Abstract
The denitrifier method is widely used as a novel pretreatment method for the determination of nitrogen and oxygen isotope ratios as it can provide quantitative and high-sensitivity measurements. Nevertheless, the method is limited by relatively low measurement accuracy for δ18O. In this study, we analyzed the factors influencing the accuracy of δ18O determination, and then systematically investigated the effects of dissolved oxygen concentrations and nitrate sample sizes on estimates of the δ15N and δ18O of nitrate reference materials. The δ18O contraction ratio was used to represent the relationship between the measured difference and true difference between two reference materials. We obtained the following main results: (1) a gas-liquid ratio of 3:10 (v/v) in ordinary triangular flasks and a shaking speed of 120 r/min produced an optimal range (1.9 to 2.6 mg/L) in the concentration of dissolved oxygen for accurately determining δ18O, and (2) the δ18O contraction ratio decreased as nitrate sample size decreased within a certain range (1.0 to 0.1 μmol). Our results suggested that δ18O contraction is influenced mainly by dissolved oxygen concentrations in pure culture, and provided a model for improving the accuracy of oxygen isotope analysis.
Keywords: Denitrifier method, Nitrate, δ15N, δ18O, Dissolved oxygen, δ18O contraction
1. Introduction
Natural abundance stable isotope signatures have been successfully used in numerous surface water and groundwater systems to trace contamination sources (Widory et al., 2005; Popescu et al., 2015). The isotopic composition of nitrate (δ15N and δ18O) can provide valuable information to characterize the dominant sources of nitrate pollution and quantitatively estimate the contributions from different sources by applying stable isotope mixing models (e.g. SIAR) (Oelmann et al., 2007; Ward et al., 2010; Rock et al., 2011; Matiatos, 2016).
A simple, rapid, low-contamination, cost-effective, and highly precise sample preparation method for isotope measurement is urgently needed.
Continuous-flow isotope ratio mass spectrometry (IRMS) requires nitrate in water to be converted to a solid (e.g. ammonium salt or AgNO3) and then to a gas (e.g. N2 and CO or N2 and CO2). Compared with other preparation methods like ion exchange chromatography (Silva et al., 2000), the diffusion method (Stark and Hart, 1996), and chemical method (Stevens and Laughlin, 1994), the denitrifier method has the advantage of simultaneously measuring nitrogen and oxygen isotopes of nitrate at concentrations as low as 0.1 μmol/L and in sample sizes of less than 10 ml (Casciotti et al., 2002). In addition, the denitrifier method can effectively reduce errors originating from atmospheric leaks, problems associated with reduction of NO3 − to NH4 +, or contamination from other N-sources (Christensen and Tiedje, 1988).
The denitrifier method was first introduced by Sigman et al. (2001) as a novel method to measure the natural abundance of nitrate isotopes in seawater and freshwater. It was based on the isotopic analysis of nitrous oxide (N2O) generated from nitrate by denitrifying bacteria (e.g. Pseudomonas aureofaciens and Pseudomonas chlororaphis) lacking N2O-reductase activity. Materials and methods like autosampler modification, water sample treatment, pre-concentration to denitrify cultures, and NO2 − removal are being constantly updated to improve the performance of the denitrifier method by increasing the precision and sensitivity of isotopic analysis (Mørkved et al., 2007; Granger and Sigman, 2009; McIlvin and Casciotti, 2011; Weigand et al., 2016). Moreover, the application of the denitrifier method is continuously expanding, and it is now used in the fields of botany, soil science, atmospheric science, and the study of the global nitrogen cycle (Dahal and Hastings, 2016).
Presently, the measurement precision of the oxygen isotope (0.5‰ to 2.0‰) is far lower than that of the nitrogen isotope (0.20‰ to 0.46‰) in nitrate dissolved in water samples (Vicars et al., 2013). This is because the conversion of nitrate to N2O maintains a mass balance reaction for nitrogen but not for oxygen. Isotope fractionation and the exchange of oxygen atoms with water may lead to δ18O in N2O differing from its original nitrate.
Experiments with 18O-labeled water were conducted by Casciotti et al. (2002). These experiments indicated that oxygen atoms exchanged with water accounted for less than 10% of those in the N2O products for P. aureofaciens. In addition, both oxygen isotope fractionation and oxygen atom exchange should be consistent within a given batch of analyses. Accordingly, the effects of isotopic fractionation, exchange, and a blank on δ18O measurements of water samples were accurately quantified by analyzing two reference materials (RMs) of known δ18O within the same batch.
Nonetheless, due to incomplete oxygen conversion and an unclear reaction process for oxygen isotope exchange, the calibration of the oxygen isotope was complicated and the measurement precision was low (Dai et al., 2017). Consequently, the limitation of the denitrifier method was the difficulty in accurately determining the δ18O of nitrate. In addition, there was a contraction in the oxygen isotopic difference between IAEA-NO-3 (International Atomic Energy Agency, Vienna, Austria, subsequently referred to as “N3”) and USUG34 (the National Institute of Standards and Technology, Gaithersburg, MD, USA) (Weigand et al., 2016).
It is known that the denitrification process is influenced by the dissolved oxygen (DO) concentration, carbon source, nitrate concentration, pH, and temperature (Deng, 2010). In addition, the experimental bacterium in the denitrifier method is an aerobic denitrifying bacterium. Therefore, the effect of DO and nitrate concentration on δ18O of N2O formed by denitrification should be noted when pH (neutral), temperature (26 °C), and carbon source are constant. In a preliminary experiment, we found that the δ18O of N2O derived from nitrate varied with the headspace in culture bottles, as noted by Xu et al. (2012).
Therefore, the objectives of this study were: (1) to examine the effects of DO concentrations and nitrate sample sizes on the precision of isotopic analysis, especially for δ18O; (2) to explore the cause of poor analytical precision and the contraction of δ18O; and (3) to identify the optimal DO concentrations and nitrate sample sizes to avoid δ18O contraction.
2. Materials and methods
2.1. Materials
P. aureofaciens (strain number: ATCC 13985; a facultative anaerobic bacterium, recently reclassified as a strain of P. chlororaphis) was selected as the experimental bacterium in this study. The nitrate isotopic RMs IAEA-NO-3, USGS32, and USGS34 were used to evaluate the analytical methods according to the accuracy and precision of measurement results (Hastings et al., 2003; Weigand et al., 2016). The isotopic composition of the RMs is shown in Table 1.
Table 1.
Reference values for the relative nitrogen and oxygen isotope ratios of the nitrate reference materials
| Reference material |
15N isotope ratio (×103) |
18O isotope ratio (×103) |
17O isotope ratio (×103) |
|||
| δ15N | Ua | δ18O | Ua | Δ17O | Ua | |
| IAEA-NO-3b | 4.7 | ±0.3 | 25.6 | ±0.4 | −0.2 | ±0.2 |
| USGS32c | 180.0 | Exact | 25.7 | ±0.4 | ||
| USGS34d | −1.8 | ±0.2 | −27.9 | ±0.6 | −0.1 | ±0.2 |
2.2. Method set-up
Based on the previously published method (Sigman et al., 2001; Mørkved et al., 2007; McIlvin and Casciotti, 2011), we focused on the oxygen isotopic ratios of nitrate. We divided the procedures (Fig. 1) of the denitrifier method into the following steps: (1) resuscitation of the denitrifying strains, (2) pure culture, (3) test bottle-making, (4) sample pretreatment and nitrate conversion, (5) N2O extraction and isolation, and (6) measurement and calibration of isotopic ratios.
Fig. 1.
Flow diagram of the experiment
First, P. aureofaciens stored in a −80 °C refrigerator was revived by daubing an agar plate. Second, a 5-ml starter was prepared from single colonies to generate inoculum overnight. The starter was transferred to a culture flask (routine lab equipment, triangular flask), sealed with a rubber stopper and Parafilm® M (#996, Pechiney Plastic Packaging, Menasha, WI, USA), and incubated using amended medium with KNO3 for 7 d. Third, the medium was concentrated 10-fold (cell slurry was dissolved using fresh amended medium without KNO3 after centrifuging) and sealed in a 20-ml headspace vial (2 to 3 ml per vial) with butyl rubber stoppers (Ht-0942, Qingdao Hiprove Medical Technologies Co., Ltd., Qingdao, China). The headspace vial was used as the test bottle after purging for 2–3 h using helium or high purity nitrogen to remove N2O and ensure anaerobic conditions.
Next, water samples were heated in a water bath for 1 h at 80 °C (Mørkved et al., 2007) to inhibit the activity of native denitrifying bacteria. The pH was adjusted to 3.5 using ascorbate to eliminate the disturbance of nitrite (Xu et al., 2012), and to neutral using NaOH solution. After samples (less than 10 ml) containing 0.2 to 0.4 μmol nitrate were injected into the test bottles overnight for NO3 − conversion, 0.1 to 0.2 ml of 10 mol/L NaOH was used to lyse the bacteria and scrub any CO2 gas in each test bottle. Next, N2O was extracted from the test bottles off-line and injected into a Thermo Scientific pre-concentrator (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) to be isolated on-line. N2O purity was analyzed using IRMS (Thermo Finnigan MAT 253).
Finally, the RMs were processed with the samples simultaneously. The nitrate concentrations of the RMs were matched to those of the samples, and the data were used to correct the nonlinearity of the mass spectrometer and blanks associated with the procedure. The natural abundances of 15N and 18O were calculated as δ15N and δ18O, respectively, expressed in per mill units (‰): natural abundance=[(R sample/R standard)−1]×1000‰, where R sample and R standard are the ratios of heavy isotope to light isotope of sample and standard, respectively (Liu et al., 2012). The standard for 15N was air, and for 18O was the Vienna Standard Means Ocean Water (VSMOW).
To facilitate analysis, we introduced the contraction ratio (C) to reflect the reliability of measurement results: C=(T−M)/T×100%, where M and T are the measured and true δ difference between RMs, respectively. Accordingly, the result is more accurate when the contraction ratio is smaller.
2.3. Experiment I: effect of DO concentrations in pure culture on δ18O measurement
The DO concentrations in medium can be affected by both gas-liquid ratios in the culture bottle and shaking speeds. To explore the optimal DO concentrations in ordinary triangular flasks and the relationship between DO concentrations in pure culture and the analytical precision of δ18O, we first conducted an experiment under both non-sealed and sealed conditions.
Generally, the ratio of gas to liquid was about 1:5 (v/v) for the culture bottle (Xu et al., 2012). The volume of the non-sealed pure culture with medium accounted for 5/6 of the actual volume (600 ml) of the 500 ml triangular flasks. The sealed conditions provided limited volume for the culture medium (375, 428, and 500 ml) in the flasks when other conditions were consistent with previously published methods (Sigman et al., 2001; Mørkved et al., 2007). For non-sealed cultures, the top of each culture flask was wrapped with six layers of gauze and four layers of newspaper. For airtight flasks, rubber stoppers and Parafilm M were used to maintain predefined gas-liquid ratios (3:5, 2:5, and 1:5; v/v).
After the optimal gas-liquid ratios in culture flasks were determined, two shaking speeds (60 and 120 r/min) were selected based on the literature (Sigman et al., 2001; McIlvin and Casciotti, 2011). Analytical pure (AR) KNO3 and two RMs (USGS34 and N3) were converted to N2O using denitrifying culture to evaluate the optimal gas-liquid ratio and shaking speed.
2.4. Experiment II: effect of nitrate sample sizes on δ18O measurement
The denitrifier method has been shown to have high sensitivity (tested down to 0.01 μmol nitrate) (Casciotti et al., 2002). To examine the effect of nitrate sample size on 18O measurement, the RM (N3 and USGS34) solutions were diluted across a gradient of nitrate concentrations (0.025 to 0.500 μmol/L), and then 2 ml of the diluted solution was injected into the test bottle to be denitrified in 2.5 ml of experimental bacteria solution. The analytical precision and contraction ratios of δ15N and δ18O in RMs were measured for different nitrate sample sizes.
3. Results
3.1. Non-sealed cultures
The average standard deviations (SDs) (n=6) of δ15N were 0.25‰ for KNO3 (AR), 0.28‰ for N3, and 0.57‰ for USGS34. The difference (6.38‰) between measured values of δ15N for USGS34 and N3 was close to the true difference of 6.5‰. However, the observed variation of measured δ18O values (the maximum SD=3.42‰) was higher than that of δ15N. The difference for measured δ18O values between the two RMs ranged from 0.63‰ to 4.90‰, which was much lower than the true difference of 53.5‰. Moreover, the δ18O contraction ratios were higher than 90% in non-sealed conditions.
3.2. Sealed cultures with different gas-liquid ratios
The measured δ18O values of RMs under sealed-culture conditions with different gas-liquid ratios are shown in Fig. 2. The results indicated that the measured δ18O differences between USGS34 and N3 increased from 7.72‰ to 47.98‰, and δ18O contraction ratios declined from 85.6% to 10.3% when the gas-liquid ratios decreased from 3:5 to 1:5 (data not shown). The contraction ratios were higher than 85.4% under the gas-liquid ratios of 3:5 and 2:5. N2O sometimes could not be detected using IRMS when the gas-liquid ratio was 1:5 with a low cell density (optical density (OD)=0.19, while OD=0.31 for a gas-liquid ratio of 2:5). To harvest sufficient cells and guarantee complete nitrate conversion in water samples, the gas-liquid ratio in the triangular flasks needed to be greater than 1:5.
Fig. 2.
Comparison of δ18O values of RMs (USGS34 and N3) at different gas-liquid ratios
Therefore, the gas-liquid ratio of 3:10 (v/v) was selected for our continuous experiments, and the measurement accuracies of δ15N and δ18O were significantly improved. The average SD values were reduced to as low as 0.11‰ (N3) and 0.16‰ (USGS34) for δ15N, and 0.20‰ (N3) and 0.22‰ (USGS34) for δ18O. The δ18O contraction ratios ranged from 4.9% to 7.3% with a mean value of 6.2%.
3.3. Nitrate sample sizes
Samples with different nitrate sample sizes were analyzed based on the case of a gas-liquid ratio of 3:10 (v/v) (Table 2). The measurement precision of δ18O for either high or low NO3 − sample sizes was lower than that for medium nitrate sample sizes in RMs (i.e. 0.2 and 0.4 μmol NO3 −, P<0.05). When the nitrate sample size was 0.05 μmol, the contraction ratios of δ15N and δ18O were higher than those of other nitrate sample sizes. When the nitrate sample sizes ranged from 0.2 to 0.4 μmol, the measured differences between USGS34 and N3 were close to the true difference values, and δ18O contraction ratios were less than the average 6.2% (Table 2).
Table 2.
Inter-batch repeatability (SD) in δ15N and δ18O, the measured difference between N3 and USGS34 under a gas to liquid ratio of 3:10 (v/v) in the culture flask and a shaking speed of 120 r/min (n=6), and the contraction ratio of δ18O
| No. of batches | RM | Nitrate sample size (μmol) | δ15N (‰) |
δ18O (‰) |
Difference (‰) |
C (%) |
||||
| Mean | SD | Mean | SD | δ15N | δ18O | δ15N | δ18O | |||
| 1 | N3 | 1.00 | 4.59 | 0.08 | 21.57 | 0.27 | 6.41 | 49.62 | 1.4 | 7.3 |
| USGS34 | −1.82 | 0.07 | −28.05 | 0.33 | ||||||
| 2 | N3 | 0.40 | 4.64 | 0.03 | 22.14 | 0.12 | 6.44 | 50.02 | 0.9 | 6.5 |
| USGS34 | −1.80 | 0.19 | −27.87 | 0.07 | ||||||
| 3 | N3 | 0.20 | 4.68 | 0.08 | 22.48 | 0.06 | 6.47 | 50.32 | 0.4 | 6.0 |
| USGS34 | −1.82 | 0.24 | −27.84 | 0.12 | ||||||
| 4 | N3 | 0.20 | 4.68 | 0.06 | 22.29 | 0.21 | 6.49 | 50.21 | 0.2 | 6.2 |
| USGS34 | −1.80 | 0.23 | −27.91 | 0.20 | ||||||
| 5 | N3 | 0.10 | 4.68 | 0.09 | 23.07 | 0.18 | 6.46 | 50.87 | 0.7 | 4.9 |
| USGS34 | −1.78 | 0.15 | −27.81 | 0.28 | ||||||
| 6 | N3 | 0.05 | 4.54 | 0.29 | 22.32 | 0.36 | 6.20 | 50.12 | 4.7 | 6.3 |
| USGS34 | −1.66 | 0.07 | −27.80 | 0.30 | ||||||
RM: reference material; SD: standard deviation; C: contraction ratio
4. Discussion
4.1. Effect of the nitrate sample sizes on δ18O measurement
The measurement accuracies of δ15N and δ18O were significantly improved using the gas-liquid ratio of 3:10, and the δ18O contraction ratios decreased as nitrate sample sizes decreased (except for 0.05 μmol). These results agree with the observations of Weigand et al. (2016). When the nitrate sample size was 0.05 μmol, it was significantly affected by the blank, resulting in a high contraction ratio for both δ15N and δ18O. Although the optimal nitrate sample sizes reduced δ18O contraction ratios, there was still a slight contraction (6.0% to 6.5%) in the measured δ18O values (Table 2). Weigand et al. (2016) suggested that a change in nitrate sample sizes was not the sole cause of the δ18O contraction. Compared with nitrogen isotopes, oxygen isotopes are more difficult to measure due to the exchange of oxygen atoms with water, isotope fractionation, blank, and other factors.
The nitrate in water samples can be completely converted to N2O in a high cell density bacterial solution, and thus no nitrogen isotope fractionation occurs according to mass balance. However, this is not the case for oxygen atoms. Xue et al. (2010) noted that only one of the six oxygen atoms in the initial nitrate pool was represented in the N2O (2NO3 −→ N2O) analysis (Fig. 3). Moreover, the intermediates (nitrite and nitric oxide; Fig. 3) during the conversion would exchange oxygen atoms with water (Ye et al., 1991). The degree of exchange may be related to the type of nitrite reductase. The copper-type nitrite reductase of P. aureofaciens incorporates fewer oxygen atoms from water into N2O than the heme-type nitrite reductase of P. chlororaphis (Glockner et al., 1993). That is, not all oxygen atoms in N2O are derived from the nitrate pool, and this issue can lead to uncertainty in isotopic analysis using the denitrifier method.
Fig. 3.
Processes of denitrification by P. aureofaciens
Nar, Nir, Nor, and Nos represent nitrate reductase, nitrite reductase, nitric oxide reductase, and nitrous oxide reductase, respectively (Morozkina and Zvyagilskaya, 2007). The gray shading in the figure indicates a process that does not occur
Moreover, there was a preferential loss of 16O in these reactions that caused a difference in δ18O between nitrate and the product N2O, even if the conversion of nitrate to N2O was complete (Casciotti et al., 2002).
The denitrifying medium may contain trace amounts of NO2 −, NO, and N2O generated from previous pure cultures. Previous studies showed that even if fresh medium was used instead of spent medium to re-suspend the cell slurry, and the fresh bacterial solution was purged by helium for more than 4 h, the denitrifying medium still resulted in lower blanks (about 0.04 nmol of N) (McIlvin and Casciotti, 2011). In addition, the δ18O contraction of RMs may be affected by addition of KH2PO4 or K2HPO4, the residence time of nitrite in the bacterial solution (Weigand et al., 2016), and the off-line headspace sampling (Mørkved et al., 2007).
4.2. Effect of the DO concentrations on δ18O measurement
Ideally, the factors (e.g. oxygen atom exchange with water, oxygen isotope fractionation, and blank size) can be eliminated by adding RMs to a batch of samples undergoing the same operation and reaction (Casciotti et al., 2002). However, the measurement precision of δ18O was low and the contraction ratios of δ18O were high under non-sealed culture conditions. Different amplitudes of δ18O contractions were observed under sealed-culture conditions with different gas-liquid ratios in culture flasks. That is, DO concentrations had a substantial influence on the growth of denitrifying bacteria and the conversion of nitrate to N2O, and this may be the most significant contributor to δ18O contraction.
During denitrification, oxygen, nitrite, and nitrate are electron acceptors for nitrate conversion to N2O in P. aureofaciens (Hu et al., 2018). If oxygen is sufficient, the bacteria can grow quickly through aerobic respiration. When oxygen drops below a certain concentration, the bacteria will switch to nitrate respiration and gain electrons from the respiratory chain (Galloway et al., 2004; Huang and Xin, 2009). McIlvin and Casciotti (2011) found that the cell density of a pure culture in an anaerobic environment was only 5% of that in an aerobic environment. It seems that the throughput of the denitrifier method can be improved using non-sealed cultures. However, higher DO concentrations may result in more disturbances to the δ18O measurement of RMs so that the differences among the δ18O values of RMs become indistinguishable (Fig. 2). When DO concentrations are high, nitrite reductase activity is inhibited, and the carbon source is broken down quickly to produce cells. This results in a lack of sufficient electron donors in subsequent denitrification and the accumulation of intermediate products. Therefore, appropriate DO concentrations in pure culture are essential for microbial growth and influence the completeness of nitrate conversion to N2O.
DO concentrations in the medium depend on the dissolution rate of oxygen, gas-liquid contact time and area, gas volume, oxygen partial pressure, and the nature of the medium (Tiedje, 1988). Thus, DO concentrations were controlled by the gas-liquid ratio of culture flasks and the shaking speed under sealed conditions. Two shaking speeds (60 and 120 r/min) were tested to explore the appropriate DO concentration under a gas-liquid ratio of 3:10 (the area of gas-liquid was about 3.7 cm2). Bacterial film and flocculent precipitation were observed at a shaking speed of 60 r/min, which made it difficult to centrifuge and blend. The measured isotope ratios varied significantly, and coenobium was broken up by vortex before centrifugation in the case of 60 r/min. By contrast, the 120 r/min shaking speed allowed the growth of bacterial turbidity, but no floc, which resulted in better measurement precision of the RMs (Table 1).
Generally, denitrification is complete when the DO concentration is less than 0.2 mg/L (Downes, 1988). Most aerobic denitrifying bacteria can tolerate DO concentrations below 3 mg/L (Zhou et al., 2007). However, nitrate may be still selected as an electron acceptor in the process of denitrification under a wider range of DO concentrations (2.3 to 11.3 mg/L) for some aerobic denitrifying bacteria (Wang et al., 2007). Our results indicated that δ18O estimates of nitrate were more precise when the DO concentrations were controlled by a gas-liquid ratio of 3:10 and a shaking speed of 120 r/min. The concentrations of DO (mg/L) were 1.9 on the second, 2.6 on the fifth, and 2.2 on the seventh day of pure culture. However, the DO concentrations were more than 3 mg/L under other oxygen conditions (i.e. non-sealed culture or gas-liquid ratios of 2:5 and 3:5), which resulted in poor performance for isotopic measurement, especially for the oxygen isotope. Accordingly, the threshold values of DO concentrations for P. aureofaciens should be below 3 mg/L.
The reason why isotopic measurement was poor at DO concentrations above 3 mg/L might be that excessive DO inhibits the activity of nitrite reductase and causes the accumulation of intermediate products (Fig. 3) (Bu et al., 2015). Kumar and Lin (2010) confirmed that the reductase in the denitrification process was sensitive to oxygen, and its activity was affected by DO concentrations. A high DO concentration may promote oxygen atom exchange between intermediate products and distilled water used for the preparation of medium in the laboratory. δ18O values of distilled water of less than 0‰ have been reported (Koehler et al., 1991). Thus, it can be inferred that the δ18O values of RMs, especially those of N3, which were lower than the true value, may be the result of oxygen atoms exchanging with distilled water. The degree of exchange was closely related to the DO concentration, and high DO concentrations may generate dilution of oxygen isotopes in intermediate products. Therefore, the effect of intermediates on the conversion of nitrate in samples to N2O was significantly greater in high than in low DO concentrations. Eventually, the measured values of δ18O in different RMs were indistinguishable due to the high DO concentration and high degree of oxygen atom exchange with water.
4.3. Precision analysis
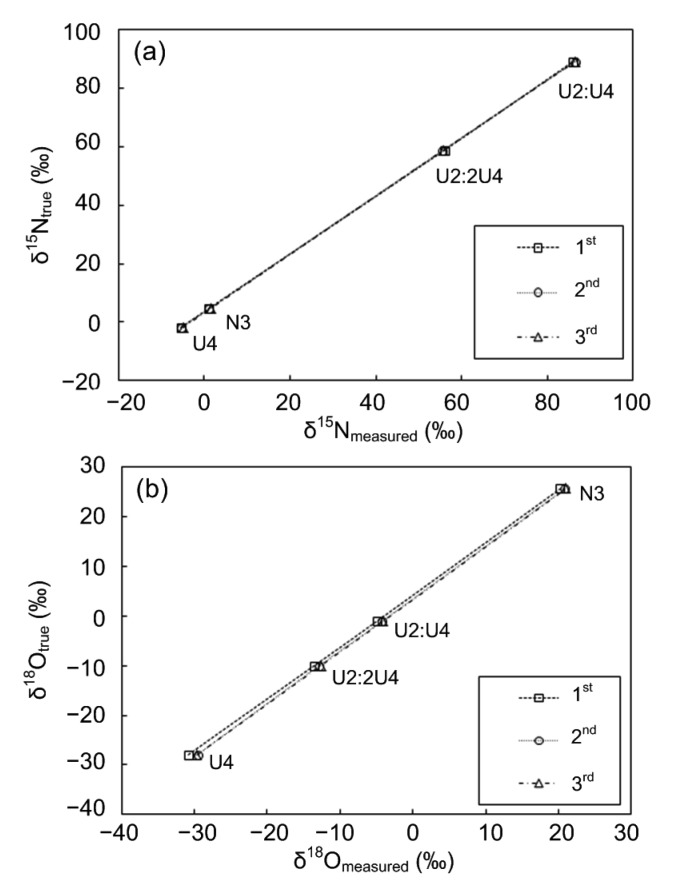
Fig. 4 shows the results of linear regression analysis of four RMs (N3, USGS34, USGS32:USGS34, and USGS32:2USGS34) (R2=1, P<0.001). Verified three times, the DO concentrations (controlled by a gas-liquid ratio of 3:10 and a shaking speed of 120 r/min) and the nitrate sample sizes (0.2 to 0.4 μmol) were effective and feasible. The slight δ18O contraction can be corrected by the standard curve.
Fig. 4.
Linear regression analysis of the measured values and true values for δ15N (a) and δ18O (b) of four reference materials
U2:U4 and U2:2U4 solutions were prepared by mixing USGS32 (U2) and USGS34 (U4) in the proportions of 1:1 and 1:2, respectively. n=3
Eight rainwater samples were collected from a watershed in northwestern Zhejiang Province, China by a rainwater collector in Jan. 2017. The δ15N-NO3 − and δ18O-NO3 − values were determined with 0.2 μmol NO3 −. The measured results are showed in Fig. 5. The δ15N-NO3 − values ranged from −0.04‰ to 4.02‰ with an average SD of 0.16‰, and the δ18O-NO3 − values ranged from 36.54‰ to 81.03‰ with an average SD of 0.25‰ (n=3). In general, δ15N-NO3 − derived from precipitation ranges from −10‰ to 8‰ (Rogers et al., 2012), and δ18O-NO3 − from 25‰ to 75‰ (Yang et al., 2013). Compared with the δ18O precision (0.5‰ to 2.0‰) of the water samples reported in previous studies, we obtained a slightly higher precision in this study.
Fig. 5.
Nitrogen and oxygen isotope composition of nitrate in rainwater samples (n=3)
The bars represent the standard deviations
5. Conclusions
In general, our study revealed a strong relationship between δ18O measurements and DO concentrations or nitrate sample sizes. DO concentration in pure culture affected the subsequent conversion of nitrate to N2O in denitrifying culture, because it promoted the accumulation of intermediate products and oxygen exchange with water. Ultimately, DO concentrations affected the measurement precision and accuracy of δ15N and δ18O, especially δ18O.
In this study, we clearly demonstrated that the measurement accuracy of δ18O can be improved and the δ18O contraction ratio can be reduced by selecting an appropriate range of DO concentrations (1.9 to 2.6 mg/L) and nitrate sample sizes (0.2 to 0.4 μmol). The appropriate range of DO concentrations was controlled by the gas-liquid ratio of 3:10 (v/v) in ordinary triangular flasks and a shaking speed of 120 r/min. The appropriateness of the DO concentrations and nitrate sample sizes was confirmed using rainwater sample analysis. The average SDs of 0.16‰ for δ15N-NO3 − and 0.25‰ for δ18O-NO3 − in rainwater were slightly higher than those of water samples previously published.
The mechanism of the effect of DO concentrations on the exchange of oxygen with water remains to be explored, and we want to focus on the cause of δ18O contraction and the determination of the concentration of oxygen isotopes.
Acknowledgments
We thank Ya-cheng CAO (School of Geography Science, Nanjing Normal University, Nanjing, China) for advising and helping us in the use of isotope mass spectrometer and the United States Department of Agriculture Culture Collection Center for providing the experimental strains.
Footnotes
Project supported by the National Key R&D Program of China (No. 2016YFD0200106) and the National Natural Science Foundation of China (No. 41571450)
Compliance with ethics guidelines: Man ZHANG, Jia-chun SHI, and Lao-sheng WU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Böhlke JK, Coplen TB. Interlaboratory comparison of reference materials for nitrogen-isotope-ratio measurements. A Consultants Meeting on Reference and Intercomparison Materials for Stable Isotopes of Light Elements; 1995. pp. 51–66. [Google Scholar]
- 2.Böhlke JK, Mroczkowski SJ, Coplen TB. Oxygen isotopes in nitrate: new reference materials for 18O:17O:16O measurements and observations on nitrate-water equilibration. Rapid Commun Mass Spectrom. 2003;17(16):1835–1846. doi: 10.1002/rcm.1123. [DOI] [PubMed] [Google Scholar]
- 3.Bu F, Hu X, Li X, et al. Cassava stillage and its anaerobic fermentation liquid as external carbon sources in biological nutrient removal. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(4):304–316. doi: 10.1631/jzus.B1400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casciotti KL, Sigman DM, Hastings MG, et al. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem. 2002;74(19):4905–4912. doi: 10.1021/ac020113w. [DOI] [PubMed] [Google Scholar]
- 5.Christensen S, Tiedje JM. Sub-parts-per-billion nitrate method: use of an N2O-producing denitrifier to convert NO3 − or 15NO3 − to N2O. Appl Environ Microbiol. 1988;54(6):1409–1413. doi: 10.1128/aem.54.6.1409-1413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahal B, Hastings MG. Technical considerations for the use of passive samplers to quantify the isotopic composition of NOx and NO2 using the denitrifier method. Atmos Environ. 2016;143:60–66. doi: 10.1016/j.atmosenv.2016.08.006. [DOI] [Google Scholar]
- 7.Dai SH, Xie LH, Peng L, et al. Determination of nitrogen and oxygen isotopes in nitrates: a minireview. Anal Lett. 2017;50(13):2045–2057. doi: 10.1080/00032719.2016.1265978. [DOI] [Google Scholar]
- 8.Deng K. Study on Denitrification and Character of Denitrifying Bacteria. MS T. South China University of Technology, Guangzhou, China; 2010. (in Chinese) [Google Scholar]
- 9.Downes MT. Aquatic nitrogen transformations at low oxygen concentrations. Appl Environ Microbiol. 1988;54(1):172–175. doi: 10.1128/aem.54.1.172-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galloway JN, Dentener FJ, Capone DG, et al. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70(2):153–226. doi: 10.1007/s10533-004-0370-0. [DOI] [Google Scholar]
- 11.Glockner AB, Jüngst A, Zumft WG. Copper-containing nitrite reductase from Pseudomonas aureofaciens is functional in a mutationally cytochrome cd 1-free background (NirS−) of Pseudomonas stutzeri . Arch Microbiol. 1993;160(1):18–26. doi: 10.1007/BF00258141. [DOI] [PubMed] [Google Scholar]
- 12.Granger J, Sigman DM. Removal of nitrite with sulfamic acid for nitrate N and O isotope analysis with the denitrifier method. Rapid Commun Mass Spectrom. 2009;23(23):3753–3762. doi: 10.1002/rcm.4307. [DOI] [PubMed] [Google Scholar]
- 13.Hastings MG, Sigman DM, Lipschultz F. Isotopic evidence for source changes of nitrate in rain at Bermuda. J Geophys Res. 2003;108(D24):4790. doi: 10.1029/2003JD003789. [DOI] [Google Scholar]
- 14.Hu X, Sobotka D, Czerwionka , K , et al. Effects of different external carbon sources and electron acceptors on interactions between denitrification and phosphorus removal in biological nutrient removal processes. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(4):305–316. doi: 10.1631/jzus.B1700064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang XL, Xin MX. Microbiology. Higher Education Press, Beijing, China; 2009. p. 193. (in Chinese) [Google Scholar]
- 16.Koehler GD, Chipley D, Kyser TK. Measurement of the hydrogen and oxygen isotopic compositions of concentrated chloride brines and brines from fluid inclusions in halite. Chem Geol. 1991;94(1):45–54. doi: 10.1016/S0009-2541(10)80016-6. [DOI] [Google Scholar]
- 17.Kumar M, Lin JG. Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal–strategies and issues. J Hazard Mater. 2010;178(1-3):1–9. doi: 10.1016/j.jhazmat.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 18.Liu XY, Koba K, Takebayashi Y, et al. Preliminary insights into δ15N and δ18O of nitrate in natural mosses: a new application of the denitrifier method. Environ Pollut. 2012;162:48–55. doi: 10.1016/j.envpol.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Matiatos I. Nitrate source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: a case study of Asopos basin (Central Greece) Sci Total Environ. 2016;541:802–814. doi: 10.1016/j.scitotenv.2015.09.134. [DOI] [PubMed] [Google Scholar]
- 20.McIlvin MR, Casciotti KL. Technical updates to the bacterial method for nitrate isotopic analyses. Anal Chem. 2011;83(5):1850–1856. doi: 10.1021/ac1028984. [DOI] [PubMed] [Google Scholar]
- 21.Mørkved PT, Dörsch P, Søvik AK, et al. Simplified preparation for the δ15N-analysis in soil NO3 − by the denitrifier method. Soil Biol Biochem. 2007;39(8):1907–1915. doi: 10.1016/j.soilbio.2007.02.004. [DOI] [Google Scholar]
- 22.Morozkina EV, Zvyagilskaya RA. Nitrate reductases: structure, functions, and effect of stress factors. Biochemistry (Moscow) 2007;72(10):1151–1160. doi: 10.1134/S0006297907100124. [DOI] [PubMed] [Google Scholar]
- 23.Oelmann Y, Kreutziger Y, Bol R, et al. Nitrate leaching in soil: tracing the NO3 − sources with the help of stable N and O isotopes. Soil Biol Biochem. 2007;39(12):3024–3033. doi: 10.1016/j.soilbio.2007.05.036. [DOI] [Google Scholar]
- 24.Popescu R, Mimmo T, Dinca OR, et al. Using stable isotopes in tracing contaminant sources in an industrial area: a case study on the hydrological basin of the Olt River, Romania. Sci Total Environ. 2015;533:17–23. doi: 10.1016/j.scitotenv.2015.06.078. [DOI] [PubMed] [Google Scholar]
- 25.Qi HP, Coplen TB, Geilmann H, et al. Two new organic reference materials for δ 13C and δ 15N measurements and a new value for the δ 13C of NBS 22 oil. Rapid Commun Mass Spectrom. 2003;17(22):2483–2487. doi: 10.1002/rcm.1219. [DOI] [PubMed] [Google Scholar]
- 26.Rock L, Ellert BH, Mayer B. Tracing sources of soil nitrate using the dual isotopic composition of nitrate in 2 M KCl-extracts. Soil Biol Biochem. 2011;43(12):2397–2405. doi: 10.1016/j.soilbio.2011.08.016. [DOI] [Google Scholar]
- 27.Rogers KM, Nicolini E, Gauthier V. Identifying source and formation altitudes of nitrates in drinking water from Réunion Island, France, using a multi-isotopic approach. J Contam Hydrol. 2012;138-139:93–103. doi: 10.1016/j.jconhyd.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Sigman DM, Casciotti KL, Andreani M, et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem. 2001;73(17):4145–4153. doi: 10.1021/ac010088e. [DOI] [PubMed] [Google Scholar]
- 29.Silva SR, Kendall C, Wilkison DH, et al. A new method for collection of nitrate from fresh water and the analysis of nitrogen and oxygen isotope ratios. J Hydrol. 2000;228(1-2):22–36. doi: 10.1016/S0022-1694(99)00205-X. [DOI] [Google Scholar]
- 30.Stark JM, Hart SC. Diffusion technique for preparing salt solutions, Kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci Soc Am J. 1996;60(6):1846–1855. doi: 10.2136/sssaj1996.03615995006000060033x. [DOI] [Google Scholar]
- 31.Stevens RJ, Laughlin RJ. Determining nitrogen-15 in nitrite or nitrate by producing nitrous oxide. Soil Sci Soc Am J. 1994;58(4):1108–1116. doi: 10.2136/sssaj1994.03615995005800040015x. [DOI] [Google Scholar]
- 32.Tiedje JM. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder AJB (Ed.), Environmental Microbiology of Anaerobes. John Wiley and Sons, New York; 1988. pp. 179–224. [Google Scholar]
- 33.Vicars WC, Morin S, Savarino J, et al. Spatial and diurnal variability in reactive nitrogen oxide chemistry as reflected in the isotopic composition of atmospheric nitrate: results from the CalNex 2010 field study. J Geophys Res: Atmos. 2013;118(18):10567–10588. doi: 10.1002/jgrd.50680. [DOI] [Google Scholar]
- 34.Wang HY, Ma F, Su JF, et al. Identification and characterization of a bacterial strain C3 capable of aerobic denitrification. Environ Sci. 2007;28(7):1548–1552. doi: 10.13227/j.hjkx.2007.07.025. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 35.Ward EJ, Semmens BX, Schindler DE. Including source uncertainty and prior information in the analysis of stable isotope mixing models. Environ Sci Technol. 2010;44(12):4645–4650. doi: 10.1021/es100053v. [DOI] [PubMed] [Google Scholar]
- 36.Weigand MA, Foriel J, Barnett B, et al. Updates to instrumentation and protocols for isotopic analysis of nitrate by the denitrifier method. Rapid Commun Mass Spectrom. 2016;30(12):1365–1383. doi: 10.1002/rcm.7570. [DOI] [PubMed] [Google Scholar]
- 37.Widory D, Petelet-Giraud E, Négrel P, et al. Tracking the sources of nitrate in groundwater using coupled nitrogen and boron isotopes: a synthesis. Environ Sci Technol. 2005;39(2):539–548. doi: 10.1021/es0493897. [DOI] [PubMed] [Google Scholar]
- 38.Xu CY, Li YZ, Hao WP, et al. Analysis of nitrogen isotopic composition of nitrate in water by denitrifier method and trace-gas/isotope ratio mass spectrometry. Chin J Anal Chem. 2012;40(9):1360–1365. doi: 10.3724/SP.J.1096.2012.20008. (in Chinese) [DOI] [Google Scholar]
- 39.Xue DM, de Baets B, Vermeulen J, et al. Error assessment of nitrogen and oxygen isotope ratios of nitrate as determined via the bacterial denitrification method. Rapid Commun Mass Spectrom. 2010;24(14):1979–1984. doi: 10.1002/rcm.4604. [DOI] [PubMed] [Google Scholar]
- 40.Yang LP, Han JP, Xue JL, et al. Nitrate source apportionment in a subtropical watershed using Bayesian model. Sci Total Environ. 2013;463-464:340–347. doi: 10.1016/j.scitotenv.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Ye RW, Toro-Suarez I, Tiedje JM, et al. H2 18O isotope exchange studies on the mechanism of reduction of nitric oxide and nitrite to nitrous oxide by denitrifying bacteria. Evidence for an electrophilic nitrosyl during reduction of nitric oxide. J Biol Chem. 1991;266(20):12848–12851. [PubMed] [Google Scholar]
- 42.Zhou Q, Takenaka S, Murakami S, et al. Screening and characterization of bacteria that can utilize ammonium and nitrate ions simultaneously under controlled cultural conditions. J Biosci Bioeng. 2007;103(2):185–191. doi: 10.1263/jbb.103.185. [DOI] [PubMed] [Google Scholar]