62‐year‐old woman with hypertension and diabetes mellitus. She had acute right thalamic hemorrhage (E) in June, 2014. Five months later, she had another episode of ICH in the left thalamus (F). Head MRI showed acute hematoma in the left thalamus (G) and diffuse white matter change in bilateral subcortical and periventricular areas (G and H).
Characteristics of IS patients with and without p.R544C mutation
Table 1 lists the characteristics of IS patients with p.R544C (+) and p.R544C (−). Compared to those with p.R544C (−) (n = 1664), patients with p.R544C (+) (n = 41) had significantly lower NIHSS score on admission, shorter length of stay, higher prevalence of medical history of TIA, different distribution of stroke subtypes, and higher prevalence of sibling history of stroke (all P < 0.05). Multivariable analysis identified SVO subtype (OR = 4.03, 95% CI = 1.26–12.92, P = 0.019) and sibling history of stroke (OR = 4.50, 95% CI = 1.67–12.14, P = 0.003) as independent factors associated with p.R544C (+) (Table 2).
Table 1.
Comparison between p.R544C (+) and p.R544C (−) in ischemic stroke patients
| p.R544C (−) | p.R544C (+) | ||
|---|---|---|---|
| (n = 1664) | (n = 41) | P | |
| Age (year) | 61.4 (13.5) | 57.8 (10.0) | 0.095 |
| Gender (male) | 70.1 | 63.4 | 0.389 |
| Length of stay (day) | 16.7(21.2) | 11.3 (12.3) | 0.011 |
| NIHSS at admission | 5 (2, 9) | 3 (2, 6) | 0.001 |
| Medical history (%) | |||
| Previous ischemic stroke | 39.0 | 45.8 | 0.529 |
| Previous transient ischemic attack | 3.5 | 10.3 | 0.052 |
| Previous intracerebral hemorrhage | 3.9 | 0 | 1.000 |
| Heart disease | 26.7 | 22.2 | 0.826 |
| Diabetes mellitus | 38.9 | 29.3 | 0.256 |
| Hypertension | 75.0 | 73.2 | 0.855 |
| Hyperlipidemia | 47.1 | 43.9 | 0.753 |
| Smoking | 47.2 | 51.9 | 0.699 |
| Ischemic stroke type (%) | <0.001 | ||
| Large artery atherosclerosis | 34.5 | 9.8 | |
| Small vessel occlusion | 28.1 | 68.3 | |
| Cardioembolism | 14.7 | 4.9 | |
| Other specific etiologies | 7.5 | 0 | |
| Undetermined | 15.2 | 17.1 | |
| Family history (parents) (%) | |||
| Hypertension | 40.0 | 44.4 | 0.693 |
| Diabetes mellitus | 24.9 | 14.8 | 0.268 |
| Coronary artery disease | 11.3 | 7.4 | 0.760 |
| Stroke | 23.2 | 25.9 | 0.818 |
| Transient ischemic attack | 1.1 | 3.8 | 0.265 |
| Family history (siblings) (%) | |||
| Hypertension | 20.7 | 18.5 | 1.000 |
| Diabetes mellitus | 13.6 | 7.4 | 0.567 |
| Coronary artery disease | 3.0 | 7.4 | 0.200 |
| Stroke | 8.4 | 22.2 | 0.025 |
| Transient ischemic attack | 0.5 | 3.8 | 0.133 |
| Good functional status at discharge (%)a | |||
| At 1 month post stroke | 51.7 | 65.4 | 0.232 |
| At 3 month post stroke | 59.2 | 64 | 0.685 |
Values are mean (standard deviation), median (interquartile range), or percentage.
NIHSS, National Institute of Health Stroke Scale.
Indicated modified Rankin Scale 0 or 1.
Table 2.
Factors related to p.R544C (+) in ischemic stroke patients
| Covariate | Odds ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Age, per year | 0.988 | 0.953–1.025 | 0.525 |
| Male gender | 0.776 | 0.320–1.879 | 0.574 |
| NIHSS, per score | 0.973 | 0.868–1.090 | 0.636 |
| Previous TIA history | 3.678 | 0.911–14.853 | 0.067 |
| Length of Stay, per day | 0.993 | 0.958–1.030 | 0.712 |
| LAA (stroke type) | 0.682 | 0.148–3.149 | 0.624 |
| SVO (stroke type) | 4.033 | 1.259–12.918 | 0.019 |
| Parents history of stroke | 0.631 | 0.227–1.752 | 0.377 |
| Sibling history of stroke | 4.496 | 1.665–12.139 | 0.003 |
NIHSS, National Institute of Health Stroke Scale; TIA, transient ischemic attack; LAA, large artery atherosclerosis; SVO, small vessel occlusion.
Regarding the stroke locations of IS patients with p.R544C (+) and having MRI examination (n = 34), there were 20 cases in supratentorial white matter (58.8%), three in basal ganglia or thalamus (8.8%), seven in brainstem or cerebellum (20.5%), one in cortex (2.9%), and three in multiple regions (8.8%). Furthermore, MRI examination in IS patients with p.R544C (+) demonstrated 100% of significant white matter change (mean Fazekas Scale ≥2), 23.5% of anterior temporal area involvement and 73.5% of external capsule involvement.
Characteristics of ICH patients with and without p.R544C mutation
Table 3 lists the characteristics of ICH patients with p.R544C (+) (n = 14) and p.R544C (−) (n = 251). Hypertension is the most common risk factor in both p.R544C (+) and p.R544C (−) patients with ICH (89.6% and 85.7% respectively). All p.R544C (+) patients with ICH were nonlobar hemorrhage including seven cases in basal ganglia (50%), four in thalamus (29%), one in subcoartical area (7%), and two in infratentorial area (14%). Compared to those with p.R544C (−), patients with p.R544C (+) had higher prevalence of medical history of TIA (P = 0.027) and sibling history of stroke (P = 0.048). Multivariable analysis confirmed that sibling history of stroke was independently associated with p.R544C (+) in ICH patients (OR = 6.03, 95% CI = 1.03–35.47, P = 0.047) (Table 4).
Table 3.
Comparison between p.R544C (+) and p.R544C (‐) in intracerebral hemorrhage patients
| p.R544C (−) | p.R544C (+) | ||
|---|---|---|---|
| (n = 251) | (n = 14) | P | |
| Age (year) | 59.9 ± 14.1 | 63.2 ± 13.9 | 0.385 |
| Gender (male) | 66.1 | 64.3 | 1.000 |
| Length of stay (day) | 32.7 ± 29.8 | 20.1 ± 26.7 | 0.122 |
| NIHSS at admission | 10.5 (4, 19) | 11.5 (6, 20) | 0.525 |
| Medical history (%) | |||
| Previous ischemic stroke | 17.9 | 37.5 | 0.174 |
| Previous transient ischemic attack | 4.1 | 21.4 | 0.027 |
| Previous intracerebral hemorrhage | 14.6 | 37.5 | 0.112 |
| Heart disease | 23.7 | 0 | 0.205 |
| Diabetes mellitus | 29.2 | 21.4 | 0.763 |
| Hypertension | 89.6 | 85.7 | 0.649 |
| Hyperlipidemia | 33.2 | 35.7 | 1.000 |
| Smoking | 40.3 | 44.4 | 1.000 |
| Intracerebral hemorrhage type (%) | 0.309 | ||
| Hypertension | 77.6 | 92.3 | |
| Nonhypertension | 22.4 | 7.7 | |
| Family history (Parents) (%) | |||
| Hypertension | 49.6 | 33.3 | 0.494 |
| Diabetes mellitus | 27.5 | 33.3 | 0.709 |
| Coronary artery disease | 12.6 | – | 0.597 |
| Stroke | 26.3 | 55.6 | 0.117 |
| Transient ischemic attack | 2.0 | 12.5 | 0.206 |
| Family history (Siblings) (%) | |||
| Hypertension | 28.6 | 25.0 | 1.000 |
| Diabetes mellitus | 16.9 | 12.5 | 1.000 |
| Coronary artery disease | 9.3 | – | 1.000 |
| Stroke | 9.2 | 37.5 | 0.048 |
| Transient ischemic attack | 2.0 | 14.3 | 0.182 |
| Good functional status at discharge (%)a | |||
| At 1 month post stroke | 41.6 | 50 | 0.694 |
| At 3 month post stroke | 49.4 | 50 | 1.000 |
Values are mean (standard deviation), median (interquartile range), or percentage.
NIHSS, National Institute of Health Stroke Scale.
Indicated modified Ranking Scale 0 or 1.
Table 4.
Factors related to p.R544C (+) in intracerebral hemorrhage patients
| Covariate | Odds ratio | 95% confidence intervals | P‐value |
|---|---|---|---|
| Age, per year | 0.981 | 0.923–1.043 | 0.538 |
| Male gender | 1.223 | 0.211–7.097 | 0.822 |
| Previous TIA history | 0.825 | 0.063–10.767 | 0.883 |
| Sibling history of stroke | 6.030 | 1.025–35.473 | 0.047 |
TIA, transient ischemic attack.
Discussion
The clinical symptoms of CADASIL were first described in 1990 and the corresponding NOTCH3 gene was discovered in 1996.4 Since then, not only the genetic heterogeneity of CADASIL, but also wide variability in its clinical symptoms, age of onset, and disease progression have been found.19, 20 Manifestations such as late onset, more benign clinical course, and even asymptomatic white matter change on neuroimaging have also increasingly been recognized.6, 7, 21
Previous studies suggested that there might be genotype–phenotype correlations of NOTCH3 mutations in patients with CADASIL.4, 7, 19, 21 Some mutations are associated with a less severe phenotype than others.7 For example, CADASIL patients with p.R544C mutation in exon 11 versus CADASIL patients with other mutations in exon 3 or 4 have rarer occurrence of migraine, older age of onset, and less frequent involvement of the anterior temporal area.4, 9, 21 Possible mechanisms underlying the vascular pathology and clinical symptoms stemming from different NOTCH3 mutations have been discussed.4, 7, 9 Because CADASIL is clinically and genetically heterogeneous, the incidence and prevalence of CADASIL are difficult to be investigated and the reported data are limited and underestimated.4, 7, 12, 22
Using patients with genetically (exons 3, 4, 5, and 6) or histologically confirmed CADASIL and their pedigree members at risk of carrying the gene, and registered in two adjacent health administrative areas of west Scotland (Greater Glasgow and Lanarkshire),22 Razvi et al. in 2005 found a probable mutation prevalence of 4.14 per 100,000 adults. In 2012, Narayan et al., using a similar method in northeast England, estimated a probable prevalence of 2.32 per 100,000 adults.23 In 2014, Moreton et al. used the same method to re‐evaluate the prevalence of CADASIL in west Scotland and found that the estimated prevalence had increased to 10.7 per 100,000 adults12 and the median age at first stroke was much higher than the 2005 estimate. More than 30% of CADASIL patients were even living independently in old age.12
The prevalence of CADASIL in stroke patients is controversial, varying between 0% and 4% according to previous studies.11, 12, 24, 25 Chong et al. observed NOTCH3 mutations in 6 of 1251 (0.48%) sporadic stroke patients with small vessel pathology (637 with ICH and 614 with SVO.11 These six cases included three with ICH (0.47%) and three with SVO (0.49%). By contrast, Choi et al. noted a much higher prevalence (six patients, 4.0%) of NOTCH3 gene mutation (all p.R544C) in 151 consecutive IS patients screened in Korea in 2013.14 Whether this discrepancy is related to phenotypic and genotypic variations in CADASIL is uncertain because of the small number of cases. Nevertheless, all the related studies including ours, pointed out the key feature of significant leukoaraiosis in stroke patients with NOTCH3 mutations. Therefore, the prevalence of NOTCH3 mutations among stroke patients with significant leukoaraiosis would be expected to be higher. Recently, Rutten et al. investigated the frequency of distinctive EGFr cysteine altering NOTCH3 mutations in the 60,706 exomes of the exome aggregation consortium (ExAC) database and identified 206 EGFr cysteine altering NOTCH3 mutations, with an estimated prevalence of 3.4/1000. Importantly, the result was much higher than previous estimates of CADASIL prevalence.13
Our study makes several valuable points. Most importantly, our data suggest that the p.R544C NOTCH3 mutation is underdiagnosed in stroke patients in Taiwan. It was found in more than 2% of IS patients (especially those with SVO subtype) and 5% of ICH patients (especially those with nonlobar ICH). In our registry, most IS and ICH patients with p.R544C (+) had been classified clinically as small vessel occlusion (68.3%) and hypertension‐related ICH (77.6%). Our study provided clinical evidence for the predominant small vessel phenotype in CADASIL patients with acute stroke.
The reports of ICH in CADASIL patients has been increased recently, especially in Asians.6, 26 History of ICH was found in 16.2% of 112 CADASIL patients in a study by Liao et al. in Taiwan.9 Cerebral microbleeds detected on head MRI and occurrence of ICH were found in 66% and 17%, respectively, of 94 genetically confirmed CADASIL cases in a study by Lee et al. in Korea27 Interestingly, both studies (Liao et al. [70.5%] and Lee et al. [95%]) identified p.R544C as the predominant NOTCH3 mutation. These results suggested that the incidence of ICH in CADASIL patients seems to be underestimated.
Second, our study indicated a possible contributing role of conventional vascular risk factors in CADASIL patients with stroke.28 Stroke patients with p.R544C (−) and stroke patients with p.R544C (+) had similar prevalence of hypertension, hyperlipidemia, diabetes mellitus, and smoking habit, etc. Especially in ICH patients with p.R544C (+), more than 80% had a history of hypertension. One recent large case series and cohort study also demonstrated the importance of systolic blood pressure in long‐term functional independence in CADASIL patients.29 As NOTCH3 mutations is currently untreatable, adequate control of conventional vascular risk factors in CADASIL patients or asymptomatic gene carriers should be emphasized, and the prophylactic effect of aggressive control of vascular risk factors on acute stroke in CADASIL patients should be studied. On the other hand, the existence of conventional vascular risk factors may prevent clinicians to suspect the possibility of NOTCH3 mutations in acute stroke patients, especially for those with underlying atrial fibrillation or large artery atherosclerosis. Our data suggested that genetic testing for NOTCH3 mutations may still be considered in IS patients with small infarct and significant leukoaraiosis, even though another risk factor as a cardioembolic source or LAA are present.
Third, our study found that sibling history of stroke rather than parental history of stroke is the independent identifier of both IS and ICH in patients with p.R544C (+). CADASIL is an autosomal dominant inherited disorder with high penetration and varying expression. Therefore, CADASIL is typically characterized by repeated episodes of stroke and a strong family history.5, 6, 30 However, tracing a family's history of stroke can be difficult or unreliable. Also, most conventional vascular risk factors involved in stroke tend to be inherited. In our study, the percentage of patients with a parental history of stroke was similar between IS patients with and without p.R544C (25.9 vs. 23.3%), and nonsignificantly higher in ICH patients with p.R544C (+) than in those with p.R544C (−) (55.6% vs. 26.3%). By contrast, the percentage of patients with a sibling history of stroke was significantly higher in both the IS and ICH p.R544C (+) groups than in the IS and ICH R544(−) groups (22.2% vs. 8.5% and 37.5% vs. 9.2% respectively). Our study emphasized the importance of sibling history of stroke in the detection of NOTCH3 mutation in stroke patients especially in clinically suspected cases.
Limitations
There are some limitations. First, to screen such a large number of stroke patients, we only screened the hot spot of p.R544C since more than 70% of NOTCH3 mutations in Taiwan are attributed to p.R544C. Because of the genetic heterogeneity of CADASIL, our findings may not be applied to other NOTCH3 mutation sites or other populations. In addition, the prevalence of CADASIL in stroke patients might have been even higher in Taiwan if we had screened all of the exons of the NOTCH3 gene, though the study expenses would have been a lot more. Second, we did not have the detailed information on how the conventional vascular risk factors were controlled, such as levels and variations in blood pressure or specific medications. Third, our study included stroke patients only. Those carrying p.R544C who were asymptomatic or had other manifestations such as headache, cognitive impairment, psychiatric disorder, or parkinsonism were not included. Therefore, there may have been some crucial determinants of stroke occurrence that we did not identify, such as specific vascular risk factors or control status, or medications. Fourth, the fact of having IS patients younger compared with previously reported large scale epidemiological study16 could cause a selection bias. Fifth, this study only analyzed clinical information but not detailed imaging data. Further study will be performed to address the value of imaging characteristics to distinguish stroke patients with p.R544C mutation from those without p.R544C mutation. Nevertheless, our epidemiological and clinical data suggest that NOTCH3 mutation may have been overlooked previously as a contributing factor in stroke, especially in patients with small vessel pathology including small vessel occlusion and deep ICH in certain geographic areas or populations. Further studies with larger sample size and more comprehensive investigations of the pathophysiological and long‐term prognosis would be encouraged for this important issue.
In conclusion, our study suggests that p.R544C NOTCH3 mutation is underdiagnosed in stroke patients in Taiwan, especially in those with SVO and having a sibling history of stroke. Investigations of the pathophysiological and prognostic roles of p.R544C NOTCH3 mutation in stroke are required.
Conflict of Interest
None reported.
Acknowledgment
This work was supported by the Ministry of Science and Technology, ROC Grants (106‐2314‐B‐002‐001). The authors thank the 3rd core facility at National Taiwan University Hospital for support and Miss Yu‐Mei, Lin at National Taipei University for statistical analysis.
Funding Information
This work was supported by the Ministry of Science and Technology, ROC Grants (106‐2314‐B‐002‐001).
Funding Statement
This work was funded by Ministry of Science and Technology grant 106‐2314‐B‐002‐001.
Contributor Information
Hung‐Yi Chiou, Email: hychiou@tmu.edu.tw.
Jiann‐Shing Jeng, Email: jsjeng@ntu.edu.tw.
References
- 1. Tang SC, Jeng JS, Lee MJ, Yip PK. Notch signaling and CADASIL. Acta Neurol Taiwanica 2009;18:81–90. [PubMed] [Google Scholar]
- 2. Joutel A, Vahedi K, Corpechot C, et al. Strong clustering and stereotyped nature of NOTCH3 mutations in CADASIL patients. Lancet (London, England) 1997;350:1511–1515. [DOI] [PubMed] [Google Scholar]
- 3. Majersik JJ. Inherited and uncommon causes of stroke. Continuum 2017;23:211–237. [DOI] [PubMed] [Google Scholar]
- 4. Joutel A, Corpechot C, Ducros A, et al. NOTCH3 mutations in CADASIL, a hereditary adult‐onset condition causing stroke and dementia. Nature 1996;383:707–710. [DOI] [PubMed] [Google Scholar]
- 5. Bersano A, Bedini G, Oskam J, et al. CADASIL: treatment and management options. Curr Treat Options Neurol 2017;19:31. [DOI] [PubMed] [Google Scholar]
- 6. Di Donato I, Bianchi S, De Stefano N, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med 2017;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rutten JW, Dauwerse HG, Gravesteijn G, et al. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol 2016;3:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang SC, Lee MJ, Jeng JS, Yip PK. Arg332cys mutation of NOTCH3 gene in the first known Taiwanese family with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neurol Sci 2005;228:125–128. [DOI] [PubMed] [Google Scholar]
- 9. Lee YC, Liu CS, Chang MH, et al. Population‐specific spectrum of NOTCH3 mutations, MRI features and founder effect of CADASIL in Chinese. J Neurol 2009;256:249–255. [DOI] [PubMed] [Google Scholar]
- 10. Liao YC, Hsiao CT, Fuh JL, et al. Characterization of CADASIL among the Han Chinese in Taiwan: distinct genotypic and phenotypic profiles. PLoS ONE 2015;10:e0136501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chong M, O'Donnell M, Thijs V, et al. Mendelian genes and risk of intracerebral hemorrhage and small‐vessel ischemic stroke in sporadic cases. Stroke 2017;48:2263–2265. [DOI] [PubMed] [Google Scholar]
- 12. Kilarski LL, Rutten‐Jacobs LC, Bevan S, et al. Prevalence of CADASIL and Fabry disease in a cohort of MRI defined younger onset lacunar stroke. PLoS ONE 2015;10:e0136352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta Neurol Scand 2014;130:197–203. [DOI] [PubMed] [Google Scholar]
- 14. Choi JC, Lee KH, Song SK, et al. Screening for NOTCH3 gene mutations among 151 consecutive Korean patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:608–614. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh YC, Seshadri S, Chung WT, et al. Association between genetic variant on chromosome 12p13 and stroke survival and recurrence: a one year prospective study in Taiwan. J Biomed Sci 2012;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsieh FI, Lien LM, Chen ST, et al. Get with the guidelines‐stroke performance indicators: surveillance of stroke care in the Taiwan stroke registry: get with the guidelines‐stroke in Taiwan. Circulation 2010;122:1116–1123. [DOI] [PubMed] [Google Scholar]
- 17. Adams HP, Jr. , Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 18. Yeh SJ, Tang SC, Tsai LK, Jeng JS. Pathogenetical subtypes of recurrent intracerebral hemorrhage: designations by SMASH‐U classification system. Stroke 2014;45:2636–2642. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt H, Zeginigg M, Wiltgen M, et al. Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age‐related cerebral small vessel disease. Brain 2011;134:3384–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tikka S, Baumann M, Siitonen M, et al. CADASIL and CARASIL. Brain Pathol 2014;24:525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen S, Ni W, Yin XZ, et al. Clinical features and mutation spectrum in Chinese patients with CADASIL: a multicenter retrospective study. CNS Neurosci Ther 2017;23:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Razvi SS, Davidson R, Bone I, Muir KW. The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) in the west of Scotland. J Neurol Neurosurg Psychiatry 2005;76:739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Narayan SK, Gorman G, Kalaria RN, et al. The minimum prevalence of CADASIL in Northeast England. Neurology 2012;78:1025–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong Y, Hassan A, Zhang Z, et al. Yield of screening for CADASIL mutations in lacunar stroke and leukoaraiosis. Stroke 2003;34:203–205. [DOI] [PubMed] [Google Scholar]
- 25. Wang T, Sharma SD, Fox N, et al. Description of a simple test for CADASIL disease and determination of mutation frequencies in sporadic ischaemic stroke and dementia patients. J Neurol Neurosurg Psychiatry 2000;69:652–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi JC, Kang SY, Kang JH, Park JK. Intracerebral hemorrhages in CADASIL. Neurology 2006;67:2042– 2044. [DOI] [PubMed] [Google Scholar]
- 27. Lee JS, Ko K, Oh JH, et al. Cerebral microbleeds, hypertension, and intracerebral hemorrhage in cerebral autosomal‐dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Front Neurol 2017;8:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viswanathan A, Guichard JP, Gschwendtner A, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two‐centre cohort study. Brain 2006;129:2375–2383. [DOI] [PubMed] [Google Scholar]
- 29. Ling Y, De Guio F, Duering M, et al. Predictors and clinical impact of incident lacunes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2017;48:283–289. [DOI] [PubMed] [Google Scholar]
- 30. Stack CA, Cole JW. A diagnostic approach to stroke in young adults. Curr Treat Opt Cardiovasc Med 2017;19:84. [DOI] [PubMed] [Google Scholar]


