Abstract
In this study, we evaluated the effect of the herbicide propyl 4-(2-(4,6-dimethoxypyrimidin-2-yloxy)benzylamino) benzoate (ZJ0273) on barley growth and explored the potential to trigger growth recovery through the application of branched-chain amino acids (BCAAs). Barley plants were foliar-sprayed with various concentrations of ZJ0273 (100, 500, or 1000 mg/L) at the four-leaf stage. Increasing either the herbicide concentration or measurement time after herbicide treatment significantly impaired plant morphological parameters such as plant height and biomass, and affected physiological indexes, i.e. maximal photochemical efficiency (F v/F m), quantum yield of photosystem II (Ф PSII), net photosynthetic rate (P n), and chlorophyll meter value (soil and plant analyzer development (SPAD)). Cellular injury of herbicide-treated plants was also evidenced by increased levels of reactive oxygen species (ROS) and antioxidative enzyme activity. Elevated levels of herbicide significantly reduced the activity of acetolactate synthase (ALS)—a key enzyme in the biosynthesis of BCAAs. In a separate experiment, growth recovery in herbicide-stressed barley plants was studied using various concentrations of BCAAs (10, 50, 100, and 200 mg/L). Increasing BCAA concentration in growth media significantly increased the biomass of herbicide-stressed barley seedlings, but had no significant effect on non-stressed plants. Further, BCAAs (100 mg/L) significantly down-regulated ROS and consequently antioxidant enzyme levels in herbicide-stressed plants. Our results showed that exogenous application of BCAAs could reverse the inhibitory effects of ZJ0273 by restoring protein biosynthesis in barley seedlings.
Keywords: Antioxidant enzyme activity, Barley, Branched-chain amino acid, Photosynthetic system, Reactive oxygen species, ZJ0273
1. Introduction
Barley (Hordeum vulgare L.) is an important cereal crop widely cultivated for food and forage purposes throughout the world. Weeds such as Alopecurus aequalis and Malachium aquaticum are the major issue for barley production systems, and can seriously inhibit the growth, yield, and quality of barley crops (Zhang et al., 2000). For example, barley yields have been reduced by 28.1% in India (Pandey et al., 1998) and 6%–79% in Canada (Watson et al., 2006), due to season-long weed competition. In China, weeds in barley production systems are managed partly by crop rotation with rapeseed. This practice leads to an overlapping of weeds in rapeseed and barley fields. In addition, herbicides such as propyl 4-(2-(4,6-dimethoxypyrimidin-2-yloxy)benzylamino) benzoate (ZJ0273), a broad-spectrum herbicide, are frequently used in oilseed rape production systems in China (Lü et al., 2004; Liu et al., 2008; Yang et al., 2008). Several advantages of this herbicide have been reported such as its high potential for targeting weed plants, low dosage, low mammalian toxicity, broad weeding spectrum and environmental compatibility across Chinese agricultural systems (Chen et al., 2005; Zhang et al., 2009; Jin et al., 2010). Yang et al. (2008) reported an extensive application of ZJ0273 across Chinese rapeseed fields with about 90% eradication efficiency for monocotyledonous and dicotyledonous weeds. This herbicide when applied at the rate of 100 mg/L can effectively reduce M. aquaticum and A. aequalis in oilseed fields (Zhang et al., 2009). In contrast, higher dosages negatively affect the photosynthetic efficiency of oilseed rape (Jin et al., 2010).
Rapeseed, a dicotyledonous crop, has been found to behave differently from rice, a monocotyledonous crop, in response to ZJ0273 application. Li et al. (2009) found that monocot species can absorb higher amounts of ZJ0273 than dicots at any given sampling time. This was supported by Guo et al. (2011), who observed a significant reduction in the volume and number of vacuoles and the mitotic index in ZJ0273-treated barley cells.
Acetolactate synthase (ALS), an enzyme that catalyzes the first step of parallel reactions in the biosynthesis of branched-chain amino acids (BCAAs) in plants (Leyval et al., 2003), has been found to be the main target of ZJ0273. Previous studies suggested that ZJ0273 arrested weed growth by inhibiting ALS activity (Zhou et al., 2007) and thus BCAA synthesis, leading to impaired protein biosynthesis (Liu et al., 2008; Tian et al., 2014). In plants, changes in the activity of enzymes which catalyze biosynthesis of BCAAs can be reflected in the BCAA content of treated plant leaves (Ortéga and Bastide, 1997; Wright et al., 1998). Considering the importance of BCAAs in plant defense mechanisms, we proposed that exogenously applied BCAAs may restore growth of herbicide-stressed plants. To our knowledge, little information is available on the morphological or physiological adaptation of barley seedlings to stress induced by ZJ0273. In this paper, we elucidate the biochemical pathways through which the herbicide ZJ0273 damages barley growth and explore the potential of BCAAs to promote growth recovery in herbicide-stressed seedlings.
2. Materials and methods
2.1. Plant materials and treatments
Seeds of barley (Hordeum vulgare L.) cultivar Zhenongda 3 were obtained from the Department of Agronomy, College of Agriculture and Biotechnology, Zhejiang University, China. Herbicide ZJ0273 was supplied by the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, China. Field trials were conducted at the Zhejiang University farm, Hangzhou, China (30°10′ N, 120°12′ E). Conventional crop management was used throughout the growing period. At the four-leaf stage, barley seedlings were foliar-sprayed once with different concentrations of ZJ0273 (0, 100, 500, or 1000 mg/L). Field trials were conducted to explore the effects of the herbicide on plant growth (plant height, biomass), chlorophyll fluorescence (maximal photochemical efficiency (F v/F m), the quantum yield of photosystem II (Ф PSII), and non-photochemical quenching (NPQ)), and physiological attributes (concentrations of ALS, glutathione transferases (GSTs), BCAAs, and total amino acids (TAAs)) of the seedlings. The experiment was arranged in a completely randomized design (CRD) with three replicates.
A hydroponic experiment was conducted to study growth recovery of ZJ0273-stressed barley seedlings following BCAA application. Mature barley seeds were surface-sterilized with 0.15 g/mL NaClO for 15 min and then washed three times with distilled water. The seeds were arrayed in a germination incubator at a temperature of 20 °C under a 16-h photoperiod (light intensity of 140 μmol/(m2·s)) and high relative humidity (70%‒80%), on a double layer of sterilized filter paper until germination. Seedlings were transplanted into plastic casks containing Hoagland solution, which was continuously aerated with an air pump. Two-week-old plantlets were exposed to hydroponic solution containing the herbicide ZJ0273 (100 mg/L) or no herbicide and with or without BCCAs (0, 10, 50, 100, or 200 mg/L) for 7 d. Then the growth parameters such as plant height and biomass were calculated to evaluate the mitigation effects contributed by BCAAs.
2.2. Determination of morphological and physiological characteristics
In the field trials, the morphological measurements were conducted at 5, 10, and 15 d after treatment (DAT) to estimate the effects of different concentrations of ZJ0273 (0, 100, 500, or 1000 mg/L) on plant growth. Six plants per treatment were harvested for measuring shoot length and fresh and dry weights. Plant samples were then oven-dried at 110 °C for 0.5 h and then at 80 °C until they reached a constant dry weight (Momoh and Zhou, 2001). In the hydroponic testing, morphological characteristics were also measured to observe the alleviatory effects of BCAAs. All measurements were made in triplicate.
The middle region of the topmost fully expanded barley leaves was used for determining ALS activity following the methods of Yang et al. (2000) and Leyval et al. (2003) with some modifications. GST activity was analyzed according to Irzyk and Fuerst (1993) and Hatton et al. (1996). The absorbance of the mixture was monitored at 340 nm for 120 s. An extinction coefficient of 9.6 L/(mmol·cm) was used to quantify conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) and glutathione (GSH).
Fresh leaf samples (0.2 g) were homogenized in 5 mL potassium phosphate buffer (50 mmol/L, pH 7.8) and then centrifuged at 10 000g for 20 min at 4 °C. The supernatant was used for analyzing antioxidant enzyme activity, reactive oxygen species (ROS), and malondialdehyde (MDA) content. The method of Zhou et al. (1997) was used to determine superoxide dismutase (SOD) activity following inhibition of photochemical reduction by nitro blue tetrazolium (NBT). Peroxidase (POD) activity was determined from variation in guaiacol absorbance of the mixture assay at 470 nm (Zhou and Leul, 1998). Catalase (CAT) activity was measured at 240 nm according to Cakmak et al. (1993). Ascorbate peroxide (APX) activity was determined according to Nakano and Asada (1981) with some modifications. Glutathione reductase (GR) activity was determined according to Jiang and Zhang (2002).
MDA content was measured using a reaction solution (0.5% thiobarbituric acid (TBA) made in 5% trichloroacetic acid (TCA)) (Zhang et al., 2008). The extract mixture in buffer solution was heated at 95 °C for 15 min and then immediately cooled on ice. The samples were centrifuged at 4800g for 10 min and the absorbance of the supernatant was measured at 532 nm. Production of ROS such as H2O2 and O2 − was determined according to Al-Aghabary et al. (2004) and Jiang and Zhang (2001), respectively.
The GSH and ascorbic acid (ASA) contents were measured according to Anderson (1985) and Law et al. (1983), respectively. Fresh leaf samples (0.2 g) were homogenized with 0.05 g/mL TCA (5 mL) in an ice bath and centrifuged at 4000g for 10 min. The resultant supernatant was used to measure GSH and ASA contents.
2.3. Photosynthesis and chlorophyll fluorescence
The middle region of the topmost fully expanded leaves was selected for estimating leaf chlorophyll fluorescence using a portable fluorometer (Mini-PAM, Walz, Effeltrich, Germany). The leaves were pre-conditioned in the dark for 30 min, and then minimal fluorescence (F 0) with all photosystem II (PSII) reaction centers opened was determined under weak light (0.04 μmol/(m2∙s)). Maximal fluorescence (F m) with all PSII reaction centers closed was measured with a 0.8-s saturating pulse at 5000 μmol/(m2∙s) in dark-adapted leaves. An actinic light (330 μmol/(m2∙s)) was applied until these parameters were at steady state with saturating flashes. From each of these, the value of F m', the maximum fluorescence in the light, was detected. The steady-state value of fluorescence immediately before a flash of light was termed F t. (F m−F 0)/F m or F v/F m was considered as a measure of the maximum quantum yield of PSII in the dark. NPQ was calculated as (F m−F m')/F m'. The Ф PSII was defined as (F m'−F t)/F m' (Maxwell and Johnson, 2000). Chlorophyll meter values (soil and plant analyzer development (SPAD) readings) were measured using a chlorophyll meter (Minolta SPAD-502, Japan). The net photosynthetic rate (P n) of leaves was measured using a portable photosynthesis system (Model Li-6400, USA). Each measurement was replicated three times.
2.4. Determination of endogenous free amino acid content
Fresh leaf samples were collected randomly to measure the concentrations of endogenous free amino acids including three BCCAs (valine (Val), leucine (Leu), and isoleucine (ILe)) and a total of 17 amino acids (TAAs) at three different time intervals, i.e. 5, 10, and 15 DAT. The samples were fixed in an oven at 110 °C for 30 min and then at 80 °C until constant weight. Dried samples were ground into powder and sieved through 60-mesh. Free amino acid content was determined according to the method of Zhu and Zhao (1994). Powder was rubbed in 1 mL of 80% alcohol until volatilization and then homogenized with 1 mL 4% sulfosalicylic acid before extracting using an ultrasonicator for 20 min. The supernatant was collected after centrifugation at 12 000g for 15 min and mixed with 1 mL 4% sulfosalicylic acid in deposition, and the extraction procedure was repeated. The two supernatants were merged and sterilized with a 0.25-μm filter. Free amino acid content was calculated by an automatic amino acid analyzer (Hitachi, L-8900, Japan).
2.5. Statistical analysis
In these experiments, three replicates were used for each measurement. Data were analyzed using the Statistical Analysis System (SAS). Significant effects were determined by analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (LSD) test to identify homogenous groups within the means at P≤0.05 level.
3. Results
3.1. Changes in free amino acid content
A significant increase in BCAAs such as Val, Leu, and ILe was recorded in response to increasing the ZJ0273 application rate or the treatment duration in this field trial (Table 1). BCAAs in barley leaves remained unaffected by the lower herbicide (100 mg/L) level at 5 DAT, but the treated plants experienced a significant increase in BCAAs at 10 and 15 DAT. No significant change in BCAAs was observed when the ZJ0273 treatment rate was increased beyond 100 mg/L at 10 DAT. BCAAs were undetectable in plant tissues under higher ZJ0273 treatments (500 and 1000 mg/L), when treatment duration was prolonged to 15 d.
Table 1.
Effects of different concentrations of ZJ0273 on the concentrations of free amino acids and BCAAs/TAAs at the second-leaf stage of barley seedlings
| DAT | Concentration of ZJ0273 (mg/L) | Concentration of free amino acids (mg/100 mg DW) |
BCAAs/TAAs (%) | |||
| Val | Leu | Ile | TAAs | |||
| 5 | 0 | 1.816b | 1.977b | 2.023b | 29.450d | 19.75a |
| 100 | 1.996ab | 2.231b | 2.244b | 32.921c | 19.66a | |
| 500 | 1.936ab | 2.256b | 2.318ab | 37.957b | 17.15c | |
| 1000 | 2.139a | 2.908a | 2.666a | 43.070a | 17.91b | |
| 10 | 0 | 1.653b | 2.239b | 1.733b | 31.065c | 18.11b |
| 100 | 2.167a | 2.660a | 2.342a | 36.059b | 19.88a | |
| 500 | 2.265a | 2.635a | 2.561a | 40.209b | 18.55b | |
| 1000 | 2.529a | 2.842a | 2.838a | 46.487a | 17.66c | |
| 15 | 0 | 1.865b | 2.133b | 2.231a | 32.403b | 19.23a |
| 100 | 2.336a | 4.376a | 2.312a | 47.293a | 19.08a | |
| 500 | ||||||
| 1000 | ||||||
Within a column, means at the same DAT followed by the same letters are not significantly different by the LSD test at P≤0.05 under completely randomized design. Data were analyzed separately at each measurement time. DAT: days after treatment; Val: valine; Leu: leucine; Ile: isoleucine; TAAs: total amino acids; BCAAs: branched-chain amino acids
TAA content in plant tissues also increased significantly in response to increasing ZJ0273 concentrations. Higher (500 and 1000 mg/L) ZJ0273 concentrations significantly increased TAAs and decreased BCAAs/TAAs compared with untreated controls at 5 and 10 DAT. Under the prolonged treatment duration (15 DAT) of ZJ0273 (500 and 1000 mg/L), TAAs were not detectable due to the death of the plants, suggesting that longtime treatment duration with higher dosages of ZJ0273 (500 and 1000 mg/L) was toxic for barley seedlings.
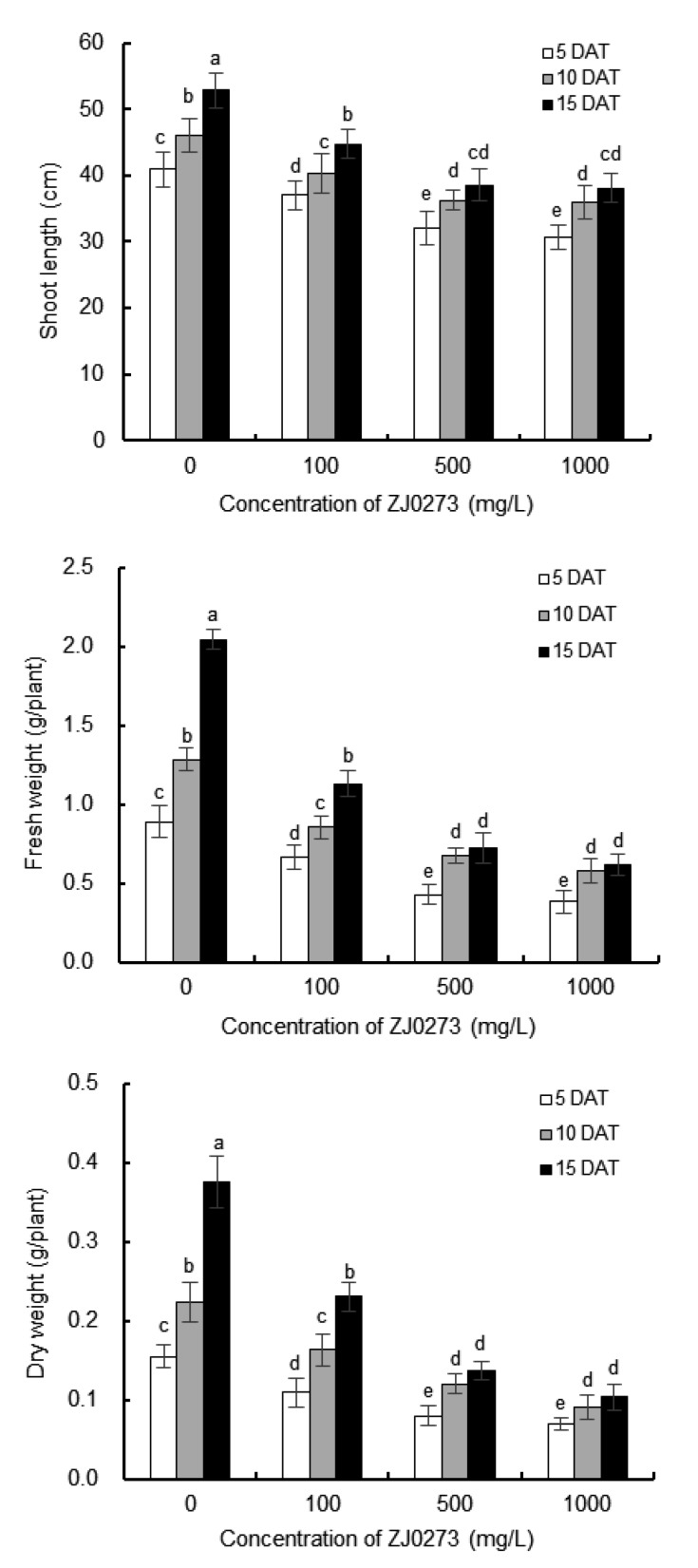
3.2. Plant growth response to different concentrations of ZJ0273
Fig. 1 shows the plant growth in response to the different concentrations of ZJ0273 (0–1000 mg/L) at the fourth-leaf stage of barley. Treatments with ZJ0273 (100–1000 mg/L) significantly inhibited plant growth, as reflected by the reductions in plant height, fresh weight, and dry weight of barley seedlings at 5 DAT. Similar trends were observed at 10 and 15 DAT, compared with controls. Plant development in the controls was obviously better than that in all treated plantlets. The growth of barley was obviously suppressed under the stress of 100 mg/L ZJ0273 compared with the control. With the successive increases of ZJ0273 concentrations from 100 to 1000 mg/L, the shoot length and biomass of barley dramatically declined. However, the barley plantlets did not show a significant difference between 500 and 1000 mg/L ZJ0273 for shoot length or biomass at all DAT (Fig. 1). When plants were treated with 1000 mg/L of ZJ0273, 24.9%, 22.2%, and 27.9% reductions in shoot length were observed at 5, 10, and 15 DAT, respectively, compared with controls. Prolonging the DAT from 5 to 15 led to growth recovery under the lower dosage of ZJ0273 (100 mg/L), as reflected by the shoot length, fresh weight, and dry weight. However, these trends were not observed with the application of higher dosages of ZJ0273 (500 and 1000 mg/L). Thus, 100 mg/L ZJ0273 was relatively safe for the barley seedlings.
Fig. 1.
Effects of different concentrations of ZJ0273 (0–1000 mg/L) on plant height and biomass of barley seedlings
Measurements were made at 5, 10, and 15 d after treatment (DAT). Values are expressed as mean±standard error (SE), n=3. Means followed by the same letters did not differ significantly by the LSD test at P≤0.05
3.3. Chlorophyll fluorescence and photosynthesis
Increasing ZJ0237 rates of application significantly reduced maximal photochemical efficiency (F v/F m) of barley leaves at 1 DAT, and this reduction grew in the subsequent measurements (1–9 DAT; Table 2). The maximum reduction in F v/F m was observed in response to 1000 mg/L ZJ0273, which caused a 14.1% reduction (compared with controls) in F v/F m at 9 DAT. Changes in leaf Ф PSII in response to ZJ0237 application followed a similar pattern to that of F v/F m. Although 100 mg/L ZJ0273 had no significant effect on Ф PSII at 1 DAT, a steep reduction in leaf Ф PSII was recorded under 1000 mg/L ZJ0273 with increasing application period. For example, Ф PSII was reduced by 13.6% at 1 DAT and by 46.4% at 9 DAT (compared with controls). In contrast to F v/F m, a significant increase in NPQ of barley leaves was observed in response to increasing the ZJ0237 application rate or the time after treatment, although the lower herbicide concentration (100 mg/L) had no significant effect on NPQ at 1 DAT. The 1000 mg/L ZJ0273 treatment increased NPQ by 72.8% at 1 DAT and by 124.4% at 9 DAT, compared with controls. No significant differences in the NPQ of the plants were detected between treatments of 500 or 1000 mg/L of ZJ0237 when measured at 5–9 DAT (Table 2).
Table 2.
Effects of different concentrations of ZJ0273 on F v/F m, Ф PSII, and NPQ in PSII at the fourth-leaf stage of barley seedlings
| DAT | Concentration of ZJ0273 (mg/L) | F v/F m | Ф PSII | NPQ |
| 1 | 0 | 0.816a | 0.550a | 0.180b |
| 100 | 0.808b | 0.534a | 0.195b | |
| 500 | 0.805bc | 0.478b | 0.316a | |
| 1000 | 0.800c | 0.475b | 0.311a | |
| 3 | 0 | 0.813a | 0.511a | 0.185d |
| 100 | 0.794b | 0.445b | 0.283c | |
| 500 | 0.788b | 0.417c | 0.397b | |
| 1000 | 0.785b | 0.409c | 0.433a | |
| 5 | 0 | 0.815a | 0.517a | 0.181c |
| 100 | 0.799b | 0.421b | 0.336b | |
| 500 | 0.762c | 0.364c | 0.411a | |
| 1000 | 0.758c | 0.361c | 0.435a | |
| 7 | 0 | 0.816a | 0.539a | 0.209c |
| 100 | 0.780b | 0.382b | 0.400b | |
| 500 | 0.750c | 0.352c | 0.443a | |
| 1000 | 0.725d | 0.338c | 0.440a | |
| 9 | 0 | 0.817a | 0.552a | 0.205c |
| 100 | 0.755b | 0.428b | 0.427b | |
| 500 | 0.729c | 0.326c | 0.448ab | |
| 1000 | 0.702c | 0.296d | 0.460a |
Within a column, means at the same DAT followed by the same letters are not significantly different by the LSD test at P≤0.05 under completely randomized design. Data were analyzed separately at each measurement time. DAT: days after treatment; F v/F m: maximal photochemical efficiency; Ф PSII: quantum yield of PSII; NPQ: non-photochemical quenching
SPAD values of barley leaves declined significantly with each successive increase in the ZJ0273 application rate (Table 3). In addition, reductions in leaf SPAD were observed as the time after treatment was increased. For example, addition of 1000 mg/L ZJ0273 caused reductions in leaf SPAD of 57.7% at 20 DAT and 65.9% at 30 DAT, compared with controls. Damage to leaf greenness resulted in reductions in leaf P n under increasing ZJ0273 concentrations and increasing measurement time after treatment. The maximum reduction in leaf P n was observed under 1000 mg/L ZJ0273, causing a 75.6% reduction compared with the control at 30 DAT (Table 3).
Table 3.
Effects of different concentrations of ZJ0273 on the SPAD value and P n at the fourth-leaf stage of barley seedlings
| DAT | Concentration of ZJ0273 (mg/L) | SPAD | P n |
| 20 | 0 | 36.37±0.96a | 24.11±1.32a |
| 100 | 22.37±1.72b | 16.11±1.98b | |
| 500 | 18.07±1.16c | 12.54±0.32c | |
| 1000 | 15.37±0.71d | 6.88±0.92d | |
| 30 | 0 | 36.90±1.10a | 24.54±1.17a |
| 100 | 19.90±0.26b | 12.69±0.34b | |
| 500 | 15.70±1.00c | 9.49±0.77c | |
| 1000 | 12.57±1.39d | 6.00±0.38d |
Within a column, means at the same DAT followed by the same letters are not significantly different by the LSD test at P≤0.05 under completely randomized design. Data were analyzed separately at each measurement time. Values are expressed as mean±SE (n=3). DAT: days after treatment; SPAD: chlorophyll meter value (soil and plant analyzer development); P n: net photosynthetic rate
3.4. Endogenous enzyme activity
No significant effect of the lower ZJ0273 concentration (100 mg/L) was observed on ALS activity of barley leaves at 5 or 10 DAT (Table 4), although higher application rates significantly reduced ALS activity. ALS activity was significantly reduced in response to all ZJ0273 application rates when measured at 15 and 20 DAT. With successive increases in ZJ0273 concentrations from 100 to 1000 mg/L, ALS activity dramatically decreased. When plants were treated with 1000 mg/L of ZJ0273, the ALS activity of barley leaves was reduced by 28.3% at 5 DAT, 36.3% at 10 DAT, 50.2% at 15 DAT, and 57.7% at 20 DAT compared with controls.
Table 4.
Effects of different concentrations of ZJ0273 on ALS and GST activity at the fourth-leaf stage of barley seedlings
| DAT | ZJ0273 (mg/L) | ALS activity (OD530/(g FW·min)) | GST activity (nmol/(min·mg protein)) |
| 5 | 0 | 1.87±0.16a | 5.85±0.31c |
| 100 | 1.83±0.36a | 7.76±0.76b | |
| 500 | 1.62±0.41b | 11.30±0.81a | |
| 1000 | 1.34±0.35c | 11.83±1.48a | |
| 10 | 0 | 1.90±0.45a | 6.61±0.53b |
| 100 | 1.81±0.42a | 8.05±0.66b | |
| 500 | 1.59±0.24b | 12.84±1.07a | |
| 1000 | 1.21±0.52c | 14.04±2.14a | |
| 15 | 0 | 2.07±0.21a | 6.59±0.50d |
| 100 | 1.69±0.19b | 10.41±0.61c | |
| 500 | 1.52±0.38c | 13.86±1.12b | |
| 1000 | 1.03±0.41d | 16.05±0.86a | |
| 20 | 0 | 2.15±0.22a | 6.62±0.46d |
| 100 | 1.65±0.25b | 15.40±1.04c | |
| 500 | 1.47±0.19c | 17.93±0.94b | |
| 1000 | 0.91±0.17d | 20.90±1.61a |
Within a column, means at the same DAT followed by the same letters are not significantly different by the LSD test at P≤0.05 under completely randomized design. Data were analyzed separately at each measurement time. Values are expressed as mean±SE (n=3). DAT: days after treatment; ALS: acetolactate synthase; GST: glutathione transferase; OD530: optical density at 530 nm; FW: fresh weight
GST activity of barley leaves increased in response to increasing herbicide application rates, although no significant change in GST activity was observed by increasing ZJ0273 concentrations from 500 to 1000 mg/L at 5 or 10 DAT (Table 4). Maximum GST activity (20.90 nmol/(min·mg protein)) was observed under 1000 mg/L ZJ0273 at 20 DAT, when it was 215.7% greater than that of the control leaves.
3.5. Growth of ZJ0273-stressed seedlings in response to exogenously applied branched-chain amino acids
A significant reduction in shoot length, fresh and dry weights was recorded in barley seedlings in response to 100 mg/L of ZJ0273 (Table 5). Plants treated with 100 mg/L ZJ0273 alone experienced maximum damage in this hydroponic study, showing reductions of 31.3% in shoot length, 30.2% in fresh weight, and 25.0% in dry weight, compared with controls. Exogenously applied BCAAs (10–200 mg/L) in the nutrient solution significantly increased the growth of barley seedlings in terms of shoot length and fresh and dry weights. Application of BCAAs to herbicide-free media also promoted growth of barley seedlings, but the effect on shoot length or dry weight was not significant compared with the control (non-BCAA, non-ZJ0237 treated). Maximum growth (shoot length, fresh and dry weights) of herbicide-stressed seedlings was observed with 200 mg/L of BCAAs. Plants treated with 200 mg/L BCAAs alone showed no significant change in growth. BCAAs significantly promoted barley growth when applied to ZJ0273 (100 mg/L)-stressed plants, causing increases of 37.0% in shoot length, 38.1% in fresh weight, and 33.9% in dry weight, compared with the 100 mg/L ZJ0273 treatment alone. The fresh and dry weights of the plants remained unaffected when treated with 100 mg/L ZJ0273+200 mg/L BCAAs. No significant changes occurred in the shoot growth of stressed seedlings with 100 or 200 mg/L of BCAAs, suggesting that 100 mg/L of BCAAs could be the best concentration for protecting barley seedlings from herbicide stress. Thus, we selected this concentration for the subsequent physiology experiments.
Table 5.
Effects of exogenous BCAAs on the shoot length, fresh weight, and dry weight of barley seedlings treated with 100 mg/L ZJ0273
| Concentration (mg/L) | Shoot length (cm) | Fresh weight (g/10 plants) | Dry weight (g/10 plants) |
| Z0 | 19.40a | 2.186bcd | 0.220abc |
| Z0+aa10 | 19.46a | 2.223bc | 0.223ab |
| Z0+aa50 | 19.49a | 2.338ab | 0.230a |
| Z0+aa100 | 19.51a | 2.398a | 0.239a |
| Z0+aa200 | 19.77a | 2.469a | 0.236a |
| Z100 | 13.32e | 1.526f | 0.165e |
| Z100+aa10 | 14.78d | 1.715e | 0.191d |
| Z100+aa50 | 16.03c | 1.814e | 0.200cd |
| Z100+aa100 | 18.08b | 2.050d | 0.210bcd |
| Z100+aa200 | 18.25b | 2.108cd | 0.221abc |
Within a column, means followed by the same letters are not significantly different by the LSD test at P≤0.05 under completely randomized design. Z0: control; Z100: 100 mg/L ZJ0273; aa10: 10 mg/L BCAAs (valine, leucine, isoleucine); aa50: 50 mg/L BCAAs; aa100: 100 mg/L BCAAS; aa200: 200 mg/L BCAAs
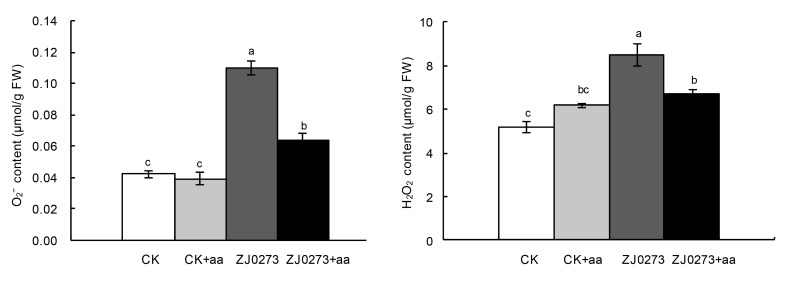
ZJ0273 treatment (100 mg/L) increased the generation of ROS such as O2 − and H2O2 in barley leaf tissues (Fig. 2). In contrast, addition of 100 mg/L BCAAs to the solution significantly decreased ROS generation in herbicide-treated seedlings. BCAA application caused reductions of 36.4% in O2 − and 24.4% in H2O2 content of herbicide-stressed plants, compared with ZJ0273 treatment alone. BCAAs applied to herbicide-free media had no significant effect on leaf O2 − or H2O2 content.
Fig. 2.
Effects of exogenous BCAAs (100 mg/L) on ROS (O2 − or H2O2) concentrations in barley seedlings treated with 100 mg/L ZJ0273
Values are expressed as mean±SE (n=3). Means followed by the same letters did not differ significantly by the LSD test at P≤0.05. CK: control, no ZJ0273; aa: BCAAs; FW: fresh weight
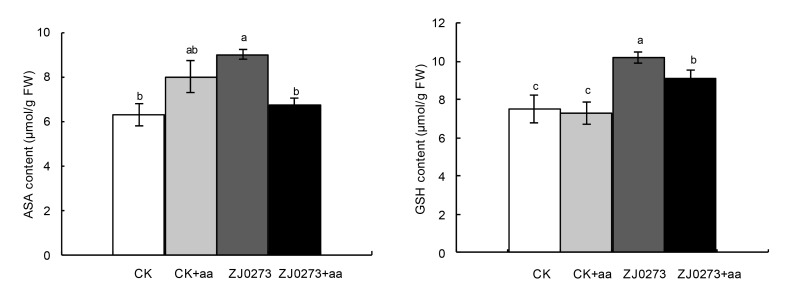
ASA and GSH content in leaf tissues increased significantly in response to 100 mg/L ZJ0273 and decreased when BCAAs were added to the growth media. BCAAs caused reductions of 20.2% in ASA content and 10.1% in GSH content of the leaves of herbicide-treated seedlings, compared with ZJ0273 treatment alone. The effect of BCAAs on herbicide-free seedlings was non-significant (Fig. 3).
Fig. 3.
Effects of exogenous BCAAs (100 mg/L) on ascorbic acid (ASA) and glutathione (GSH) concentrations in barley seedlings treated with 100 mg/L ZJ0273
Values are expressed as mean±SE (n=3). Means followed by the same letters did not significantly differ by the LSD test at P≤0.05. CK: control, no ZJ0273; aa: BCAAs; FW: fresh weight
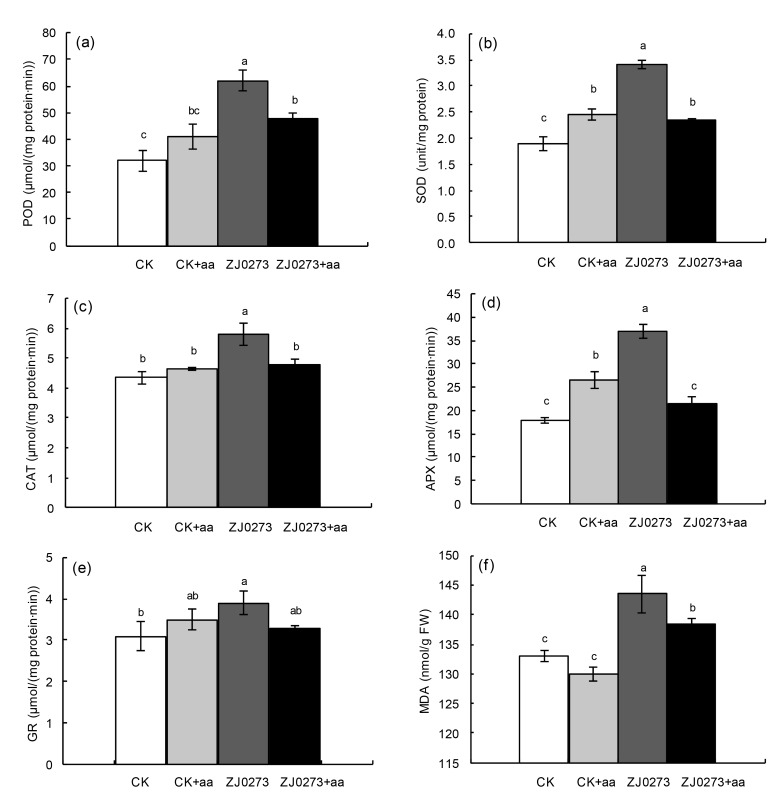
The activity of various antioxidant enzymes (POD, SOD, CAT, APX, and GR) and leaf peroxidation (MDA) increased significantly in response to ZJ0273 treatment (Fig. 4). BCAAs applied exogenously to herbicide-stressed seedlings, significantly reduced antioxidant enzyme activity and MDA content. For example, BCAAs applied to herbicide-stressed plants decreased the activity of POD by 19.4%, SOD by 35.3%, CAT by 17.2%, and APX by 48.6%, compared with ZJ0273 treatment alone, although GR activity remained unaffected. No significant effect of BCAA application was observed on antioxidant enzymes or the MDA content of barley seedlings in herbicide-free media, except for SOD and APX activity, which increased significantly under BCAA treatment under both herbicide-stressed and herbicide-free conditions.
Fig. 4.
Effects of exogenous BCAAs (100 mg/L) on biochemical changes in barley seedlings treated with 100 mg/L ZJ0273
(a) Peroxidase (POD) activity; (b) Superoxide dismutase (SOD) activity; (c) Catalase (CAT) activity; (d) Ascorbate peroxide (APX) activity; (e) Glutathione reductase (GR) activity; (f) Malondialdehyde (MDA) content. Values are expressed as mean±SE (n=3). Means followed by the same letters did not significantly differ by the LSD test at P≤0.05. CK: control, no ZJ0273; aa: BCAAs; FW: fresh weight
4. Discussion
The first objective of these experiments was to identify the mechanism by which herbicide ZJ0273 inhibits barley growth. A significant reduction in SPAD and chlorophyll fluorescence of herbicide-stressed leaves indicated that ZJ0273 can impair chlorophyll structure and function. Ralph et al. (2005) indicated that inhibition curves of chlorophyll fluorescence parameters varied with different herbicide concentrations. Damage to photosynthetic machinery leads to an overall inhibition of photosynthesis and biomass production (Anderson, 1985; Cobb and Kirkwood, 2000; Abbaspoor et al., 2006; Ali et al., 2018).
Various biochemical pathways for stress-induced P n reduction have been proposed. The interrupted biosynthesis of proteins associated with the carbon assimilation process has been suggested by Halliwell and Gutteridge (1999) and Romero-Puertas et al. (2004). Similarly, Orcaray et al. (2010) indicated that glyphosate can inhibit biosynthesis of aromatic amino acids by blocking the shikimate pathway and arresting the photosynthetic process in leaf tissues. Herbicide-induced chlorophyll impairment in this study could have been associated with an increased production of ROS and lipid membrane damage (MDA production), which are the major causes of cellular injury in plants. In contrast to a significant recovery in leaf P n at 28 DAT in 100 and 500 mg/L ZJ0273-treated Brassica plants (Jin et al., 2010), no photosynthesis recovery in barley seedlings suggested a relatively high herbicide sensitivity for monocotyledons. Although stressed barley plants up-regulated defense-related primary antioxidants (POD, SOD, CAT, APX, and GR) and secondary antioxidants (ASA and GSH), no significant growth recovery was observed in this study. This suggests that ZJ0273 can cause permanent cellular damage in barley seedlings and the plants are unable to mitigate this damage through internal antioxidative defense mechanisms. Up-regulation of antioxidant enzymes has also been linked with increased cellular injury to ZJ0273-treated Brassica plants (Law et al., 1983; Dixon et al., 1998; Frankart et al., 2003; Jin et al., 2010). Studies of the key enzymes in the biosynthesis of natural products have been important in revealing the mechanism pathways (Yang et al., 2012; Liang et al., 2013; Xia et al., 2015, 2016; Zhang et al., 2017).
Herbicide-induced growth suppression in barley seedlings in this study was associated with inhibited activity of ALS, an enzyme associated with BCAA production. Previous studies indicated that ALS inhibitors can block the biosynthesis of BCAAs and cell division, and eventually cause plant death (Ray, 1984; Shaner and Singh, 1993). Chen et al. (2005) suggested that ZJ0273 can inhibit ALS activity by altering the biosynthesis of amino acids. Similarly, using near-infrared spectroscopy (NIRS) techniques, Tian et al. (2014) confirmed a gradual reduction in free amino acids (BCAAs and TAAs) and ALS activity in oilseed rape leaves in response to increasing ZJ0273 concentrations. Growth inhibition in ALS inhibitor-treated plants has been linked with a dramatic decrease in specific BCAAs (Rost and Reynolds, 1985; Hofgen et al., 1995). In contrast, a significant increase in BCAA content in this study under herbicide application could be the result of protein transformation to satisfy new protein requirements (Royuela et al., 2000; Orcaray et al., 2010). Similarly, Royuela et al. (2000) and Liu et al. (2008) suggested an increase in BCAA content in response to herbicide application as a result of degradation of some proteins to maintain the balance of new protein synthesis. A significant increase in BCAAs/TAAs of ZJ0273-stressed barley leaves in this study supports the suggestion that amino acid composition and thus protein biosynthesis had been altered.
The second objective of this study was to examine the growth recovery of herbicide-stressed barley seedlings following BCAA application. A significant growth recovery in ZJ0273-stressed barley was achieved in response to BCAA application. No significant effect of BCAAs on unstressed plants suggested that the primary role of these amino acids was to restore growth of stressed plants without affecting normal plant growth patterns. Exogenously applied amino acids had already been found to be effective in alleviating the negative effects of ALS inhibitors in pea (Rost and Reynolds, 1985) and corn (Shaner and Reider, 1986). Therefore, the herbicide ZJ0273 may block the biosynthetic pathway of BCAAs by inhibiting ALS, and exogenous application of BCAAs may compensate for the shortage of BCAAs induced by the herbicide (Tang et al., 2006; Xia et al., 2006).
5. Conclusions
In this study, we confirmed that tolerance to herbicide increased with the growth of barley. The lower dosage (100 mg/L) of ZJ0273 was safer for barley than the higher dosages (500 and 1000 mg/L). The inhibitory effects of herbicide ZJ0273 on the photosystem and enzymes might be related to the metabolic mechanism of this herbicide. Based on our observations, the lower dosage of ZJ0273 (100 mg/L) could be recommended for weed eradication purposes in barley fields. This study also explored the prospects of using BCAAs for improving the growth of barley under herbicide stress.
Footnotes
Project supported by the Zhejiang Science and Technology Department (Nos. LGN18C130007 and 2016C02050-8), the Jiangsu Collaborative Innovation Center for Modern Crop Production, and the Science Foundation of Zhejiang Sci-Tech University (No. 14042216-Y), China
Contributors: Ling XU, Ullah NAJEEB, and Wei-jun ZHOU designed and conceived the study. Ling XU, Jian-yao SHOU, and Xiang GUO performed the experiments. Ling XU, Jian-yao SHOU, Rafaqat Ali GILL, and Ullah NAJEEB analyzed the data and wrote the manuscript. All authors revised and approved the paper.
Compliance with ethics guidelines: Ling XU, Jian-yao SHOU, Rafaqat Ali GILL, Xiang GUO, Ullah NAJEEB, and Wei-jun ZHOU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abbaspoor M, Teicher HB, Streibig JC. The effect of root-absorbed PSII inhibitors on Kautsky curve parameters in sugar beet. Weed Res. 2006;46(3):226–235. doi: 10.1111/j.1365-3180.2006.00498.x. [DOI] [Google Scholar]
- 2.Al-Aghabary K, Zhu ZJ, Shi QH. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr. 2004;27(12):2101–2115. doi: 10.1081/PLN-200034641. [DOI] [Google Scholar]
- 3.Ali E, Hussain N, Shamsi IH, et al. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(2):130–146. doi: 10.1631/jzus.B1700191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Meth Enzymol. 1985;113:548–570. doi: 10.1016/S0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 5.Cakmak I, Strbac D, Marschner H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J Exp Bot. 1993;44(1):127–132. doi: 10.1093/jxb/44.1.127. [DOI] [Google Scholar]
- 6.Chen J, Yuan J, Liu JD, et al. Mechanism of action of the novel herbicide ZJ0273. Acta Phytophy Sin. 2005;32(1):48–52. doi: 10.3321/j.issn:0577-7518.2005.01.010. (in Chinese) [DOI] [Google Scholar]
- 7.Cobb AH, Kirkwood RC. Herbicides and Their Mechanisms of Action. Sheffield Academic Press, Sheffield, England; 2000. pp. 73–82. [Google Scholar]
- 8.Dixon DP, Cummins I, Cole DJ, et al. Glutathione-mediated detoxification systems in plants. Curr Opin Plant Biol. 1998;1(3):258–266. doi: 10.1016/S1369-5266(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 9.Frankart C, Eullaffroy P, Vernet G. Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor . Environ Exp Bot. 2003;49(2):159–168. doi: 10.1016/S0098-8472(02)00067-9. [DOI] [Google Scholar]
- 10.Guo X, Zhang F, Jin ZL, et al. Physiological effect and cytological characterization regarding susceptible response of new herbicide ZJ0273 in barley. Sci Agric Sin. 2011;44(18):3750–3758. doi: 10.3864/j.issn.0578-1752.2011.18.006. (in Chinese) [DOI] [Google Scholar]
- 11.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, 3rd Ed. Oxford University Press, Oxford; 1999. [Google Scholar]
- 12.Hatton PJ, Dixon D, Cole DJ, et al. Glutathione transferase activities and herbicide selectivity in maize and associated weed species. Pestic Manage Sci. 1996;46(3):267–275. doi: 10.1002/(SICI)1096-9063(199603)46:3<267::AID-PS347>3.0.CO;2-N. [DOI] [Google Scholar]
- 13.Hofgen R, Streber WR, Pohlenz HD. Antisense gene expression as a tool for evaluating molecular herbicide targets. Pestic Sci. 1995;43(2):175–177. doi: 10.1002/ps.2780430216. [DOI] [Google Scholar]
- 14.Irzyk GP, Fuerst EP. Purification and characterization of a glutathione S-transferase from benoxacor-treated maize (Zea mays) Plant Physiol. 1993;102(3):803–810. doi: 10.1104/pp.102.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang MY, Zhang JH. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001;42(11):1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- 16.Jiang MY, Zhang JH. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot. 2002;53(379):2401–2410. doi: 10.1093/jxb/erf090. [DOI] [PubMed] [Google Scholar]
- 17.Jin ZL, Zhang F, Ahmed ZI, et al. Differential morphological and physiological responses of two oilseed Brassica species to a new herbicide ZJ0273 used in rapeseed fields. Pestic Biochem Physiol. 2010;98(1):1–8. doi: 10.1016/j.pestbp.2010.04.002. [DOI] [Google Scholar]
- 18.Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem J. 1983;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyval D, Uy D, Delaunay S, et al. Characterisation of the enzyme activities involved in the valine biosynthetic pathway in a valine-producing strain of Corynebacterium glutamicum . J Biotechnol. 2003;104(1-3):241–252. doi: 10.1016/S0168-1656(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Han AL, Zhang YF, et al. Uptake, translocation, and distribution of root-applied [C ring-U-14C]-ZJ0273 in plants of oilseed rape and rice. J Nucl Agric Sci. 2009;23(4):676–680. (in Chinese) [Google Scholar]
- 21.Liang ZS, Ma YN, Xu T, et al. Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza Bunge hairy roots. PLoS ONE. 2013;8(9):e72806. doi: 10.1371/journal.pone.0072806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Zhang F, Jin ZL, et al. Determination of acetolactate synthase activity and protein content of oilseed rape (Brassica napus L.) leaves using visible/near-infrared spectroscopy. Anal Chim Acta. 2008;629(1-2):56–65. doi: 10.1016/j.aca.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Lü L, Chen J, Wu J, et al. 2004
- 24.Maxwell K, Johnson GN. Chlorophyll fluorescence–a practical guide. J Exp Bot. 2000;51(345):659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 25.Momoh EJJ, Zhou W. Growth and yield responses to plant density and stage of transplanting in winter oilseed rape (Brassica napus L.) J Agron Crop Sci. 2001;186(4):253–259. doi: 10.1046/j.1439-037x.2001.00476.x. [DOI] [Google Scholar]
- 26.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- 27.Orcaray L, Igal M, Marino D, et al. The possible role of quinate in the mode of action of glyphosate and acetolactate synthase inhibitors. Pest Manage Sci. 2010;66(3):262–269. doi: 10.1002/ps.1868. [DOI] [PubMed] [Google Scholar]
- 28.Ortéga F, Bastide J. Inhibition of acetolactate synthase isozyme II from Escherichia coli by a new azido-photoaffinity sulfonylurea. Bioorg Chem. 1997;25(4):261–274. doi: 10.1006/bioo.1997.1071. [DOI] [Google Scholar]
- 29.Pandey AK, Prasad K, Singh P, et al. Comparative yield loss assessment and crop-weed association in major winter crops of mid hills of N-W Himalayas. Indian J Weed Sci. 1998;30(1-2):54–57. [Google Scholar]
- 30.Ralph PJ, Macinnis-Ng CMO, Frankart C. Fluorescence imaging application: effect of leaf age on seagrass photokinetics. Aquat Bot. 2005;81(1):69–84. doi: 10.1016/j.aquabot.2004.11.003. [DOI] [Google Scholar]
- 31.Ray TB. Site of action of chlorsulfuron: inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984;75(3):827–831. doi: 10.1104/pp.75.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Puertas MC, McCarthy I, Gómez M, et al. Reactive oxygen species-mediated enzymatic systems involved in the oxidative action of 2,4-dichlorophenoxyacetic acid. Plant, Cell Environ. 2004;27(9):1135–1148. doi: 10.1111/j.1365-3040.2004.01219.x. [DOI] [Google Scholar]
- 33.Rost TL, Reynolds T. Reversal of chlorsulfuron-induced inhibition of mitotic entry by isoleucine and valine. Plant Physiol. 1985;77(2):481–482. doi: 10.1104/pp.77.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Royuela M, Gonzalez A, Gonzalez EM, et al. Physiological consequences of continuous, sublethal imazethapyr supply to pea plants. J Plant Physiol. 2000;157(3):345–354. doi: 10.1016/S0176-1617(00)80057-7. [DOI] [Google Scholar]
- 35.Shaner DL, Reider ML. Physiological responses of corn (Zea mays) to AC 243 997 in combination with valine, leucine, and isoleucine. Pestic Biochem Physiol. 1986;25(2):248–257. doi: 10.1016/0048-3575(86)90051-9. [DOI] [Google Scholar]
- 36.Shaner DL, Singh BK. Phytotoxicity of acetohydroxyacid synthase inhibitors is not due to accumulation of 2-ketobutyrate and/or 2-aminobutyrate. Plant Physiol. 1993;103(4):1221–1226. doi: 10.1104/pp.103.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang QH, Chen J, Shen GH, et al. Research on application techniques for a novel herbicide 10% ZJ0273 EC on transplanted rapes. Acta Phytophy Sin. 2006;33(3):328–332. doi: 10.3321/j.issn:0577-7518.2006.03.021. (in Chinese) [DOI] [Google Scholar]
- 38.Tian T, Jin ZL, Ali B, et al. The influence of new herbicide ZJ0273 on the total- and branched-chain amino acids in oilseed rape (Brassica napus L.) leaves as revealed by near-infrared spectroscopy. Acta Physiol Plant. 2014;36(8):2149–2156. doi: 10.1007/s11738-014-1591-z. [DOI] [Google Scholar]
- 39.Watson PR, Derksen DA, van Acker RC. The ability of 29 barley cultivars to compete and withstand competition. Weed Sci. 2006;54(4):783–792. doi: 10.1614/WS-05-020R3.1. [DOI] [Google Scholar]
- 40.Wright TR, Bascomb NF, Sturner SF, et al. Biochemical mechanism and molecular basis for ALS-inhibiting herbicide resistance in sugarbeet (Beta vulgaris) somatic cell selections. Weed Sci. 1998;46(1):13–23. [Google Scholar]
- 41.Xia PG, Li JZ, Wang RL, et al. Comparative study on volatile oils of four Panax genus species in Southeast Asia by gas chromatography-mass spectrometry. Ind Crop Prod. 2015;74:478–484. doi: 10.1016/j.indcrop.2015.05.059. [DOI] [Google Scholar]
- 42.Xia PG, Guo HB, Zhao HG, et al. Optimal fertilizer application for Panax notoginseng and effect of soil water on root rot disease and saponin contents. J Gins Res. 2016;40(1):38–46. doi: 10.1016/j.jgr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia XJ, Huang YY, Wang L, et al. Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pestic Biochem Physiol. 2006;86(1):42–48. doi: 10.1016/j.pestbp.2006.01.005. [DOI] [Google Scholar]
- 44.Yang DF, Ma PD, Liang X, et al. Metabolic profiles and cDNA-AFLP analysis of Salvia miltiorrhiza and Salvia castanea Diel f. tomentosa Stib. PLoS ONE. 2012;7(1):e29678. doi: 10.1371/journal.pone.0029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang YT, Peredelchuk M, Bennett GN, et al. Effect of variation of Klebsiella pneumoniae acetolactate synthase expression on metabolic flux redistribution in Escherichia coli . Biotechnol Bioeng. 2000;69(2):150–159. doi: 10.1002/(SICI)1097-0290(20000720)69:2<150::AID-BIT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 46.Yang ZM, Ye QF, Lu L. Synthesis of herbicidal ZJ0273 labeled with tritium and carbon-14. J Labelled Compd Rad. 2008;51(4):182–186. doi: 10.1002/jlcr.1498. [DOI] [Google Scholar]
- 47.Zhang F, Jin ZL, Naeem MS, et al. Spatial and temporal changes in acetolactate synthase activity as affected by new herbicide ZJ0273 in rapeseed, barley and water chickweed. Pestic Biochem Physiol. 2009;95(2):63–71. doi: 10.1016/j.pestbp.2009.06.005. [DOI] [Google Scholar]
- 48.Zhang JL, Wang LL, Zheng YP, et al. Effects of Bemisia tabaci (Gennadius) infestation and squash silverleaf disorder on Cucurbita pepo L. leaf. Sci Hortic. 2017;217:8–16. doi: 10.1016/j.scienta.2017.01.017. [DOI] [Google Scholar]
- 49.Zhang RJ, He JH, Zheng JY, et al. Weed species and their damage in wheat, barley, and rapeseed fields in Zhejiang. Acta Agric Zhejiang. 2000;12(6):308–316. doi: 10.3969/j.issn.1004-1524.2000.06.002. (in Chinese) [DOI] [Google Scholar]
- 50.Zhang WF, Zhang F, Raziuddin R, et al. Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul. 2008;27(2):159–169. doi: 10.1007/s00344-008-9042-y. [DOI] [Google Scholar]
- 51.Zhou QY, Liu WP, Zhang YS, et al. Action mechanisms of acetolactate synthase-inhibiting herbicides. Pestic Biochem Physiol. 2007;89(2):89–96. doi: 10.1016/j.pestbp.2007.04.004. [DOI] [Google Scholar]
- 52.Zhou W, Zhao D, Lin X. Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.) J Plant Growth Regul. 1997;16(1):47–53. doi: 10.1007/PL00006974. [DOI] [Google Scholar]
- 53.Zhou WJ, Leul M. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul. 1998;26(1):41–47. doi: 10.1023/A:1006004921265. [DOI] [Google Scholar]
- 54.Zhu SD, Zhao SH. Analysis of amino acids by high liquid chromatography. Chin J Chromatogr. 1994;12(1):20–24. (in Chinese) [Google Scholar]