Abstract
Globally, peptide-based anticancer therapies have drawn much attention. Marine organisms are a reservoir of anticancer peptides that await discovery. In this study, we aimed to identify cytotoxic oligopeptides from Sarcophyton glaucum. Peptides were purified from among the S. glaucum hydrolysates produced by alcalase, chymotrypsin, papain, and trypsin, guided by a methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay on the human cervical cancer (HeLa) cell line for cytotoxicity evaluation. Purification techniques adopted were membrane ultrafiltration, gel filtration chromatography, solid phase extraction (SPE), and reversed-phase high-performance liquid chromatography (RP-HPLC). Purified peptides were identified by de novo peptide sequencing. From papain hydrolysate, three peptide sequences were identified: AGAPGG, AERQ, and RDTQ (428.45, 502.53, and 518.53 Da, respectively). Peptides synthesized from these sequences exhibited cytotoxicity on HeLa cells with median effect concentration (EC50) values of 8.6, 4.9, and 5.6 mmol/L, respectively, up to 5.8-fold stronger than the anticancer drug 5-fluorouracil. When tested at their respective EC50, AGAPGG, AERQ, and RDTQ showed only 16%, 25%, and 11% cytotoxicity to non-cancerous Hek293 cells, respectively. In conclusion, AERQ, AGAPGG, and RDTQ are promising candidates for future development as peptide-based anticancer drugs.
Keywords: Anticancer therapy, Bioactive peptide, Cytotoxicity, HeLa cells, Sarcophyton glaucum, Soft coral
1. Introduction
Cancer is one of the leading causes of morbidity and mortality worldwide. There were about 14 million new cancer cases and 8.2 million cancer deaths in 2012 globally. It was projected that in the next 20 years, new cases will increase by about 63% (Ferlay et al., 2018). Unfortunately, chemotherapy, a common cancer treatment, often incurs side effects, including nausea, hair loss, fatigue, and loss of appetite. Many of these side effects take place due to nonspecific actions of the anticancer compounds on non-cancerous tissues, thus reducing the effectiveness of the treatment (Sutradhar and Amin, 2014). Therefore, there is an urgent need to develop more cancer-specific cytotoxic drugs.
More than 60% of cancer therapeutic agents currently used in chemotherapy originate from natural sources (Pangestuti and Kim, 2017). This has driven further interest among the scientific community to bioprospecting of natural resources for new anticancer agents. Marine-derived bioactive peptides are an invaluable resource for anticancer drug development (Zheng et al., 2013). Some marine bioactive peptides, such as kahalalide F, HTI-286, dehydrodidemnin B, dolastatin 10, and synthadotin, have already made it to preclinical or clinical trials for cancer treatment (Simmons et al., 2005). Bioactive peptides, usually consisting of residues with 2–20 amino acids, are inactive within the sequence of the parent proteins and can be liberated by enzymatic hydrolysis (Kim et al., 2013; Chai et al., 2017). Proteases often used for such a purpose include alcalase, α-chymotrypsin, papain, trypsin, and pepsin (He et al., 2005; Qian et al., 2007; Ngo et al., 2012). Separation techniques, such as membrane ultrafiltration (UF), gel filtration (GF) chromatography, fast protein liquid chromatography, and reversed-phase high-performance liquid chromatography (RP-HPLC), are often employed to purify the bioactive peptides before they can be identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) or Edman degradation (Chai et al., 2017). Anticancer peptides have been identified from various marine organisms, including sponges (Quah et al., 2017), tunicates (Jumeri and Kim, 2011), fish (Picot et al., 2006; Hsu et al., 2011; Hung et al., 2014), and oysters (Wang et al., 2010). However, only a few studies have evaluated the cytotoxicity of those peptides on normal or non-cancerous cells to characterize selectivity of the peptides (Chen et al., 2013; Pan et al., 2016; Daliri et al., 2017).
Sarcophyton glaucum is a marine soft coral that belongs to the family Alcyoniidae, phylum Cnidaria, and class Anthozoa (Cordeiro et al., 2010). S. glaucum has received much attention in the last decade for its diverse bioactive secondary metabolites, including cembranoids (Fahmy et al., 2006; Hegazy et al., 2011), bicembranoids (Huang et al., 2015), and steroids (Chao et al., 2017). A number of non-peptide compounds from S. glaucum showed cytotoxicity towards some cancer cell lines, e.g. human breast cancer (MCF-7) (Al-Lihaibi et al., 2014; Abdel-Lateff et al., 2015), lymphoma human liver cancer (HepG2) (Hegazy et al., 2011; Al-Lihaibi et al., 2014; Abdel-Lateff et al., 2015), human colon cancer (HCT-116) (Hegazy et al., 2011; Abdel-Lateff et al., 2015), and human cervical cancer (HeLa) (Hegazy et al., 2011) cell lines. However, the discovery of cytotoxic peptides from S. glaucum has not been documented in the literature. Therefore, this study aimed to purify and identify cytotoxic peptides from S. glaucum. Guided by a cytotoxicity assay on HeLa cells and using UF, GF, solid phase extraction (SPE), RP-HPLC, and de novo peptide sequencing, three peptides AGAPGG, AERQ, and RDTQ were identified from a papain hydrolysate of S. glaucum.
2. Materials and methods
2.1. Reagents and materials
Ultrafiltration centrifugal units with molecular weight cut-off (MWCO) 3 kDa and HPLC-grade acetonitrile (ACN) were purchased from Merck (Darmstadt, Germany). RPMI 1640 medium was bought from Gibco, Life Technologies (Paisley, UK), and HPLC-grade trifluoroacetic acid (TFA) was from Fisher Chemical (Loughborough, UK). Methylthiazolyldiphenyl-tetrazolium bromide (MTT) was purchased from Amresco (Ohio, USA), 5-fluorouracil (5-FU) from Biobasic (Ontario, Canada), and o-phthalaldehyde (OPA) from Nacalai Tesque (Kyoto, Japan). Strata® C18-E SPE cartridges (55 µm, 70 Å, 1000 mg/6 mL) were purchased from Phenomenex, Inc. (Torrance, CA, USA), and Acrodisc® syringe filters with Supor® membranes (0.2 µm pore size) from Pall Life Sciences (East Hills, New York, USA). Other reagents used were of analytical grade.
2.2. Preparation of S. glaucum protein isolate and hydrolysates
S. glaucum was sampled at Nanga Kecil Island, Malaysia, in July 2013. The specimens were obtained using self-contained underwater breathing apparatus (SCUBA) at 3 m depth. Species were identified by reference to Fabricius and Alderslade (2001). The samples were brought back to the laboratory on ice, stored in a freezer at −20 °C, and lyophilized before use. Proteins from S. glaucum were isolated as described by Quah et al. (2017). The protein content of the isolate was determined with Bradford’s assay (Bradford, 1976), using a standard curve constructed with bovine serum albumin.
Hydrolysis was performed as previously described (Quah et al., 2017). Briefly, the lyophilized protein isolate was dissolved in 50 mmol/L sodium phosphate buffer at a mass (g):volume (mL) ratio of 1:200. The pH of the buffer was adjusted to 7, 7, 6, and 8, for the hydrolysis with alcalase, chymotrypsin, papain, and trypsin, respectively. The proteases were applied according to an enzyme (g):protein (g) ratio of 1:10. Hydrolysis was carried out in water baths at the optimum temperature for each protease (50 °C for alcalase; 37 °C for trypsin, chymotrypsin, and papain). Aliquoted hydrolysate samples were lyophilized before use in an MTT cytotoxicity test as described below. For each protease treatment, the degree of hydrolysis (DH) was measured as reported previously (Chen et al., 2009).
2.3. MTT assay
The MTT assay was performed as reported previously (Quah et al., 2017). Briefly, the HeLa human cervical cancer cell line (ATCC CCL-2) was grown at 37 °C in a humidified incubator in 5% CO2, in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Filtered samples (100 µL) of various concentrations were added to each microplate well, followed by 24 h of incubation. For a negative control, the sample was substituted with sterile, deionized water. Upon addition of MTT (5 mg/mL) and after 4-h incubation, the microplate was centrifuged at 1000g. To solubilize the purple formazan, dimethyl sulfoxide (200 µL) was added to each well and the contents of each well were mixed thoroughly prior to measurement of absorbance at 570 nm. 5-FU was used as a positive control.
2.4. Fractionation by membrane UF
Papain hydrolysate was fractionated by membrane UF as reported previously (Quah et al., 2017), with modifications. Briefly, two fractions designated as “<3 kDa UF” and “>3 kDa UF”, respectively, were obtained using a 3-kDa MWCO ultrafiltration centrifugal unit. The two UF fractions were lyophilized before being tested for cytotoxicity. Peptide content was quantified using the OPA method (Nielsen et al., 2001).
2.5. Fractionation by GF chromatography
The <3 kDa UF fraction was fractionated using a Sephadex G-25 column (1.6 cm×70.0 cm) as described previously (Quah et al., 2017). Briefly, sample fractions were eluted with deionized water and the elution profile was monitored at 280 nm. The pooled fractions obtained, designated as GF1, GF2, and GF3, were lyophilized and tested for cytotoxicity. Peptide content was determined as described above.
2.6. Fractionation by SPE
Pooled fraction GF3 was further purified using SPE cartridges. GF3 (50 mg/mL, 2 mL) was applied to Strata® C18-E cartridges which were preconditioned with methanol (6 mL), washed with 100% ACN containing 0.1% TFA (6 mL), and equilibrated with deionized water containing 0.1% TFA (12 mL). GF3 was fractionated using a stepwise elution (6 mL per step) with increasing ACN concentrations (0%, 10%, 20%, 30%, 40%, 50%, 80%, and 100%) containing 0.1% TFA. This produced a series of fractions designated as SPE-F1, SPE-F2, SPE-F3, SPE-F4, SPE-F5, SPE-F6, SPE-F7, and SPE-F8, respectively. Absorbance of each fraction was monitored at 214 nm. The peptide content and cytotoxicity of the SPE fractions were determined as described above.
2.7. Analysis of SPE-F7 by RP-HPLC
SPE-F7 (10 µL) was separated by RP-HPLC (Shimadzu LC-20D dual binary pumps and Shimadzu Prominence SPD-M20A PDA detector, Kyoto, Japan) using a Kinetex C18 column (100 Å, 5 µm, 4.6 mm×250.0 mm). The mobile phase consisted of deionized water with 0.1% TFA as solvent A and ACN containing 0.1% TFA as solvent B at a flow rate of 0.5 mL/min. The column was eluted with a gradient elution as follows: 0–10 min, 5%–35% of solvent B; 10–35 min, 35%–95% solvent B; 35–41 min, 95% solvent B; 41–50 min, 5% solvent B. The elution profile was monitored at 214 nm.
2.8. De novo peptide sequencing
Online LC-MS/MS analysis was performed at the Proteomics Core Facility, Malaysia Genome Institute, National Institutes of Biotechnology, Malaysia to identify the peptide sequences in SPE-F7. Briefly, the peptide was analyzed using a Waters nanoACQUITY UPLC system (Waters, Milford, MA, USA), coupled to a Waters Synapt G2 HDMS-Q-TOF mass spectrometer (Waters, Manchester, UK). De novo peptide sequencing was carried out using data directed analysis (DDA) with a positive electrospray ionization mode. ProteinLynx Global Server Software (Version 2.4, Waters, Milford, MA, USA) was used for data analysis.
2.9. Validation of cytotoxicity of synthetic peptides
Synthetic peptides (>95% purity) were manufactured by and purchased from Bio Basic Inc., Canada, based on the sequences identified (AGAPGG, AERQ, and RDTQ). The cytotoxicity of the synthetic peptides was tested on HeLa and Hek293 cell lines. Hek293 is a non-cancer human embryonic kidney cell line.
2.10. Data analysis
Data are expressed as mean±standard error (SE; n=3). Statistical Analysis System (SAS; Version 9.4, North Carolina, USA) was used for statistical analysis. Data were analyzed by one-way analysis of variance (ANOVA), and where appropriate, followed by Fisher’s least significant difference (LSD) test to separate means of significant differences. Student’s t-test was used to compare two mean values. P values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Protein isolation and hydrolysis
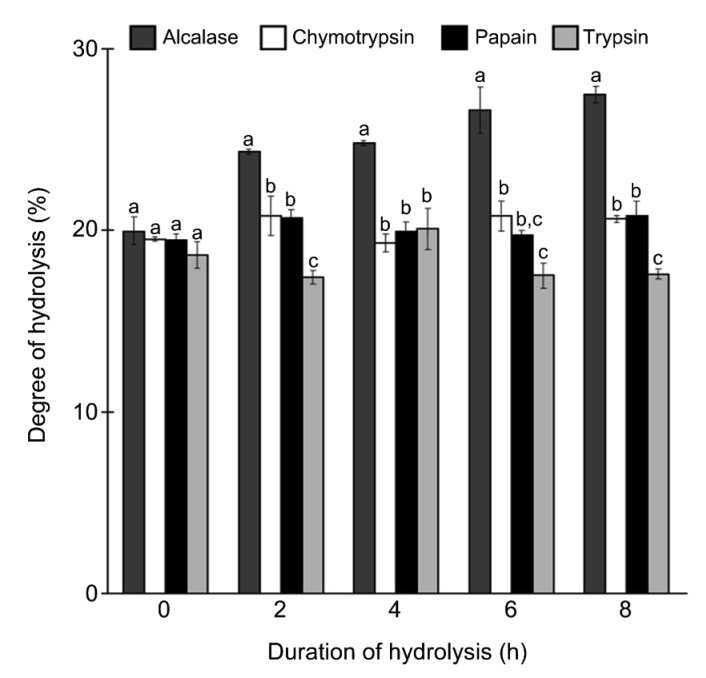
Protein isolate prepared from S. glaucum yielded 443.1 mg proteins/g dry weight. Hydrolysis of the protein isolate by alcalase, chymotrypsin, papain, and trypsin was monitored for up to 8 h (Fig. 1). Yield percentages of the four hydrolysates were 26.4%, 22.9%, 22.2%, and 28.1%, respectively. Overall, hydrolysis using alcalase produced the highest DH value ((27.5±0.4)% after 8 h of hydrolysis) among all protease treatments. A clear-cut rising trend in DH was not observed in the protein hydrolysates produced using chymotrypsin, papain, or trypsin. Chymotrypsin and papain treatments produced very similar DH values over the 8 h period. After 8 h of hydrolysis, the highest DH values were (20.7±0.2)% for chymotrypsin hydrolysate and (20.8±0.8)% for papain hydrolysate. For hydrolysis using trypsin, the maximum DH value of (20.1±1.1)% was obtained after 4 h. In this study, the optimal hydrolysis duration was defined as the duration required to achieve the maximum DH value. Therefore, the optimum hydrolysis duration for alcalase, chymotrypsin, and papain was 8 h, whereas for trypsin, was 4 h.
Fig. 1.
Degree of hydrolysis of S. glaucum protein hydrolysates
Data are expressed as mean±SE (n=3). Data for the same hydrolysis duration that are labelled with different letters are significantly different (P<0.05) according to the Fisher’s LSD test
3.2. Cytotoxic activity of S. glaucum protein hydrolysates
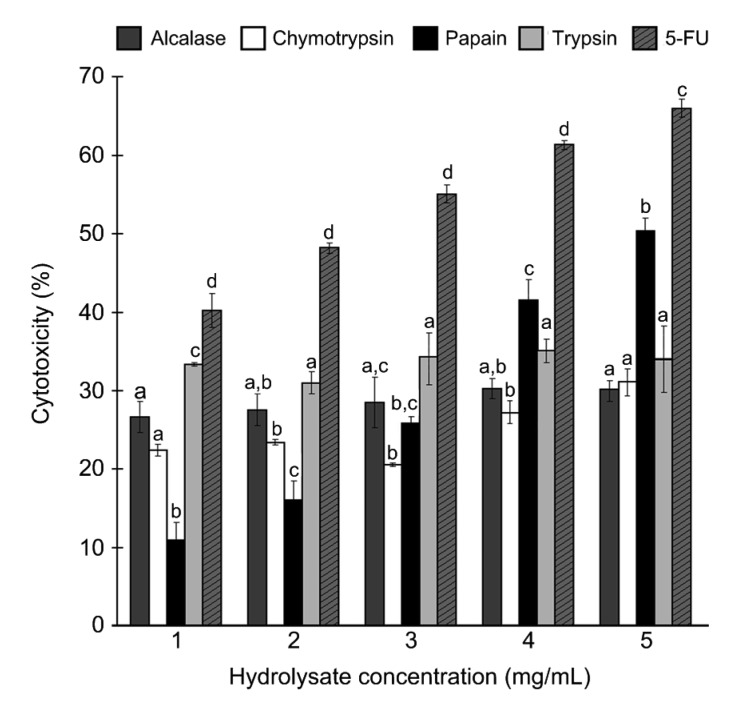
Hydrolysates obtained after their respective optimum hydrolysis durations were tested for cytotoxicity against the HeLa cell line. The four hydrolysates were cytotoxic at doses of 1–5 mg/mL (Fig. 2). Notably, papain hydrolysate was the only hydrolysate that showed a dose-dependent increase in cytotoxicity. By contrast, the cytotoxicity of the alcalase, chymotrypsin, and trypsin hydrolysates fluctuated slightly over the range of concentrations tested. 5-FU, an anticancer drug, was used as the positive control in our assay. 5-FU was more cytotoxic than all four hydrolysates tested. When the sample concentration tested was 5 mg/mL, the cytotoxicity of alcalase, chymotrypsin, papain, and trypsin hydrolysates was 30%, 31%, 50%, and 34%, respectively. Owing to its high cytotoxicity, papain hydrolysate was further fractionated using membrane UF.
Fig. 2.
Cytotoxicity of S. glaucum hydrolysates against HeLa cells
Data are expressed as mean±SE (n=3). Data for the same hydrolysate concentration that are labelled with different letters are significantly different (P<0.05) according to the Fisher’s LSD test
3.3. Purification of cytotoxic peptides
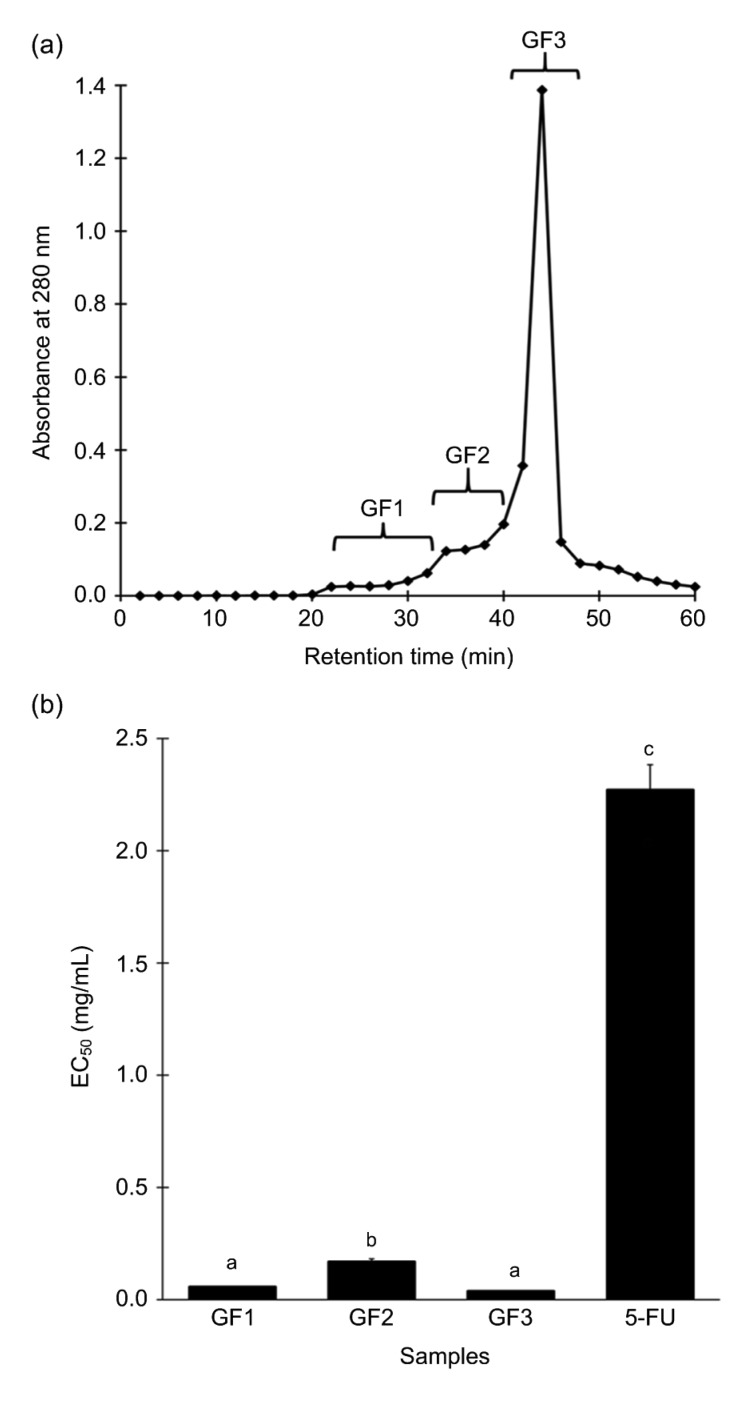
Membrane UF separated papain hydrolysate into two fractions: <3 kDa UF and >3 kDa UF. Yield percentages of <3 kDa UF and >3 kDa UF were 11.5% and 3.2%, respectively. When evaluated for cytotoxic activity against HeLa cells, the median effect concentration (EC50) values of <3 kDa UF and >3 kDa UF were (0.16±0.01) and (0.14±0.01) mg/mL, respectively. There were no significant differences between the EC50 values of the two fractions (P>0.05, Student’s t-test). Considering that previous reports (Hsu et al., 2011; Hung et al., 2014; Song et al., 2014) often found cytotoxic marine peptides to be smaller than 3 kDa, <3 kDa UF was selected to be further fractionated. A representative elution profile of <3 kDa UF following separation on a Sephadex G-25 column is presented in Fig. 3a. Yield percentages of GF1, GF2, and GF3 were 3.2%, 9.3%, and 0.8%, respectively. Pooled fractions GF1, GF2, and GF3 were evaluated for cytotoxicity on HeLa cells. Among the three GF fractions, GF2 had the highest EC50 value (0.17 mg/mL; Fig. 3b), which was similar to that of <3 kDa UF (P>0.05, Student’s t-test). The EC50 values of GF1 (0.06 mg/mL) and GF3 (0.04 mg/mL) were about 2.7-and 4.0-fold lower, respectively, than that of <3 kDa UF (P<0.05, Student’s t-test). Overall, all three GF fractions had much lower EC50 values than 5-FU. In comparison to 5-FU, the EC50 values of GF1 and GF3 were about 37.8-and 56.8-fold lower, respectively. GF3 was, therefore, further purified by reversed-phase SPE.
Fig. 3.
Gel filtration chromatography of <3 kDa UF
(a) A representative elution profile. Eluates were divided into three pooled fractions, GF1, GF2, and GF3. (b) Cytotoxicity of the GF fractions and 5-FU, expressed as EC50 values. For (b), data are expressed as mean±SE (n=3). Data labelled by different letters are significantly different (P<0.05) according to the Fisher’s LSD test
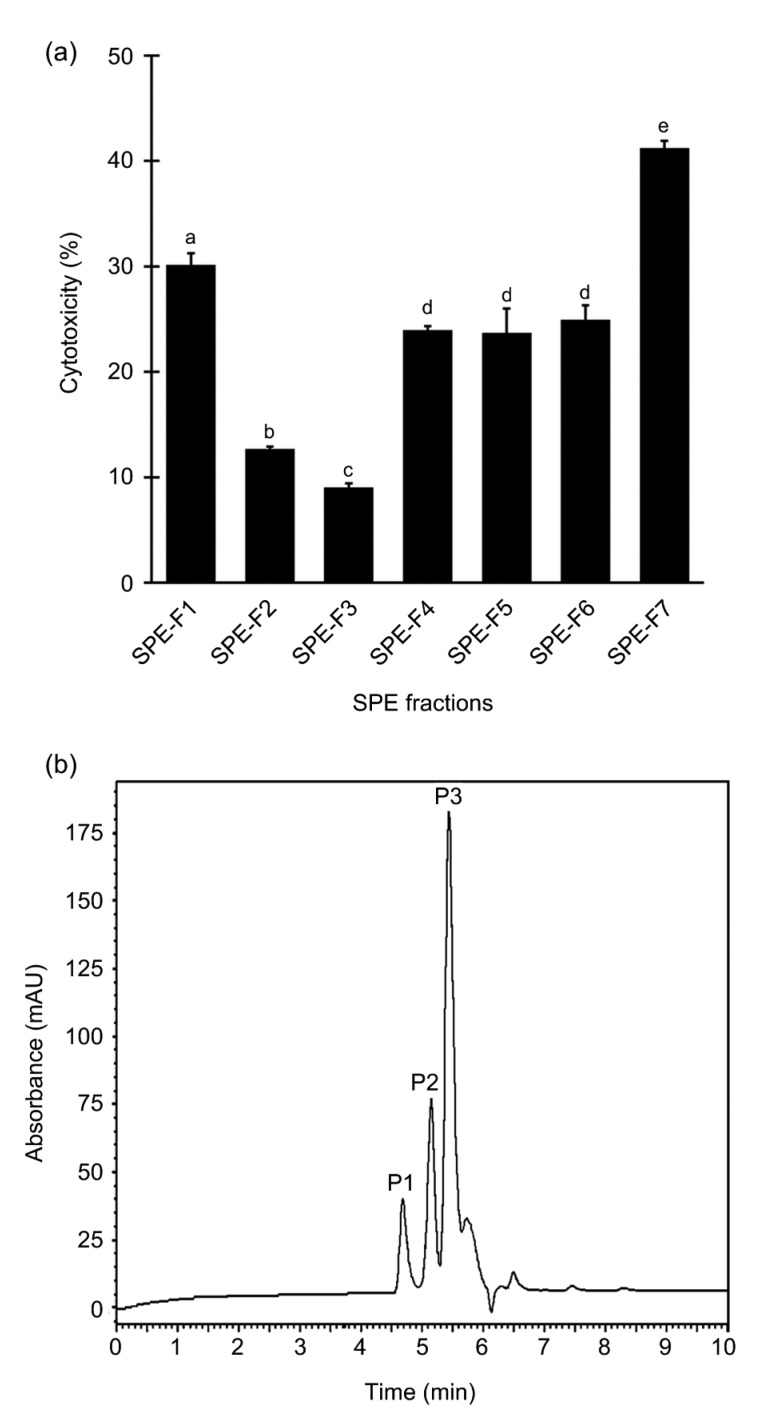
A representative SPE elution profile of GF3, monitored at 214 nm, is presented in Fig. S1. Among the eight fractions, an absorbance reading was undetectable for SPE-F8. Thus, the peptide contents of only seven fractions SPE-F1 to SPE-F7 were quantified. Except for SPE-F1, the peptide contents of most SPE fractions ranged between 0.01 and 0.37 mg/mL (Fig. S2). The cytotoxicity of the seven SPE fractions was then tested at 0.04 mg peptide/mL. This standardized concentration was selected according to the EC50 of GF3. Although all the SPE fractions showed cytotoxic activity against HeLa cells, SPE-F7 stood out as the most active ((41.2±0.7)%; Fig. 4a). When analyzed using RP-HPLC, the chromatogram for SPE-F7 revealed three major peaks (Fig. 4b), namely P1, P2, and P3, which constituted 8.2%, 12.6%, and 31.8% of the relative peak area in relation to total area of all peaks in the chromatogram. These peaks were eluted at retention time of 4.677, 5.147, and 5.434 min, respectively. The SPE-F7 fraction was taken for peptide sequencing by de novo sequencing.
Fig. 4.
Cytotoxicity of SPE fractions and RP-HPLC profile of SPE-F7
(a) Cytotoxicity of SPE fractions tested at 0.04 mg peptide/mL on HeLa cells. Data are expressed as mean±SE (n=3). Data labelled by different letters are significantly different (P<0.05) according to the Fisher’s LSD test. (b) A representative RP-HPLC profile of SPE-F7 monitored at 214 nm
3.4. Identification of cytotoxic peptides
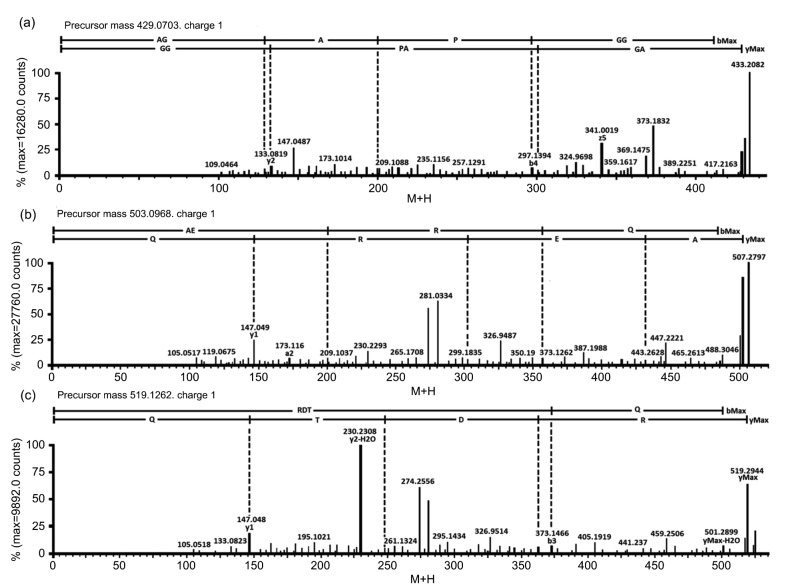
One 6-residue peptide AGAPGG and two 4-residue peptides AERQ and RDTQ were identified from SPE-F7. Fig. 5 shows the MS/MS spectra of the three peptides. The m/z values of these peptides, as single-charged ions, ranged between 429 and 519. The detected molecular mass of AGAPGG (428.2019 Da), AERQ (502.2500 Da), and RDTQ (518.2449 Da) agreed well with the theoretical molecular mass of the three peptides (428.2013, 502.2492, and 518.2441 Da), as calculated by PepDraw (http://www.tulane.edu/~biochem/WW/PepDraw). These peptide sequences were synthesized and used for validation of their cytotoxicity.
Fig. 5.
MS/MS spectra of AGAPGG (a), AERQ (b), and RDTQ (c)
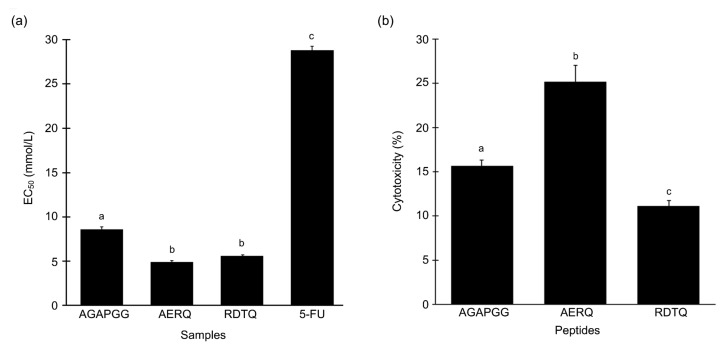
Among the three synthetic peptides, AERQ exhibited the highest cytotoxic activity on HeLa cells, closely followed by RDTQ. Both these peptides exhibited about 90% cytotoxic activity at 5 mg/mL, surpassing that of 5-FU (data not shown). EC50 values of the three peptides, when tested on HeLa cells, were 8.6, 4.9, and 5.6 mmol/L, respectively (Fig. 6a). Based on EC50 comparison, AGAPGG, AERQ, and RDTQ were 3.3-, 5.8-, and 5.1-fold more potent than 5-FU. Statistically, the EC50 values of AERQ and RDTQ were not significantly different (P>0.05, Fisher’s LSD).
Fig. 6.
Cytotoxicity of the synthetic peptides
(a) Cytotoxicity of the synthetic peptides and 5-FU on HeLa cells, expressed as EC50 values. (b) Cytotoxic activity of synthetic peptides tested at their respective EC50 on Hek293 cells. Data are expressed as mean±SE (n=3). Data labelled by different letters are significantly different (P<0.05) according to the Fisher’s LSD test
To evaluate their selectivity, AGAPGG, AERQ, and RDTQ were further assessed for their cytotoxicity on the Hek293 cell line based on their respective EC50 against HeLa cells. The relative cytotoxicity of the three peptides against Hek293 was RDTQ<AGAPGG<AERQ (Fig. 6b). The cytotoxicity of the peptides against Hek293 cells, tested at their respective EC50 against HeLa cells, ranged from 11% to 25% (Fig. 6b). Notably, whereas 5.6 mmol/L RDTQ induced 50% cytotoxicity on HeLa cells (Fig. 6a), the same peptide concentration induced only 11% cytotoxicity on Hek293 cells (Fig. 6b).
4. Discussion
The bioprospecting of edible marine fishes, other seafoods, and their by-products for bioactive peptides is frequently accomplished via bioassay-guided purification and identification of such peptides from protein hydrolysates (Wang et al., 2013; Chai et al., 2017; Nurdiani et al., 2017). Adopting the same strategy, we demonstrated for the first time that protease-mediated hydrolysis can also be used to release potent cytotoxic peptides from S. glaucum, a non-food marine animal. In this study, papain hydrolysate exhibited the most prominent dose-dependent cytotoxic effect against HeLa cells (Fig. 2). This result is consistent with those of other studies which reported papain hydrolysates having stronger cytotoxicity than other enzyme hydrolysates prepared from tuna dark muscle (Hsu et al., 2011), tuna cooking juice (Hung et al., 2014), and the marine sponge Xestospongia testudinaria (Quah et al., 2017). Our findings suggest that papain hydrolysis likely liberated more potent cytotoxic peptides encrypted in the proteins of S. glaucum than did the other three proteases.
In this study, no correlation was found between DH and cytotoxicity. Hsu et al. (2011) reported no correlation between the DH of hydrolysates prepared from tuna dark muscle and their antiproliferative activities against the MCF-7 cell line. Similarly, Picot et al. (2006) observed no correlation between the DH of several fish hydrolysates and their antiproliferative activities. Thus, our findings and those of others (Picot et al., 2006; Hsu et al., 2011) imply that although DH may be useful as an indicator of the progress of protein hydrolysis, its value as an indicator of cytotoxicity appears limited.
Our results revealed that the <3 kDa UF and >3 kDa UF fractions exhibited similar levels of cytotoxicity against the HeLa cells. Similar levels of cytotoxicity were also observed when <3 kDa UF and >3 kDa UF fractions of a Porphyra haitanesis hydrolysate were tested on the A549 lung cancer and SGC-7901 gastric cancer cell lines (de Lumen, 2005; Fan et al., 2017). Nevertheless, the <3 kDa UF fraction of X. testudinaria papain hydrolysate exhibited about seven times stronger cytotoxicity than the 3–10 kDa UF fraction (Quah et al., 2017). When compared with fractions of larger molecular weight (MW) ranges, the <3 kDa UF fraction prepared from loach papain hydrolysate also exhibited stronger antiproliferative activity against the HepG2, MCF-7, and Caco-2 colon cancer cell lines (You et al., 2011). Furthermore, according to Fan et al. (2017), short peptides can be identified easily by MS and are easier to synthesize. Small peptides are also less costly to synthesize (Chai et al., 2017). Therefore, in this study, <3 kDa UF was chosen for further purification.
Following separation on a Sephadex G-25 gel filtration column, three active peaks were detected in the elution profile (Fig. 3a). This implies that multiple peptides of variable MWs were found in the <3 kDa UF fraction. Notably, the three GF fractions had EC50 values that were drastically lower than that of 5-FU, indicating that they were considerably more potent than 5-FU. This result is interesting in the light of some other studies in which GF fractions were found to have poorer cytotoxicity than 5-FU. For example, the GF fractions derived from Spirulina (Arthrospira) platensis (Wang and Zhang, 2016) and P. haitanesis (Fan et al., 2017) exhibited weaker cytotoxicity on the MCF-7, HepG2, SGC-7901, A549, and HT-29 cell lines than 5-FU.
Among the three GF fractions in this study, GF3, which corresponded to the lowest MW range, displayed the strongest cytotoxicity against HeLa cells. Similar trends were also reported for GF fractions prepared from X. testudinaria hydrolysate (Quah et al., 2017) and half-fin anchovy hydrolysate (Song et al., 2014). Working on the alcalase hydrolysate of a solitary tunicate, Jumeri and Kim (2011) reported that F2, the GF fraction of a lower MW range, was more cytotoxic to HeLa cells than F1, the GF fraction of a higher MW range. The authors proposed that the enhanced anticancer activity of F2 may be due to its higher molecular mobility and diffusivity compared with F1, which may have enabled better interaction between F2 and cancer cells (Jumeri and Kim, 2011). Thus, in this study, favorable mobility and diffusivity characteristics may have underlain the potency of GF3 as a cytotoxic agent against HeLa cells.
Further separation of GF3 using SPE revealed that SPE-F7, the most cytotoxic peptide fraction, was eluted with 80% ACN (Fig. 4a). Thus, peptides with relatively high hydrophobicity may have contributed to the strong cytotoxic effect of SPE-F7 against HeLa cells. Supporting this suggestion are previous observations that peptides with greater hydrophobicity showed stronger anticancer activity against HeLa, MCF-7, and other cancer cell lines (Huang et al., 2011; Shan et al., 2012). RP-HPLC analysis of SPE-F7 revealed three major peaks (Fig. 4b), implying the presence of at least three peptides with different polarities in the fraction. Furthermore, the chromatogram suggests that SPE-F7 was sufficiently purified to be subjected to the determination of peptide sequences using LC-MS/MS analysis.
De novo peptide sequencing led to the identification of three potential cytotoxic peptides: AGAPGG, AERQ, and RDTQ (Fig. 5). When these peptides were chemically synthesized and tested on HeLa cells, all showed cytotoxicity (Fig. 6a). To our knowledge, this study reports for the first time the identification of cytotoxic peptides from S. glaucum. Moreover, AGAPGG, AERQ, and RDTQ are cytotoxic peptides that have not been previously reported. A search of the BIOPEP database (Minkiewicz et al., 2008) (accessed on Sept. 19, 2017) also found these three peptides not documented for any bioactivities. Importantly, AGAPGG, AERQ, and RDTQ were more powerful cytotoxic agents than 5-FU. Hence, S. glaucum should be exploited more intensively in future as a source of novel cytotoxic peptides.
The structure–activity relationship of anticancer peptides is still not fully understood (Gabernet et al., 2016). Nevertheless, amphiphilicity is believed to be important to the ability of anticancer peptides to not only bind to but also penetrate cancer cell membranes (Dennison et al., 2006; Li and Yu, 2015). Interestingly, two of the three peptides identified in this study were amphiphilic. The amphiphilicity of AGAPGG is indicated by hydrophobic (A and P) and hydrophilic (G) amino acid residues, with a calculated hydrophobic ratio of 50%. The amphiphilicity of AERQ is indicated by the presence of hydrophobic (A) and hydrophilic (E, R, Q) residues, with a calculated hydrophobic ratio of 25%. Although AGAPGG and AERQ are both amphiphilic, AERQ was about 1.5-fold more cytotoxic than AGAPGG (Fig. 6a). Song et al. (2014) reported that upon the replacement of an H residue with a G residue in the peptide YALPAH, the modified peptide YALPAG showed weaker inhibitory activity on PC-3 prostate cancer cells. Thus, the presence of three G residues in AGAPGG could have lowered its cytotoxic effect. The strong cytotoxicity of AERQ may also be associated with the presence of an R residue within the peptide (Schmidt et al., 2010). Tada et al. (2011) demonstrated that replacement of an H residue by an R residue in an EGFR-lytic hybrid peptide enhanced the ability of the peptide to bind to cancer cells, hence increasing its anticancer activity. Among the three peptides identified in this study, RDTQ is not amphiphilic. When tested on HeLa cells, RDTQ was more cytotoxic than AGAPGG and similarly cytotoxic as AERQ. Hence, our results suggest that, in contrast to peptide amphiphilicity, the presence of specific amino acid residues and/or their arrangement in a peptide sequence may be a more important determinant of cytotoxicity.
In this study, although AERQ and RDTQ were similarly cytotoxic to HeLa cells, RDTQ was less toxic than AERQ to the non-cancerous Hek293 cells. In other words, our results suggest that RDTQ was more selectively toxic to HeLa cells than AERQ. Based on this finding, RDTQ seems to be a more promising candidate for future development of selective anticancer therapeutics. Future research to unravel the cellular and molecular mechanisms that underlie the cytotoxicity of RDTQ and whether it also exerts toxicity against other cancer and non-cancer cell lines is of great interest.
According to the BIOPEP database (Minkiewicz et al., 2008), AGAPGG shares part of its sequence with 14 previously identified peptides, including those possessing antiamnestic (PG), angiotensin-converting enzyme (ACE) inhibitory (AP, GA, AG, GG, and PG), and dipeptidyl peptidase IV (DPP IV) inhibitory (AP, APG, GA, AG, GG, and PG) activities. AERQ shares partial homology with a previously identified DPP IV inhibitory dipeptide (AE), whereas RDTQ shares partial homology with an ACE and DPP IV inhibitory dipeptide (TQ). In the light of this information, future investigations on the potential multifunctionality of AGAPGG, AERQ, and RDTQ are warranted. If confirmed to have multiple bioactivities, the three S. glaucum-derived peptides would have a wider range of applications in the future development of therapeutics.
5. Conclusions
In this study, three novel cytotoxic peptides, AGAPGG, AERQ, and RDTQ, were successfully purified and identified from the papain hydrolysate of S. glaucum. The three peptides displayed relatively high cytotoxicity on HeLa cells, but low cytotoxicity on non-cancerous Hek293 cells. Significantly, the cytotoxic activity of AGAPGG, AERQ, and RDTQ was 3.3-, 5.8-, and 5.1-fold stronger than that of the anticancer drug 5-FU. The findings of this study demonstrate that these peptides have remarkable potential for development as anticancer agents in future.
Acknowledgments
We thank Ms. Nina Ann Jin HO of the Institute of Biological Sciences, Faculty of Science, University of Malaya, Malaysia, for SCUBA diving and sampling assistance in the field.
List of electronic supplementary materials
A representative SPE elution prolife of GF3
Peptide content of SPE fractions
Footnotes
Project supported by the Fundamental Research Grant Scheme of the Ministry of Higher Education, Malaysia (FRGS/1/2013/ST04/UTAR/02/1)
Contributors: Yixian QUAH, Nor Ismaliza MOHD ISMAIL, Jillian Lean Sim OOI, and Yang Amri AFFENDI performed the sampling and experimental work. Yixian QUAH, Fazilah ABD MANAN, Lai-Kuan TEH, Fai-Chu WONG, and Tsun-Thai CHAI conceived the ideas, designed the experiments, wrote different parts of the manuscript, and performed the statistical analysis.
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B1700586) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Yixian QUAH, Nor Ismaliza MOHD ISMAIL, Jillian Lean Sim OOI, Yang Amri AFFENDI, Fazilah ABD MANAN, Lai-Kuan TEH, Fai-Chu WONG, and Tsun-Thai CHAI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abdel-Lateff A, Alarif WM, Ayyad SEN, et al. New cytotoxic isoprenoid derivatives from the Red Sea soft coral Sarcophyton glaucum . Nat Prod Res. 2015;29(1):24–30. doi: 10.1080/14786419.2014.952637. [DOI] [PubMed] [Google Scholar]
- 2.Al-Lihaibi SS, Alarif WM, Abdel-Lateff A, et al. Three new cembranoid-type diterpenes from Red Sea soft coral Sarcophyton glaucum: isolation and antiproliferative activity against HepG2 cells. Eur J Med Chem. 2014;81:314–322. doi: 10.1016/j.ejmech.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Chai TT, Law YC, Wong FC, et al. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: a review. Mar Drugs. 2017;15(2):42. doi: 10.3390/md15020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao CH, Li WL, Huang CY, et al. Isoprenoids from the soft coral Sarcophyton glaucum . Mar Drugs. 2017;15(7):202. doi: 10.3390/md15070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LL, Song LY, Li TF, et al. A new antiproliferative and antioxidant peptide isolated from Arca subcrenata . Mar Drugs. 2013;11(6):1800–1814. doi: 10.3390/md11061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YC, Chang HS, Wang CT, et al. Antioxidative activities of hydrolysates from duck egg white using enzymatic hydrolysis. Asian Austral J Anim Sci. 2009;22(11):1587–1593. doi: 10.5713/ajas.2009.90119. [DOI] [Google Scholar]
- 8.Cordeiro , R. , van Ofwegen, L. , Williams , G. World List of Octocorallia. Sarcophyton glaucum (Quoy & Gaimard, 1833) http://wwwmarinespeciesorg/aphiaphp?p=taxdetails&id=209621 [Accessed on Sept 25, 2017] 2010 [Google Scholar]
- 9.Daliri EBM, Oh DH, Lee BH. Bioactive peptides. Foods. 2017;6(5):32. doi: 10.3390/foods6050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lumen BO. Lunasin: a cancer-preventive soy peptide. Nutr Rev. 2005;63(1):16–21. doi: 10.1111/j.1753-4887.2005.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 11.Dennison SR, Whittaker M, Harris F, et al. Anticancer α-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr Protein Pept Sci. 2006;7(6):487–499. doi: 10.2174/138920306779025611. [DOI] [PubMed] [Google Scholar]
- 12.Fabricius KE, Alderslade P. Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science, Townsville, Australia; 2001. p. 264. [Google Scholar]
- 13.Fahmy H, Zjawiony JK, Konoshima T, et al. Potent skin cancer chemopreventing activity of some novel semi-synthetic cembranoids from marine sources. Mar Drugs. 2006;4(2):28–36. doi: 10.3390/md402028. [DOI] [Google Scholar]
- 14.Fan XD, Bai L, Mao XL, et al. Novel peptides with anti-proliferation activity from the Porphyra haitanesis hydrolysate. Process Biochem. 2017;60:97–107. doi: 10.1016/j.procbio.2017.05.018. [DOI] [Google Scholar]
- 15.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2018, Cancer tomorrow: estimated number of incident cases from 2018 to 2040, all cancers, both sexes, all ages. International Agency for Research on Cancer, Lyon, France. http://gco.iarc.fr/tomorrow/graphic-line?type=0&population=900&mode=population&sex=0&cancer=39& age_group=value&apc_male=0&apc_female=0 [Accessed on Dec. 20, 2018].2018. [Google Scholar]
- 16.Gabernet G, Müller AT, Hiss JA, et al. Membranolytic anticancer peptides. Med Chem Commun. 2016;7(12):2232–2245. doi: 10.1039/C6MD00376A. [DOI] [Google Scholar]
- 17.He GQ, Xuan GD, Ruan H, et al. Optimization of angiotensin I-converting enzyme (ACE) inhibition by rice dregs hydrolysates using response surface methodology. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2005;6(6):508–513. doi: 10.1631/jzus.2005.B0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegazy ME, El-Beih AA, Moustafa AY, et al. Cytotoxic cembranoids from the Red Sea soft coral Sarcophyton glaucum . Nat Prod Commun. 2011;6(12):1809–1812. [PubMed] [Google Scholar]
- 19.Hsu KC, Li-Chan ECY, Jao CL. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011;126(2):617–622. doi: 10.1016/j.foodchem.2010.11.066. [DOI] [Google Scholar]
- 20.Huang CY, Sung PJ, Uvarani C, et al. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum . Sci Rep, 5:15624. 2015 doi: 10.1038/srep15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YB, Wang XF, Wang HY, et al. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol Cancer Ther. 2011;10(3):416–426. doi: 10.1158/1535-7163.mct-10-0811. [DOI] [PubMed] [Google Scholar]
- 22.Hung CC, Yang YH, Kuo PF, et al. Protein hydrolysates from tuna cooking juice inhibit cell growth and induce apoptosis of human breast cancer cell line MCF-7. J Funct Foods. 2014;11:563–570. doi: 10.1016/j.jff.2014.08.015. [DOI] [Google Scholar]
- 23.Jumeri , Kim SM. Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava) Food Sci Biotechnol. 2011;20(4):1075–1085. doi: 10.1007/s10068-011-0146-y. [DOI] [Google Scholar]
- 24.Kim EK, Kim YS, Hwang JW, et al. Purification and characterization of a novel anticancer peptide derived from Ruditapes philippinarum . Process Biochem. 2013;48(7):1086–1090. doi: 10.1016/j.procbio.2013.05.004. [DOI] [Google Scholar]
- 25.Li Y, Yu JM. Research progress in structure-activity relationship of bioactive peptides. J Med Food. 2015;18(2):147–156. doi: 10.1089/jmf.2014.0028. [DOI] [PubMed] [Google Scholar]
- 26.Minkiewicz P, Dziuba J, Iwaniak A, et al. BIOPEP database and other programs for processing bioactive peptide sequences. J AOAC Int. 2008;91(4):965–980. [PubMed] [Google Scholar]
- 27.Ngo DH, Vo TS, Ngo DN, et al. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int J Biol Macromol. 2012;51(4):378–383. doi: 10.1016/j.ijbiomac.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen PM, Petersen D, Dambmann C. Improved method for determining food protein degree of hydrolysis. J Food Sci. 2001;66(5):642–646. doi: 10.1111/j.1365-2621.2001.tb04614.x. [DOI] [Google Scholar]
- 29.Nurdiani R, Vasiljevic T, Yeager T, et al. Bioactive peptides with radical scavenging and cancer cell cytotoxic activities derived from Flathead (Platycephalus fuscus) by-products. Eur Food Res Technol. 2017;243(4):627–637. doi: 10.1007/s00217-016-2776-z. [DOI] [Google Scholar]
- 30.Pan X, Zhao YQ, Hu FY, et al. Anticancer activity of a hexapeptide from skate (Raja porosa) cartilage protein hydrolysate in HeLa cells. Mar Drugs. 2016;14(8):153. doi: 10.3390/md14080153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pangestuti R, Kim SK. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar Drugs. 2017;15(3):67. doi: 10.3390/md15030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picot L, Bordenave S, Didelot S, et al. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem. 2006;41(5):1217–1222. doi: 10.1016/j.procbio.2005.11.024. [DOI] [Google Scholar]
- 33.Qian ZJ, Je JY, Kim SK. Antihypertensive effect of angiotensin I converting enzyme-inhibitory peptide from hydrolysates of bigeye tuna dark muscle, Thunnus obesus . J Agric Food Chem. 2007;55(21):8398–8403. doi: 10.1021/jf0710635. [DOI] [PubMed] [Google Scholar]
- 34.Quah Y, Ismail NIM, Ooi JLS, et al. Identification of novel cytotoxic peptide KENPVLSLVNGMF from marine sponge Xestospongia testudinaria, with characterization of stability in human serum. Int J Pept Res Ther. 2017;24(1):189–199. doi: 10.1007/s10989-017-9604-6. [DOI] [Google Scholar]
- 35.Schmidt N, Mishra A, Lai GH, et al. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584(9):1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 36.Shan YP, Huang JF, Tan JJ, et al. The study of single anticancer peptides interacting with HeLa cell membranes by single molecule force spectroscopy. Nanoscale. 2012;4(4):1283–1286. doi: 10.1039/c2nr11541g. [DOI] [PubMed] [Google Scholar]
- 37.Simmons TL, Andrianasolo E, McPhail K, et al. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4(2):333–342. [PubMed] [Google Scholar]
- 38.Song R, Wei RB, Luo HY, et al. Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty) J Funct Foods. 2014;10:104–111. doi: 10.1016/j.jff.2014.06.010. [DOI] [Google Scholar]
- 39.Sutradhar KB, Amin ML. Nanotechnology in cancer drug delivery and selective targeting. Int Sch Res Notices, 2014:939378. 2014 doi: 10.1155/2014/939378. [DOI] [Google Scholar]
- 40.Tada N, Horibe T, Haramoto M, et al. A single replacement of histidine to arginine in EGFR-lytic hybrid peptide demonstrates the improved anticancer activity. Biochem Biophys Res Commun. 2011;407(2):383–388. doi: 10.1016/j.bbrc.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Li L, Chi CF, et al. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013;138(2-3):1713–1719. doi: 10.1016/j.foodchem.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Wang YK, He HL, Wang GF, et al. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar Drugs. 2010;8(2):255–268. doi: 10.3390/md8020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang ZJ, Zhang XW. Inhibitory effects of small molecular peptides from Spirulina (Arthrospira) platensis on cancer cell growth. Food Funct. 2016;7(2):781–788. doi: 10.1039/C5FO01186H. [DOI] [PubMed] [Google Scholar]
- 44.You LJ, Zhao MM, Liu RH, et al. Antioxidant and antiproliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. J Agric Food Chem. 2011;59(14):7948–7953. doi: 10.1021/jf2016368. [DOI] [PubMed] [Google Scholar]
- 45.Zheng LH, Lin XK, Wu N, et al. Targeting cellular apoptotic pathway with peptides from marine organisms. Biochim Biophys Acta. 2013;1836(1):42–48. doi: 10.1016/j.bbcan.2013.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A representative SPE elution prolife of GF3
Peptide content of SPE fractions