Abstract
Introduction: The purpose of this article is to report our institution's 10-year experience on palliative radiotherapy for the treatment of leptomeningeal carcinomatosis (LC), assessing survival, neurologic outcome, and prognostic factors.
Patients and methods: We retrospectively analyzed 110 patients who received palliative radiotherapy for LC between 2008 and 2018. The most common histologies were breast cancer (n = 43, 39.1%) and non-small cell lung cancer (NSCLC) (n = 31, 28.2%). Radiotherapy was administered as whole-brain radiotherapy (WBRT) (n = 51, 46.4%), focal spinal RT (n = 11, 10.0%) or both (n = 47, 42.7%). Twenty-five patients (22.7%) were selected for craniospinal irradiation. Clinical performance and neurologic function were quantified on the neurologic function scale (NFS) before and in response to therapy. A Cox Proportional Hazards model with univariate and multivariate analysis was fitted for survival.
Results: Ninety-eight patients (89.1%) died and 12 (10.9%) were alive at the time of analysis. Median OS from LC diagnosis and from the beginning of RT was 13.9 weeks (IQR: 7.1–34.0) and 9.9 weeks (IQR: 5.3–26.3), respectively. In univariate analysis, prognostic of longer OS were a Karnofsky performance scale index (KPI) of ≥70% (HR 0.20, 95%-CI: [0.13; 0.32], p < 0.001), initially moderate neurological deficits (NFS ≤2) (HR 0.32, 95% CI: [0.19; 0.52], p < 0.001), symptom response to RT (HR 0.41, 95%-CI: [0.26; 0.67], p < 0.001) and the administration of systemic therapy (HR 0.51, 95%-CI: [0.33; 0.78], p = 0.002). Prognostic of inferior OS were high-grade myelosuppression (HR 1.78, 95% CI: [1.06; 3.00], p = 0.03) and serum LDH levels >500 U/l (HR 3.62, 95% CI: [1.76; 7.44], p < 0.001). Clinical performance, symptom response and serum LDH stayed independently prognostic for survival in multivariate analysis. RT was well-tolerated and except for grade III myelosuppression in 19 cases (17.3%), no high-grade acute toxicities were observed. Neurologic symptom stabilization was achieved in 83 cases (75.5%) and a sizeable improvement in 39 cases (35.5%).
Conclusion: Radiotherapy is a well-tolerated and efficacious means of providing symptom palliation for patients with LC, delaying neurologic deterioration while probably not directly influencing survival. Prognostic factors such as clinical performance, neurologic response and serum LDH can be used for patient stratification to facilitate treatment decisions.
Keywords: leptomeningeal metastases, carcinomatous meningitis, neurologic function, palliative, radiotherapy, whole-brain radiotherapy, craniospinal irradiation
Introduction
Leptomeningeal carcinomatosis (LC), as defined by the current EANO-ESMO guidelines, refers to a usually multifocal spread of tumor cells to the leptomeninges (pia or arachnoidea) and/or the cerebrospinal fluid (CSF) (1). Incidence amounts to approximately 5–10% of patients presenting with metastatic cancer (1–3). The most common histologies to develop LC are breast cancer (12–35%), lung cancer (10–26%), and melanoma (5–25%). The overall incidence of LC shows an upward tendency, possibly attributable to an increase in the availability of efficacious systemic therapies and consequently prolonged survival. Advances in imaging and diagnostic capabilities may also contribute to an increasing number of LC diagnoses (4–7).
Clinical presentation can vary among a wide range of neurological symptoms, most frequently headaches and nausea (25–39%), motoric or sensory dysfunction (17–21%), altered mental status (16%) or cranial nerve deficits (11–14%) (5, 8). Notably, in 50–80% of the cases, LC is associated with additional parenchymal brain metastases (5, 8–10).
With reported median overall survival (OS) of 10–15 weeks, prognosis is dismal and therapeutic options are limited. Several approaches have been established to provide symptom palliation or prolong survival for selected subgroups (8, 11–13). Frequently, focal radiotherapy of symptomatic spinal lesions is performed to preserve neurologic function and control symptoms (13). This approach is often combined with whole brain radiotherapy (WBRT), which can positively affect survival, especially in the presence of additional brain metastases (13, 14). In addition to supportive corticosteroids, medicamentous treatment has been established primarily in the form of intrathecal chemotherapy (15–17). With the rising diversity of available small-molecule targeted therapies and the advances in molecularly informed decision-making, efficacy in the CNS has been evident for several of those drugs (18, 19).
While providing questionable benefit, many of the abovementioned therapeutical approaches are associated with substantial toxicity, most frequently critical myelosuppression and potential worsening of pre-existing symptoms (20, 21). Consequently, careful clinical assessment and patient selection, as well as interdisciplinary decision-making, are warranted before determining a course of action. It is the aim of this report to discuss our institution's experience over a period of 10 years with the radiotherapeutical treatment of 110 patients with LC and evaluate outcome, toxicity, and predictive clinical factors.
Patients and Methods
To assess the palliative outcome and efficacy of radiotherapy (RT) in the setting of LC, we performed a retrospective analysis of 110 patients, who received radiotherapy for LC between 2008 and 2018 at our institution. Patient data were extracted from an oncologic research database maintained at our institution, as well as from the patients' detailed medical records. All reviews were performed following institutional guidelines and the Declaration of Helsinki of 1975 in its most recent version. Ethics approval for the study and a waiver of written informed consent was granted by the Heidelberg University ethics committee on April 12th, 2018 (#S-172/2018). Patient confidentiality was maintained by anonymizing patient data to remove any identifying information.
Patient Characteristics
Median patient age at LC diagnosis was 59 years, and median interval from primary diagnosis was 29.5 months. Detailed patient characteristics are illustrated in Table 1. Primary histology varied: Breast cancer was most frequent (n = 43, 39.1%), followed by lung cancer (n = 31, 28.2%).
Table 1.
Patient characteristics.
| AGE(YEARS) | |
| Mean | 57.9 |
| SD | 12.27 |
| Median | 59 |
| Q1–Q3 | 52–68 |
| Min.–Max. | 17.0–78.0 |
| GENDER | |
| Female | 63 (57.3%) |
| Male | 47 (42.7%) |
| PRIMARY HISTOLOGY | |
| Breast cancer | 43 (39.1%) |
| Lung cancer | 31 (28.2%) |
| Gastrointestinal cancers | 9 (8.2%) |
| Melanoma | 7 (6.4%) |
| Prostate cancer | 6 (5.5%) |
| Others | 14 (12.7%) |
| INTERVAL FROM PRIMARY DIAGNOSIS TO LC DIAGNOSIS (MONTHS) | |
| Mean | 60.4 |
| SD | 76.81 |
| Median | 29.5 |
| Q1–Q3 | 10–86 |
| Min.–Max. | 0.0–459.0 |
| KPI AT LC DIAGNOSIS (%) | |
| Mean | 60 |
| SD | 14 |
| Median | 60 |
| Q1–Q3 | 60–70 |
| Min.–Max. | 30–90 |
| METASTASES OUTSIDE CNS | |
| Yes | 82 (74.5%) |
| No | 28 (25.5%) |
| PARENCHYMAL BRAIN METASTASES | |
| Yes | 82 (74.5%) |
| No | 28 (25.5%) |
| LAST THERAPY BEFORE RT | |
| None | 33 (30.0%) |
| Systemic chemotherapy | 30 (27.3%) |
| Targeted therapy | 20 (18.2%) |
| Antihormonal therapy | 11 (10.0%) |
| Intrathecal chemotherapy | 9 (8.2%) |
| Combination therapy | 7 (6.3%) |
| THERAPY AFTER RT | |
| None | 68 (61.8%) |
| Systemic chemotherapy | 16 (14.5%) |
| Targeted therapy | 14 (12.7%) |
| Antihormonal therapy | 5 (4.5%) |
| Intrathecal chemotherapy | 4 (3.6%) |
| Combination therapy | 3 (2.7%) |
| DIAGNOSTICS | |
| MRI | 110 (100.0%) |
| Lumbar puncture | 64 (58.2%) |
| Both | 64 (58.2%) |
| LC SPREAD | |
| Spine | 65 (59.1%) |
| Brain | 79 (71.8%) |
| Both | 46 (41.8%) |
| NEUROLOGIC FUNCTION (NFS) BEFORE TREATMENT | |
| 0 | 4 (3.6%) |
| 1 | 26 (23.6%) |
| 2 | 57 (51.8%) |
| 3 | 23 (20.9%) |
| LDH LEVEL (U/L) | |
| Mean | 371.9 |
| SD | 217.03 |
| Median | 315 |
| Q1–Q3 | 245.75–419 |
| Min.–Max. | 134.0–1267.0 |
| CRP LEVEL (MG/L) | |
| Mean | 19.4 |
| SD | 37.53 |
| Median | 3.2 |
| Q1–Q3 | 1.9–15.6 |
| Min.–Max. | 1.9–249.5 |
| HEMOGLOBIN LEVEL (U/L) | |
| Mean | 12.5 |
| SD | 1.92 |
| Median | 12.9 |
| Q1–Q3 | 11.2–13.9 |
| Min.–Max. | 7.9–16.1 |
| RPA CLASS (82 PATIENTS WITH BRAIN METASTASES) | |
| 1 | 11 (13.4%) |
| 2 | 29 (35.4%) |
| 3 | 42 (51.2%) |
| GPA SCORE (82 PATIENTS WITH BRAIN METASTASES) | |
| 0–1 | 71 (64.5%) |
| 1.5–2.5 | 10 (9.1%) |
| 3.0 | 1 (0.9%) |
| 3.5–4.0 | 0 (0.0%) |
LC, leptomeningeal carcinomatosis; CNS, central nervous system; RT, radiotherapy; KPI, Karnofsky performance scale index; MRI, magnetic resonance imaging; NFS, neurologic function scale; CRP, C-reactive protein; LDH, lactate dehydrogenase; RPA, recursive partitioning analysis; GPA, graded prognostic assessment.
Symptoms and overall clinical performance (Karnofsky performance scale index, KPI) at the beginning of RT and during follow-up were extracted from the patients' medical records and quantified regarding symptom control, improvement or worsening after therapy. Based on the documented symptoms, a neurologic function status (NFS) was derived and used to assess the palliative effect achieved by RT (22). Neurologic function was quantified in accordance with the validated functional outcome measure of two past RTOG trials that has since been adopted in several clinical analyses (22–24). It was classified on the five-point NFS scale as follows: asymptomatic (0), minor neurological symptoms (1), moderate neurological symptoms (2), neurologically seriously limited, requiring hospitalization (3), and requiring hospitalization and constant nursing care (4). The outcome of NFS was assessed as either stable, improved or worsened, according to documented symptoms. Symptom control was defined as a constant value of the NFS at therapy completion or first follow-up if available, whereas improvement was defined as a reduction of the NFS by at least one point from baseline. Recursive partitioning analysis (RPA) class and graded prognostic assessment (GPA) score were calculated for all patients who showed parenchymal brain metastases (25, 26). Date of death was obtained from medical and official records. Treatment-related toxicity was rated according to the Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0 (27). Detailed patient characteristics are illustrated in Table 1.
Treatment
Treatment indication was discussed interdisciplinarily in the context of our institution's comprehensive cancer center. Palliative treatment was indicated for symptomatic spinal lesions. Additionally, if previously untreated parenchymal brain metastases or radiographic intracerebral LC were present, WBRT including the uppermost two cervical vertebrae (WBRT-C2) was performed. Twenty-five patients were selected for craniospinal irradiation (CSI) based on good performance and estimated clinical benefit.
For cranial RT, an individual head fixation mask was fitted for each patient. Treatment planning was performed using a 3 mm computed tomography (CT) and Gadolinium enhanced MR-imaging when available. The most commonly prescribed dose for WBRT was 30 Gy in 10 fractions. An additional dose of most commonly 9 Gy in 3 fractions to large brain metastases was applied after three-dimensional conformal (3DCRT) treatment planning in 9 cases. Treatment was delivered at a linear accelerator using two laterally opposing fields for WBRT and multi-field technique for 3DCRT, as has been previously described (28, 29).
For spinal RT, the symptomatic spinal segments were identified using clinical assessment and Gadolinium enhanced MR-imaging. Treatment planning was performed based on a 3- or 5-mm CT. The target segments, commonly including a safety margin of one vertebra in upward and downward direction, but at least of 5 mm were defined as PTV. Detailed aspects of target volume delineation for spinal irradiation at our institution have been described earlier (28). The most commonly prescribed dose for spinal irradiation was 30 Gy in 10 fractions. Treatment for segmental irradiation was most commonly delivered at a linear accelerator using multi-field technique for 3DCRT. For CSI, if clinically feasible, vertebral bodies were spared to reduce hematologic toxicity. CSI was delivered as helical intensity-modulated radiotherapy (IMRT) at a TomoTherapy machine (Accuray Inc., Sunnyvale, California). All aspects of TomoTherapy planning for CSI have been described earlier (30). Details on treatment parameters are illustrated in Table 2.
Table 2.
Prior radiotherapy and detailed treatment parameters for current irradiation.
| PRIOR RADIOTHERAPY OUTSIDE CNS | |
| No | 68 (61.8%) |
| Yes | 42 (38.2%) |
| PRIOR RADIOTHERAPY TO PARTS OF THE CNS | |
| None | 63 (57.3%) |
| Spinal | 23 (20.9%) |
| SRS | 11 (10.0%) |
| WBRT | 16 (14.5%) |
| Combination of the above | 3 (2.7%) |
| EXTENT OF CURRENT IRRADIATION | |
| Only WBRT | 51 (46.4.0%) |
| Only focal spinal irradiation | 11 (10.0%) |
| Both | 47 (42.7%) |
| Total or subtotal craniospinal irradiation | 25 (22.7%) |
| PHYSICAL DOSE PER FRACTION FOR CURRENT RT (GY) | |
| Mean | 2.7 |
| SD | 0.73 |
| Median | 3.0 |
| Q1–Q3 | 2.4–3.0 |
| Min.–Max. | 2.0–8.0 |
| PHYSICAL CUMULATIVE DOSE FOR CURRENT RT (GY) | |
| Mean | 30.3 |
| SD | 6.1 |
| Median | 30.0 |
| Q1–Q3 | 30–35 |
| Min.–Max. | 8–51.0 |
| BIOLOGICALLY EQUIVALENT CUMULATIVE DOSE FOR CURRENT RT (GY) | |
| Mean | 38.2 |
| SD | 7.19 |
| Median | 39 |
| Q1–Q3 | 39–42.5 |
| Min.–Max. | 11.7–61.7 |
Biologically equivalent dose (BED) is calculated with an underlying assumed α/β = 10 for malignant cells. CNS, central nervous system; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy
Statistical Analysis
For baseline analyses descriptive statistics are used, continuous variables are given as means (SD) and/or median (IQR and range, as appropriate) and categorical variables as absolute and relative frequencies. The quantiles of the follow-up time were calculated using the reverse Kaplan–Meier method (31), additionally the naïve median follow-up over all patients was calculated. OS was calculated independently from the date of LC diagnosis and from the beginning of RT to last follow-up or death. Overall survival (OS) was investigated using the method of Kaplan–Meier. Survival curves for prognostic factors were compared using a two-sided log rank test. To identify prognostic factors on overall survival, univariate and multivariate Cox regression were used. Variables with significance in univariate analysis and meeting statistical quality criteria for multivariate modeling (applicable to all patients, not severely imbalanced) were considered in multivariate Cox regression. Since this was a retrospective exploratory data analysis, p-values are of descriptive nature. A descriptive p-value of <0.05 was considered as statistically significant. Statistical analyses were performed with the software R Version 3.4.3.
Results
Survival and Prognostic Factors
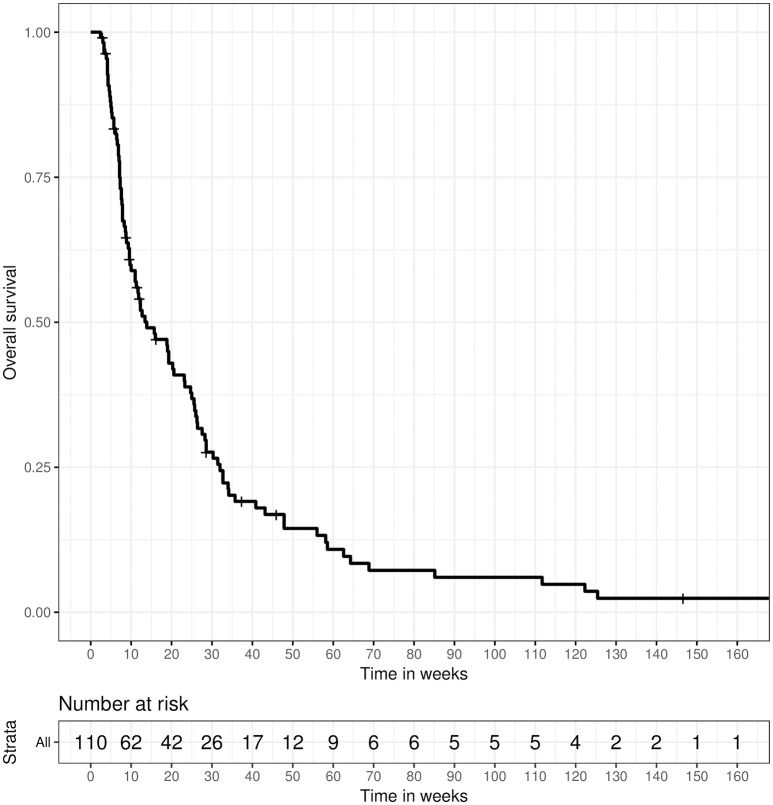
The 80%-quantile of the follow-up time, as estimated by the reverse Kaplan-Meier method, was 45.86 weeks (90%-quantile: 16.14 weeks). Directly calculating the naive median follow-up over all patients resulted in a median follow-up of 11.93 weeks (IQR: 7.14–28.57). Ninety-eight (89.1%) patients had died at the time of analysis and 12 (10.9%) patients were still alive. Median overall survival (OS) from LC diagnosis in all treated patients was 13.86 weeks (IQR: 7.14–32.00) (Figure 1). Median OS from the beginning of RT was 9.86 weeks (IQR 5.29–26.29).
Figure 1.
Overall survival for 110 patients receiving radiotherapy for the treatment of LC, calculated from the date of LC diagnosis until death or last follow-up.
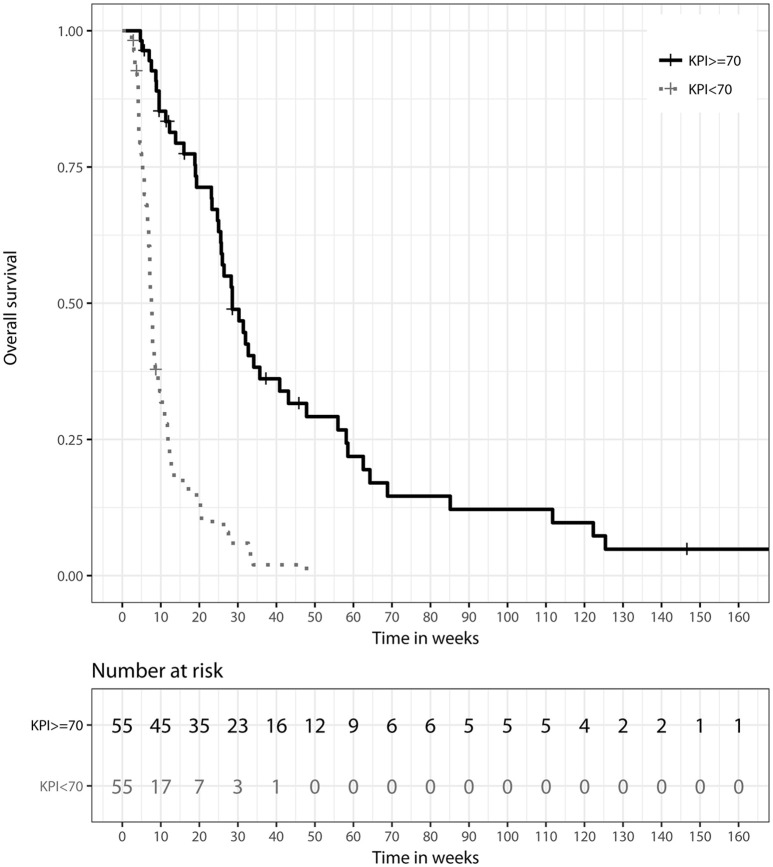
A Karnofsky performance scale index (KPI) of ≥70% was associated with a longer median OS of 28.57 weeks (IQR: 19.00–58.14), compared to 7.57 weeks (IQR: 5.29–11.86) for patients with reduced clinical performance and a KPI of <70%. This difference showed significant prognostic relevance in univariate (HR 0.20, 95%-CI: [0.13; 0.32], p < 0.0001) and multivariate analyses (HR 0.25, 95%-CI: [0.11; 0.54], p < 0.001), as illustrated in Figure 2.
Figure 2.
Kaplan–Meier survival curves stratified by Karnofsky performance scale index (KPI) at LC diagnosis, p < 0.001 (two-sided log rank test).
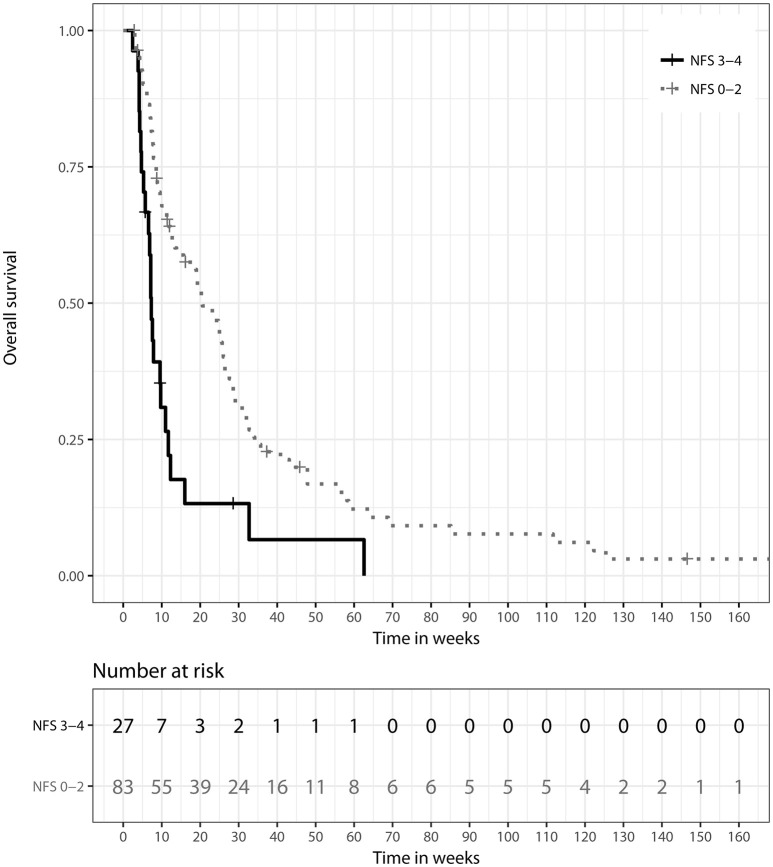
Patients showing only mild or moderate neurologic symptoms at initial presentation (NFS ≤2) had a longer median OS of 20.57 weeks (IQR: 8.57–35.71) than patients presenting with severe neurologic deficits (NFS > 2). For those patients, median OS was 7.14 weeks (IQR: 4.29–12.71), and prognostic impact of a better NFS score was detectable in univariate analysis (HR 0.32, 95% CI: [0.19; 0.52], p < 0.001). Overall symptom response to treatment was defined as a stabilization or improvement of the patient's NFS score at treatment completion or first follow-up. Symptom response was a significant prognostic factor for longer OS in univariate (HR 0.41, 95%-CI: [0.26; 0.66], p < 0.001) and multivariate analyses (HR 0.41, 95%-CI: [0.21; 0.80], p = 0.009). Respective figures for median OS were 20.57 weeks (IQR: 8.57–35.71) vs. 7.14 weeks (IQR: 4.29–12.71), as illustrated in Figure 3. Patients classified class 3 according to RPA showed significantly inferior OS when compared to class 1 in univariate analysis (HR 4.93, 95%-CI: [2.16–11.25], p < 0.001). Comparison of RPA class 2 to RPA class 1 yielded no statistical significance. GPA score showed no significant impact on OS.
Figure 3.
Kaplan–Meier survival curves stratified by neurologic function scale (NFS) response to treatment, p = 0.009 (two-sided log rank test).
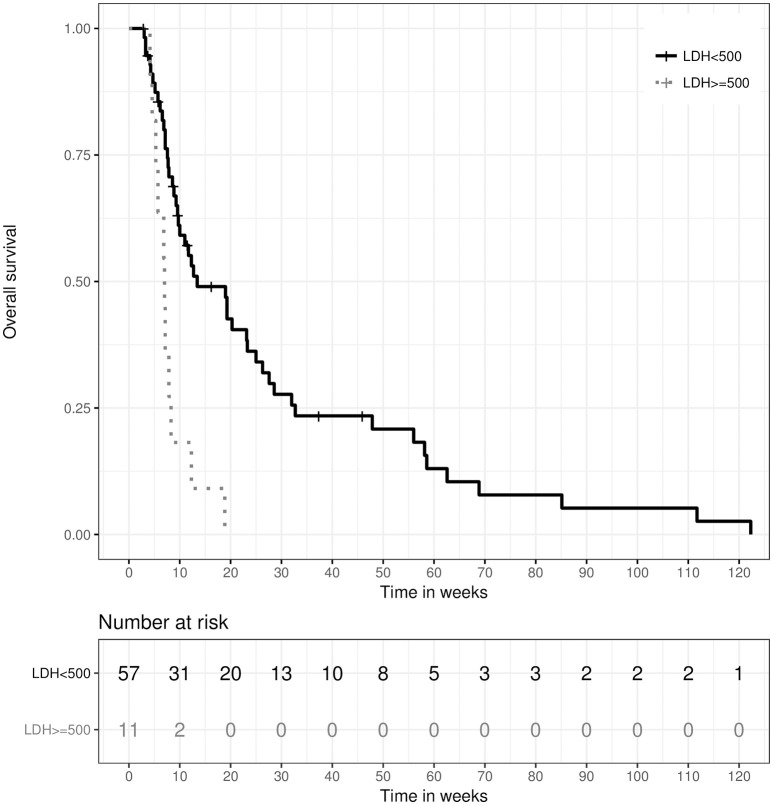
Baseline inflammatory markers and hemoglobin levels were determined out of bloodwork done within the period of 2 weeks prior to until 3 days after the beginning of RT. Furthermore, patients were regularly screened for the early detection of high-grade radiogenic myelosuppression. As such were considered any one of following: anemia with hemoglobin levels < 8 g/dl, neutropenia with leucocyte levels < 1.0/nl or thrombopenia with thrombocyte levels < 50/nl. In univariate analysis, a lactate dehydrogenase (LDH) level of >500 U/l was strongly associated with inferior median OS of 7.0 weeks (IQR: 5.29–8.29) vs. 13.43 weeks (IQR: 7.57–32.71), HR 3.62, 95%-CI: [1.8; 7.4], p < 0.001, as were higher C-reactive protein (CRP) levels >50 mg/l, though significance for CRP was not reached (6.86 weeks (IQR: 4.14–7.57) vs. 18.86 weeks (IQR: 7.71–32.71), HR 1.93, 95%-CI: [0.96; 3.88], p = 0.063). In multivariate analysis, LDH >500 U/l stayed an independent prognostic factor for inferior OS (HR 3.59, 95%-CI: [1.61; 8.01], p = 0.002), as illustrated in Figure 4. A higher hemoglobin level showed a trend toward longer OS in univariate analysis, but did not reach statistical significance (HR 0.90, 95%-CI: [0.80; 1.01], p = 0.079). However, the occurrence of high-grade myelosuppression during RT was significantly associated with inferior OS in univariate analysis (HR 1.78, 95%-CI: [1.06; 3.00], p = 0.030).
Figure 4.
Kaplan–Meier survival curves stratified by serum lactate dehydrogenase (LDH) levels > 500 U/l, p = 0.002 (two-sided log rank test).
The application of systemic therapy was assessed before and after radiotherapy, and in univariate analysis, significantly improved OS could be observed in patients who received systemic therapy after the beginning of RT (26.0 weeks (IQR: 9.57–58.14) vs. 11.28 weeks (IQR: 6.86–25.57), HR 0.51, 95%-CI: [0.33; 0.78], p = 0.002).
No prognostic significance regarding OS could be detected for any single primary histology, when tested against all other histologies. Similarly, the radiographic extent of leptomeningeal tumor spread, affecting only intracranial, only spinal or both meninges, did not significantly impact OS. Neither did the extent of the radiotherapy field(s), when comparing patients receiving only WBRT, only focal spinal RT or both. No significant difference in OS was detected for the subgroup of 25 patients who received total or subtotal craniospinal irradiation. Additional factors without statistically significant impact on OS were age at LC diagnosis, gender, the presence of parenchymal brain metastases and the application of corticosteroids. All factors examined for univariate and multivariate analyses, corresponding p-values and hazard ratios are listed in Tables 3, 4.
Table 3.
Factors analyzed in univariate cox regression with corresponding hazard ratios and p-values.
| Factor analyzed for association with overall survival | Hazard ratio | 95% CI | p |
|---|---|---|---|
| BASELINE CHARACTERISTICS | |||
| Age at LC diagnosis | 0.718 | 0.475–1.080 | 0.115 |
| Gender | 1.150 | 0.766–1.720 | 0.505 |
| Interval primary diagnosis to LC diagnosis | 1.000 | 0.998–1.000 | 0.313 |
| Metastases outside CNS present | 0.767 | 0.485–1.210 | 0.256 |
| Breast cancer histology* | 0.956 | 0.635–1.440 | 0.830 |
| Lung cancer histology* | 1.160 | 0.727–1.850 | 0.533 |
| RPA class 3 (reference = 1) | 4.935 | 2.165–11.250 | <0.001 |
| RPA class 2 (reference = 1) | 1.334 | 0.592–3.006 | 0.487 |
| GPA score 1.5–2.5 | 0.426 | 0.756–3.096 | 0.236 |
| CLINICAL PERFORMANCE | |||
| Initial KPI | 0.203 | 0.128–0.322 | <0.001 |
| Initial NFS | 0.315 | 0.192–0.517 | <0.001 |
| EXTENT OF CNS METASTASES | |||
| Intracranial LC | 0.944 | 0.622–1.430 | 0.786 |
| Spinal LC | 0.959 | 0.600–1.530 | 0.861 |
| Tumor cells in CSF | 1.150 | 0.768–1.730 | 0.493 |
| Parenchymal brain metastases | 0.856 | 0.538–1.360 | 0.512 |
| TREATMENT AND RESPONSE | |||
| Systemic therapy before start of RT | 0.809 | 0.521–1.260 | 0.345 |
| Systemic therapy after start of RT | 0.507 | 0.331–0.777 | 0.002 |
| Only spinal irradiation | 0.809 | 0.406–1.610 | 0.546 |
| Only WBRT | 0.985 | 0.661–1.470 | 0.943 |
| Both focal spinal and cranial irradiation | 1.080 | 0.724–1.620 | 0.697 |
| Total or subtotal craniospinal irradiation | 0.804 | 0.495–1.300 | 0.376 |
| Prophylactic or therapeutic corticosteroids | 1.010 | 0.552–1.860 | 0.965 |
| Neurologic response to treatment | 0.412 | 0.255–0.665 | <0.001 |
| High-grade myelosuppression after RT | 1.780 | 1.060–3.000 | 0.030 |
| SERUM AND INFLAMMATORY MARKERS | |||
| CRP > 50 mg/l | 1.930 | 0.964–3.880 | 0.063 |
| Higher hemoglobin level | 0.900 | 0.801–1.010 | 0.079 |
| Serum LDH > 500 U/l | 3.620 | 1.760–7.440 | <0.001 |
Significant p-values indicated in bold type.
No single histology reached significance in univariate analysis; for better readability only the two largest subgroups are listed. #Reference group: GPA score ≤ 1; only one patient had a GPA score ≥ 3, consequently this group was not considered. LC, Leptomeningeal carcinomatosis; KPI, Karnofsky performance scale index; CNS, central nervous system; NFS, Neurologic function scale; CSF, cerebrospinal fluid; RT, radiotherapy; WBRT, whole-brain radiotherapy; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Table 4.
Factors analyzed in multivariate cox regression with corresponding hazard ratios and p-values.
| Factor analyzed for association with overall survival | Hazard ratio | 95% CI | p |
|---|---|---|---|
| Initial NFS | 0.756 | 0.366–1.561 | 0.450 |
| Initial KPI | 0.246 | 0.112–0.541 | <0.001 |
| Neurologic response to treatment | 0.407 | 0.207–0.798 | 0.009 |
| High-grade myelosuppression after RT | 1.273 | 0.608–2.664 | 0.522 |
| Serum LDH > 500 U/l | 3.592 | 1.611–8.012 | 0.002 |
| Systemic therapy after start of RT | 0.738 | 0.364–1.495 | 0.399 |
Significant p-values indicated in bold type. All variables significant in univariate analysis were included in multivariate modeling. KPI, Karnofsky performance scale index; NFS, Neurologic function scale; RT, radiotherapy; WBRT, whole-brain radiotherapy; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Toxicity and Symptom Response
Palliative treatment was adequately tolerated, and overall, acute treatment-associated toxicity was manageable, falling under grades I and II according to CTCAE v4.03. It consisted mainly of fatigue, most commonly observed in 50.9% (n = 56) of the patients, and nausea, observed in 30.0% (n = 33) of the patients. High-grade myelosuppression, as defined above, occurred in 17.3% (n = 19) of the cases and was controllable by medical intervention. Ninety-four patients (85.5%) completed treatment, and in 16 cases (14.5%) treatment was discontinued due to disease-associated general deterioration or patient wish. Prophylactic or therapeutic corticosteroids were administered in 96 cases (87.3%). An overview of acute treatment-related toxicities is presented in Table 5.
Table 5.
Acute treatment-associated toxicity.
| Grade I | Grade II | Grade III | ||||
|---|---|---|---|---|---|---|
| Toxicity | n | % | n | % | n | % |
| Nausea | 17 | 15.5 | 15 | 13.6 | – | – |
| Fatigue | 13 | 11.8 | 55 | 50.0 | – | – |
| Skin erythema | 7 | 6.4 | – | – | – | – |
| High-grade myelosuppression | – | – | – | – | 19 | 17.3 |
Patients initially presented with a variety of LC-associated symptoms, most common among which were motor and sensory deficits, observed in 76 (69.1%) and 60 (54.5%) patients, respectively. Headaches manifested in 41 (37.3%) and visual impairments in 34 (30.9%) patients. Summarizing pre-existing neurologic symptoms on the five-point neurologic function scale, as described above, most patients (n = 83, 75.5%) presented with mild or moderate symptoms (NFS 1-2). Twenty-three patients (20.9%) showed severe neurologic deficits at initial presentation, and 4 patients (3.6%) were asymptomatic. Neurologic symptom response to treatment in the form of NFS score stabilization or improvement, as defined above, could be achieved in a total of 83 cases (75.5%); 39 (35.5%) of those cases showed a sizeable improvement. Twenty-seven patients (24.5%) showed further neurologic deterioration, irrespective of treatment. Detailed information on individual symptoms, as well as NFS, and their respective response to treatment are provided in Table 6.
Table 6.
Response of individual symptoms and neurologic function scale (NFS) to treatment.
| Before palliative RT | Clinical outcome after palliative RT | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical symptoms and NFS | Improvement | Stabilization | Worsening | |||||
| n | % | n | % | n | % | n | % | |
| Headache | 41 | 37.3 | 31 | 28.2 | 71 | 64.5 | 8 | 7.3 |
| Vomiting | 13 | 11.8 | 12 | 10.9 | 92 | 83.6 | 6 | 5.5 |
| Visual impairment | 34 | 30.9 | 14 | 12.7 | 91 | 82.7 | 5 | 4.5 |
| Seizures | 19 | 17.3 | 16 | 14.5 | 92 | 83.6 | 2 | 1.8 |
| Motor deficits | 76 | 69.1 | 35 | 31.8 | 64 | 58.2 | 11 | 10.0 |
| Sensory deficits | 60 | 54.5 | 31 | 28.2 | 71 | 64.5 | 8 | 7.3 |
| NFS 0 | 4 | 3.6 | 0 | 0.0 | 3 | 2.7 | 1 | 0.9 |
| NFS 1–2 | 83 | 75.5 | 32 | 29.1 | 31 | 28.2 | 20 | 18.2 |
| NFS 3–4 | 23 | 20.9 | 7 | 6.4 | 10 | 9.1 | 6 | 5.5 |
NFS 0: asymptomatic, NFS 1–2: mild or moderate neurologic symptoms, NFS 3–4: severe neurologic symptoms, requiring hospitalization.
Discussion
We have conducted a retrospective analysis of 110 patients with LC, treated with palliative radiotherapy at our institution over a 10-year period. Treatment was well-tolerated and achieved a palliative effect regarding symptom stabilization or even improvement in the majority (75.5%) of patients. Overall survival at 13.9 weeks was comparable to figures published in recent literature for similar patient cohorts, as will be discussed. We were able to identify initial clinical performance and symptom response to treatment as favorable and elevated LDH levels as unfavorable independent prognostic factors for OS.
High-level evidence on the treatment of LC is scarce, and most current clinical guidelines are largely based on small retrospective series or expert consensus (1, 32). One reason is the relative rarity of LC diagnosis and until recently, the exclusion of patients with LC from most prospective clinical trials (33). The current EANO-ESMO as well as the NCCN guidelines recommend a multidisciplinary approach with regard to diagnostics and treatment of patients with LC (1, 32). This approach is based on exact MRI imaging, complemented by pathological confirmation in CSF cytology (34–36). Radiotherapy is recommended in the form of WBRT to treat additional brain metastases and symptomatic intracranial lesions. Furthermore, focal spinal irradiation is performed to alleviate pain and neurologic symptoms and in the case of nodular disease to restore CSF flow (37). Regarding medicamentous therapy, systemic and intrathecal chemotherapy application have previously shown efficacy (38–40). Recently, molecularly targeted substances such as tyrosine kinase inhibitors (TKI) have produced encouraging results with proven activity in the CNS (41). In this context, primary histology and molecular analyses play an important role in recent data, since they decisively influence the range of available substances for systemic and LC treatment (33, 42, 43).
For patients with CNS metastases from breast cancer, commonly employed systemic cytotoxic agents include methotrexate (MTX) and 5-fluorouracil (5-FU) (1, 44). Trastuzumab has yielded promising results in patients with HER2-positive tumors, although evidence on the efficacy of trastuzumab-emtansine (T-DM1) is limited (45, 46). Regarding the use of TKI, lapatinib might be a promising novel agent when combined with capecitabine (47, 48). Several recent analyses have retrospectively evaluated the outcome of reasonably sized cohorts of between 60 and 100 patients (11, 21, 33). Those patients most commonly received a combination of radiotherapy and ITC with methotrexate, thiotepa or liposomal cytarabine. Estimated OS ranged from 10 to 17 weeks, and the application of ITC, as well as the combination with systemic chemo- or antihormonal therapy, was prognostic for a favorable outcome. On the other hand, high-grade histology, poor clinical performance and triple-negative tumors had a significant negative impact on survival (11, 43, 49).
Several retrospective analyses of cohorts sized between 100 and 150 patients evaluated survival in patients with LC from NSCLC. OS estimates in those series ranged between 13 and 15 weeks and were thus similar to those previously discussed for breast cancer (8, 12, 50). Favorable prognostic impact was detected for the use of ITC, WBRT, and concurrent chemotherapy, while poor clinical performance and uncontrolled intracranial pressure yielded inferior OS (8, 12, 50). Notably, patients who received EGFR-TKI therapy showed a median OS of up to 14 months in several cohorts (12, 51). Molecularly targeted substances have played a role of rising importance in the treatment of CNS metastases in patients with correspondingly mutated adeno-NSCLC. Recent developments on this horizon have been comprehensively reviewed by Cheng and colleagues (42). Although most of the reviewed data featured only small retrospective cohorts of up to 35 patients, results were conclusive in demonstrating superior OS for EGFR- or anaplastic lymphoma kinase (ALK)-mutated patients receiving corresponding TKI therapy when compared to non-mutated patients. Median OS in those cohorts ranged from 3.5 to 12 months. Prospective evidence regarding CNS response is currently emerging for newer TKIs such as osimertinib: In the AURA extension trial, median duration of CNS response was 15.2 months and progression-free survival (PFS) was 12.3 months for patients with brain metastases receiving osimertinib. A recent subgroup analysis of the FLAURA trial reported a CNS PFS rate of 77% after 12 months for patients with brain metastases and an objective response rate of 60% for a smaller subgroup of 5 patients with radiological LC, treated with osimertinib (52). Several smaller case series have reported similarly promising efficacy in patients with LC, achieving a median OS of up to 18 months (53, 54).
The primary role of radiotherapy for LC patients is symptom control and the treatment of bulky or nodular disease (1, 55). The more extensive approach of craniospinal irradiation aims at tumor cell eradication in all CNS compartments and is frequently employed for the treatment of pediatric and primary CNS tumors such as medulloblastomas or ependymomas (30, 56). Its role in the treatment of LC is marginal due to the substantial toxicity, particularly hematologic. Modern irradiation techniques, such as intensity-modulated radiotherapy with helical dose delivery or proton therapy have allowed for a significant reduction of the toxicity caused by CSI (30, 57, 58). Nevertheless, the scarce evidence available on this approach suggests, only few and highly selected patients with good clinical performance might benefit from CSI for the treatment of LC (20, 59). Our findings concerning the 25 patients, who received CSI in the current collective, confirm this rationale, although OS in this subgroup did not significantly differ from the rest of the collective and the occurrence and intensity of treatment-associated toxicity was not elevated in comparison. Regarding only the CSI-subgroup, younger age at LC diagnosis, good clinical performance and symptom response to treatment were prognostic of superior OS. Detailed clinical and technical aspects of this subgroup and an in-depth analysis of factors possibly relevant for patient selection have been discussed separately (60).
Regarding the efficacy of WBRT, several reports have found it to be associated with prolonged survival (12, 13), while other reports, as well as our current data could not show a statistically significant effect on OS (8). However, in our collective, WBRT was primarily performed in patients with additional symptomatic brain metastases and poor clinical performance, with those strong clinical prognosticators largely defining oncologic outcome, irrespective of WBRT. On the other hand, we could achieve a stabilization of intracranial pressure-associated symptoms such as headaches and vomiting in up to 83.6% of the patients and an improvement in up to 28.2%, emphasizing the palliative value of WBRT in LC patients. Neurologic function, quantified in the form of the NFS score, has proven a useful tool in the current analysis, providing better comparability and facilitating the assessment of treatment-induced effects. Two past RTOG trials, as well as several recent publications have confirmed the role and applicability of the NFS for the clinical assessment of patients with CNS metastases (22–24). Like any form of purely clinical assessment, the NFS is to a certain extent prone to subjectivity and inter-observer variability. While this may limit its applicability as an objective outcome measure, it is a tradeoff in return for simplicity and ease of clinical use. In general, however, structured analyses on the functional outcome and palliative effect of RT in LC patients are scarce, and no comparable works providing an assessment of individual symptoms as well as general neurologic function are available.
Patient selection is evidently crucial in the context of LC to avoid the burden of over-treatment in a highly palliative situation with severely limited prognosis: The body of literature discussed above widely agrees on the prognostic value of initial clinical performance, represented by the KPI (12–14, 49, 61, 62). We could confirm the impact of this prognosticator in our analysis, while additionally providing a quantitative functional assessment in the form of the NFS. Statistical significance in multivariate analysis was not reached for the initial NFS score, possibly due to correlation with the KPI. However, NFS outcome in response to treatment was prognostic for overall survival, potentially aiding in the decision about the pursuit or omission of further oncologic therapy after palliative RT. A high tumor burden, represented by strongly elevated serum LDH levels, was prognostic of inferior OS. Consequently and in light of the prospective evidence available on brain metastases, LC patients with low performance status and high tumor burden might be candidates for treatment limitation to corticosteroids and best supportive care (63).
Histology had no significant impact on survival in our current collective, for which several reasons are conceivable. As discussed above, with the availability of new histology-dependent and molecularly targeted therapies, oncologic prognosis is critically influenced by medicamentous treatment, while the focus of radiotherapy lies on symptom control (41). Accordingly, prolonged survival may be observed in histologies for which efficacious substances are available, such as breast cancer and adeno-NSCLC (42, 43). As the patients in our current collective have been treated over a period of 10 past years, only a minority received treatment with newer substances for which a substantial impact on survival can be expected. Additionally, only a small fraction of the analyzed patients received any systemic therapy in combination with or after the completion of RT. For this subgroup, a significant survival benefit could be shown, although it comprised tumors of different histologies.
In the context of LC, the safety of combining novel targeted substances with palliative radiotherapy is a question of rising importance. A recently published meta-analysis examined seven studies including patients with brain metastases, who received sequential or simultaneous TKI treatment and radiotherapy to the CNS (WBRT or stereotactic irradiation). No significant increase in neurotoxicity was observed for erlotinib and gefitinib (64, 65). Data on newer substances such as afatinib or osimertinib is scarce at best, consisting of small series or case reports (66–68). Reliable data regarding the safety and efficacy of combined treatment might in recent future prove of great importance in the treatment of LC, where fast and effective symptom palliation is just as crucial as an uninterrupted administration of effective systemic therapy (41, 68).
Limitations of the current study include its retrospective nature and relatively small number of patients. Furthermore, the heterogeneity of the patient collective, regarding histology and systemic treatment, makes it difficult to reliably identify treatment-related factors with a significant impact on survival. The fraction of patients receiving LC treatment with novel, molecularly targeted agents at 12.7% was relatively small, limiting the extent to which those treatments' effects on survival can be assessed. However, the palliative value of radiotherapy, providing symptom control or improvement in the majority of cases was evident by the current data and should be considered independently of overall prognosis.
Conclusion
To the best of our knowledge, this is the largest cohort in current literature to focus on the palliative efficacy of radiotherapy in LC patients of different histologies, while providing detailed analysis of symptom control and neurologic function. We could identify initial clinical performance, symptom response to treatment and serum LDH levels as independent prognostic factors for survival. Although general prognosis nowadays is decisively influenced by the availability of efficacious systemic treatment, radiotherapy is invaluable in providing effective palliation of neurological symptoms. Due to the limited life expectancy associated with LC, patient selection is mandatory to avoid over-treatment.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
RE, DB, JD, and SR planned and supervised this analysis as part of the neuro-radiooncological research group. KB performed data collection and review. DW performed all statistical analysis. RE reviewed data analysis and drafted the manuscript. KL, FS, SA, AP, MH, SK, JH-R, and PH contributed patient data and participated in reviewing and improving analysis and manuscript. SL provided neurologic review and guidance on respective aspects. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Footnotes
Funding. This work was supported by Heidelberg University young investigator grants to RE, DB, and JH-R.
References
- 1.Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, et al. EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. (2017) 28:iv84–99. 10.1093/annonc/mdx221 [DOI] [PubMed] [Google Scholar]
- 2.Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin. (2003) 21:25–66. 10.1016/S0733-8619(02)00032-4 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. (1990) 9:225–9. 10.1007/BF02341153 [DOI] [PubMed] [Google Scholar]
- 4.Nugent JL, Bunn PA, Matthews MJ, Ihde DC, Cohen MH, Gazdar A, et al. CNS metastases in small cell bronchogenic carcinoma. Increasing frequency and changing pattern with lengthening survival. Cancer (1979) 44:1885–93. [DOI] [PubMed] [Google Scholar]
- 5.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology (2010) 74:1449–54. 10.1212/WNL.0b013e3181dc1a69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer (1982) 49:759–72. [DOI] [PubMed] [Google Scholar]
- 7.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. (1999) 25:103–19. 10.1053/ctrv.1999.0119 [DOI] [PubMed] [Google Scholar]
- 8.Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. (2012) 7:382–5. 10.1097/JTO.0b013e3182398e4f [DOI] [PubMed] [Google Scholar]
- 9.Umemura S, Tsubouchi K, Yoshioka H, Hotta K, Takigawa N, Fujiwara K, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: okayama lung cancer study group. Lung Cancer (2012) 77:134–9. 10.1016/j.lungcan.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Lara-Medina F, Crismatt A, Villarreal-Garza C, Alvarado-Miranda A, Flores-Hernández L, González-Pinedo M, et al. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. (2012) 18:233–41. 10.1111/j.1524-4741.2012.01228.x [DOI] [PubMed] [Google Scholar]
- 11.Le Rhun E, Taillibert S, Zairi F, Kotecki N, Devos P, Mailliez A, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. (2013) 113:83–92. 10.1007/s11060-013-1092-8 [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Lee J-I, Nam D-H, Ahn YC, Han JH, Sun J-M, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. (2013) 8:185–91. 10.1097/JTO.0b013e3182773f21 [DOI] [PubMed] [Google Scholar]
- 13.Brower J V., Saha S, Rosenberg SA, Hullett CR, Ian Robins H. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. (2016) 27:130–7. 10.1016/j.jocn.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 14.Ozdemir Y, Yildirim BA, Topkan E. Whole brain radiotherapy in management of non-small-cell lung carcinoma associated leptomeningeal carcinomatosis: evaluation of prognostic factors. J Neuro Oncol. (2016) 129:329–35. 10.1007/s11060-016-2179-9 [DOI] [PubMed] [Google Scholar]
- 15.Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern cooperative oncology group. J Clin Oncol. (1993) 11:561–9. [DOI] [PubMed] [Google Scholar]
- 16.Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. (1987) 5:1655–62. 10.1200/JCO.1987.5.10.1655 [DOI] [PubMed] [Google Scholar]
- 17.Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. (1999) 5:3394–402. [PubMed] [Google Scholar]
- 18.Ceresoli GL. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. (2004) 15:1042–7. 10.1093/annonc/mdh276 [DOI] [PubMed] [Google Scholar]
- 19.Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-Naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. (2017) 35:1070–7. 10.1200/JCO.2016.69.7144 [DOI] [PubMed] [Google Scholar]
- 20.Hermann B, Hültenschmidt B, Sautter-Bihl ML. Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlenther Onkol. (2001) 177:195–9. 10.1007/PL00002398 [DOI] [PubMed] [Google Scholar]
- 21.Kak M, Nanda R, Ramsdale EE, Lukas RV. Treatment of leptomeningeal carcinomatosis: current challenges and future opportunities. J Clin Neurosci. (2015) 22:632–7. 10.1016/j.jocn.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 22.Bezjak A, Adam J, Barton R, Panzarella T, Laperriere N, Wong CS, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer (2002) 38:487–96. 10.1016/S0959-8049(01)00150-2 [DOI] [PubMed] [Google Scholar]
- 23.Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases: final results of the first two studies by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. (1980) 6:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Bernhardt D, Bozorgmehr F, Adeberg S, Opfermann N, von Eiff D, Rieber J, et al. Outcome in patients with small cell lung cancer re-irradiated for brain metastases after prior prophylactic cranial irradiation. Lung Cancer (2016) 101:76–81. 10.1016/j.lungcan.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 25.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. (2008) 70:510–4. 10.1016/j.ijrobp.2007.06.074 [DOI] [PubMed] [Google Scholar]
- 26.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. (1997) 37:745–51. 10.1016/S0360-3016(96)00619-0 [DOI] [PubMed] [Google Scholar]
- 27.National Institutes of Health NCI . Common Terminology Criteria for Adverse Events V4.0 (CTCAE). (2010) Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (Accessed December 19, 2016).
- 28.Rief H, Bischof M, Bruckner T, Welzel T, Askoxylakis V, Rieken S, et al. The stability of osseous metastases of the spine in lung cancer – a retrospective analysis of 338 cases. Radiat Oncol. (2013) 8:200. 10.1186/1748-717X-8-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharp M, Hauswald H, Bischof M, Debus J, Combs SE. Re-irradiation in the treatment of patients with cerebral metastases of solid tumors: retrospective analysis. Radiat Oncol. (2014) 9:4. 10.1186/1748-717X-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiopu SRI, Habl G, Häfner M, Katayama S, Herfarth K, Debus J, et al. Craniospinal irradiation using helical tomotherapy for central nervous system tumors. J Radiat Res. (2017) 58:238–46. 10.1093/jrr/rrw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials (1996) 17:343–6. 10.1016/0197-2456(96)00075-X [DOI] [PubMed] [Google Scholar]
- 32.Bower M, Waxman J. Central nervous system cancers. Lect Notes Oncol. (2016) 2016:96–7. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf [Google Scholar]
- 33.Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: review and update on management. Cancer (2018) 124:21–35. 10.1002/cncr.30911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamberlain M, Junck L, Brandsma D, Soffietti R, Rudà R, Raizer J, et al. Leptomeningeal metastases : a RANO proposal for response criteria. Neuro Oncol. (2017) 19:484–92. 10.1093/neuonc/now183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahendru G, Chong V. Meninges in cancer imaging. Cancer Imaging (2009) 9:S14–21. 10.1102/1470-7330.2009.9004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh SK, Leeds NE, Ginsberg LE. MR imaging of leptomeningeal metastases: comparison of three sequences. AJNR Am J Neuroradiol. (2002) 23:745–6. [PMC free article] [PubMed] [Google Scholar]
- 37.Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 38:135–40. 10.1023/A:1005982826121 [DOI] [PubMed] [Google Scholar]
- 38.Glantz MJ, Cole BF, Recht L, Akerley W, Mills P, Saris S, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. (1998) 16:1561–7. 10.1200/JCO.1998.16.4.1561 [DOI] [PubMed] [Google Scholar]
- 39.Siegal T. Leptomeningeal metastases: rationale for systemic chemotherapy or what is the role of intra-CSF-chemotherapy? J Neurooncol. (1998) 38:151–7. 10.1023/A:1005999228846 [DOI] [PubMed] [Google Scholar]
- 40.Lassman AB, Abrey LE, Shah GD, Shah GG, Panageas KS, Begemann M, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. (2006) 78:255–60. 10.1007/s11060-005-9044-6 [DOI] [PubMed] [Google Scholar]
- 41.Thomas KH, Ramirez RA. Leptomeningeal disease and the evolving role of molecular targeted therapy and immunotherapy. Ochsner J. (2017) 17:362–78. [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. (2018) 19:e43–e55. 10.1016/S1470-2045(17)30689-7 [DOI] [PubMed] [Google Scholar]
- 43.Niwinska A, Pogoda K, Michalski W, Kunkiel M, Jagiełło-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM). J Neurooncol. (2018) 138:191–8. 10.1007/s11060-018-2790-z [DOI] [PubMed] [Google Scholar]
- 44.Eano N, Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. (2017) 19:162–74. 10.1093/neuonc/now241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacot W, Pons E, Frenel J-S, Guiu S, Levy C, Heudel PE, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat (2016) 157:307–18. 10.1007/s10549-016-3828-6 [DOI] [PubMed] [Google Scholar]
- 46.Keith KC, Lee Y, Ewend MG, Zagar TM, Anders CK. Activity of trastuzumab-emtansine (TDM1) in HER2-positive breast cancer brain metastases: a case series. Cancer Treat Commun. (2016) 7:43–6. 10.1016/j.ctrc.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. (2008) 26:1993–9. 10.1200/JCO.2007.12.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. (2013) 14:64–71. 10.1016/S1470-2045(12)70432-1 [DOI] [PubMed] [Google Scholar]
- 49.De Azevedo CRAS, Cruz MRS, Chinen LTD, Peres SV, Peterlevitz MA, De Azevedo Pereira AE, et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. (2011) 104:565–72. 10.1007/s11060-010-0524-y [DOI] [PubMed] [Google Scholar]
- 50.Gwak HS, Joo J, Kim S, Yoo H, Shin SH, Han JY, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol. (2013) 8:599–605. 10.1097/JTO.0b013e318287c943 [DOI] [PubMed] [Google Scholar]
- 51.Liao BC, Lee JH, Lin CC, Chen YF, Chang CH, Ho CC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non–small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. (2015) 10:1754–61. 10.1097/JTO.0000000000000669 [DOI] [PubMed] [Google Scholar]
- 52.Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. (2018) 36 10.1200/JCO.2018.78.3118. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 53.Saboundji K, Auliac J, Pérol M, François G, Janicot H, Marcq M, et al. Efficacy of osimertinib in EGFR - mutated non-small cell lung cancer with leptomeningeal metastases pretreated with EGFR-tyrosine kinase inhibitors. Target Oncol. (2018) 13:501–7. 10.1007/s11523-018-0581-2 [DOI] [PubMed] [Google Scholar]
- 54.Li J, Liu X, Yuan C. Treatment response to osimertinib in a patient with leptomeningeal metastasis from lung adenocarcinoma following failure of gefitinib and erlotinib: a case report. Mol Clin Oncol. (2018) 9:321–4. 10.3892/mco.2018.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Network NCC. Central Nervous System Cancers (Version 1.2017). (2017) Available at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed February 24, 2018).
- 56.Tsang DS, Murphy ES, Ezell SE, Lucas JT, Tinkle C, Merchant TE. Craniospinal irradiation for treatment of metastatic pediatric low-grade glioma. J Neurooncol. (2017) 134:317–24. 10.1007/s11060-017-2525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugie C, Shibamoto Y, Ayakawa S, Mimura M, Komai K, Ishii M, et al. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat. (2011) 10:187–95. 10.7785/tcrt.2012.500194 [DOI] [PubMed] [Google Scholar]
- 58.Peñagarícano J, Moros E, Corry P, Saylors R, Ratanatharathorn V. Pediatric craniospinal axis irradiation with helical tomotherapy: patient outcome and lack of acute pulmonary toxicity. Int J Radiat Oncol. (2009) 75:1155–61. 10.1016/j.ijrobp.2008.12.083 [DOI] [PubMed] [Google Scholar]
- 59.Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol. (2010) 22:627–35. 10.1097/CCO.0b013e32833de986 [DOI] [PubMed] [Google Scholar]
- 60.El Shafie R, Böhm K, Weber D, Lang K, Schlaich F, Adeberg S, et al. (in press). Outcome and prognostic factors following palliative craniospinal irradiation for leptomeningeal carcinomatosis. Cancer Manage. Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris Z, Whiteley WN, Longstreth WT, Weber F, Lee YC, Tsushima Y, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ (2009) 339:b3016–b3016. 10.1136/bmj.b3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gani C, Müller AC, Eckert F, Schroeder C, Bender B, Pantazis G, et al. Outcome after whole brain radiotherapy alone in intracranial leptomeningeal carcinomatosis from solid tumors. Strahlenther Onkol. (2012) 188:148–53. 10.1007/s00066-011-0025-8 [DOI] [PubMed] [Google Scholar]
- 63.Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority. Lancet (2016) 388:2004–14. 10.1016/S0140-6736(16)30825-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of up-front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: a meta-analysis. Lung Cancer (2018) 122:94–9. 10.1016/j.lungcan.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Yang J, Li X, Hao D, Wu X, Yang Y, et al. First-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain radiotherapy for brain metastases in patients with EGFR-mutated lung adenocarcinoma. Cancer Sci. (2016) 107:1800–5. 10.1111/cas.13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eze C, Hegemann N-S, Roengvoraphoj O, Dantes M, Manapov F. Concurrent afatinib and whole-brain radiotherapy in exon 19-del-egfr mutant lung adenocarcinoma: a case report and mini review of the literature. Front Oncol. (2017) 7:88. 10.3389/fonc.2017.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bot I, Blank CU, Brandsma D. Clinical and radiological response of leptomeningeal melanoma after whole brain radiotherapy and ipilimumab. J Neurol. (2012) 259:1976–8. 10.1007/s00415-012-6488-4 [DOI] [PubMed] [Google Scholar]
- 68.Kroeze SGC, Fritz C, Hoyer M, Lo SS, Ricardi U, Sahgal A, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy : a systematic review. Cancer Treat Rev. (2017) 53:25–37. 10.1016/j.ctrv.2016.11.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.