Highlights
-
•
Adolescent abstinent cannabis users showed significantly greater activation in the dorsolateral and ventrolateral prefrontal and posterior parietal cortices compared to controls.
-
•
Adolescent users showed increased activation in regions involved in executive functioning, attentional control and the default mode network compared to non-using controls.
-
•
No significant group differences in brain activation observed between abstinent and current adolescent cannabis users.
Keywords: Cannabis, THC, Functional magnetic resonance imaging, Meta-analysis, Abstinence
Abstract
Whether the effects of cannabis use on brain function persist or recover following abstinence remains unclear. Therefore, using meta-analytic techniques, we examined whether functional alterations measured using fMRI persist in cannabis users abstinent for over 25 days (or 600 h) as evidence suggests that the effects on cognitive performance no longer persist beyond this period. Systematic literature search identified 20 studies, of which, 12 examined current cannabis users (CCU) (361 CCU versus 394 non-cannabis using controls (NU)) and 3 examined abstinent cannabis users (ACU) in 5 separate comparisons (98 ACU versus 106 NU). Studies in ACU were carried out in adolescents and suggest significantly greater activation in components of the central executive and default mode networks in adolescent ACU compared to NU. While this evidence is to be interpreted with caution because studies were carried out in overlapping samples, they indicate a pressing need for independent confirmation whether certain neurofunctional alterations in adolescent cannabis users may persist even after cannabis and its metabolites are likely to have left their bodies.
1. Introduction
Cannabis use has been associated with changes in cognitive task performance (Curran et al., 2002; Jacobus et al., 2009; Schoeler et al., 2016a; Schreiner and Dunn, 2012a; Scott et al., 2018; Solowij and Pesa, 2010) and altered brain function (Batalla et al., 2013; Tapert et al., 2007) involving various cognitive domains. Impaired task performance (Bhattacharyya et al., 2015a; Curran et al., 2002; D’Souza et al., 2004; Hindocha et al., 2015, 2017; Lawn et al., 2016; Ramaekers et al., 2006) and brain functional alterations (Batalla et al., 2014; Bhattacharyya et al., 2017, 2015b) involving different cognitive domains have also been observed during acute intoxication. Recent meta-analyses employing different analytic approaches have shown that persistent long-term use of cannabis is associated with functional alterations in key brain regions across different cognitive tasks (Blest-Hopley et al., 2018; Yanes et al., 2018; Yücel et al., 2008).
One of the key issues that can potentially confound the interpretation of current evidence is whether the effects of cannabis use on cognition and underlying brain function abstinence persist or recover following a period of abstinence. Following abstinence, cognitive performance has been found to improve in cannabis users (CU) (Hanson et al., 2010) to the level of controls after longer periods of abstinence (Schulte et al., 2014), with cognitive deficits possibly only detectable within the first 25 days of abstinence. Meta-analysis of cognitive task performance in continuing CU has shown significant impairment over a wide range of tasks, while abstinent users showed no significant difference to controls in any specific or global cognitive domain (Schreiner and Dunn, 2012b). In contrast, structural changes in CU have been observed (Batalla et al., 2013), in particular decreased volume in the hippocampus (Chye et al., 2018; Cousijn et al., 2012; Matochik et al., 2005), that persisted after a prolonged abstinence in some (Ashtari et al., 2011) but not all studies (Koenders et al., 2017).
However, whether the effects of recreational cannabis use on brain function persist not only beyond the acute intoxication stage typically lasting 2–3 h (Grotenhermen, 2003)(when used by the inhalation route), but even after the key metabolites of delta-9-tetrahydrocannabinol (THC) with psychotropic effects have been excreted from the body, is less well known. THC and its metabolites(11-hydroxy-Δ9-tertrahydrocannabinol and 11-nor-9-carboxy-Δ9-tertrahrdrocannabinol (Sharma et al., 2012) are of particular interest, as it is the main psychotropic ingredient in cannabis known to be associated with harmful effects on various cognitive domains(Pertwee, 2008). It is worth noting that the half-life of THC in frequent users is 5–13 days (Smith-Kielland et al., 1999) and THC is detectable in urine for up-to 2–4 weeks(Lowe et al., 2009). Interestingly, the upper limit for the period of detection of metabolites in urine is consistent with the period over which cognitive deficits are detectable following abstinence (Schreiner and Dunn, 2012b).
We have recently examined the residual effects of recreational cannabis use on brain function in adult and adolescent cannabis users by meta-analytically combining the data from 20 published studies employing functional MRI techniques (Blest-Hopley et al., 2018). While some of these studies investigated cannabis-using participants after a period of abstinence, several others allowed cannabis use up until, as short a period as, 3 h prior to scanning. Therefore, interpretation of the results of these studies may be confounded by residual acute effects of THC and its metabolites that may still be left in cannabis-using participants as well as effects of withdrawal from cannabis. On the other hand, brain functional alteration following a sustained period of abstinence has also been investigated, though the results of these studies are less consistent, with users showing both increased (Chang et al., 2006; De Bellis et al., 2013; Jacobsen et al., 2004; Schweinsburg et al., 2008; Tapert et al., 2007) and decreased (Chang et al., 2006; Schweinsburg et al., 2008) activation compared to controls. Studies comparing cannabis users with different periods of abstinence have found greater activation in the prefrontal cortex and insula in recently abstinent users compared to users with longer (at least 27 days) periods of abstinence, who in turn had greater activation in the precentral gyrus (Schweinsburg et al., 2010). Another study that investigated cannabis users at multiple time-points following abstinence, reported that 28 days of abstinence resulted in reduced activation difference to controls in some regions, but some differences in brain activation persisted (Pillay et al., 2008). Therefore, understanding differences in brain activation between currently using (or non-abstinent) and abstinent cannabis users (ACU), is of particular interest. However, to our knowledge existing evidence in this regard has not been systematically reviewed and summarized using meta-analytic approaches. Hence, we have carried out a meta-analysis complementary to that previously reported by us (Blest-Hopley et al., 2018) to investigate whether altered brain function associated with regular cannabis use persists even after a sustained period of abstinence from cannabis. Consistent with our approach previously (Blest-Hopley et al., 2018), we included fMRI studies that employed a wide range of cognitive activation paradigms engaging various cognitive processes rather than focusing only on task-specific approaches as in other work (Yanes et al., 2018). Our strategy was driven by two key considerations. Firstly, only a limited number of available studies have specifically employed comparable activation paradigms limiting our ability to meaningfully investigate the question of interest here. More importantly, as we have argued before (Blest-Hopley et al., 2018), the effects of cannabis use are unlikely to be limited to only those brain regions that sub-serve cognitive processes examined in studies conducted hitherto. Rather they are more likely to be widely distributed, consistent with ubiquitous distribution of cannabinoid receptors in the brain (Iversen, 2003). Therefore, we included fMRI studies employing a range of cognitive activation paradigms to investigate using a meta-analytic approach whether brain functional alterations associated with cannabis use persist even after periods of abstinence sufficiently long such that cannabis metabolites are no longer detectable in urine.
2. Methods
2.1. Study identification
A systematic search was competed on the 13/12/2017 following the Cochrane Handbook (JPT, 2011) and the MOOSE (Stroup et al., 2000) guidelines, using the database PubMed. Two categories of search terms were used: 1) cannabis, marijuana, marihuana, THC, tetrahydrocannabinol and 2) imaging, fMRI, functional activation, BOLD. Following screening through abstract to meet inclusion criteria.
Inclusion criteria were:
-
•
A published peer-reviewed manuscript reported in English
-
•
A data-based publication
-
•
Comparison of cannabis users to a non-cannabis using control group (NU) using fMRI
-
•
Reported whole-brain imaging analysis results and not just region-of-interest analysis results
-
•
Used a cognitive or emotional activation task with no cannabis-related stimuli
Manuscripts were separated into three categories, based on the type of cannabis-using groups reported. Those that reported at least one comparison with current cannabis users (CCU) or with abstinent cannabis users (ACU) were included while those that did not report either of the previous comparisons were excluded (flow-chart in Fig. 1; number of manuscripts indicated by ‘N’). CCU were identified as those in whom the time interval between the last cannabis smoke and scanning was a maximum of 48 h (so as to avoid peak withdrawal symptoms (Budney et al., 2004)) with inclusion criteria requiring at least an average weekly use of cannabis or a positive test for THC or its metabolites at urine drug screening. ACU were required to have a monitored period of abstinence from cannabis use for at least 600 h/25 days based on previous evidence of upper limit of period of detection of THC metabolites in urine (Schreiner and Dunn, 2012b) and to have provided a negative urine test for THC.
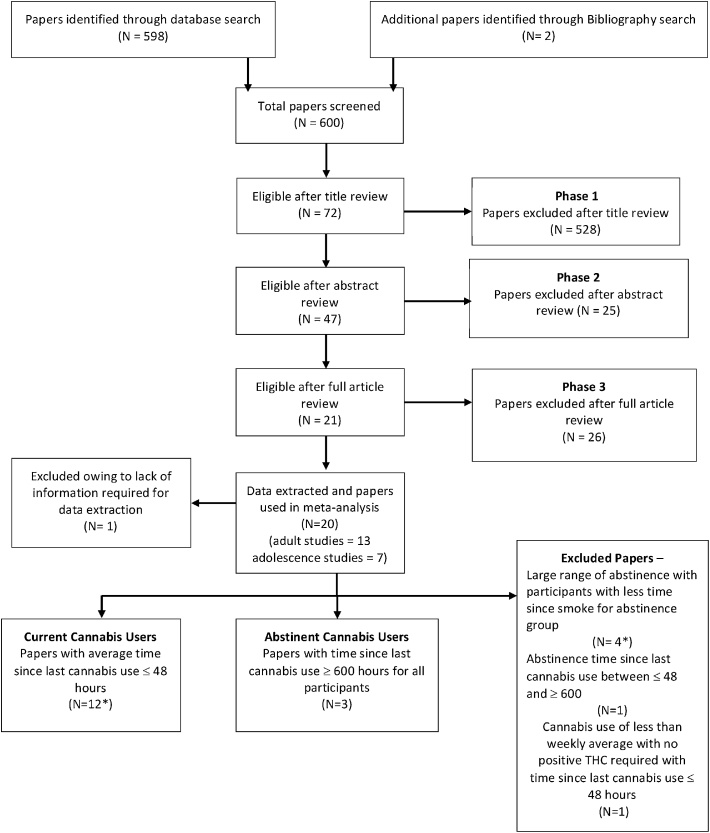
Fig. 1.
Flow-chart showing the identification, classification and inclusion of papers selected for meta-analysis.
*One paper included two studies, one of which was eligible for inclusion, one of which was excluded.
2.2. Data extraction
Data extraction and data analysis using seed-based d mapping (SDM)(Sdmproject.com, 2017) was conducted as outlined previously (Blest-Hopley et al., 2018) and are described here in brief. Significant peak coordinates were extracted from included studies along with their t-statistic. In papers reporting z- values or p –values, t- statistics were computed using a converter provided with SDM (www.sdmproject.com/utilities /?show = statistics). In case of studies where no inferential statistical values were reported, a ‘p’ or ‘n’ was used to indicate a positive or negative peak, respectively. As per protocol for SDM meta-analysis (Radua et al., 2012) a study-specific text file was created for each study included in the meta-analysis, including the coordinates reported, t-value, and the number of participants for each group. In this file, the sign of the t-statistic was positive or negative, depending on the comparisons of interest. For example, for comparisons between CCU and healthy controls, greater activation in CCU compared to healthy controls was indicated as a positive t-statistic and vice versa. Similarly, for comparison of ACU with healthy controls, greater activation in ACU compared to healthy controls was indicated as a positive t-statistic and vice versa. Following this we tested whole-brain differences in activation between CCU and ACU by calculating the difference between both groups in each voxel and determining its statistical significance using a randomization test (Radua et al., 2010).
Information regarding the brain template used to report cluster coordinates (for example, Montreal Neurological Institute (MNI) or Talairach) was included in each study-specific file text file created as above. Any study that reported no significantly different activation peaks was also included. Each contrast completed between CU (CCU or ACU) and controls was extracted and treated as a separate studies, for example if a study reported the results of separate encoding and recall conditions in a memory task, these were treated as two separate studies, following established protocol (Radua et al., 2012). Each study was then assigned as a study with current (CCU) or abstinent (ACU) users.
2.3. Data analysis
Meta-analysis was carried out using seed based-d-mapping (Sdmproject.com, 2017), using the methods previously outlined (Radua et al., 2014). For voxels that contained a peak coordinate, the unbiased effect-size and variance were computed using standard formulae (Hedges and Olkin, 1985), while for all other voxels, the effect-size was estimated based on their distance to nearby peaks, using a 20 mm full-width-at-half-maximum non-normalized Gaussian kernel (Radua and Mataix-Cols, 2009). For voxels that were assigned a value from more than one peak coordinate, an average value was estimated by weighting by the square of the distance to each close peak. To reduce bias from a publication reporting numerous closely located peaks, a study maximum value was employed. Both the positive and negative activations were assigned to the same map. For coordinates with no t-value, a threshold-based imputation of effect-size was carried out by estimating the mean effect-size of peaks from studies that did report t-values, separately for each significance threshold. Individual effect-size maps were created for each study and a random effects model meta-analytically combined the data from each study, by weighting each study with the inverse of the sum of its variance plus the between-study variance as obtained by the DerSimonian-Laird estimator (DerSimonian and Laird, 1986). This has been shown to be statistically comparable to the restricted maximum likelihood (Viechtbauer, 2005). All maps were then included in to a meta-analysis seed-based d map, where a null distribution of the meta-analytic values was created to test which voxels had studies reporting activation difference around them by chance, using monte-carlo randomizations. Due to previous work yielding highly stable results with 20 randomizations (Radua et al., 2012), we carried out 20 randomisations for each meta-analysis. The co-ordinates of cluster peaks were then reported using MNI coordinates.
Three initial meta-analyses were completed comparing CCU to NU; comparing ACU to NU and, comparing activation in CCU to ACU. Two further meta-analyses were completed, as the ACU group contained only adolescent participants, comparing adolescent CCU compared to adolescent NU and comparing adolescent CCU to the adolescent ACU. Results have been thresholded to ensure voxel threshold = p < 0.005, peak height threshold: peak SDM-Z < 1, and a cluster-size threshold of clusters ≥ 10 voxels. The co-ordinates of cluster peaks were reported using MNI coordinates.
2.4. Assessment of study heterogeneity and publication bias
Heterogeneity Q statistic was assessed in terms of a chi-squared distribution after conversion to standard z values and reported. Between–study heterogeneity was assessed by comparison of heterogeneity maps. Funnel plots were created for each cluster peak and Egger’s test performed in order to asses publication bias (Sedgwick and Marston, 2015).
2.5. Assessment of study quality
Quality assessment of each study was completed using criteria previously used for fMRI studies (Radua et al., 2015), which we have reported before (Blest-Hopley et al., 2018).The quality assessment reported here has been amended to also include assessment of the extent to which studies accounted for use of substances other than cannabis. No studies were excluded from analysis based on this quality assessment. Studies that matched groups on use of substances other than cannabis scored 2 points, while those that did not study matched groups but instead used statistical methods to control for group differences in use of substances other than cannabis scored 1 point. Finally, certain studies controlled for only some substances and were rated 0.5 and studies that neither matched participant groups based on use of substances other than cannabis, nor controlled for them analytically were rated 0.
3. Results
3.1. Included studies
Twenty studies were identified as meeting study inclusion criteria as detailed earlier. Of those, twelve manuscripts met inclusion criteria as reporting CCU, with 22 separate comparisons, comparing 361 CCU to 394 NU (Table 1). Three manuscripts were identified as having studied ACU with 5 separate comparisons, comparing 98 ACU to 106 NU (Table 1). Five manuscripts and one comparison from another manuscript were excluded as they had large ranges of abstinence within their cannabis using group (Chang et al., 2006; Heitzeg et al., 2015; Jager et al., 2013; Nestor et al., 2010, 2008; van Hell et al., 2010) or had abstinent periods (between 48 and 600 h) that were not consistent with our pre-defined abstinence criteria for inclusion in the ‘abstinent users’ group. Number of studies included for each comparison are indicated by ‘k’ and number of participants for each comparison are indicated by ‘n’ henceforth in the text as well as in the relevant sections of tables or figures.
Table 1.
Studies included in meta-analysis.
| Current Cannabis User Studies | fMRI activation task | CU M/F | NU M/F | Age of CU (years) |
Age of NU (years) |
Quantity of cannabis used by CU | Time between scan and last smoke * | Age of onset of cannabis use for CU (years) | Average years of cannabis use by CU | Task condition | Results whole brain analysis | Task Performance results | Number of task comparisons | Tesla |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdullaev et al., 2010 | Attention Network Task | 10/4 | 10/4 | 19.5 (0.8) (SD) |
19.7 (1.4) (SD) |
71-196 days per year | 48 | 12-16 | N/A | Executive task; Alerting task; Orienting task. | CU > NU R-LPFC, supplementary motor cortex, Lateral parietal cortex; No difference for alerting & orientation task. | Longer reaction time for CU. More errors made for executive task. | 3 | 3T |
| Use Generation Task | 5/2 | 5/2 | 19.6 (0.9) (SD) |
20 (0.2) (SD) |
71-196 days per year | 48 | 12-16 | N/A | Generating nouns versus reading nouns; difficult words versus easy words. | CU > NU R-VPFC NU > CU Bi -ACG to L-PFC, L- TPC; CU > NU R - ACC, R FOC, L frontal pole & L precuneus. | N/A | 2 | 3T | |
| Smith et al., 2011 | Go/NoGo Task | 6/4 | 9/5 | 19-21 | 19-21 | > 1 joints per week | 3 | N/A | 4.55 years |
Press all but X; Press X | No significant differences in both tasks after including covariates. | No Significant difference | 2 | 1.5T |
| Chang et al., 2006 | Visual- Attention Task | 9/3 | 11/8 | 27.91 6 3.13 (SEM) |
30.57 6 1.83 (SEM) |
≥5 days per week | 4 | 9–20 | 36–448 months | Visual attention | CU > NU small clusters of L precuneus, L-LG & L limbic uncus. NU > CU R-FC, Bi- dorsal parietal and R cerebella. | No Significant difference | 1 | 4T |
| Cousijn et al., 2012 | Iowa Gambling Task | 21/11 | 26/15 | 21.4 (2.3) (SD) |
22.2 (2.4) (SD) |
> 10 days per month | 38.4 | N/A | 2.5 (1.9) (SD) |
Win > Loss; Loss > Win | CU > NU R-OFC, R insula, L-STG; No activation difference. | No Significant difference | 2 | 3T |
| Gruber, Rogowska and Yurgelun-Todd, 2009 | Facial effect task | 14/1 | 14/1 | 25 (±8.8) | 26 (±9.0) | 4-7 days per week | 12 | 14.9 (±2.50) | N/A | Viewing Angry; Viewing Happy | CU > NU R-IFG, R-precuneus, R-paracetral lobe, L-SFG, cerebellar, R-MiTG, NU > CU L-SPL, interhemispheric precuneus, L- CG ; CU > NU cerebella, NU > CU L -STG & sub.lobular space. | No Performance data | 2 | 3T |
| Heitzeg et al., 2015 | Emotional arousal word task | 12/8 | 14/6 | 19.84 (1.45) (SD) |
20.51 (1.26) (SD) |
>100 time (average 618.12) | 48 | N/A | 13.4 (2.7) (SD) |
Negative words; Positive words. | NU > CU R- MiFG, R- DLSFG, R- MiTG, R-STG, R calcarine fissure, R- L, insula CU > NU R Dorsolateral SFG, NU > CU R-IPL. | No Significant difference | 2 | 3T |
| King et al., 2011 | Checker-board task | 16/14 | 16/14 | M = 21 F = 22.5 |
M = 23 F= 24.5 |
6-7 days per week | 12 | M = 14.5 F = 16 (years) |
M = 78 F = 63 (months) |
2HZ frequency; 4HZ frequency | CU > NU SFG, NU > CU LG & cuneus; L- postcentral gyrus, Bi- MiFG, R-SPG, R- frontal pole, NU > CU R- postcentral gyrus, R- precentral gyrus & L- LG. | None Taken | 2 | 3T |
| Kanayama et al., 2004 | Spacial working memory Task | 10/2 | 6/4 | 37.9 (7.4) (SD) |
27.8 (7.9) (SD) |
5100-54000 life time use | 21 | N/A | >5000 lifetime use | Short- delay task minus perception task. | CU > NU R-SFG, L-MiFG, IFG, R-STG, Bi. ACG. R. precentral gyrus, Bi -caudate & R-putamen. NU > CU Bi -MiFC. | No Significant difference | 1 | 1.5T |
| Wesley, Hanlon and Porrino, 2011 | Iowa Gambling Task | 9/7 | 6/10 | 26.4 (3.6) (SD) |
26.6 (6.1) (SD) |
Mean 29.4 days per month | 12 | 16.3 (2.1) (SD) |
9.6 (4.1) (SD) |
Win; Lose | No difference in Win; NU > CU Bi. MFG, R ACC, R-Precuneus & R- SPL, L declive. |
More loss events for CU | 2 | 1.5T |
| Adolescent Current Cannabis Users | Task | CU M/F | NU M/F | Age of CU (years) |
Age of NU (years) |
Quantity of cannabis used by CU | Time between scan and last smoke * | Age of onset of cannabis use for CU (years) | Average years of cannabis use by CU | Trials | Results whole brain analysis | Task Performance results | Number of task comparisons | Tesla |
| Acheson et al., 2015 | Win/Lose Gambling Task | 11/3 | 11/3 | 17.3 (1.3) (SEM) |
17.6 (1.0) (SEM) |
>5 uses per week | 12 | N/A | N/A | Win ; Loss | CU > NU Bi- MiFG, caudate claustrum; CU > NU R- MiFG, R- PCC R- ACC, L-Insula, Bi. claustrum Bi- declive. | Not Reported | 2 | 3T |
| Behan et al., 2014 | Go/NoGo Task | 16/1 | 17/1 | 16.5 (0.2) (SEM) |
16.1 (0.4) (SEM) |
42.9 mean joints per week | 12 | 13 (0.2) (SEM) |
N/A | Successful inhibition | NU > CU Bi. white matter adjacent to ACC. | CU significantly worse at inhibition task. | 1 | 3T |
| Lopez-Larson et al., 2012 | Finger Tapping | 22/12 | 17/7 | 18.2 (0.7) (SD) |
18.0 (1.9) (SD) |
Mean use of 10.3 joints per week | 24 | 15.3 (1.4) (SD) |
N/A | Finger taping | NU > CU R- CG | Not Reported | 1 | 3T |
| Abstinent Cannabis User Studies |
Task | CU M/F | NU M/F | Age of CU (years) |
Age of NU (years) |
Quantity of cannabis used by CU | Time between scan and last smoke * | Age of onset of cannabis use for CU (years) | Average years of cannabis use by CU | Trials | Results whole brain analysis | Task Performance results | Number of task comparisons | Tesla |
| Schweins-burg et al., 2011 | Verbal Encoding Task | 27/9 | 29/9 | 18.1 (0.9) 18.0 (1.0) (SD) |
17.6 (0.8) 18.1 (0.7) (SD) |
480.7 (277.2 SD) life time use | 600 | 14.5 (2.5) 14.9 (3.4) (SD) |
N/A | Novel encoding | No significant difference. | No Significant Difference | 1 | 3T |
| Schweins-burg et al., 2008 | Spacial working memory Task | 11/4 | 12/5 | 18.1 (0.7) (SD) |
17.9 (1.0) (SD) |
480.7 (277.2 SD) life time use | 672 | N/A | 4.0 (1.6) (SD) |
SWM> Viligance; Viligance> SWM. |
CU > NU R- SPL NU > CU R- DLPFC; CU > NU R - Inferior cuneus. |
No Significant Difference | 2 | 1.5T |
| Tapert, et al, 2007 | Go/NoGo Task | 12/4 | 12/5 | 18.1 (0.7) (SD) |
17.9 (1.0) (SD) |
>60 times | 672 | 14.0 (1.6) (SD) |
N/A | Inhibition; Go | CU > NU Bi- SFG, Bi- MiFG, R-Insula, L- MPFC, Bi- PPC, R- LG, CU > NU R-IFG, R- insula, R-SFG, R-SPL, R-IPL, R medial precuneus. | No Significant Difference | 2 | 1.5T |
*Time between scan and last smoke reported here as the mean or median estimate (number of hours) reported in the manuscript, or based on the inclusion/exclusion criterion related to minimum period of abstinence reported in the manuscript.
CU = Cannabis users NU = Non-using controls, R = Right, L = Left, Bi = Bilateral, LPFC = Lateral Prefrontal Cortex, VPFC = Ventrolateral Prefrontal Cortex, DLPFC = Dorsolateral Prefrontal Cortex, MPFC = Medial Prefrontal Cortex, PFC = Prefrontal Cortex, OFC = Orbitofrontal Cortex, FC = Frontal Cortex, MFG = Medial Frontal Cortex, MiFG = Middle Frontal Gyrus, SFG = Superior Frontal Gyrus, DLSFG = Dorsolateral Superior Frontal Gyrus, FOC = Frontal Orbital Cortex, IFG = Inferior Frontal Gyrus, PPC = Posterior Parietal Cortex, IPL = Inferior Parietal Lobe, TPC = Temporo-Parietal Cortex, SPL = Superior Parietal Lobe, SPG = Superior Parietal Gyrus, MTG = Medial Temporal Gyrus, MiTG = Middle Temporal Gyrus, STG = Superior Temporal Gyrus, LG = Lingual Gyrus, CG = Cingulate Gyrus, ACC = Anterior Cingulate Cortex, ACG = Anterior Cingulate Gyrus.
Summary of quality assessment of studies included in the present meta-analyses are reported in Table 2. Although, no studies were excluded from analysis based on this quality assessment, as is evident from the summary, studies did not always control for the effect of substances other than cannabis that may have been used by study participants. However, it is worth noting that all of the adolescent ACU studies controlled for these effects statistically.
Table 2.
Quality assessment.
| Study | Sample size | Inclusion criteria | Exclusion criteria | Control for other substance use | Match for age/sex/ handedness/ education |
Control for motion artefacts | Co-registration with anatomical image | Software and statistical test applied | Correction for multiple testing | Sum of the scores & category |
|---|---|---|---|---|---|---|---|---|---|---|
| Current Cannabis Users | ||||||||||
| Abdullaev et al., 2010 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 14 |
| Smith et al., 2011 | 0.5 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 13 |
| Chang et al., 2006 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 15 |
| Cousijn et al., 2012 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 17 |
| Gruber, Rogowska and Yurgelun-Todd, 2009 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 0 | 13 |
| Heitzeg et al., 2015 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 16 |
| King et al., 2011 | 2 | 2 | 2 | 0.5 | 1 | 2 | 2 | 2 | 2 | 15.5 |
| Kanayama et al., 2004 | 0.5 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 0 | 11.5 |
| Wesley, Hanlon and Porrino, 2011 | 1 | 1 | 2 | 0.5 | 2 | 2 | 2 | 2 | 2 | 14.5 |
| Adolescent Current Cannabis Users | ||||||||||
| Acheson et al., 2015 | 1 | 2 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 13 |
| Behan et al., 2014 | 1 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 13 |
| Jager et al., 2013 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 12 |
| Adolescent Abstinent Cannabis User Studies | ||||||||||
| Schweinsburg et al., 2011 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 15 |
| Schweinsburg et al., 2008 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 13 |
| Tapert, Schweinsburg and Brown, 2008 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 14 |
Rating criteria:Sample size: n1 < 12,n2 < 12: 0 point; n1 < 12,n2 = 12-20: 0.5 point; n1 < 12,n2 > 20: 1 point; n1 = 12–20,n2 < 12: 0.5 point; n1 = 12–20,n2 = 12-20: 1 point; n1 = 12–20,n2 > 20: 1.5 point; n1 > 20,n2 < 12: 1 point; n1 > 20,n2 = 12-20: 1.5 point; n1 > 20,n2 > 20: 2 point. Inclusion criteria: 0 (not reported), 1 (partly reported), 2 (reported). Exclusion criteria 0 (not reported), 1 (only one reported), 2 (reported). Control for other substance use: Groups not matched for other substance use and not statistically controlled for 0 points; groups not matched for other substance use and only some substances statistically controlled for 0.5 points; groups not matched for other substance use, but statistically controlled for 1 point; groups matched for other substance use 2 points. Matched for age/sex/handedness/education: 0 (for no parameter), 1 (partly), 2 (for all parameters). Control for motion artefacts: 0 (not performed), 2 (performed). Co-registration with anatomical image: 0 (not performed), 2 (performed) Software and statistical test applied: 0 (not reported), 1 (partly reported), 2 (reported).
Correction for multiple testing: 0 (not corrected), 2 (corrected).
All studies qualifying for the ACU group, were carried out in adolescent users. Further meta-analysis comparing ACU and CCU, using only adolescent studies was completed. Data from three manuscripts reporting on current adolescent users, with four separate comparisons, with 69 CCU and 70 NU were used for comparison with the abstinent adolescent user group described above. Results for all meta-analyses are reported in Table 3 and Fig. 2.
Table 3.
Results from meta-analyses.
| x | y | z | Voxels | p | SDM-Z | Egger’s test p value | Brain regions |
| Meta-analysis: CCU vs NU (k = 22; CU n = 361, NU n = 394) | |||||||
| CCU > NU | |||||||
| −4 | −4 | 62 | 177 | 0.001409173 | 1.617 | 0.872 | Left medial frontal gyrus extending bilaterally |
| 38 | 18 | 2 | 340 | 0.000125647 | 1.942 | 0.406 | Right insula extending to ipsilateral inferior frontal gyrus |
| CCU < NU | |||||||
| −10 | −98 | −8 | 684 | 0.000094950 | −1.664 | 0.302 | Left cuneus extending to ipsilateral superior, middle, and inferior occipital gyri |
| 30 | −18 | 56 | 165 | 0.001311898 | −1.288 | 0.595 | Right precentral gyrus |
| Meta-analysis: ACU vs NU (only adolescent studies) (k = 5; CU n = 98, NU n = 106) | |||||||
| ACU > NU | |||||||
| 46 | −46 | 50 | 669 | 0.00013572 | 1.554 | 0.418 | Right inferior parietal lobule extending to ipsilateral superior parietal and angular gyri |
| 38 | 52 | 10 | 142 | 0.001023412 | 1.162 | 0.851 | Right middle frontal gyrus extending to ipsilateral superior frontal gyrus |
| 26 | −92 | 6 | 86 | 0.00138104 | 1.121 | 0.652 | Right middle occipital gyrus extending to ipsilateral superior occipital gyrus and cuneus |
| −34 | 58 | −6 | 67 | 0.00138104 | 1.121 | 0.724 | Left middle frontal gyrus extending to ipsilateral superior frontal gyrus |
| 8 | −60 | 68 | 56 | 0.000980318 | 1.170 | 0.816 | Right precuneus extending to ipsilateral superior parietal gyrus |
| −42 | −52 | 58 | 30 | 0.00138104 | 1.121 | 0.577 | Left inferior parietal lobule extending to ipsilateral superior parietal gyrus |
| 60 | 16 | 8 | 19 | 0.001511097 | 1.110 | 0.132 | Right inferior frontal gyrus |
| Meta-analysis: CCU vs ACU (adult as well as adolescent studies) (CCU: k = 22, n = 361; ACU: k = 5, n = 98) | |||||||
| ACU > CCU | |||||||
| 46 | −48 | 50 | 390 | 0.000501096 | 1.244 | 0.443 | Right inferior parietal lobule extending to ipsilateral inferior parietal, superior parietal and angular gyri |
| −10 | −98 | −8 | 263 | 0.000559688 | 1.228 | 0.320 | Left lingual gyrus extending to ipsilateral middle and superior occipital gyri |
| 6 | −60 | 68 | 100 | 0.000584483 | 1.220 | 0.898 | Precuneus extending to ipsilateral postcentral and superior parietal gyri |
| −34 | 58 | −4 | 53 | 0.002155423 | 1.019 | 0.753 | Left middle frontal gyrus extending to ipsilateral superior frontal gyrus |
| −38 | −52 | 60 | 46 | 0.001968861 | 1.033 | 0.753 | Left inferior parietal lobule extending to ipsilateral superior parietal gyrus |
| Meta-analysis: Adolescent CCU vs NU (k = 4; CU n = 69, NU n = 70) | |||||||
| Adolescent CCU > NU | |||||||
| 44 | 28 | 34 | 220 | 0.000734389 | 1.061 | 0.238 | Right middle frontal gyrus extending to ipsilateral inferior frontal gyrus. |
| 40 | −86 | 20 | 71 | 0.000344992 | 1.211 | 0.678 | Right middle occipital gyrus |
CCU: Current cannabis users; ACU: Abstinent cannabis users; NU: Non-user healthy controls.
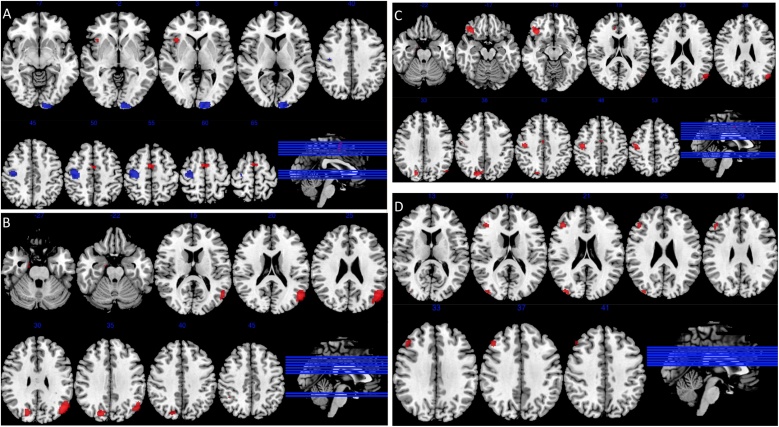
Fig. 2.
Maps of statistically significant differences in activation (Voxel threshold = p < 0.005, peak height threshold: peak SDM-Z < 1, clusters ≥ 10). Axial brain slice position shown on a sagittal view bottom right, with slices arranged from left to right in the different panels showing brain slices in ascending order from bottom to top.
A - Activation of current CU compared to non-using control subjects, increased activation in CU shown in red, decreased activation in CU shown in blue (k = 22; CU n = 361, NU n = 394).
B - Activation of adolescent abstinent CU compared to non-using controls, increased activation in CU shown in red (k = 5; CU n = 98, NU n = 106).
C - Activation of adolescent abstinent CU compared to current adult and adolescent CU, increased activation in abstinent users shown in red (CCU: k = 22, n = 361; ACU: k = 5, n = 98).
D - Adolescent current CU compared to non-using controls, increased activation in CU shown in red (k = 4; CU n = 69, NU n = 70).
3.2. Adult and adolescent CCU compared to NU
Meta-analysis of CCU revealed that CCU had increased activation when compared to NU in the medial frontal gyrus bilaterally and right insula, extending ipsilaterally to the inferior frontal gyrus. A decrease in activation was found in CCU compared to NU in the left cuneus, extending ipsilaterally to the superior, middle, and inferior occipital gyri; and in the right precentral gyrus, (k = 22; CU n = 361, NU n = 394).
3.3. Adolescent ACU compared to NU
Meta-analysis of adolescent ACU compared to NU revealed that ACU had increased activation in the right inferior frontal gyrus; right precuneus, extending ipsilaterally to the superior parietal gyrus; and the right middle occipital gyrus, extending ipsilaterally to the superior occipital gyrus and cuneus; the middle frontal gyrus extending to the superior frontal gyrus bilaterally and the inferior parietal gyri bilaterally, extending to the superior parietal gyrus bilaterally and the right angular gyrus. There were no areas where brain activation was significantly decreased in ACU compared to NU (k = 5; CU n = 98, NU n = 106).
3.4. Adult and adolescent CCU compared to adolescent ACU
Meta-analysis comparing CCU including both adult and adolescent studies to adolescent ACU, revealed no areas where brain activation was significantly increased in CCU compared to ACU, However, ACU had increased activation in the right precuneus, extending ipsilaterally to postcentral and superior parietal gyrI; the left lingual gyrus, extending ipsilaterally to superior and middle occipital gyri; the left middle frontal gyrus extending ipsilaterally to superior frontal gyrus; and the inferior parietal lobule bilaterally extending to superior parietal gyrus bilaterally and to the right angular gyrus, (CCU: k = 22; n = 361; ACU: k = 5; n = 98).
3.5. Adolescent CCU compared to NU
Meta-analysis of adolescent CCU revealed that adolescent CCU had increased activation when compared to NU in the right middle frontal gyrus extending ipsilaterally to the inferior frontal gyrus ; and the middle occipital gyrus on the right side. There were no areas where brain activation was significantly decreased in adolescent CCU compared to (k = 4; CU n = 69, NU n = 70).
3.6. Adolescent CCU compared to adolescent ACU
Meta-analysis of adolescent CCU compared to adolescent ACU, revealed that there were no areas where brain activation was significantly different between adolescent CCU and adolescent ACU NU (CCU: k = 4; n = 69; ACU: k = 5; n = 98).
3.7. Study heterogeneity
Funnel plots were created and examined for each cluster from each meta-analysis. Egger’s tests were used with no cluster reaching significance, indicating no publication bias (see Table 3). SDM-Z scores for each peak are reported in Table 3, no between-study heterogeneity was seen in the adolescent only ACU vs NU, CCU vs NU and ACU vs CCU meta-analyses. In the mixed age group comparison of ACU vs CCU, some heterogeneity was seen in the cluster spanning the left superior frontal gyrus.
4. Discussion
The key comparisons of interest in the present set of analyses relate to the brain activation differences between ACU and NU and that between ACU and CCU. Although the ACU versus NU comparison was limited to only studies in adolescent cannabis users (as no studies in adult users met our stringent inclusion criteria for ACU), we found significantly greater activation in the dorsolateral and ventrolateral prefrontal and posterior parietal cortices, which are part of the central executive network and are known to be involved in higher order cognitive processes such as attentional control, executive function and working memory (Seeley et al., 2007; Sridharan et al., 2008). These findings are similar to those found by a study in adolescent cannabis users following an abstinence period of average 5 weeks (Jager et al., 2010). Furthermore, ACU also displayed greater activation compared to NU in regions that are part of the default mode network (Buckner et al., 2008) such as the cuneus, inferior parietal cortex and angular gyrus as well as the visual cortex. Comparison of ACU and CCU across all studies (both adult and adolescent) revealed greater activation in ACU across regions within the central executive (dorsolateral prefrontal and posterior parietal cortices) and default mode (inferior parietal cortex and precuneus) network as well as lingual and precentral gyri. However, these differences were likely a result of CCU group including both adult and adolescent studies with ACU group including only adolescent studies. Direct comparison between ACU and CCU limited to studies in adolescent users alone revealed no significant group differences in brain activation. Further comparisons between CCU and NU across all eligible studies revealed activation differences in certain brain regions (such as inferior frontal gyrus where CCU > NU; and superior, middle and inferior occipital and precentral gyri where CCU < NU) that were broadly consistent with results from comparisons between all cannabis users and non-users in our previous meta-analysis (Blest-Hopley et al., 2018). Although, no comparably consistent patterns emerged from meta-analysis of adolescent studies alone, it is worth noting the limited number of studies eligible for inclusion for this comparison. Nevertheless, the robustness of our findings was supported by the results of the heterogeneity analysis, which showed no between-study heterogeneity in these clusters, as well as the results of the Egger’s test, which also showed that none of the reported clusters were significantly affected by publication bias.
Collectively, results of the present meta-analysis clearly suggest that at least in adolescent cannabis users, brain functional alterations persist even after periods of abstinence equivalent to around 25 days, by when cannabis metabolites are no longer detectable in urine. One cannot be certain on the basis of present analyses whether similar functional alterations also persist in adult regular cannabis users after comparable periods of abstinence. However, functional alterations in similar brain areas as reported here have also been observed in adult occasional cannabis users compared to non-users after prolonged abstinence (Colizzi et al., 2018a, b). Adolescence is a period of particular vulnerability to the effects of exogenous insults (Andersen, 2003; Belue et al., 1995; Rice and Barone, 2000; Spear, 2007) such as from use of drugs like cannabis, especially in light of progressive change in the density of cannabinoid receptors (Biegon and Kerman, 2001; Glass et al., 1997; Mato et al., 2003). Therefore, results presented here specifically underscore the particular vulnerability of the adolescent brain to residual effects of long-term cannabis use even after the drug and its metabolites have been fully excreted from the body.
What might underlie these functional differences between abstinent adolescent cannabis users and non-users? Evidence from two independent studies suggest downregulation of cannabinoid receptor 1 density in regular cannabis users (D’Souza et al., 2016; Hirvonen et al., 2012), which returns to normal levels following comparable periods of abstinence (D’Souza et al., 2016; Hirvonen et al., 2012), with normalisation starting as early as following 2 days of abstinence (D’Souza et al., 2016). This may suggest that functional alterations in abstinent adolescent cannabis users are unlikely to be related to altered availability of cannabinoid type 1 receptors. Whether these effects are related to longer-term alterations in glutamatergic (Colizzi et al., 2016) or dopaminergic (Sami et al., 2015) neurotransmitter systems also known to be affected by cannabis remains to be tested. The cross-sectional nature of the studies included in the present meta-analyses precludes inference regarding the precise nature of changes detected, whether they are a cause or consequence of cannabis use. Future, studies adopting longitudinal and genetically informed designs are necessary to disentangle causal factors from consequential effects (Paul and Bhattacharyya, 2018). Additional limitations need to be carefully considered while interpreting these results. Most importantly, this meta-analysis was limited by the number of studies available for inclusion. As a result, all studies meeting criteria for the ACU group contained only adolescent participants. As no adult studies met our criteria for inclusion to the abstinent group, it is therefore difficult to infer that functional alterations in adult users similarly persist following longer periods of abstinence. Results of comparisons between CCU and NU are also limited by inclusion of both adult and adolescent studies rather than separate analyses, in light of potentially different effects in these age groups (Blest-Hopley et al., 2018). However, this does not affect the key results of ACU vs NU and ACU vs CCU in adolescent only studies. Furthermore, the three papers investigating adolescent ACU were reported from the same research group, with almost complete overlap in the cohorts reported in two of the studies (Schweinsburg et al., 2008; Tapert et al., 2007) and around 45% overlap with a much larger cohort reported by Schweinsburg et al. (2011). Therefore, it may be argued that the meta-analysis results are inflated on account of non-trivial overlap of the samples investigated. However, it is also worth noting that these 3 studies reported on three distinct fMRI activation paradigms (Schweinsburg et al., 2008, 2011; Tapert et al., 2007). While this does not address the issue of overlapping samples, it suggests some degree of consistency across different cognitive tasks. Nevertheless, this highlights the limited nature of evidence currently available regarding longer term effects of cannabis that persist even after its metabolites are no longer detectable in the system, and underscore the need for further studies in this area. Another important limitation worth noting relates to the effects of withdrawal symptoms confounding comparisons including CCU. Again this is unlikely to have affected comparisons between ACU and NU as most withdrawal symptoms typically return to baseline by 2 weeks (Budney et al., 2004), while participants were abstinent for longer duration (25 days) in studies included in the ACU group. While some studies controlled for the potential confounding effects of other psychoactive substances (e.g. nicotine, alcohol) either statistically or by including study groups matched for use of these substances, others did not. Therefore, we cannot be completely certain that results of our meta-analyses were not influenced by potential effects of these substances on brain function, when they incorporated such studies that did not consider the confounding effect of comorbid exposure to other substances. Cannabis use parameters, such as age of onset and frequency of use, have been previously found to be important in relation to neurological changes (Broyd et al., 2016). A limited number of studies in the different sub-groups also precluded systematic examination of the relationship with the extent of previous cannabis use. The cannabinoid compositions of cannabis with regard to THC and cannabindiol levels has been found produce difference effects (Colizzi and Bhattacharyya, 2017), with high THC cannabis linked to increases in extreme psychological outcomes (Di Forti et al., 2009), no study has yet have reported cannabis type. Future studies may wish to further explore cannabis composition as a measure, alongside cannabis use parameters. Further to this, only certain types of cognitive processes, due to the limited number of studies, may have biased the results based on the cognitive processes used. The tasks employed by the adolescent ACU and CCU both included go/no go task, but the ACU group studies reported on two memory-related tasks as well, whereas the CCU had gambling and finger tapping tasks. While this may have introduced bias (Ganzer et al., 2016), it is worth noting that comparison groups (NU) were studied with the same tasks. Nevertheless, we cannot rule out this possibility. Methodological heterogeneity between studies, such as cannabis use levels, selective reporting of only results that reached statistical significance as is common practice, and studies with small sample sizes, are also caveats that are worth considering while interpreting these results.
Notwithstanding limitations highlighted above, results from the present meta-analyses suggest that certain neurofunctional alterations in components of the central executive and default mode networks in adolescent cannabis users may persist even after cannabis and its metabolites are likely to have left their bodies. However, given the overlap in the samples that support the key conclusions reported here, there is an urgent need for independent studies investigating whether brain function in abstinent cannabis users differ significantly from non-users following a sustained period of monitored abstinence, both in adolescents as well as in adults. Furthermore, whether these persistent brain functional alterations underlie short-term or longer-term risks of mental, social and behavioural disturbances (Boden et al., 2017; Fergusson et al., 2015; Sami and Bhattacharyya, 2018; Schoeler et al., 2018, 2016b; Silins et al., 2015, 2018), particularly in young people remains to be tested.
Role of the funding source
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. All authors have approved the final version of the paper.
Financial disclosures
None.
Acknowledgements
SB has been funded by the National Institute for Health Research (NIHR), UK through a Clinician Scientist award (NIHR-CS-11-001) and also supported by the NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Psychology and Neuroscience, King’s College London and the South London and Maudsley Trustees. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Ashtari M., Avants B., Cyckowski L., Cervellione K.L., Roofeh D., Cook P., Gee J., Sevy S., Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J. Psychiatr Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A., Bhattacharyya S., Yucel M., Fusar-Poli P., Crippa J.A., Nogue S., Torrens M., Pujol J., Farre M., Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055821. e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A., Crippa J.A., Busatto G.F., Guimaraes F.S., Zuardi A.W., Valverde O., Atakan Z., McGuire P.K., Bhattacharyya S., Martin-Santos R. Neuroimaging studies of acute effects of THC and CBD in humans and animals: a systematic review. Curr. Pharm. Des. 2014;20:2168–2185. doi: 10.2174/13816128113199990432. [DOI] [PubMed] [Google Scholar]
- Belue R.C., Howlett A.C., Westlake T.M., Hutchings D.E. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Atakan Z., Martin-Santos R., Crippa J.A., Kambeitz J., Malhi S., Giampietro V., Williams S., Brammer M., Rubia K., Collier D.A., McGuire P.K. Impairment of inhibitory control processing related to acute psychotomimetic effects of cannabis. Eur. Neuropsychopharmacol. 2015;25:26–37. doi: 10.1016/j.euroneuro.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Falkenberg I., Martin-Santos R., Atakan Z., Crippa J.A., Giampietro V., Brammer M., McGuire P. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015;40:1343–1352. doi: 10.1038/npp.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Egerton A., Kim E., Rosso L., Riano Barros D., Hammers A., Brammer M., Turkheimer F.E., Howes O.D., McGuire P. Acute induction of anxiety in humans by delta-9-tetrahydrocannabinol related to amygdalar cannabinoid-1 (CB1) receptors. Sci. Rep. 2017;7:15025. doi: 10.1038/s41598-017-14203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A., Kerman I.A. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage. 2001;14:1463–1468. doi: 10.1006/nimg.2001.0939. [DOI] [PubMed] [Google Scholar]
- Blest-Hopley G., Giampietro V., Bhattacharyya S. Residual effects of cannabis use in adolescent and adult brains - a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2018;88:26–41. doi: 10.1016/j.neubiorev.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Boden J.M., Lee J.O., Horwood L.J., Grest C.V., McLeod G.F. Modelling possible causality in the associations between unemployment, cannabis use, and alcohol misuse. Soc. Sci. Med. 2017;175:127–134. doi: 10.1016/j.socscimed.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., van Hell H.H., Beale C., Yucel M., Solowij N. Acute and chronic effects of cannabinoids on human cognition-a systematic review. Biol. Psychiatry. 2016;79:557–567. doi: 10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. New. York Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Budney A.J., Hughes J.R., Moore B.A., Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am. J. Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Chang L., Yakupov R., Cloak C., Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chye Y., Lorenzetti V., Suo C., Batalla A., Cousijn J., Goudriaan A.E., Jenkinson M., Martin-Santos R., Whittle S., Yucel M., Solowij N. Alteration to hippocampal volume and shape confined to cannabis dependence: a multi-site study. Addict. Biol. 2018;(July) doi: 10.1111/adb.12652. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Colizzi M., Bhattacharyya S. Does Cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr. Addict. Rep. 2017;4:62–74. doi: 10.1007/s40429-017-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizzi M., McGuire P., Pertwee R.G., Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: a systematic review of human and animal evidence. Neurosci. Biobehav. Rev. 2016;64:359–381. doi: 10.1016/j.neubiorev.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Colizzi M., McGuire P., Giampietro V., Williams S., Brammer M., Bhattacharyya S. Modulation of acute effects of delta-9-tetrahydrocannabinol on psychotomimetic effects, cognition and brain function by previous cannabis exposure. Eur. Neuropsychopharmacol. 2018;28:850–862. doi: 10.1016/j.euroneuro.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Colizzi M., McGuire P., Giampietro V., Williams S., Brammer M., Bhattacharyya S. Previous cannabis exposure modulates the acute effects of delta-9-tetrahydrocannabinol on attentional salience and fear processing. Exp. Clin. Psychopharmacol. 2018 doi: 10.1037/pha0000221. [DOI] [PubMed] [Google Scholar]
- Cousijn J., Wiers R.W., Ridderinkhof K.R., van den Brink W., Veltman D.J., Goudriaan A.E. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Curran H.V., Brignell C., Fletcher S., Middleton P., Henry J. Cognitive and subjective dose-response effects of acute oral delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- D’Souza D.C., Perry E., MacDougall L., Ammerman Y., Cooper T., Wu Y.T., Braley G., Gueorguieva R., Krystal J.H. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D’Souza D.C., Cortes-Briones J.A., Ranganathan M., Thurnauer H., Creatura G., Surti T., Planeta B., Neumeister A., Pittman B., Normandin M., Kapinos M., Ropchan J., Huang Y., Carson R.E., Skosnik P.D. Rapid changes in CB1 receptor availability in Cannabis dependent males after abstinence from Cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:60–67. doi: 10.1016/j.bpsc.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Wang L., Bergman S.R., Yaxley R.H., Hooper S.R., Huettel S.A. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug. Alcohol. Depend. 2013;133:134–145. doi: 10.1016/j.drugalcdep.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Di Forti M., Morgan C., Dazzan P., Pariante C., Mondelli V., Marques T.R., Handley R., Luzi S., Russo M., Paparelli A., Butt A., Stilo S.A., Wiffen B., Powell J., Murray R.M. High-potency cannabis and the risk of psychosis. Br. J. Psychiatry. 2009;195:488–491. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D.M., Boden J.M., Horwood L.J. Psychosocial sequelae of cannabis use and implications for policy: findings from the Christchurch health and development study. Soc. Psychiatry Psychiatr. Epidemiol. 2015;50:1317–1326. doi: 10.1007/s00127-015-1070-x. [DOI] [PubMed] [Google Scholar]
- Ganzer F., Bröning S., Kraft S., Sack P.M., Thomasius R. Weighing the evidence: a systematic review on Long-term neurocognitive effects of Cannabis use in abstinent adolescents and adults. Neuropsychol. Rev. 2016;26:186–222. doi: 10.1007/s11065-016-9316-2. [DOI] [PubMed] [Google Scholar]
- Glass M., Dragunow M., Faull R.L. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Hanson K.L., Winward J.L., Schweinsburg A.D., Medina K.L., Brown S.A., Tapert S.F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L.V., Olkin I. Academic Press; San Diego: 1985. Statistical Methods for Meta-Analysis; pp. 1–14. [Google Scholar]
- Heitzeg M.M., Cope L.M., Martz M.E., Hardee J.E., Zucker R.A. Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev. Cogn. Neurosci. 2015;16:71–83. doi: 10.1016/j.dcn.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C., Freeman T.P., Schafer G., Gardener C., Das R.K., Morgan C.J., Curran H.V. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur. Neuropsychopharmacol. 2015;25:325–334. doi: 10.1016/j.euroneuro.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C., Freeman T.P., Xia J.X., Shaban N.D.C., Curran H.V. Acute memory and psychotomimetic effects of cannabis and tobacco both’ joint’ and individually: a placebo-controlled trial. Psychol. Med. 2017;47:2708–2719. doi: 10.1017/S0033291717001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J., Goodwin R.S., Li C.T., Terry G.E., Zoghbi S.S., Morse C., Pike V.W., Volkow N.D., Huestis M.A., Innis R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobsen L.K., Mencl W.E., Westerveld M., Pugh K.R. Impact of cannabis use on brain function in adolescents. Ann. N.Y. Acad. Sci. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobus J., Bava S., Cohen-Zion M., Mahmood O., Tapert S.F. Functional consequences of marijuana use in adolescents. Pharmacol. Biochem. Behav. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G., Block R.I., Luijten M., Ramsey N.F. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child. Adolesc. Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. 572.e561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G., Block R.I., Luijten M., Ramsey N.F. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: a cross-sectional multicenter fMRI study. J. Psychoact. Drugs. 2013;45:156–167. doi: 10.1080/02791072.2013.785837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JPT H. The cochrane collaboration. In: Green S., editor. Cochrane Handbook for Systematic Reviews of Interventions. 2011. [Google Scholar]
- Koenders L., Lorenzetti V., de Haan L., Suo C., Vingerhoets W., van den Brink W., Wiers R.W., Meijer C.J., Machielsen M., Goudriaan A.E., Veltman D.J., Yucel M., Cousijn J. Longitudinal study of hippocampal volumes in heavy cannabis users. J. Psychopharmacol. 2017;31:1027–1034. doi: 10.1177/0269881117718380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn W., Freeman T.P., Pope R.A., Joye A., Harvey L., Hindocha C., Mokrysz C., Moss A., Wall M.B., Bloomfield M.A., Das R.K., Morgan C.J., Nutt D.J., Curran H.V. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis’ amotivational’ hypotheses. Psychopharmacology (Berl) 2016;233:3537–3552. doi: 10.1007/s00213-016-4383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R.H., Abraham T.T., Darwin W.D., Herning R., Cadet J.L., Huestis M.A. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug. Alcohol. Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S., Del Olmo E., Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- Matochik J.A., Eldreth D.A., Cadet J.L., Bolla K.I. Altered brain tissue composition in heavy marijuana users. Drug. Alcohol. Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Nestor L., Roberts G., Garavan H., Hester R. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage. 2008;40:1328–1339. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- Nestor L., Hester R., Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Bhattacharyya S. Does thinner right entorhinal cortex underlie genetic liability to cannabis use? Psychol. Med. 2018:1–10. doi: 10.1017/S0033291718000417. [DOI] [PubMed] [Google Scholar]
- Pertwee R.G. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict. Biol. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Pillay S.S., Rogowska J., Kanayama G., Gruber S., Simpson N., Pope H.G., Yurgelun-Todd D.A. Cannabis and motor function: fMRI changes following 28 days of discontinuation. Exp. Clin. Psychopharmacol. 2008;16:22–32. doi: 10.1037/1064-1297.16.1.22. [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Radua J., van den Heuvel O.A., Surguladze S., Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch. Gen. Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips M.L., El-Hage W., Kronhaus D.M., Cardoner N., Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua J., Rubia K., Canales-Rodríguez E.J., Pomarol-Clotet E., Fusar-Poli P., Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., Schmidt A., Borgwardt S., Heinz A., Schlagenhauf F., McGuire P., Fusar-Poli P. Ventral striatal activation during reward processing in psychosis: aA neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- Ramaekers J.G., Kauert G., van Ruitenbeek P., Theunissen E.L., Schneider E., Moeller M.R. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Rice D., Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108(Suppl. 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami M.B., Bhattacharyya S. Are cannabis-using and non-using patients different groups? Towards understanding the neurobiology of cannabis use in psychotic disorders. J. Psychopharmacol. 2018 doi: 10.1177/0269881118760662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami M.B., Rabiner E.A., Bhattacharyya S. Does cannabis affect dopaminergic signaling in the human brain? A systematic review of evidence to date. Eur. Neuropsychopharmacol. 2015;25:1201–1224. doi: 10.1016/j.euroneuro.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Schoeler T., Kambeitz J., Behlke I., Murray R., Bhattacharyya S. The effects of cannabis on memory function in users with and without a psychotic disorder: findings from a combined meta-analysis. Psychol. Med. 2016;46:177–188. doi: 10.1017/S0033291715001646. [DOI] [PubMed] [Google Scholar]
- Schoeler T., Theobald D., Pingault J.B., Farrington D.P., Jennings W.G., Piquero A.R., Coid J.W., Bhattacharyya S. Continuity of cannabis use and violent offending over the life course. Psychol. Med. 2016;46:1663–1677. doi: 10.1017/S0033291715003001. [DOI] [PubMed] [Google Scholar]
- Schoeler T., Theobald D., Pingault J.B., Farrington D.P., Coid J.W., Bhattacharyya S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. Psychol. Med. 2018:1–8. doi: 10.1017/S0033291717003658. [DOI] [PubMed] [Google Scholar]
- Schreiner A.M., Dunn M.E. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp. Clin. Psychopharmacol. 2012;20:420–429. doi: 10.1037/a0029117. [DOI] [PubMed] [Google Scholar]
- Schreiner A.M., Dunn M.E. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp. Clin. Psychopharmacol. 2012;20:420–429. doi: 10.1037/a0029117. [DOI] [PubMed] [Google Scholar]
- Schulte M.H., Cousijn J., den Uyl T.E., Goudriaan A.E., van den Brink W., Veltman D.J., Schilt T., Wiers R.W. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin. Psychol Rev. 2014;34:531–550. doi: 10.1016/j.cpr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Schweinsburg A.D., Nagel B.J., Schweinsburg B.C., Park A., Theilmann R.J., Tapert S.F. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg A.D., Schweinsburg B.C., Medina K.L., McQueeny T., Brown S.A., Tapert S.F. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J. Psychoact. Drugs. 2010;42:401–412. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg A.D., Schweinsburg B.C., Nagel B.J., Eyler L.T., Tapert S.F. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.C., Slomiak S.T., Jones J.D., Rosen A.F.G., Moore T.M., Gur R.C. Association of Cannabis with cognitive functioning in adolescents and Young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585–595. doi: 10.1001/jamapsychiatry.2018.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdmproject.com . 2017. Seed-Based D Mapping (Formerly Signed Differential Mapping) - Meta-Analyses of Neuroimaging Data. [Google Scholar]
- Sedgwick P., Marston L. How read. Funnel plot in meta-analysis. BMJ. 2015;351 doi: 10.1136/bmj.h4718. h4718. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Murthy P., Bharath M.M. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran. J. Psychiatry. 2012;7:149–156. [PMC free article] [PubMed] [Google Scholar]
- Silins E., Fergusson D.M., Patton G.C., Horwood L.J., Olsson C.A., Hutchinson D.M., Degenhardt L., Tait R.J., Borschmann R., Coffey C., Toumbourou J.W., Najman J.M., Mattick R.P., Cannabis Cohorts Research C. Adolescent substance use and educational attainment: an integrative data analysis comparing cannabis and alcohol from three Australasian cohorts. Drug. Alcohol. Depend. 2015;156:90–96. doi: 10.1016/j.drugalcdep.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Silins E., Horwood L.J., Patton G.C., Fergusson D.M., Olsson C.A., Hutchinson D.M., Spry E., Toumbourou J.W., Degenhardt L., Swift W., Coffey C., Tait R.J., Letcher P., Copeland J., Mattick R.P., Cannabis Cohorts Research, C Young adult sequelae of adolescent cannabis use: an integrative analysis. The lancet. Psychiatry. 2014;1:286–293. doi: 10.1016/S2215-0366(14)70307-4. [DOI] [PubMed] [Google Scholar]
- Smith-Kielland A., Skuterud B., Morland J. Urinary excretion of 11-nor-9-carboxy-delta9-tetrahydrocannabinol and cannabinoids in frequent and infrequent drug users. J Anal. Toxicol. 1999;23:323–332. doi: 10.1093/jat/23.5.323. [DOI] [PubMed] [Google Scholar]
- Solowij N., Pesa N. Cognitive abnormalities and cannabis use. Rev. Bras. Psiquiatr. 2010;32(Suppl. 1):S31–S40. [PubMed] [Google Scholar]
- Spear L.P. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol. Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Tapert S.F., Schweinsburg A.D., Drummond S.P., Paulus M.P., Brown S.A., Yang T.T., Frank L.R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hell H.H., Vink M., Ossewaarde L., Jager G., Kahn R.S., Ramsey N.F. Chronic effects of cannabis use on the human reward system: an fMRI study. Eur. Neuropsychopharmacol. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J. Educ. Behav. Stat. 2005;30:261–293. [Google Scholar]
- Yanes J.A., Riedel M.C., Ray K.L., Kirkland A.E., Bird R.T., Boeving E.R., Reid M.A., Gonzalez R., Robinson J.L., Laird A.R., Sutherland M.T. Neuroimaging meta-analysis of cannabis use studies reveals convergent functional alterations in brain regions supporting cognitive control and reward processing. J. Psychopharmacol. 2018;32:283–295. doi: 10.1177/0269881117744995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M., Solowij N., Respondek C., Whittle S., Fornito A., Pantelis C., Lubman D.I. Regional brain abnormalities associated with long-term heavy cannabis use. Arch. Gen. Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]