Abstract
Interferon-γ (IFN-γ) has been used to control cancers in clinical treatment. However, an increasing number of reports have suggested that in some cases effectiveness declines after a long treatment period, the reason being unclear. We have reported previously that long-term IFN-γ treatment induces malignant transformation of healthy lactating bovine mammary epithelial cells (BMECs) in vitro. In this study, we investigated the mechanisms underlying the malignant proliferation of BMECs under IFN-γ treatment. The primary BMECs used in this study were stimulated by IFN-γ (10 ng/mL) for a long term to promote malignancy. We observed that IFN-γ could promote malignant cell proliferation, increase the expression of cyclin D1/cyclin-dependent kinase 4 (CDK4), decrease the expression of p21, and upregulate the expression of cellular-abelsongene (c-Abl) and histone deacetylase 2 (HDAC2). The HDAC2 inhibitor, valproate (VPA) and the c-Abl inhibitor, imatinib, lowered the expression level of cyclin D1/CDK4, and increased the expression level of p21, leading to an inhibitory effect on IFN-γ-induced malignant cell growth. When c-Abl was downregulated, the HDAC2 level was also decreased by promoted proteasome degradation. These data suggest that IFN-γ promotes the growth of malignant BMECs through the c-Abl/HDAC2 signaling pathway. Our findings suggest that long-term application of IFN-γ may be closely associated with the promotion of cell growth and even the carcinogenesis of breast cancer.
Keywords: Interferon-γ(IFN-γ), Cellular-abelsongene (c-Abl), Histone deacetylase 2 (HDAC2), Malignant cell growth
1. Introduction
Interferon-γ (IFN-γ), a multifunctional cytokine, is capable of participating in antiviral defense, the host response during infection, tumor immune surveillance, and the control of cellular function and replication (Xia et al., 2016a). IFN-γ can exert anti-tumor effects in many tumor cells by activating cells of the immune system, such as natural killer cells (Zhu et al., 2010; Sarhan et al., 2013). It can also directly affect the process of oncogenesis (Matsuzaki et al., 2015) through inhibition of cell proliferation and its anti-apoptosis, anti-angiogenesis, and immunoregulation activities (Horikoshi et al., 1995). However, it has been reported that IFN-γ has the dark side (Mojic et al., 2018). It was discovered that highly purified IFN-γ was ineffective for the treatment of metastatic breast cancer and caused undesirable symptoms such as nausea, vomiting, and anorexia in clinical trials of breast cancer patients (Muss et al., 1986). In stages II to III clinical trials of melanoma patients, the prognosis of patients was good, but no useful effect of IFN-γ could be detected, and most importantly, the function of helper T cells was significantly inhibited (Schiller et al., 1996). The failure of these IFN-γ treatments demonstrates the two-sided role of IFN-γ. Mutation and inactivation of the IFN-γ pathway have been found in about one-third of melanoma and lung adenocarcinoma cell lines (Kaplan et al., 1998), suggesting that tumor cells may evade immune surveillance through IFN-γ insensitivity. The effect of IFN-γ is limited and transient in the treatment of many cancers, such as breast cancer (Carpi et al., 2009) and melanoma (Creagan et al., 1990). Furthermore, IFN-γ has been found to be able to promote the development of cancer under certain conditions (Zaidi and Merlino, 2011; Zuo et al., 2014). It was also reported that IFN-γ could induce the malignant transformation of bovine mammary epithelial cells (BMECs) through autophagy activated by general control nonderepressible 2 (GCN2) (Xia et al., 2016b). Abnormal regulation of the cell cycle is the first step in the malignant transformation of cells, and unlimited proliferation is a biological characteristic typical of tumor cells. Thus, we wanted to study how IFN-γ affects the malignant growth of BMECs.
Cellular-abelsongene (c-Abl), a non-receptor tyrosine kinase distributed in both the cytoplasm and the nucleus, is involved in cell proliferation, the cell cycle and cell migration by regulating multiple signaling pathways, and plays an important role in tumorigenesis and development (Brightbill and Schlissel, 2009). It is subject to strict regulation in normal cells and can regulate the physiological balance and homeostasis of normal mammary epithelial cells (MECs). However, this regulation is destroyed when cells are stimulated by growth factors, cell adhesion molecules, and stress, especially genotoxic or oxidative stress (Bradley and Koleske, 2009). Abnormal regulation of c-Abl contributes to tumor development. c-Abl overexpression is a common event in breast cancer (about 40% of cases) and plays a significant role in advanced breast cancer (Zhao et al., 2012). In breast cancer, activated c-Abl promotes cell survival and invasion as well as resistance to anti-estrogen therapy, thereby promoting cancer progression, whereas the knockout of c-Abl induces apoptosis and inhibits cell proliferation to block transformation (Zhao et al., 2013).
Histone and non-histone proteins are acetylated to regulate gene expression and cell signaling. Histone deacetylases (HDACs) are important epigenetic modulators that catalyze the deacetylation of ε-N-acetylated lysine residues in many proteins (Hildmann et al., 2007). HDAC2, belonging to class I of four classes (Barneda-Zahonero and Parra, 2012), is essential for embryonic development, affects the cytokine signals involved in the immune response, and is often highly elevated in solid or hematologic tumors (Krämer, 2009). It is an important enzyme that promotes tumor formation at an early stage. Nam et al. (2005) found that HDAC2 expression increased gradually in cells from non-tumor to apparent cancer stages. A recent study found that the expression of HDAC2 was positively correlated with the histological grade of breast cancer, with 10% in low, 22.8% in medium, and 43.6% in high grade tumors. More than half (56.7%) of low-grade tumors had only a small proportion of cells with HDAC2 expression (Müller et al., 2013). The knockout of HDAC2 significantly inhibited tumor growth and induced apoptosis (Yamaguchi et al., 2010). These results suggest that HDAC2 plays an important role in the development and progression of cancers (Weichert et al., 2008; Noh et al., 2011).
c-Abl and HDAC2 are important participants in the process of tumorigenesis, but the role they play in the IFN-γ regulation of malignant growth of BMECs has not been reported. In our study, we found that there was an increase in both the expression of c-Abl and HDAC2 and malignant cell growth when cells were treated with IFN-γ for a long time. However, inhibition of c-Abl or HDAC2 could antagonize the promotion of malignant cell growth by IFN-γ. In addition, c-Abl inhibition could inhibit HDAC2 expression by increasing proteasome degradation. Our results suggest that c-Abl/HDAC2 may be a therapeutic target for preventing the malignant growth of mammary epithelial cells induced by IFN-γ.
2. Materials and methods
2.1. Materials
The main antibodies used in this study were specific for β-actin (Affinity Biosciences; AF7018), HDAC2 and c-Abl (Cell Signaling Technology; Cat. 5113P and Cat.2684), cyclin D1 (Abcam; ab95281), cyclin-dependent kinase 4 (CDK4) (Affinity Biosciences; AF4034) and p21 (Santa Cruz Biotechnology Inc., sc-397). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies were purchased from Proteintech Group, Inc. (Chicago, IL, USA). 4',6-Diamidino-2-phenylindole (C1005) was purchased from Beyotime Institute of Biotechnology (Shanghai, China). Bovine interferon was purchased from the Kingfisher Biotech, Inc. (Saint Paul, MN, USA). Valproate (VPA), imatinib, and MG132 were purchased from LC Laboratories (Woburn, MA, USA).
2.2. Cell culture
A primary culture cell-separation technique was used to isolate BMECs as previously described (Xia et al., 2016b). The cells were grown in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12) with 10% fetal bovine serum (HyClone, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin, and incubated at 37 °C in a humidified atmosphere with 5% CO2. The primary BMECs were treated with 10 ng/mL IFN-γ for a long term. The cultures were subcultured every 3 d (3 d/cycle), and the treatments were repeated for 8 weeks.
2.3. Cell viability assays
The cells were inoculated in a 96-well plate at 1×104 cells in 100 μL per well for 24 h, and then incubated with stimulators for 0, 24, 48, and 96 h. After 20 μL methylthiazolyldiphenyl-tetrazolium bromide (MTT) solution was added to each well for another 2 h, 150 μL dimethyl sulfoxide (DMSO) was added to each well and shaken at low speed for 10 min. The absorbance values were measured at 490 nm. Each group contained three duplicates and a blank. The experiment was carried out three times.
2.4. Real-time quantitative PCR
Cells were treated with IFN-γ or IFN-γ in combination with imatinib for 24 h and then washed twice with phosphate-buffered saline (PBS). The total RNA was extracted using TRIzol reagent (Invitrogen, USA) according to the instructions. Reverse transcription was performed using the Prime Script™ RT Master Mix Kit (TaKaRa, Dalian, China). In this study, β-actin was used as an internal reference gene for quantitative analysis, and the relative transcript levels for the target genes were determined using the 2−ΔΔ C T method (Bougarn et al., 2011). Fluorescent quantitative PCR experiments were performed using FastStart Universal SYBR Green Master Kit (Roche, Basel, Switzerland). The special primers were as follows: HDAC2: sense, AGTGTGGTGCAGACTCCCTA, antisense, TTGTGTATCCACCTCCCCCA; β-actin: sense, GCCCTGAGGCTCTCTTCCA, antisense, GC GGATGTCGACGTCACA. For each experiment, at least three parallel measurements were carried out.
2.5. Western blotting
The bicinchoninic acid (BCA) method (Thermo, USA) was used to measure the concentration of protein. Proteins separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to polyvinylidene fluoride membranes. Each membrane was sealed in 5% (0.05 g/mL) bovine serum albumin (BSA) solution and shaken at room temperature for 40 min. The primary antibody, in Tris-buffered saline containing 0.05% Tween 20 (TBST) solution at a ratio of 1:1000, was added to the membranes. After incubation at 4 °C overnight, the membranes were washed four times (10 min per wash) in TBST, and then treated with anti-rabbit IgG (Proteintech, China) for 45 min at room temperature (secondary antibody:TBST=1:5000–1:7000), washed again four times (10 min per wash). The relative levels of the target proteins were estimated using densitometry (ImageJ), and β-actin was used as an internal control. Each group of samples was carried out three times.
2.6. Immunofluorescence
The cells were washed twice with PBS, fixed with 4% (0.04 g/mL) paraformaldehyde for 15 min, and again washed twice with PBS. Next, they were treated with 0.5% Triton-X-100 for 20 min (except for membrane protein analysis) and blocked with normal goat serum for 30 min at room temperature. The cells were incubated with primary antibodies (c-Abl, 1:50) overnight at 4 °C. Afterwards, the cells were washed three times with TBST and incubated with secondary antibodies for 1 h in the dark at 37 °C. Finally, the cells were counterstained with 4'6-diamidino-2-phenylindole (DAPI) to stain the nuclei. A laser confocal microscope (Olympus, FV300, Tokyo, Japan) was used to obtain fluorescence images.
2.7. Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) software (SPSS 16.0). The mean and standard error of mean (SEM) values and sample sizes are indicated in each figure. Values of P<0.05 were considered significant, P<0.01 highly significant, and P<0.001 extremely significant, as indicated by the superscripts *, **, and #, respectively.
3. Results
3.1. Effect of IFN-γ on the malignant growth of BMECs
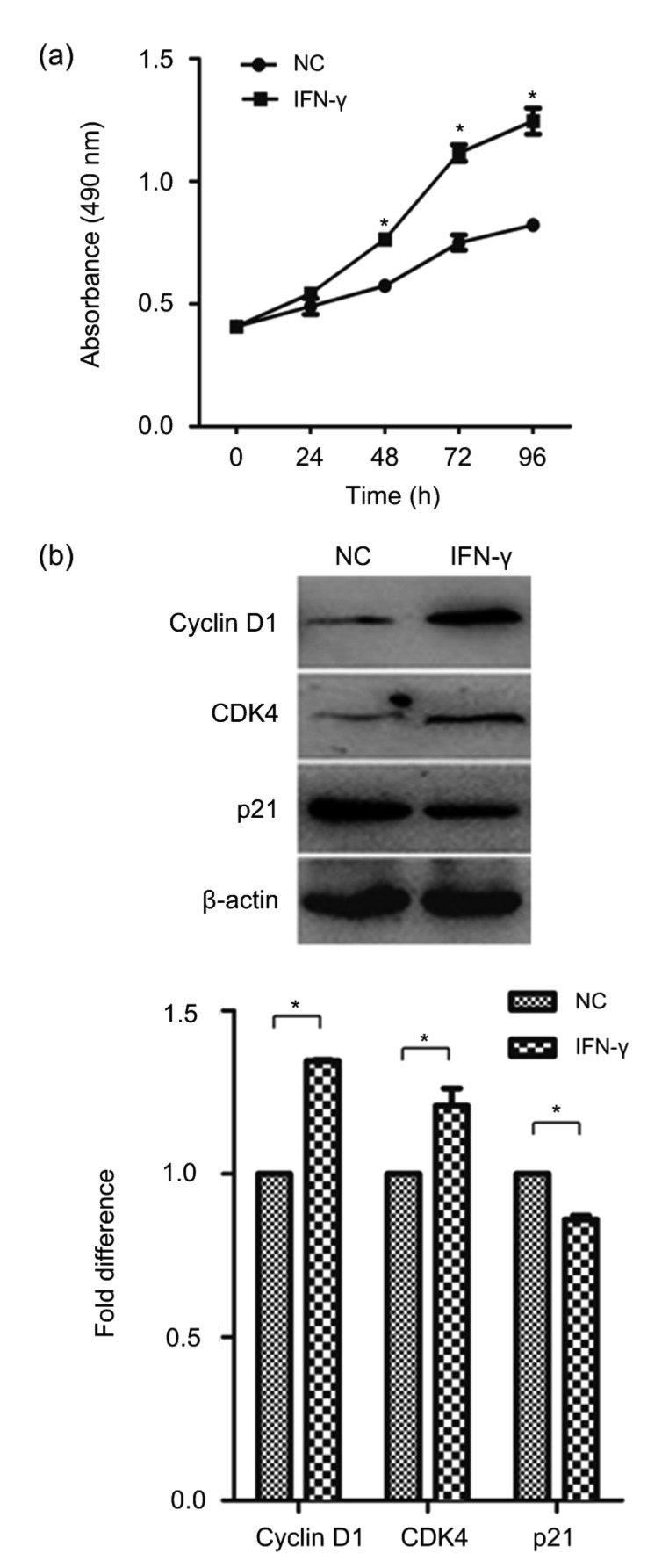
To determine the effect of IFN-γ on malignant cell growth, primary BMECs were exposed to 10 ng/mL IFN-γ for 8 weeks as described by Xia et al. (2016b). BMECs treated by short-term IFN-γ have remained non-transformed in our experiment (results not shown). We then assessed the growth rate of BMECs and malignantly transformed BMECs via prolonged treatment with IFN-γ (γ-BMECs) using an MTT assay. The result showed that the γ-BMECs had higher cell viability than the BMECs. This indicated that IFN-γ could promote cell proliferation (Fig. 1a). Then, we examined the expression of cell cycle-related proteins by Western blotting. We found that the expression of cyclin D1 and CDK4 was markedly increased, while the expression of p21, a cell cycle-negative regulator, was significantly decreased by IFN-γ (Fig. 1b). Thus, IFN-γ can promote the cell cycle progression of BMECs. Taken together, these data confirmed that IFN-γ plays an important role in the process of BMEC malignant growth.
Fig. 1.
Cell viability and levels of cell growth-related markers after IFN-γ treatment
(a) BMECs were incubated with 10 ng/mL IFN-γ for a long term, until the cells went through malignant transformation. Cell counting MTT was used to detect cell viability. (b) Expression of cyclin D1, CDK4, and p21 was detected using Western blotting. The data represent the mean±SEM of three independent experiments. * P<0.05 vs. NC. NC, negative control (BMECs)
3.2. Effect of IFN-γ on malignant growth in BMECs via an HDAC2-dependent mechanism
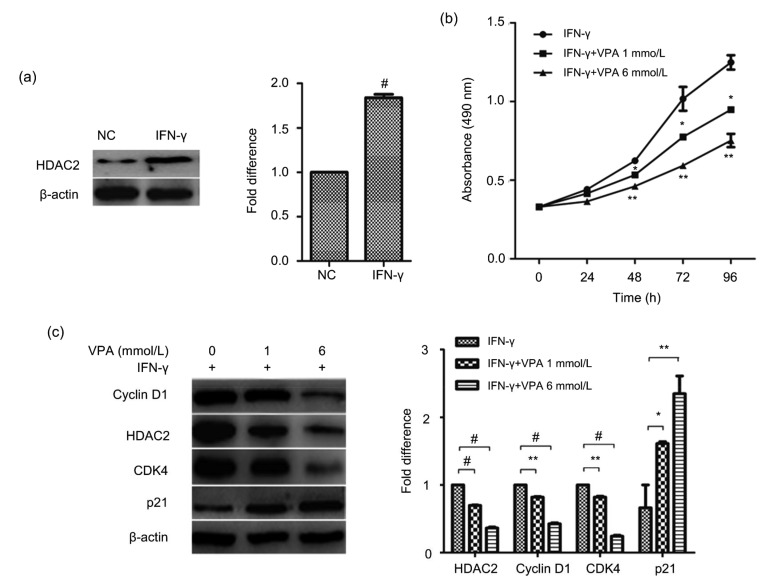
We next attempted to investigate the mechanisms underlying IFN-γ-induced malignant cell growth in BMECs. HDAC2, which can regulate cell proliferation and the cell cycle, is one of the important enzymes that promote tumor formation at an early stage. Therefore, we measured the expression of HDAC2 using Western blotting. HDAC2 levels were significantly increased in γ-BMECs (Fig. 2a). Then, we sought to determine the role of HDAC2 in IFN-γ-induced cell proliferation. We continued to assess cells that had undergone malignant transformation. We found that VPA, an HDAC2 inhibitor, could decrease the cell viability of IFN-γ-treated BMECs (Fig. 2b). The levels of the cell growth-related proteins cyclin D1 and CDK4 markedly decreased (Fig. 2c), while the amount of p21 increased in cells treated with VPA in combination with IFN-γ, compared to those treated with IFN-γ alone. These results indicated that activation of HDAC2 contributed to the promoted growth rate in γ-BMECs.
Fig. 2.
Influence of HDAC2 on IFN-γ-induced cell malignant growth
(a) HDAC2 expression was detected by Western blotting in the negative control and IFN-γ groups. # P<0.001 vs. NC. (b) An MTT assay was used to detect cell viability after valproate (VPA (HDAC2 inhibitor), 1 and 6 mmol/L) treatment. * P<0.05, ** P<0.01 vs. IFN-γ. (c) BMECs were separately treated with IFN-γ and different concentrations of VPA for 24 h. The expression of cell growth-related markers was detected by Western blotting. The data represent the mean±SEM of three independent experiments. * P<0.05, ** P<0.01, # P<0.001. NC, negative control (BMECs)
3.3. Effect of IFN-γ on c-Abl expression in BMECs
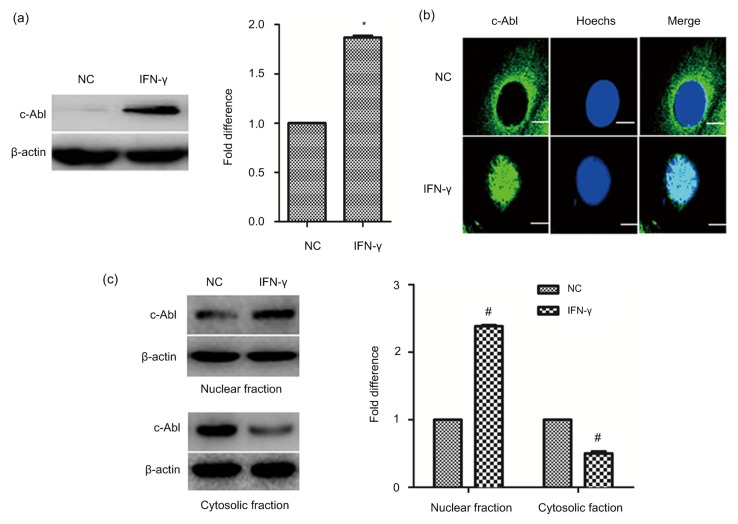
c-Abl, a non-receptor tyrosine kinase, can regulate a wide variety of cellular activities, such as cell proliferation, cell cycle, and cell adhesion through multiple signaling pathways. It plays an important role in the development and progression of tumors. In this study, we found that the expression of c-Abl was increased in γ-BMECs (Fig. 3a). c-Abl is distributed in the cell membrane, cytoplasm, and nucleus, and whether it translocases when induced by IFN-γ is unknown. Thus, the subcellular distribution of the c-Abl protein in BMECs was first analyzed by immunofluorescence. c-Abl was predominantly expressed in the cytoplasm of BMECs, while IFN-γ treatment enhanced the distribution of c-Abl in the nucleus (Fig. 3b). The Western blotting results also showed the same results in that c-Abl was significantly elevated in the nucleus when BMECs were treated continuously with IFN-γ (Fig. 3c).
Fig. 3.
Levels and translocation of c-Abl after IFN-γ treatment
(a) c-Abl expression was detected by Western blotting. (b) BMECs and γ-BMECs were stained with c-Abl, fixed, and viewed under a fluorescence microscope. Bar=3 μm. (c) c-Abl protein levels in the cytosol and nucleus were analyzed by Western blotting. The data represent the mean±SEM of three independent experiments. * P<0.05, # P<0.001 vs. NC. NC, negative control (BMECs)
3.4. Effect of c-Abl on the malignant growth of BMECs induced by IFN-γ
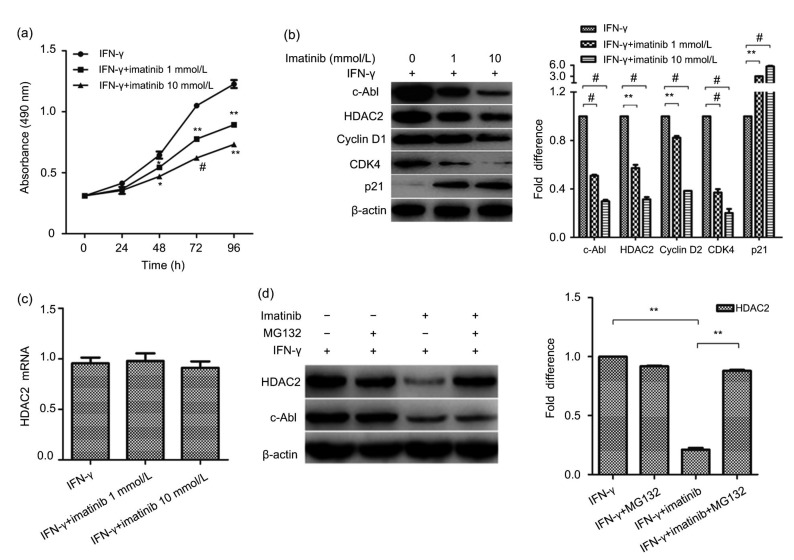
c-Abl and HDAC2 not only can participate in cell growth, but also are linked to some diseases. To further study the mechanism of IFN-γ in the regulation of the malignant growth of BMECs, the relationship between c-Abl and HDAC2 was examined. Imatinib, an inhibitor of c-Abl, had a suppressive effect on cell proliferation (Fig. 4a), upregulated the expression of CDK4 and cyclin D1 and downregulated the expression of p21. Imatinib also inhibited the increased expression of the HDAC2 protein induced by IFN-γ (Fig. 4b), but surprisingly, HDAC2 mRNA levels did not change significantly (Fig. 4c). These results indicate that the increase in c-Abl induced by IFN-γ might upregulate the expression of HDAC2 in BMECs at the post-translational level. It has been proposed that c-Abl phosphorylation prevents protein ubiquitination and proteasomal degradation of HDAC2 (Gonzalez-Zuñiga et al., 2014). To investigate whether c-Abl regulates the proteasome degradation of HDAC2, we treated cells first with imatinib to repress c-Abl activity, and then with MG132, a proteasome inhibitor. As described previously, when c-Abl was downregulated, we found that the HDAC2 levels were also decreased. MG132 treatment prevented degradation of HDAC2 (Fig. 4d). The results showed that c-Abl upregulated HDAC2 by preventing proteasome-mediated protein degradation. These data suggest that IFN-γ promotes malignant BMEC growth through the c-Abl/HDAC2 signaling pathway.
Fig. 4.
Influence of c-Abl on IFN-γ-induced cell malignant growth
(a) Imatinib had a suppressive effect on cell proliferation. * P<0.05, ** P<0.01, # P<0.001 vs. IFN-γ. (b) The expression of CDK4, cyclin D1, p21 and HDAC2 proteins in γ-BMECs treated with IFN-γ alone or in combination with imatinib (1 and 10 mmol/L) for 24 h detected by Western blotting. (c) Quantitative PCR results for HDAC2 mRNA in BMECs treated with IFN-γ alone or in combination with imatinib (1 and 10 mmol/L) for 24 h. (d) Proteasome inhibition prevents the decrease in the HDAC2 protein levels. The data represent the mean±SEM of three independent experiments. * P<0.05, ** P<0.01, # P<0.001
4. Discussion
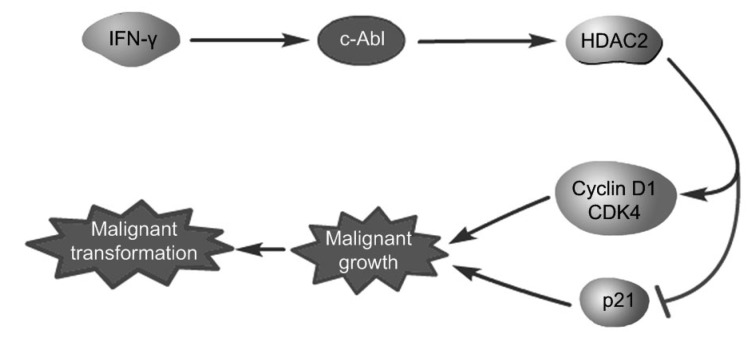
In previous studies, we found that IFN-γ induces the malignant transformation of BMECs by GCN2, a nutrient receptor (Xia et al., 2016b; Ren et al., 2018). However, among the phenotypes of the malignant transformation, including cell cycle shortening, increased cell proliferation, and the occurrence of cell migration and invasion, the abnormal regulation of cell growth is the first step in cell malignant transformation. Therefore, we investigated the molecular mechanism of IFN-γ-induced malignant growth of cells. In our study, we used γ-BMECs as an experimental group. The results showed that epithelial cells subjected to long-term treatment with IFN-γ had higher proliferative rates than primary epithelial cells (Fig. 1). We also found that both HDAC2 and c-Abl, which are proteins involved in the development of cancer, were highly expressed after IFN-γ treatment (Figs. 2 and 3). We also studied whether IFN-γ exerted its malignant effect through the HDAC2 or c-Abl signaling pathway. We found that the HDAC2 inhibitor, VPA, reduced the expression of the cyclin D1/CDK4 complex, but increased the expression of p21 after IFN-γ treatment, and then decreased the proliferation of the cells (Fig. 2). We further demonstrated that imatinib had an influence on cell growth similar to that of VPA. It reduced the expression of HDAC2 in a dose-dependent manner in malignantly transformed BMECs, but did not affect HDAC2 mRNA levels. This suggests that c-Abl might influence HDAC2 expression through a post-translational mechanism. HDAC2 ubiquitination mediated by the Ubc8 E2 binding enzyme and RING finger LIM domain-binding protein (RLIM) E3 ligase can induce degradation by its proteasomes (Krämer et al., 2003). Moreover, the tyrosine phosphorylation of c-Abl can promote protein stability by preventing its proteasome degradation (Gonzalez-Zuñiga et al., 2014). Further studies have shown that the addition of the protease inhibitor MG132 can antagonize HDAC2 inhibited by imatinib, suggesting that decreased c-Abl could promote HDAC2 proteasome degradation (Fig. 4). c-Abl travels between the cytoplasm and the nucleus and interacts with various proteins, playing a different role in many processes (Wang, 2014). HDAC2 is located in the nucleus, so c-Abl must be translocated to the nucleus to interact with HDAC2. This study showed that IFN-γ promoted the nuclear translocation of c-Abl (Fig. 3). Therefore, IFN-γ promoted the effect of c-Abl on the expression of HDAC2 in the nucleus. In conclusion, we demonstrated that IFN-γ contributed to the stability of HDAC2 by increasing the expression and translocation of c-Abl. This led to the upregulation of CDK4 and cyclin D1 and downregulation of p21, thereby accelerating the cell cycle and promoting cell proliferation (Fig. 5).
Fig. 5.
Schematic representation of the signal pathway
IFN-γ contributed to the stability of HDAC2 by increasing the expression and translocation of c-Abl, thus upregulating CDK4 and cyclin D1 and downregulating the expression of p21, thereby accelerating the cell cycle and promoting cell proliferation in malignant BMECs
It has been reported that BMECs are similar to human mammary epithelial cells and can be used to study mammary gland development, breastfeeding mechanisms and the development of breast cancer. Therefore, they are a cell model used to study the development of breast cancer (Xia et al., 2016c). γ-BMECs represent a new research tool used for basic research on the occurrence of breast cancer. We selected malignant cell growth to investigate in detail the mechanism of action of IFN-γ, providing direct evidence of the close relationship among IFN-γ, malignant cell growth, and even breast cancer.
The role of IFN-γ, as a pleiotropic cytokine, in cancer has generated substantial controversy. Although it has been used in the treatment of some clinical cancers, understanding of its role has become blurred because of the appearance of its side effects and the limitations of its efficacy (Carpi et al., 2009). Thus, we studied the ability of IFN-γ to induce malignant transformation of normal epithelial cells. As little is known about this subject, this represents an innovation in the study of malignant transformation.
HDAC2 and c-Abl are well-known oncogenic proteins. Many studies have shown that c-Abl is involved in a variety of cell activities, including cell proliferation, survival, normal development, gene expression, morphology, and migration (Zhao et al., 2013). Although it has been previously reported that c-Abl is a negative regulator of the tumor phenotype and epithelial-mesenchymal transition (EMT) (Allington et al., 2009), it has long been considered a factor promoting cancer, so its multi-faceted effects require more research. Gene suppression mediated by HDAC2 can lead to uncontrolled cell growth because HDAC2 inhibits the transcription of cyclin-dependent kinase inhibitors (CDKIs) (Noh et al., 2011). HDAC2 mediates the deacetylation of the transcription factor Gata4, decreasing the transactivation of genes associated with cell proliferation (Trivedi et al., 2010). Pharmacological or transcriptional inhibition of HDAC1/2 increases p19INK4d and p21Waf1/Cip1 expression, decreases CDK expression and arrests hepatocellular carcinoma cell (HCC) growth (Zhou et al., 2018). HDAC1/2 plays an essential role in mouse growth and development, and redundant roles in the regulation of cell proliferation (Montgomery et al., 2007; Yamaguchi et al., 2010). These studies showed that HDAC2 plays important roles in cell malignant growth. Our study was limited to the signaling pathway in which IFN-γ induced the malignant growth of cells, the c-Abl/HDAC2 pathway, but the mechanism may be complex and worthy of deeper study. Although our study provided new insights for basic research on breast cancer, it focused on only one of the many causes of breast cancer, and more attention is needed for breast cancer research.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31772715)
Compliance with ethics guidelines: Wen-bo REN, Xiao-jing XIA, Jing HUANG, Wen-fei GUO, Yan-yi CHE, Ting-hao HUANG, and Lian-cheng LEI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Allington TM, Galliher-Beckley AJ, Schiemann WP. Activated Abl kinase inhibits oncogenic transforming growth factor-β signaling and tumorigenesis in mammary tumors. Faseb J. 2009;23(12):4231–4243. doi: 10.1096/fj.09-138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6(6):579–589. doi: 10.1016/j.molonc.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bougarn S, Cunha P, Gilbert FB, et al. Technical note: validation of candidate reference genes for normalization of quantitative PCR in bovine mammary epithelial cells responding to inflammatory stimuli. J Dairy Sci. 2011;94(5):2425–2430. doi: 10.3168/jds.2010-3859. [DOI] [PubMed] [Google Scholar]
- 4.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009;122(19):3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brightbill H, Schlissel MS. The effects of c-Abl mutation on developing B cell differentiation and survival. Int Immunol. 2009;21(5):575–585. doi: 10.1093/intimm/dxp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpi A, Nicolini A, Antonelli A, et al. Cytokines in the management of high risk or advanced breast cancer: an update and expectation. Curr Cancer Drug Tar. 2009;9(8):9888–9903. doi: 10.2174/156800909790192392. [DOI] [PubMed] [Google Scholar]
- 7.Creagan ET, Schaid DJ, Ahmann DL, et al. Disseminated malignant melanoma and recombinant interferon: analysis of seven consecutive phase II investigations. J Invest Dermatol. 1990;95(S6):S188–S192. doi: 10.1111/1523-1747.ep12875512. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Zuñiga M, Contreras PS, Estrada LD, et al. c-Abl stabilizes HDAC2 levels by tyrosine phosphorylation repressing neuronal gene expression in Alzheimer’s disease. Mol Cell. 2014;56(1):163–173. doi: 10.1016/j.molcel.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Hildmann C, Riester D, Schwienhorst A. Histone deacetylases–an important class of cellular regulators with a variety of functions. Appl Microbiol Biotechnol. 2007;75(3):487–497. doi: 10.1007/s00253-007-0911-2. [DOI] [PubMed] [Google Scholar]
- 10.Horikoshi T, Fukuzawa KF, Hanada N, et al. In vitro comparative study of the antitumor effects of human interferon-α, β and γ on the growth and invasive potential of human melanoma cells. J Dermatol. 1995;22(9):631–636. doi: 10.1111/j.1346-8138.1995.tb03889. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95(13):7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krämer OH. HDAC2: a critical factor in health and disease. Trends Pharmacol Sci. 2009;30(12):647–655. doi: 10.1016/j.tips.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Krämer OH, Zhu P, Ostendorff HP, et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003;22(13):3411–3420. doi: 10.1093/emboj/cdg315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki J, Tsuji T, Luescher IF, et al. Direct tumor recognition by a human CD4+ T-cell subset potently mediates tumor growth inhibition and orchestrates anti-tumor immune responses. Sci Rep, 5:14896. 2015 doi: 10.1038/srep14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: its role in promoting cancer immunoeevasion. Int J Mol Sci. 2018;19(1):89. doi: 10.3390/ijms19010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery RL, Davis CA, Potthoff MJ, et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Gene Dev. 2007;21(14):1790–1802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller BM, Jana L, Kasajima A, et al. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer-overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer, 13:215. 2013 doi: 10.1186/1471-2407-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muss HB, Caponera M, Zekan PJ, et al. Recombinant gamma interferon in advanced breast cancer: a phase II trial. Invest New Drugs. 1986;4(4):377–381. doi: 10.1007/BF00173511. [DOI] [PubMed] [Google Scholar]
- 19.Nam SW, Park JY, Ramasamy A, et al. Molecular changes from dysplastic nodule to hepatocellular carcinoma through gene expression profiling. Hepatology. 2005;42(4):809–818. doi: 10.1002/hep.20878. [DOI] [PubMed] [Google Scholar]
- 20.Noh JH, Jung KH, Kim JK, et al. Aberrant regulation of HDAC2 mediates proliferation of hepatocellular carcinoma cells by deregulating expression of G1/S cell cycle proteins. PLoS ONE. 2011;6(11):e28103. doi: 10.1371/journal.pone.0028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren WB, Li Y, Xia XJ, et al. Arginine inhibits the malignant transformation induced by interferon-gamma through the NF-κB-GCN2/eIF2α signaling pathway in mammary epithelial cells in vitro and in vivo. Exp Cell Res. 2018;368(2):236–247. doi: 10.1016/j.yexcr.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Sarhan D, D'Arcy P, Wennerberg E, et al. Activated monocytes augment TRAIL-mediated cytotoxicity by human NK cells through release of IFN-γ. Euro J Immunol. 2013;43(1):249–257. doi: 10.1002/eji.201242735. [DOI] [PubMed] [Google Scholar]
- 23.Schiller JH, Pugh M, Kirkwood JM, et al. Eastern cooperative group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin Cancer Res. 1996;2(1):29–36. [PubMed] [Google Scholar]
- 24.Trivedi CM, Zhu WT, Wang QH, et al. Hopx and Hdac2 interact to modulate Gata4 acetylation and embryonic cardiac Myocyte proliferation. Dev Cell. 2010;19(3):450–459. doi: 10.1016/j.devcel.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JYJ. The capable ABL: what is its biological function? Mol Cell Biol. 2014;34(7):1188–1197. doi: 10.1128/MCB.01454-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weichert W, Röske A, Gekeler V, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98(3):604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia XJ, Che YY, Gao YY, et al. Arginine supplementation recovered the IFN-γ-mediated decrease in milk protein and fat synthesis by inhibiting the GCN2/eIF2α pathway, which induces autophagy in primary bovine mammary epithelial cells. Mol Cell. 2016;39(5):410–417https://doiorg/1014348/molcells20162358. doi: 10.14348/molcells.2016.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia XJ, Gao YY, Zhang J, et al. Autophagy mediated by arginine depletion activation of the nutrient sensor GCN2 contributes to interferon-γ-induced malignant transformation of primary bovine mammary epithelial cells. Cell Death Discov, 2:15065. 2016 doi: 10.1038/cddiscovery.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia XJ, Che YY, Zhang J, et al. Diet-driven interferon-γ enhances malignant transformation of primary bovine mammary epithelial cells through nutrient sensor GCN2-activated autophagy. Cell Death Dis. 2016;7(3):e2138. doi: 10.1038/cddis.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Cubizolles F, Zhang Y, et al. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 2010;24(5):455–469. doi: 10.1101/gad.552310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17(19):6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao HJ, Ho PC, Lo YH, et al. Interaction of proliferation cell nuclear antigen (PCNA) with c-Abl in cell proliferation and response to DNA damages in breast cancer. PLoS ONE. 2012;7(1):e29416. doi: 10.1371/journal.pone.0029416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao HJ, Chen MS, Lo YH, et al. The Ron receptor tyrosine kinase activates c-Abl to promote cell proliferation through tyrosine phosphorylation of PCNA in breast cancer. Oncogene. 2013;33(11):1429–1437. doi: 10.1038/onc.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou HY, Cai Y, Liu DN, et al. Pharmacological or transcriptional inhibition of both HDAC1 and 2 leads to cell cycle blockage and apoptosis via p21Waf1/Cip1 and p19INK4d upregulation in hepatocellular carcinoma. Cell Proliferat. 2018;51(3):e12447. doi: 10.1111/cpr.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu JF, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo H, Tell GS, Vollset SE, et al. Interferon-γ-induced inflammatory markers and the risk of cancer: the hordaland health study. Cancer. 2014;120(21):3370–3377. doi: 10.1002/cncr.28869. [DOI] [PMC free article] [PubMed] [Google Scholar]