Abstract
Unsatisfactory therapeutic effects of currently used antidepressants force to search for new pharmacological treatment strategies. Recent research points to the relationship between depressive disorders and the adenosinergic system. Therefore, the main goal of our studies was to evaluate the effects of DMPX (3 mg/kg, i.p.), which possesses selectivity for adenosine A2A receptors versus A1 receptors, on the activity of imipramine (15 mg/kg, i.p.), escitalopram (2.5 mg/kg, i.p.), and reboxetine (2 mg/kg, i.p.) given in subtherapeutic doses. The studies carried out using the forced swim and tail suspension tests in mice showed that DMPX at a dose of 6 and 12 mg/kg exerts antidepressant-like effect and does not affect the locomotor activity. Co-administration of DMPX at a dose of 3 mg/kg with the studied antidepressant drugs caused the reduction of immobility time in both behavioral tests. The observed effect was not associated with an increase in the locomotor activity. To evaluate whether the observed effects were due to a pharmacokinetic/pharmacodynamic interaction, the levels of the antidepressants in blood and brain were measured using high-performance liquid chromatography. It can be assumed that the interaction between DMPX and imipramine was exclusively pharmacodynamic in nature, whereas an increased antidepressant activity of escitalopram and reboxetine was at least partly related to its pharmacokinetic interaction with DMPX.
Keywords: DMPX, Antidepressants, Forced swim test, Tail suspension test, Mice
Introduction
Adenosine is widely distributed throughout the central nervous system (CNS). It functions as an endogenous agonist active at purinoreceptors (Berk et al. 2001). Adenosine modulates neuronal function via controlling the release of various neurotransmitters (Sebastião and Ribeiro 2009), e.g., it highly inhibits dopamine (DA), γ-aminobutyric acid (GABA), glutamate (Glu), acetylcholine (ACh), serotonin (5-HT), and noradrenaline (NA) release (Sebastião and Ribeiro 1996). The adenosine receptors (ARs) belong to the G protein coupled receptor superfamily (Fredholm et al. 2000; Olah and Stiles 2000). At least four ARs subtypes are known: A1, A2A, A2B, and A3, which are d istributed in different organ systems and control various physiological functions in the human body (Olah and Stiles 1992, 2000; Fredholm et al. 2000, 2001). A1R and A2AR are mainly located in the CNS (especially in the cortex and striatum, respectively), whereas both A2BR and A3R are present in the brain in low amount (Corset et al. 2000).
Studies aimed at unraveling the neurobiological basis of depression and the mechanisms underlying antidepressant effects of drugs emphasize the role of the adenosinergic system in the regulation of mood (Cunha et al. 2008; Lara 2010; Gomes et al. 2011). Because the adenosinergic system modulates neurotransmission its impact on depressive behavior is complex (Scaccianoce et al. 1989; Chau et al. 1999; Okada et al. 2001; Yamato et al. 2002). Depressant-like effect of adenosine and its analogues has been demonstrated in animal behavioral despair tests (Minor et al. 1994; Hunter et al. 2003). Moreover, the administration of classic antidepressants counteracted this effect (Kulkarni and Mehta 1985). The results of preclinical investigations highlight in particular the relationship between the manipulation of A2AR and depression and suggest that A2AR antagonists may constitute a novel strategy for the treatment of depressive disorders (Cunha 2008; Gomes et al. 2011). El Yacoubi et al. (2001, 2003) demonstrated that A2AR knockout mice are predisposed to the occurrence of antidepressant-like behavior. Likewise, Cunha et al. (2006) observed that blockade of these receptors inhibits changes in the hippocampus caused by one of the major environmental factors conducive to depression, i.e., stress. Furthermore, the therapeutic strategies currently used in depressed patients also affect the adenosinergic system (e.g., desipramine, chlorimipramine, nortriptiline, tricyclic antidepressant drugs (TCAs), are able to bind to the ARs) (Deckert and Gleiter 1989).
Because of an increasing evidence that adenosine neurotransmission is engaged in the development of psychiatric disorders, including major depressive disorder (Kaster et al. 2015; Ortiz et al. 2015; Ali-Sisto et al. 2016), and in the mechanisms underlying antidepressant effects (Deckert and Gleiter 1989; Fredholm et al. 1999; Cunha et al. 2008; Lara 2010; Gomes et al. 2011), while the number of prescribed drugs is growing (Murray and Lopez 1997; Tondo et al. 2003; Hashimoto 2011), the possible interaction between selective A2AR antagonist and commonly used antidepressants should be examined. Therefore, we assessed the antidepressant-like effect of DMPX, a synthetic analog of caffeine, which possesses higher selectivity for A2A receptors versus A1 receptors (Armentero et al. 2011), in two widely used preclinical screening tests, the forced swim test (FST) (Porsolt et al. 1977) and tail suspension test (TST) (Steru et al. 1985). In the next step, we examined the effects of DMPX on the antidepressant activity of a TCA, a selective 5-HT reuptake inhibitor (SSRI), and a selective NA reuptake inhibitor (SNRI), imipramine, escitalopram, and reboxetine, respectively. To exclude false positive outcomes obtained in the FST and TST, animal’s spontaneous locomotor activity was measured. Further, to evaluate whether the observed effects during short-term exposure to inescapable and uncontrollable stress were due to a pharmacokinetic/pharmacodynamic interaction, the levels of the studied antidepressants in the collected biological material (blood and brain tissue) were measured using a high-performance liquid chromatography (HPLC) method.
Materials and Methods
Animals
The experiment was carried out on 336 naïve adult male albino Swiss mice weighing 25–30 g, purchased from the licensed breeder (Kołacz, Warsaw, Poland). The animals were housed in the environmentally controlled rooms (temperature maintained at 21–25 °C and humidity 40–60%) in standard cages in groups of 10 with unlimited access to water and food. The rooms were illuminated with a 12-h light/dark cycle. The procedures began after at least 1-week acclimation period in the laboratory conditions and were performed between 8 a.m. and 3 p.m. to minimize circadian influences. Behavioral tests were video recorded and then analyzed by two blind experimenters. All procedures were conducted in accordance with the European Communities Council Directive and Polish legislation acts concerning animal experimentations. The procedures and protocols were approved by the First Local Ethics Committee at the Medical University of Lublin (license no. 5/2015).
Drug Administration
DMPX (3,7-dimethyl-1-propargylxanthine, 3, 6, and 12 mg/kg, Sigma-Aldrich, Poznań, Poland) was suspended in a 1% aqueous solution of Tween 80 (POCH S.A., Gliwice, Poland). Imipramine hydrochloride (15 mg/kg, Sigma-Aldrich), reboxetine mesylate (2.5 mg/kg, Ascent Scientific, Cambridge, UK), and escitalopram oxylate (2 mg/kg, Sigma-Aldrich) were dissolved in 0.9% NaCl. All solutions of antidepressants were administered intraperitoneally (i.p.) 60 min, whereas DMPX suspension was injected i.p. 30 min prior behavioral testing. The volume of all administered solutions/suspension was 0.01 ml/g. The doses and treatment schedules were selected on the basis of literature and the results of our previous experiments (Poleszak 2007; Poleszak et al. 2005, 2011, 2013, 2016a,b; Szewczyk et al. 2002, 2009; Szopa et al. 2016). Animals from the control groups received i.p. injections of saline.
FST
FST was carried out according to the method of Porsolt et al. (1977). Each mouse was placed individually for 6 min into a glass cylinder (height 25 cm, diameter 10 cm) with 15 cm of water at 23–25 °C. After the first 2 min of the test, total duration of immobility (in seconds) was measured. An animal was judged to be immobile when it ceased struggling and remained floating motionless and making only movements allowing to keep the head just above the surface of water.
TST
TST was carried out according to the method of Steru et al. (1985). Each mouse was suspended for 6 min by the tail (2 cm from the end of the tail) using adhesive tape. After the first 2 min of the test, total duration of immobility (in seconds) was measured. An animal was judged to be immobile when it ceased moving limbs and body, making only movements allowing to breathe.
Spontaneous Locomotor Activity
Spontaneous locomotor activity was assessed using Opto-Varimex-4 Auto-Track (Columbus Instruments, Columbus, OH, USA). Plexiglas cages with lids (43 × 43 × 32 cm) were equipped with a set of four infrared emitters and four detectors monitoring mice movements. Each animal was placed individually for 6 min into a cage to measure the distance (in cm) traveled between the second and the sixth minutes, which corresponds to the time interval analyzed in the FST and TST.
Determination of Antidepressant Levels in Serum and Brain Homogenates
To acquire blood and brain for pharmacokinetic studies, mice were decapitated in an appropriate time after injection of examined drugs with or without DMPX. The blood was collected into Eppendorf tubes and allowed to cloth. Then the samples were centrifuged for 10 min at 1000 rpm and the serum was collected into polyethylene tubes and frozen at − 25 °C. Brains, just after decapitation, were dissected from the skulls, rinsed with 0.9% NaCl, and frozen at − 25 °C.
Brain and serum concentrations of antidepressants were assayed by HPLC method as it was described previously (Poleszak et al. 2016a; Szopa et al. 2016).
Calibration curves that were developed on the basis of the ratio of the peak heights of the tested compounds to internal standard versus the concentration of the drug were linear in the tested concentration ranges. No interfering peaks were observed in the chromatograms. The assays were reproducible with low intra- and interday variation (a coefficient of variation less than 10%). The extraction efficiencies of the analyzed compounds and internal standards ranged from 66 to 97%. Concentrations of antidepressants were expressed in ng/ml of serum and ng/g of wet brain tissue.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) with Dunnett’s post hoc test, two-way ANOVA with Bonferroni’s post hoc test, or Student’s t test, depending on the study design. All results are presented as mean ± SEM for each experimental group. P values < 0.05 were considered statistically significant.
Results
FST
DMPX Dose-Effect Relationship in FST
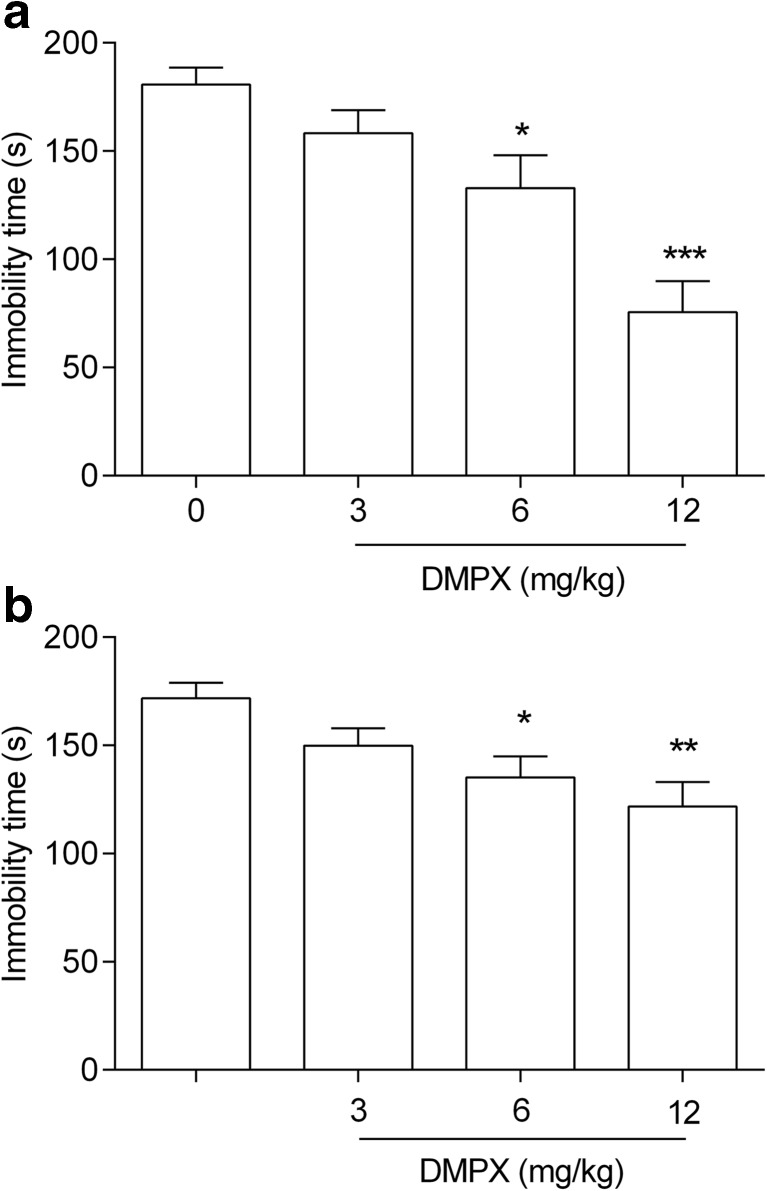
In dose-effect studies, DMPX was used in three different doses: 3, 6, and 12 mg/kg (Fig. 1a). The statistical analysis of the FST results showed that DMPX at doses of 6 and 12 mg/kg exhibited an antidepressant-like activity. DMPX at a dose of 3 mg/kg had no statistically significant influence on mice behavior in the FST (one-way ANOVA: F(3,36) = 13.48, *p < 0.05, ***p < 0.001, p > 0.05, respectively).
Fig. 1.
The antidepressant-like activity of DMPX in the FST (a) and TST (b) in mice. DMPX and saline were administered i.p. 30 min before the test. Each experimental group consisted of 10 animals. The data are presented as the means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 versus control group (one-way ANOVA followed by Dunnett’s post hoc test)
Effect of Combined Administration of DMPX and Tested Antidepressants in FST
Effect of Combined Administration of DMPX and Imipramine in FST
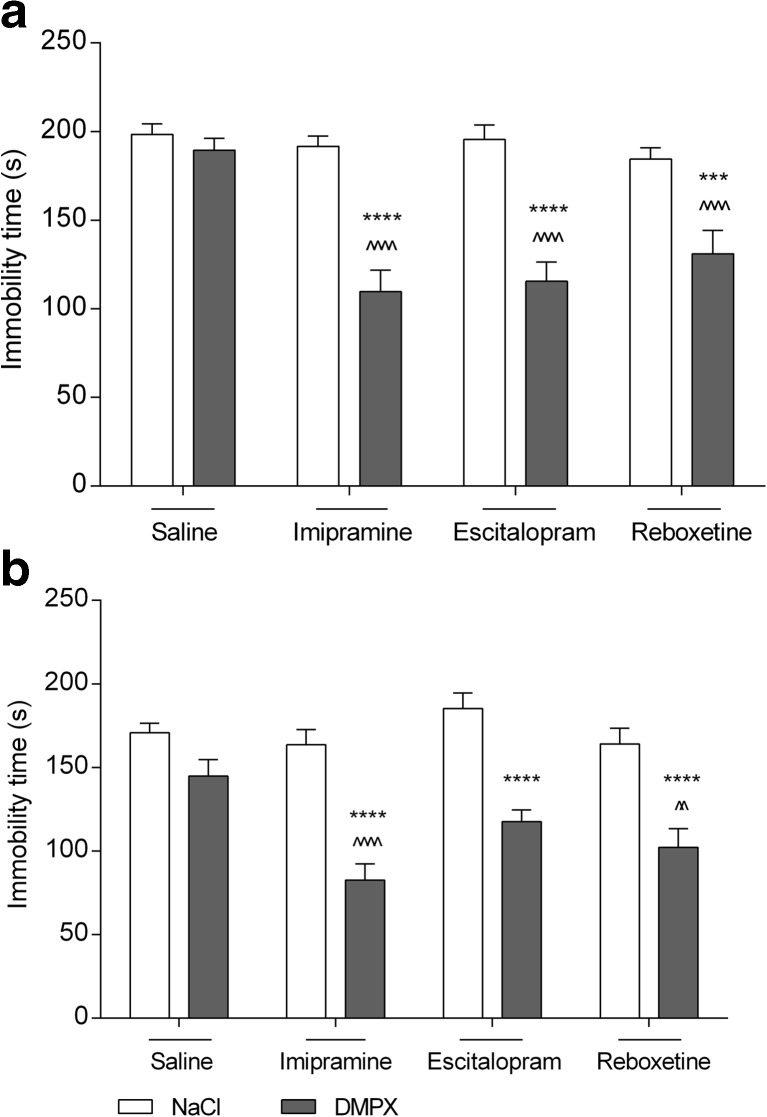
DMPX (3 mg/kg) and imipramine (15 mg/kg), administered alone, did not affect the immobility in the FST (p > 0.05) in mice. However, when given together, the duration of mouse immobility was significantly shortened (p < 0.0001) (Fig. 2a).
Fig. 2.
Effect of combined administration of DMPX and antidepressants in the FST (a) and TST (b) in mice. Antidepressants and saline were administered i.p. 60 min, whereas DMPX i.p. 30 min before the test. Each experimental group consisted of 10 animals. The data are presented as the means ± SEM. ***p < 0.001, ****p < 0.0001 versus respective antidepressant-treated group; ^^p < 0.01, ^^^^p < 0.0001 versus DMPX-treated group (two-way ANOVA followed by Bonferroni’s post hoc test)
Two-way ANOVA showed a significant effect of imipramine (F(1,36) = 29.18, p < 0.001), a significant effect of DMPX (F(1,36) = 32.23, p < 0.001), and a significant interaction between imipramine and DMPX (F(1,36) = 20.83, p < 0.001).
Effect of Combined Administration of DMPX and Escitalopram in FST
DMPX (3 mg/kg) and escitalopram (2 mg/kg), administered alone, did not affect the immobility in the FST (p > 0.05) in mice. However, when given together, the duration of mouse immobility was significantly shortened (p < 0.001) (Fig. 2a).
Two-way ANOVA showed a significant effect of escitalopram (F(1,35) = 23.20, p < 0.001), a significant effect of DMPX (F(1,35) = 31.16, p < 0.001), and no interaction between escitalopram and DMPX (F(1,35) = 19.94, p < 0.001).
Effect of Combined Administration of DMPX and Reboxetine in FST
DMPX (3 mg/kg) and reboxetine (2.5 mg/kg), administered alone, did not affect the immobility in the FST (p > 0.05) in mice. However, when given together, the duration of mouse immobility was significantly shortened (p < 0.0001) (Fig. 2a).
Two-way ANOVA demonstrated a significant effect of reboxetine (F(1,36) = 17.88, p = 0.0002), a significant effect of DMPX (F(1,36) = 13.27, p = 0.0008), and a significant interaction between reboxetine and DMPX (F(1,36) = 6.772, p = 0.0134).
TST
DMPX Dose-Effect Relationship in TST
In dose-effect studies, DMPX was used in three different doses: 3, 6, and 12 mg/kg (Fig. 1b). The statistical analysis of the TST results showed that DMPX at a dose of 6 and 12 mg/kg had an antidepressant-like activity, which was manifested by the shortening of the duration of immobility. DMPX at a dose of 3 mg/kg had no statistically significant influence on mice behavior in the FST (one-way ANOVA: F(3,36) = 13.48, *p < 0.05, **p < 0.01, p > 0.05, respectively).
Effect of Combined Administration of DMPX and Tested Antidepressants in TST
Effect of Combined Administration of DMPX and Imipramine in TST
DMPX (3 mg/kg) and imipramine (15 mg/kg), administered alone, did not affect immobility in the TST (p > 0.05) in mice. However, when given together, the duration of mouse immobility was significantly shortened (p < 0.001) (Fig. 2b).
Two-way ANOVA indicated a significant effect of imipramine (F(1,32) = 15.54, p = 0.0004), a significant effect of DMPX (F(1,32) = 36.92, p < 0.0001), and a significant interaction between imipramine and DMPX (F(1,32) = 9.736, p = 0.0038).
Effect of Combined Administration of DMPX and Escitalopram in TST
DMPX (3 mg/kg) and escitalopram (2 mg/kg), administered alone, did not affect immobility in the TST (p > 0.05) in mice. However, when given together, the duration of mouse immobility was significantly shortened (p < 0.001) (Fig. 2b).
Two-way ANOVA showed no effect of escitalopram (F(1,33) = 0.5905, p = 0.4477), a significant effect of DMPX (F(1,33) = 31.61, p < 0.0001), and a significant interaction between escitalopram and DMPX (F(1,33) = 6.251, p = 0.0176).
Effect of Combined Administration of DMPX and Reboxetine in TST
DMPX (3 mg/kg) and reboxetine (2.5 mg/kg), administered alone, did not affect immobility in the TST (p > 0.05) in mice. However, when given together, the duration of mouse immobility was significantly shortened (p < 0.001) (Fig. 2b).
Two-way ANOVA showed a significant effect of reboxetine (F(1,34) = 6.851, p = 0.0131), a significant effect of DMPX (F(1,34) = 21.82, p < 0.0001), and no interaction between reboxetine and DMPX (F(1,34) = 3.640, p = 0.0649).
Spontaneous Locomotor Activity
Effect of DMPX on Locomotor Activity in Mice
The effect of DMPX (3, 6, and 12 mg/kg) on the spontaneous locomotor activity in mice is shown in Table 1. Statistical analysis of the results demonstrated that DMPX at doses of 3, 6, and 12 mg/kg had no statistically significant effects on animal’s locomotor activity versus the control group (one-way ANOVA: F(3,28) = 1.467, p > 0.05).
Table 1.
Effect of DMPX on spontaneous locomotor activity in mice
| Treatment (mg/kg) | Distance traveled (cm) |
|---|---|
| Saline (control group) | 525.1 ± 79.04 |
| DMPX 3 | 754.0 ± 72.81 |
| DMPX 6 | 690.8 ± 62.92 |
| DMPX 12 | 687.1 ± 102.5 |
DMPX and saline were administered i.p. 30 min before the test. Distance traveled was recorded between the second and the sixth min of the test. Each experimental group consisted of eight animals. The data are presented as the means ± SEM. The results were considered statistically significant if p < 0.05 (one-way ANOVA followed by Dunnett’s post hoc test)
Effect of Combined Administration of DMPX and Tested Drugs on Locomotor Activity in Mice
The effect of combined administration of DMPX and the antidepressants on locomotor activity is shown in Table 2. DMPX, imipramine, escitalopram, and reboxetine given alone or in combination had no statistically significant effects on mice locomotor activity.
Table 2.
Effect of treatments on spontaneous locomotor activity in mice
| Treatment (mg/kg) | Distance traveled (cm) |
|---|---|
| Saline + saline (control group) | 640.7 ± 74.67 |
| DMPX 3 + saline | 955.8 ± 121.8 |
| Imipramine 15 + saline | 524.8 ± 96.23 |
| DMPX 3 + imipramine 15 | 685.2 ± 102.9 |
| Escitalopram 2 + saline | 706.5 ± 110.8 |
| DMPX 3 + escitalopram 2 | 1007 ± 145.2 |
| Reboxetine 2.5 + saline | 611.1 ± 163.9 |
| DMPX 3 + reboxetine 2.5 | 755.2 ± 81.50 |
Antidepressants and saline were administered i.p. 60 min, whereas DMPX i.p. 30 min before the test. Distance traveled was recorded between the second and the sixth min of the test. Each experimental group consisted of eight animals. Data are presented as the means ± SEM (two-way ANOVA followed by Bonferroni’s post hoc test)
Two-way ANOVA demonstrated:
(A): no effect of imipramine (F(1,26) = 3.396, p = 0.0768), a significant effect of DMPX (F(1,26) = 5.144, p = 0.0319), and no interaction (F(1,26) = 0.5452, p = 0.4669).
(B): no effect of escitalopram (F(1,26) = 0.2310, p = 0.6348), a significant effect of DMPX (F(1,26) = 6.356, p = 0.0182), and no interaction (F(1,26) = 0.003379, p = 0.9541).
(C): no effect of reboxetine (F(1,26) = 0.08845, p = 0.3556), no effect of DMPX (F(1,26) = 3.523, p = 0.0718), and no interaction (F(1,26) = 0.4887, p = 0.4907).
Pharmacokinetic Studies
Pharmacokinetic studies outcomes are shown in Table 3. In the case of combined administration of DMPX with imipramine, no significant changes in drugs concentration were observed in murine serum and brain homogenates. DMPX increased the concentrations of escitalopram and reboxetine (t test: p < 0.05) in serum without significant changes in brain tissue.
Table 3.
Effect of DMPX on the concentration of antidepressants in mouse serum and brain
| Treatment (mg/kg) |
Antidepressant concentration in serum (ng/ml) | Antidepressant concentration in brain (ng/g) | ||
|---|---|---|---|---|
| Imipramine 15 + saline (metabolite-desipramine) Imipramine 15 + DMPX 3 (metabolite-desipramine) |
229.5 ± 31.92 (39.20 ± 4.72) 215.0 ± 38.64 (40.66 ± 8.23) |
p = 0.7767 p = 0.8826 |
6193 ± 466.5 (299.0 ± 42.22) 7166 ± 1523 (183.0 ± 39.19) |
p = 0.5490 p = 0.3968 |
| Escitalopram 2 + saline Escitalopram 2 + DMPX 3 |
54.87 ± 3.76 77.55 ± 6.72 * |
p = 0.0109 | 639.1 ± 69.29 780.5 ± 103.9 |
p = 0.2721 |
| Reboxetine 2.5 + saline Reboxetine 2.5 + DMPX 3 |
91.90 ± 7.60 126.3 ± 11.70 * |
p = 0.0278 | 226.7 ± 30.82 159.0 ± 11.59 |
p = 0.0546 |
Antidepressants were administered i.p. 60 min, whereas DMPX i.p. 30 min before decapitation. Each experimental group consisted of 10 animals. Results are presented as mean values ± SEM. *p < 0.05 versus the respective control group (Student’s t test)
Discussion
Adenosine system’s capability to regulate a large number of physiological processes including the CNS activity is well-documented (Jenner 2003; Jenner et al. 2009; Perez-Lloret and Merello 2014). The amount of adenosine determines whether adenosine suppresses or intensifies neurotransmission in the CNS. Under physiological conditions, adenosine levels in the brain are maintained at 25–250 nM. The A1Rs and A2ARs are the main subtypes involved in the regulation of mental disorders, including anxiety or depression (Ruby et al. 2011). A1Rs and A2ARs have a greater binding affinity for adenosine than the A2BR and A3R (10–100 nM, 1–5 mM, respectively) (Fredholm et al. 1999, 2005a, b; Fredholm 2010; Ruby et al. 2011). The highest density of A1Rs is found in neurons of cortex, hippocampus, cerebellum, as well as in dorsal horn of spinal cord (Mahan et al. 1991; Dixon et al. 1996; Fredholm et al. 2000). These receptors participate in the tonic inhibition of neuronal activity. Presynaptic A1Rs regulate the release of neurotransmitters, whereas postsynaptic A1Rs modulate the activity of K+ channels (Ebersolt et al. 1983; Linden 1991; Linden et al. 1991; Heurteaux et al. 1995). Adenosine A2ARs are expressed mainly in the dorsal and ventral striatum and olfactory tubercle (Schiffmann et al. 1991a, b; Fink et al. 1992; Svenningsson et al. 1997a, b; Rosin et al. 1998). They exert a stimulating effect on neurons, increasing the level of cAMP in the CNS. It is also known that A2ARs bind to other neurotransmitter receptors, including dopamine (mainly D2) and glutamate receptors. Such receptor-receptor interaction seems to be necessary for striatal function and may be impaired in mental diseases (Ferré et al. 1992, 1996a, b, 1997; Ferré and Fuxe 1992). A growing evidence supports their influence on behavior, mood, and cognitive function, which results from a close relationship between the adenosine modulation and the dopaminergic and glutamatergic transduction (Fredholm et al. 2005a).
It was found that both adenosine and its analogs (e.g., 2-chloroadenosine) increased the immobility time in the FST, which equates to behavioral despair (Duman 2010). On the contrary, the non-selective inhibition of ARs in the CNS seems to induce antidepressant-like behavior in animals. One of the tested non-selective AR antagonist was caffeine, which in a dose-dependent manner effectively prolonged a mobility period in the FST (El Yacoubi et al. 2003; Szopa et al. 2016). Research aimed at determining whether selective genetic or pharmacological inhibition of A2ARs will contribute to the antidepressant-like effect has been carried out recently (El Yacoubi et al. 2001, 2003; Yamada et al. 2013). The suppression of the behavioral despair was observed by El Yacoubi et al. (2001) in mice with the genetically inactive A2ARs. In turn, Coelho et al. (2014) demonstrated that the rodents overexpressing A2ARs in the CNS structures, such as cortex, striatum, and hippocampus, exhibit symptoms of depression. The period of immobility in the FST and TST was shortened, inter alia, by the acute oral administration of istradefylline (Yamada et al. 2013) and i.p. injection of single dose of ZM241385 or SCH58261 (El Yacoubi et al. 2001, 2003). Here, the dose-dependent antidepressant-like effect, in both the FST and TST, has been demonstrated for another A2AR antagonist, i.e., DMPX, which displays higher selectivity for A2A receptors over A1 receptors. Of the three tested doses (3, 6, and 12 mg/kg), only the lowest dose did not change the mice behavior. Also, subchronic and chronic administration of a selective antagonist of A2AR, istradefylline, is characterized by a dose-dependent effect in the FST (studied dose range 0.16–2.5 mg/kg, per os) (Yamada et al. 2013) and the learned helplessness test (LH) in the rat (studied dose range 0.31–5.0 mg/kg, per os) (Yamada et al. 2014).
However, there is lack of information about the interaction between A2AR antagonists and therapeutic agents commonly used in the treatment of patients with depression. Monoamine hypothesis of depression assumes that the essential reason of depressive symptoms is a diminution of NA, 5-HT, and DA level in the CNS (Akiskal and McKinney 1973; Delgado 2000). Therapeutic agents that elevate the levels of these monoamine transmitters have been shown to be effective in the depressive disorders (Gillman 2007). The stimulation of the monoaminergic system, notably noradrenergic and serotonergic, by A2AR blockade may also produce a similar antidepressant effect (Yamada et al. 2014). In our studies, we have demonstrated the synergism of the antidepressant-like activity of the A2AR antagonist, DMPX, and selected antidepressants from various therapeutic groups. DMPX, imipramine, escitalopram, and reboxetine were injected at doses that did not affect the animals’ behavior in the FST, TST, and locomotor activity test. Concomitant administration of DMPX with these agents resulted in a significant stimulation of mice motility either in the FST or TST. The reliability of the obtained data is supported by the fact that observed effects did not correspond with increase in the mice spontaneous locomotor activity. This means that observed antidepressant-like activity of the studied agents is not a false positive.
It has been previously demonstrated that non-selective AR antagonist, caffeine, at a non-effective dose (5 mg/kg) potentiated the activity of antidepressants belonging to different classes (i.e., fluoxetine, paroxetine, escitalopram, imipramine, desipramine, reboxetine, venlafaxine, moclobemide, mianserin, milnacipran, bupropion, and agomelatine) (Kale and Addepalli 2014; Poleszak et al. 2015, 2016b; Szopa et al. 2016, 2017). The antidepressant-like effect of the analyzed drug-drug combinations (DMPX-imipramine, DMPX-escitalopram, and DMPX-reboxetine) observed in our study is probably the result of the sum of their action on the monoaminergic transmission. Moreover, it is well-known that stress-induced illnesses, such as depression, are closely related to the hypothalamic-pituitary-adrenal axis (HPA) (Pariante and Miller 2001; Pariante and Lightman 2008). For example, maternal separation, which is a model of depression (Vetulani 2013), was shown to increase corticosterone plasma levels (Biagini et al. 1998). The modification of this hormonal system activity plays an essential role in the action of TCAs and SSRIs (Reul et al. 1993, 1994). The release of steroid stress hormone, e.g., cortisol, corticosterone, is controlled by the adenosinergic system (Scaccianoce et al. 1989; Chau et al. 1999). Yamada et al. (2013) showed that injection of corticosterone suppressed the antidepressant-like behavior observed in rats after administration of A2AR antagonists. Accordingly, A2AR antagonists through direct and/or indirect modulation of the HPA axis can influence stress-induced diseases (Yamada et al. 2014). Therefore, the effects on the HPA axis may be another underpinning of the behavioral effects following concomitant administration of DMPX and antidepressants. Because antidepressants were shown to produce different effects on the HPA axis (depending on the primary mechanism of action/acute or chronic administration), it may be hypothesized that adding adenosine-mediated impact on the stress axis to the effects of antidepressant drugs on this axis, may be beneficial in terms of depression treatment.
Because DMPX is a caffeine analog, it is probable that its pharmacokinetics is similar to that of other xanthines. DMPX exhibits 100% bioavailability after both i.p. and per os administration (Yang et al. 2007). The main xanthine-metabolizing isoenzyme is CYP1A2. This isoenzyme is also engaged in the biotransformation of most drugs, including psychotropics (Caccia 1998; Nelson et al. 2004; Guengerich 2008; Zanger et al. 2008). Therefore, there was a high expectation of an interaction between DMPX and antidepressants: imipramine, escitalopram, and reboxetine. There is no information on the metabolism of DMPX either in rodents or in humans. Based on the data collected during the HPLC analysis, we found that DMPX does not significantly affect the level of imipramine, as well as desipramine (an active metabolite of imipramine) in mice. Similarly, in our previous studies, in which caffeine was used in the same scheme, there were no changes in serum concentration of imipramine (Szopa et al. 2016). The combined use of caffeine and reboxetine or escitalopram also did not contribute to the altered levels of these antidepressants in serum of mice (Szopa et al. 2016). Notwithstanding, in the case of a concomitant administration of DMPX with reboxetine and escitalopram, a small, but statistically significant enhancement in their concentrations in serum was found. Escitalopram and reboxetine levels in brain tissue did not increase/decrease statistically when administered with DMPX. It is astounding that the changes in levels of escitalopram/reboxetine in murine serum did not reflect the changes in their levels in brain homogenates. The reason for this may be a delay in the transport of these drugs across the blood-brain barrier (Burke and Preskorn 2004). The source of DMPX-escitalopram and DMPX-reboxetine interactions shown in our study is not entirely clear. The results imply that the interaction between DMPX and imipramine probably takes place in the pharmacodynamic phase, i.e., at the neurotransmitter or receptor level, etc.
Conclusions
Based on our and quoted in this paper outcomes, it can be inferred that the adenosine system plays a role in several animal tests for depression and the selective inhibitors of A2AR reverse this behavior. The present study supports the possibility of augmenting the antidepressant pharmacotherapy with DMPX or different A2AR antagonists. The interaction between the tested xanthine analog and imipramine seems to be exclusively pharmacodynamic in nature, whereas an increased antidepressant activity of escitalopram/reboxetine was at least partly related to its pharmacokinetic interaction with DMPX. In summary, the presented results indicate that A2AR may be a useful target for the therapy of depressive disorders. A2AR antagonist can be an advantageous complement to the current antidepressant pharmacotherapy, but it is necessary to perform further studies, which shall explore this issue.
Acknowledgments
The authors wish to thank the Chair and Department of Hygiene of Medical University in Lublin for access to an animal activity meter Opto-Varimex-4 Auto-Track.
Funding Information
This study was supported by the Funds for Statutory Activity of Medical University of Lublin, Poland.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) and Polish legislation acts concerning animal experimentations. The experimental procedures and protocols were approved by the First Local Ethics Committee at the Medical University of Lublin.
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Ewa Poleszak, Phone: +48 81 448 70 40, Email: ewa.poleszak@umlub.pl.
Aleksandra Szopa, Phone: +48 81 448 70 40, Email: aleksandra.szopa@umlub.pl.
References
- Akiskal HS, McKinney WT. Depressive disorders: toward a unified hypothesis. Science. 1973;182:20–29. doi: 10.1126/science.182.4107.20. [DOI] [PubMed] [Google Scholar]
- Ali-Sisto T, Tolmunen T, Toffol E, Viinamaki H, Mantyselka P, Valkonen-Korhonen M, Honkalampi K, Ruusunen A, Velagapudi V, Lehto SM. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology. 2016;70:25–32. doi: 10.1016/j.psyneuen.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Armentero MT, Pinna A, Ferré S, Lanciego JL, Müller CE, Franco R. Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol Ther. 2011;132:280–299. doi: 10.1016/j.pharmthera.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Plein H, Ferreira D, Jersky B. Blunted adenosine A2a receptor function in platelets in patients with major depression. Eur Neuropsychopharmacol. 2001;11:183–186. doi: 10.1016/s0924-977x(01)00074-8. [DOI] [PubMed] [Google Scholar]
- Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosc. 1998;16:187–197. doi: 10.1016/s0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Burke MJ, Preskorn SH. Therapeutic drug monitoring of antidepressants. In: Preskorn SH, Feigher JP, Stanga CY, Ross R, editors. Antidepressants: past, present and future. Heidelberg: Springer-Verlag; 2004. pp. 87–117. [Google Scholar]
- Caccia S. Metabolism of the newer antidepressants. An overview of the pharmacological and pharmacokinetic implications. Clin Pharmacokinet. 1998;34:281–302. doi: 10.2165/00003088-199834040-00002. [DOI] [PubMed] [Google Scholar]
- Chau A, Rose JC, Koos BJ. Adenosine modulates corticotropin and cortisol release during hypoxia in fetal sheep. Am J Obstet Gynecol. 1999;180:1272–1277. doi: 10.1016/s0002-9378(99)70628-9. [DOI] [PubMed] [Google Scholar]
- Coelho JE, Alves P, Canas PM, Valadas JS, Shmidt T, Batalha VL, Ferreira DG, Ribeiro JA, Bader M, Cunha RA, do Couto FS, Lopes LV. Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Front Psychiatry. 2014;5:67. doi: 10.3389/fpsyt.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chédotal A, Mehlen P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- Cunha GM, Canas PM, Oliveira CR, Cunha RA. Increased density and synapto-protective effect of adenosine A2A receptors upon sub-chronic restraint stress. Neuroscience. 2006;141:1775–1781. doi: 10.1016/j.neuroscience.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ferré S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Gleiter CH. Adenosinergic psychopharmaceuticals? Trends Pharmacol Sci. 1989;10:99–100. doi: 10.1016/0165-6147(89)90203-4. [DOI] [PubMed] [Google Scholar]
- Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH (2010) Models of depression. Vitam Horm 82:1–21 [DOI] [PubMed]
- Ebersolt C, Premont J, Prochiantz A, Perez M, Bockaert J. Inhibition of brain adenylate cyclase by A1 adenosine receptors: pharmacological characteristics and locations. Brain Res. 1983;267:123–129. doi: 10.1016/0006-8993(83)91045-4. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Costentin J, Vaugeois JM. Adenosine A2A receptors and depression. Neurology. 2003;61:S82–S87. doi: 10.1212/01.wnl.0000095220.87550.f6. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Bertorelli R, Ongini E, Costentin J, Vaugeois JM. Adenosine A2A receptor antagonists are potential antidepressants: evidence based on pharmacology and A2A receptor knockout mice. Br J Pharmacol. 2001;134:68–77. doi: 10.1038/sj.bjp.0704240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fuxe K. Dopamine denervation leads to an increase in the intramembrane interaction between adenosine A2 and dopamine D2 receptors in the neostriatum. Brain Res. 1992;594:124–130. doi: 10.1016/0006-8993(92)91036-e. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fuxe K, von Euler G, Johansson B, Fredholm BB (1992) Adenosine-dopamine interactions in the brain. Neuroscience 51:501–512 [DOI] [PubMed]
- Ferré S, O'Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, Stromberg I, Goldstein M, Ogren SO, Ungerstedt U, Fredholm BB, Fuxe K. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 1996;8:1545–1553. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Tinner-Staines B, Fuxe K (1996b) Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neurosci Lett 19:109–112 [DOI] [PubMed]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316:1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedeberg's Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. The role of glutamate on the action of antidepressants. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:1558–1568. doi: 10.1016/j.pnpbp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci U S A. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AM, Balleine BW, Minor TR. Helplessness and escape performance: glutamate-adenosine interactions in the frontal cortex. Behav Neurosci. 2003;117:123–135. doi: 10.1037//0735-7044.117.1.123. [DOI] [PubMed] [Google Scholar]
- Jenner P. A2A antagonists as novel non-dopaminergic therapy for motor dysfunction in PD. Neurology. 2003;61:S32–S38. doi: 10.1212/01.wnl.0000095209.59347.79. [DOI] [PubMed] [Google Scholar]
- Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A2A antagonists, and Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:406–413. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kale PP, Addepalli V. Augmentation of antidepressant effects of duloxetine and bupropion by caffeine in mice. Pharmacol Biochem Behav. 2014;124:238–244. doi: 10.1016/j.pbb.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, Baqi Y, Muller CE, Rodrigues AL, Porciuncula LO, Chen JF, Tome AR, Agostinho P, Canas PM, Cunha RA. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112:7833–7838. doi: 10.1073/pnas.1423088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Mehta AK. Purine nucleoside-mediated immobility in mice: reversal by antidepressants. Psychopharmacology. 1985;85:460–463. doi: 10.1007/BF00429665. [DOI] [PubMed] [Google Scholar]
- Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis. 2010;20(Suppl 1):S239–S248. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- Linden J, Tucker AL, Lynch KR. Molecular cloning of adenosine A1 and A2 receptors. Trends Pharmacol Sci. 1991;12:326–328. doi: 10.1016/0165-6147(91)90589-k. [DOI] [PubMed] [Google Scholar]
- Mahan LC, McVittie LD, Smyk-Randall EM, Nakata H, Monsma FJ, Gerfen CR, Sibley DR. Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol. 1991;40:1–7. [PubMed] [Google Scholar]
- Minor TR, Winslow JL, Chang WC. Stress and adenosine: II. Adenosine analogs mimic the effect of inescapable shock on shuttle-escape performance in rats. Behav Neurosci. 1994;108:265–276. doi: 10.1037//0735-7044.108.2.265. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, Kaneko S. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci. 2001;21:628–640. doi: 10.1523/JNEUROSCI.21-02-00628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. Adenosine receptors. Annu Rev Physiol. 1992;54:211–225. doi: 10.1146/annurev.ph.54.030192.001235. [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. The role of receptor structure in determining adenosine receptor activity. Pharmacol Ther. 2000;85:55–75. doi: 10.1016/s0163-7258(99)00051-0. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Ulrich H, Zarate CA, Jr, Machado-Vieira R. Purinergic system dysfunction in mood disorders: a key target for developing improved therapeutics. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;57:117–131. doi: 10.1016/j.pnpbp.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Perez-Lloret S, Merello M. Two new adenosine receptor antagonists for the treatment of Parkinson’s disease: istradefylline versus tozadenant. Expert Opin Pharmacother. 2014;15:1097–1107. doi: 10.1517/14656566.2014.903924. [DOI] [PubMed] [Google Scholar]
- Poleszak E. Modulation of antidepressant-like activity of magnesium by serotonergic system. J Neural Transm (Vienna) 2007;114:1129–1134. doi: 10.1007/s00702-007-0714-8. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Stasiuk W, Szopa A, Wyska E, Serefko A, Oniszczuk A, Wośko S, Świąder K, Wlaź P. Traxoprodil, a selective antagonist of the NR2B subunit of the NMDA receptor, potentiates the antidepressant-like effects of certain antidepressant drugs in the forced swim test in mice. Metab Brain Dis. 2016;31:803–814. doi: 10.1007/s11011-016-9810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak E, Szopa A, Wyska E, Kukuła-Koch W, Serefko A, Wośko S, Bogatko K, Wróbel A, Wlaź P. Caffeine augments the antidepressant-like activity of mianserin and agomelatine in forced swim and tail suspension tests in mice. Pharmacol Rep. 2016;68:56–61. doi: 10.1016/j.pharep.2015.06.138. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Szopa A, Wyska E, Wośko S, Serefko A, Wlaź A, Pieróg M, Wróbel A, Wlaź P. The influence of caffeine on the activity of moclobemide, venlafaxine, bupropion and milnacipran in the forced swim test in mice. Life Sci. 2015;136:13–18. doi: 10.1016/j.lfs.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Wlaź P, Szewczyk B, Kędzierska E, Wyska E, Librowski T, Szymura-Oleksiak J, Fidecka S, Pilc A, Nowak G. Enhancement of antidepressant-like activity by joint administration of imipramine and magnesium in the forced swim test: behavioral and pharmacokinetic studies in mice. Pharmacol Biochem Behav. 2005;81:524–529. doi: 10.1016/j.pbb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Wlaź P, Szewczyk B, Wlaź A, Kasperek R, Wróbel A, Nowak G. A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J Neural Transm. 2011;118:1535–1546. doi: 10.1007/s00702-011-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak E, Wośko S, Serefko A, Szopa A, Wlaź A, Szewczyk B, Nowak G, Wlaź P. Effects of ifenprodil on the antidepressant-like activity of NMDA ligands in the forced swim test in mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;46:29–35. doi: 10.1016/j.pnpbp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Reul JM, Labeur MS, Grigoriadis DE, De Souza EB, Holsboer F. Hypothalamic-pituitary-adrenocortical axis changes in the rat after long-term treatment with the reversible monoamine oxidase-A inhibitor moclobemide. Neuroendocrinology. 1994;60:509–519. doi: 10.1159/000126788. [DOI] [PubMed] [Google Scholar]
- Reul JM, Stec I, Soder M, Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology. 1993;133:312–320. doi: 10.1210/endo.133.1.8391426. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Ruby CL, Adams CA, Mrowicka M, Choi DS. Adenosine signaling in anxiety. In: Kalinin V, editor. Anxiety disorders. New Jersey: InTech; 2011. pp. 52–68. [Google Scholar]
- Scaccianoce S, Navarra D, Di SA, Angelucci L, Endroczi E. Adenosine and pituitary-adrenocortical axis activity in the rat. Neuroendocrinology. 1989;50:464–468. doi: 10.1159/000125264. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Libert F, Vassart G, Vanderhaeghen JJ. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci Lett. 1991;130:177–181. doi: 10.1016/0304-3940(91)90391-6. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA. Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol. 1996;48:167–189. doi: 10.1016/0301-0082(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009;193:471–534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997;27:322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB (1997b) Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience 80:1171–1185 [DOI] [PubMed]
- Szewczyk B, Brański P, Wierońska JM, Pałucha A, Pilc A, Nowak G (2002) Interaction of zinc with antidepressants in the forced swimming test in mice. Pol J Pharmacol 54:681–685 [PubMed]
- Szewczyk B, Poleszak E, Wlaź P, Wróbel A, Blicharska E, Cichy A, Dybała M, Siwek A, Pomierny-Chamioło L, Piotrowska A, Brański P, Pilc A, Nowak G. The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:323–329. doi: 10.1016/j.pnpbp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Szopa A, Doboszewska U, Herbet M, Wośko S, Wyska E, Świąder K, Serefko A, Korga A, Wlaź A, Wróbel A, Ostrowska M, Terlecka J, Kanadys A, Poleszak E, Dudka J, Wlaź P. Chronic treatment with caffeine and its withdrawal modify the antidepressant-like activity of ve serotonin reuptake inhibitors in the forced swim and tail suspension tests in mice. Effects on Comt, Slc6a15 and Adora1 gene expression. Toxicol Appl Pharmacol. 2017;337:95–103. doi: 10.1016/j.taap.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Szopa A, Poleszak E, Wyska E, Serefko A, Wośko S, Wlaź A, Pieróg M, Wróbel A, Wlaź P. Caffeine enhances the antidepressant-like activity of common antidepressant drugs in the forced swim test in mice. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:211–221. doi: 10.1007/s00210-015-1189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondo L, Isacsson G, Baldessarini R. Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs. 2003;17:491–511. doi: 10.2165/00023210-200317070-00003. [DOI] [PubMed] [Google Scholar]
- Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep. 2013;65:1451–1461. doi: 10.1016/s1734-1140(13)71505-6. [DOI] [PubMed] [Google Scholar]
- Yamada K, Kobayashi M, Kanda T. Involvement of adenosine A2A receptors in depression and anxiety. Int Rev Neurobiol. 2014;119:373–393. doi: 10.1016/B978-0-12-801022-8.00015-5. [DOI] [PubMed] [Google Scholar]
- Yamada K, Kobayashi M, Mori A, Jenner P, Kanda T. Antidepressant-like activity of the adenosine A(2A) receptor antagonist, istradefylline (KW-6002), in the forced swim test and the tail suspension test in rodents. Pharmacol Biochem Behav. 2013;114-115:23–30. doi: 10.1016/j.pbb.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Yamato T, Yamasaki S, Misumi Y, Kino M, Obata T, Aomine M. Modulation of the stress response by coffee: an in vivo microdialysis study of hippocampal serotonin and dopamine levels in rat. Neurosci Lett. 2002;332:87–90. doi: 10.1016/s0304-3940(02)00828-5. [DOI] [PubMed] [Google Scholar]
- Yang M, Soohoo D, Soelaiman S, Kalla R, Zablocki J, Chu N, Leung K, Yao L, Diamond I, Belardinelli L, Shryock JC. Characterization of the potency, selectivity, and pharmacokinetic profile for six adenosine A2A receptor antagonists. Naunyn Schmiedeberg's Arch Pharmacol. 2007;375:133–144. doi: 10.1007/s00210-007-0135-0. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Turpeinen M, Klein K, Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem. 2008;392:1093–1108. doi: 10.1007/s00216-008-2291-6. [DOI] [PubMed] [Google Scholar]