Highlights
-
•
Cellular and molecular mechanisms underlying increases in regional blood flow in response to neuronal activity are not fully understood.
-
•
We have compared the effects of 79 in vivo and 36 in vitro experimental attempts to inhibit the neurovascular response.
-
•
Blockade of neuronal NO synthase (nNOS) has the largest effect of any individual target, reducing the neurovascular response by 64%.
-
•
This points to the existence of an unknown key signalling mechanism which accounts for approximately one third of the neurovascular response.
Keywords: Brain blood flow, Functional hyperaemia, Neurovascular coupling, Systematic review, Functional MRI
Abstract
The mechanisms of neurovascular coupling contribute to ensuring brain energy supply is sufficient to meet demand. Despite significant research interest, the mechanisms underlying increases in regional blood flow that follow heightened neuronal activity are not completely understood. This article presents a systematic review and analysis of published data reporting the effects of pharmacological or genetic blockade of all hypothesised signalling pathways of neurovascular coupling. Our primary outcome measure was the percent reduction of the neurovascular response assessed using in vivo animal models. Selection criteria were met by 50 primary sources reporting the effects of 79 treatments. Experimental conditions were grouped into categories targeting mechanisms mediated by nitric oxide (NO), prostanoids, purines, potassium, amongst others. Blockade of neuronal NO synthase was found to have the largest effect of inhibiting any individual target, reducing the neurovascular response by 64% (average of 11 studies). Inhibition of multiple targets in combination with nNOS blockade had no further effect. This analysis points to the existence of an unknown signalling mechanism accounting for approximately one third of the neurovascular response.
1. Introduction
Constant and optimal nutrient and oxygen supply as well as effective removal of metabolic waste products (e.g. CO2) are ensured by several mechanisms controlling cerebral blood flow. Control of global cerebral circulation is provided by metabolic factors, cerebral autoregulation, and autonomic mechanisms (Willie et al., 2014). Fine-tuning and continuous adjustment of local cerebral blood flow in accord with the levels of neuronal activity and metabolism are achieved by operation of the neurovascular coupling mechanism. The functional significance of this mechanism arises from 1) the inability of the brain to store significant energy reserves; 2) highly dynamic and regionally heterogeneous metabolism; 3) volume constraints imposed by the cranium; and 4) the need to maximise the efficacy of energy use.
The mechanisms underlying neurovascular coupling have been the subject of intense research interest for the past ∼25 years for two important reasons. Firstly, changes in local brain blood flow which follow changes in neuronal activity provide the physiological basis of blood oxygen level dependent (BOLD) functional MRI (fMRI) imaging (Buxton and Frank, 1997). Regional cerebral blood flow changes in humans can, therefore, be measured non-invasively and used as a surrogate to assess changes in the neuronal activity (Attwell and Iadecola, 2002). Secondly, impaired mechanisms controlling brain blood flow may contribute to cognitive impairment and potentially precipitate the development of dementia/ neurodegenerative disease (Iadecola, 2017). However, despite continued experimental scrutiny by many research groups, the cellular and molecular mechanisms of the neurovascular response are not fully understood and/or remain controversial. Here, using meta-analysis, we evaluated the results of experimental studies that aimed to identify the signalling mechanism(s) of the neurovascular coupling response. We analysed the relative efficacy of experimental treatments targeting all the hypothesised pathways. Our analysis suggests that a substantial proportion of the neurovascular response is mediated by an as yet unknown mechanism.
2. Metabolic feedback mechanisms
The physiological significance of the neurovascular response is predicated on the fact that neuronal activity requires copious amounts of energy substrates. Oxygen and glucose are used to generate ATP, which is required to maintain ion gradients and provide energy to fuel the mechanisms of neurotransmitter release/recycling that make neuronal communication and brain information processing possible (Attwell and Laughlin, 2001; Howarth et al., 2012). As levels of neuronal activity are believed to be highly heterogeneous across the brain (which has no significant stores of metabolic substrates), blood flow is redirected to support regions of heightened activity.
Feedback mechanisms are fundamental for the operation of all physiological systems, including those which maintain energy homeostasis. A metabolic feedback hypothesis of brain blood flow regulation was proposed by Roy and Sherrington (1890) who observed that products of cerebral metabolism released during brain asphyxia (hypoxia/hypercapnia) dilate cerebral vasculature. These observations led to the hypothesis that signals increasing local brain blood flow are generated in response to a reduction in supply of metabolic substrates and/or accumulation of metabolic waste products (Kety, 1950).
A reduction in the local concentration of oxygen and/or glucose might, therefore, serve as a suitable negative feedback trigger to initiate the neurovascular response. Brain metabolism is argued to be significantly more sensitive to the decreases of oxygen delivery over that of glucose (Leithner and Royl, 2014). Indeed, experimental studies in anesthetised rats involving raising the arterial concentration of glucose up to ∼18 mmol L−1 demonstrated no effect of excess glucose on the neurovascular response (Wolf et al., 1997).
Earlier studies suggested that neurovascular response might be triggered by brain tissue hypoxia associated with the increased neuronal activity (Malonek and Grinvald, 1996; Offenhauser et al., 2005; Thompson et al., 2003). A study conducted in rat brain slices demonstrated that parenchymal oxygen tension determines the polarity of cerebrovascular responses that follow activation of perivascular astrocytes by intracellular Ca2+ uncaging (Gordon et al., 2008). More recently astrocytes themselves have been shown to be acutely sensitive to hypoxia (Angelova et al., 2015). Red blood cells have also been proposed to function as oxygen sensors responding with conformational changes to decreases in brain PO2 during neuronal activation (Wei et al., 2016). Freeman and Li (2016) reported faithful coupling between neuronal activity and local tissue PO2 decreases upon visual stimulation in the anesthetised cat. However, the magnitude of the neuronal activity-induced transient brain parenchymal PO2 decreases is small (∼1-5 mmHg) (Freeman and Li, 2016; Parpaleix et al., 2013; Wei et al., 2016) and neurovascular responses are not affected in conditions of normobaric (Wolf et al., 1997) or hyperbaric hyperoxia in rats (Lindauer et al., 2010). In conditions of hyperbaric hyperoxia, enhanced neuronal oxygen consumption would be exceeded by the amount of physically dissolved oxygen. That the neurovascular response is unaffected under hyperbaric hyperoxia would suggest it is unlikely to be triggered by (small) associated decreases in local tissue oxygenation.
Could a build up of metabolic waste products be mediating the neurovascular response? CO2, protons and lactate have powerful effects on cerebral vasculature and are generated proportionately with increased brain tissue metabolism.
Astrocytes have been shown to detect local increases in CO2 or proton concentrations responding to these stimuli with increases in intracellular [Ca2+] (Gourine et al., 2010; Howarth et al., 2017), which may lead to the release of vasoactive signalling molecules. Release of lactate via several parallel mechanisms, involving certain membrane channels (Karagiannis et al., 2016; Sotelo-Hitschfeld et al., 2015) is coupled to metabolic signalling pathways (Fernandez-Moncada et al., 2018) and follows enhanced neuronal activity. Yet, the roles of locally produced CO2, H+ and lactate as potential triggers of the neurovascular response have not been experimentally addressed. There is evidence that brain parenchymal pH does not change or even becomes alkaline due to neuronal activity-induced increases in flow leading to rapid CO2 washout and activation of the membrane Ca2+-ATPase rapidly removing the excess of extracellular protons (Chen and Chesler, 1992; Makani and Chesler, 2010; Ueki et al., 1988).
Taken together, these lines of evidence argue against the importance of metabolic feedback mechanisms in triggering changes in regional cerebral blood flow which follow changes in the neuronal activity. These mechanisms will not be considered further because of the insufficient number of relevant studies meeting our inclusion criteria for meta-analysis (described in detail below).
3. Activity-dependent feed-forward mechanisms
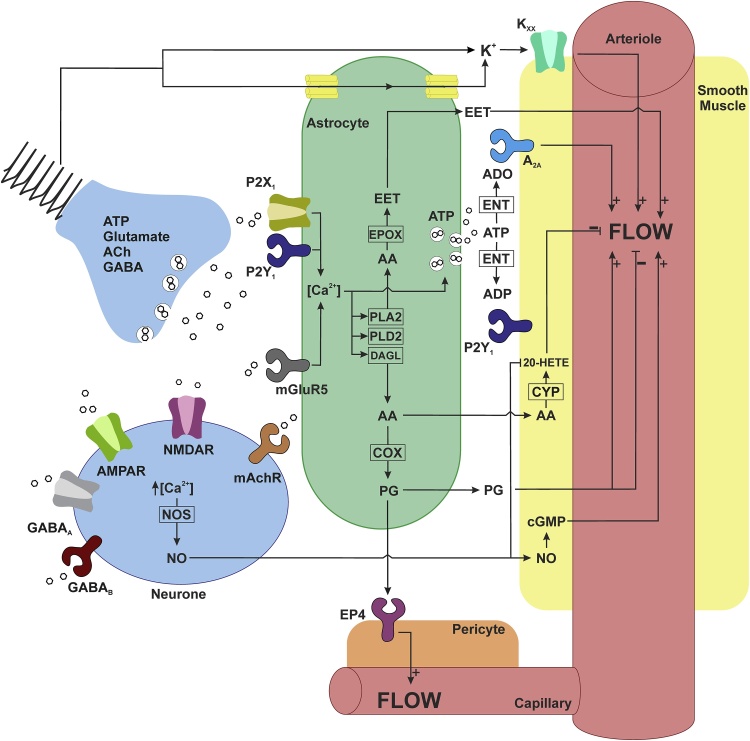
An alternative hypothesis to a feed-forward mechanism centres on the idea that neuronal activity-induced changes in the brain neurochemical milieu mediate neurovascular coupling (Attwell et al., 2010). According to this hypothesis, neurotransmitter(s) released during synaptic activity and/or extracellular K+ accumulating as a result of neuronal activity initiate and maintain the neurovascular response (Longden et al., 2017). Downstream mechanisms have been proposed to involve recruitment of intermediate cell types, including interneurons and astrocytes, which signal to vascular smooth muscle cells and pericytes (Mishra et al., 2016). Significant experimental evidence suggests that signalling within the neurovascular unit could be mediated by several sequential, parallel, competing and/or redundant pathways involving nitric oxide (NO), prostanoids, purines, amongst others (Attwell et al., 2010; Iadecola, 2017). Hypothesized signalling pathways responsible for the increases in local brain blood flow in response to enhanced neuronal activity are summarized in Fig. 1.
Fig. 1.
Hypothesized signalling mechanisms of the neurovascular coupling. Schematic illustration of all hypothesized pathways mediating the neurovascular response suggested by the results of studies summarized in Figures 2 and 3. All pathways are depicted assuming equal weighting. 20-HETE, 20-Hydroxyeicosatetraenoic acid; A2A, adenosine receptor 2A; AA, arachidonic acid; ACh, acetylcholine; ADO, adenosine; ADP, adenosine diphosphate; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ATP, adenosine triphosphate; cGMP, cyclic guanosine monophosphate; COX, cyclooxygenase; CYP, cytochrome P450; DAGL, diacylglycerol lipase; EET, epoxyeicosatrienoic acid; ENT, ectonucleotidase; EP4, prostaglandin E2 receptor 4; EPOX, cytochrome P450 epoxygenase; GABAA, γ-aminobutyric acid (GABA) A receptor; GABAB, γ-aminobutyric acid B receptor; Kxx, potassium channels; mAchR, muscarinic acetylcholine (ACh) receptor; mGluR5, metabotropic glutamate receptor 5; NMDAR, N-methyl-d-aspartate receptor; NO, nitric oxide; NOS, nitric oxide synthase; P2X1, P2X purinoceptor 1; P2Y1, P2Y purinoceptor 1; PG, prostaglandin; PLA2, phospholipase A2; PLD2, phospholipase D2.
As newcomers to this field we found no up-to-date systematic review of experimental data attempting to analyse the relative significance of the proposed signalling pathways. Addressing this need, we conducted a meta-analysis of publications reporting the results of the experimental studies targeting all hypothesized pathways of neurovascular coupling either pharmacologically or genetically.
Initial searches returned hundreds of relevant studies published since the feedforward hypothesis was proposed. In an attempt to distil critical information, meta-analysis was performed using the selection criteria outlined next. Our primary outcome measure was the percent reduction of the cerebrovascular response induced by increased neuronal activity assessed using in vivo animal models. The necessary criteria for inclusion were enhanced neuronal activity, recruited by either electrical (e.g. forepaw or direct input pathway) or natural (e.g. whisker deflection, visual or motor task) stimulation, evoked the neurovascular response measured by recording changes in cerebral vessel diameter, cerebral blood flow, BOLD fMRI or local brain tissue PO2 in experimental animals. While it is accepted that not all of these measures are exclusively dependent on changes in blood flow only, all have been used as measures of the neurovascular response. Our secondary measure was percent reduction of the cerebral vessel response studied in brain slice preparations in vitro. Studies of neurovascular responses evoked by application of receptor agonists (e.g. (Busija et al., 2007)), high K+ (e.g. (Filosa et al., 2006)), direct stimulation of the brain area from which the response was measured or involving pathological models (e.g. cortical spreading depression (Ostergaard et al., 2015)) were not included in the analysis. A minimum of two experimental studies in vivo targeting the same signalling pathway under the above criteria were required for inclusion. Experimental conditions were grouped into categories targeting hypothesised signalling pathways mediated by NO, prostanoids, ATP, adenosine, K+ and those aimed at blocking several targets. Means were taken from the control and experimental group data and the effect of a pharmacological agent or genetic manipulation was expressed as a percentage of the control response. The individual data points were then pooled per targeted signalling pathway and plotted with their respective 95% confidence intervals (Fig. 2, Fig. 3). This analysis standardizes the relative effectiveness of blocking each target or a combination of targets, allowing direct comparisons. Individual data points are tabulated with the original source referenced in Supplementary Table 1 for the primary (in vivo) outcome measure.
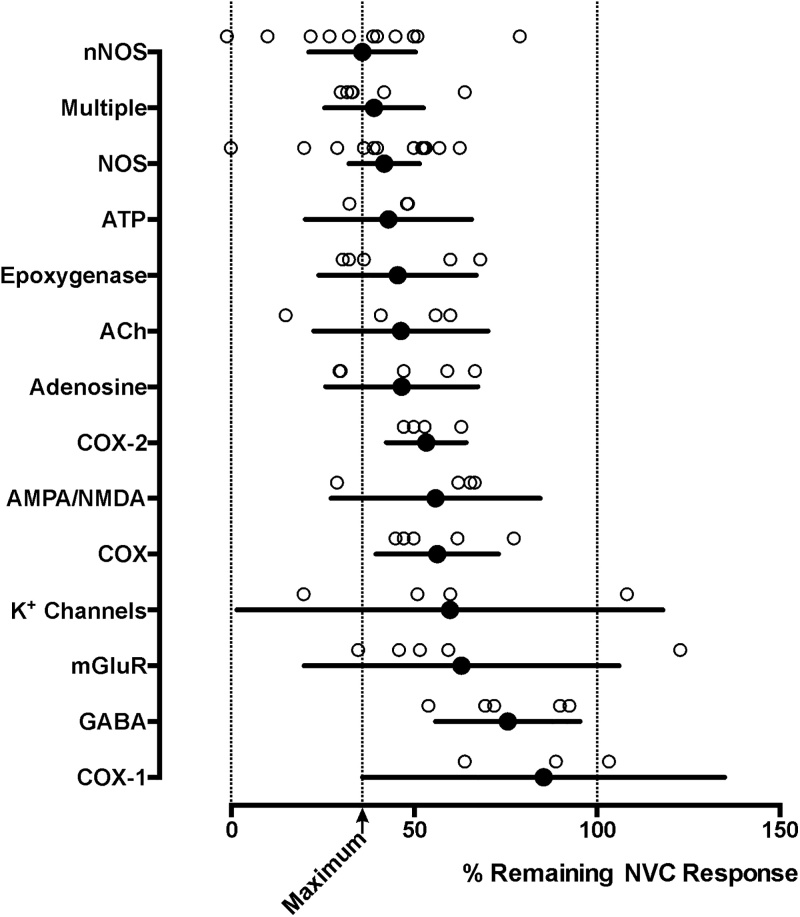
Fig. 2.
The effects of blocking hypothesized signalling mechanisms of neurovascular coupling in vivo. Summary plot illustrating the percentage means (with 95% confidence intervals) of neurovascular coupling (NVC) responses that remain in conditions of pharmacological or genetic blockade of hypothesized signalling pathways in vivo. Individual data points illustrate the magnitude of the effects of the 79 experimental treatments reported in the publications referenced. *Multiple category includes the results of experimental studies that combined neuronal NOS (nNOS) inhibition with blockade of at least one other target. References: nNOS; (Bonvento et al., 2000; Burke and Buhrle, 2006; Cholet et al., 1997; Iadecola et al., 1993; Kitaura et al., 2007; Lindauer et al., 1999; Offenhauser et al., 2005; Stefanovic et al., 2007; Yang et al., 1999; Yang and Iadecola, 1997; Yang et al., 2003), Multiple; (Dirnagl et al., 1994; Golanov and Reis, 1994; Leithner et al., 2010; Peng et al., 2004; Petzold et al., 2008; Tarantini et al., 2015), ATP; (Mishra et al., 2016; Toth et al., 2015; Wells et al., 2015), NOS; (Adachi et al., 1992; Akgoren et al., 1997; Dirnagl et al., 1993; Golanov and Reis, 1994; Iadecola et al., 1993; Ido et al., 2004; Irikura et al., 1994; Kitaura et al., 2007; Ngai et al., 1995; Peng et al., 2004; Raszkiewicz et al., 1992; Toth et al., 2015; Yang and Iadecola, 1997; Zhang et al., 1995), Epoxygenase; (Lecrux et al., 2011; Peng et al., 2002, 2004), Ach; (Arneric et al., 1987; Biesold et al., 1989; Kocharyan et al., 2008; Zhang et al., 1995), Adenosine; (Dirnagl et al., 1994; Ko et al., 1990; Meno et al., 2005), COX-2; (Bakalova et al., 2002; Lecrux et al., 2011; Niwa et al., 2000), AMPA/NMDA; (Calcinaghi et al., 2011; Lecrux et al., 2011; Petzold et al., 2008; Yang et al., 1999), COX; (Bakalova et al., 2002; Bruhn et al., 2001; Golanov and Reis, 1994; St Lawrence et al., 2003), K+ Channels; (Hosford et al., 2018; Longden et al., 2017, 2011), mGluR; (Calcinaghi et al., 2011; Lecrux et al., 2011; Petzold et al., 2008; Sloan et al., 2010; Zonta et al., 2003), GABA; (Kocharyan et al., 2008; Lecrux et al., 2011), COX-1; (Niwa et al., 2001; Petzold et al., 2008).
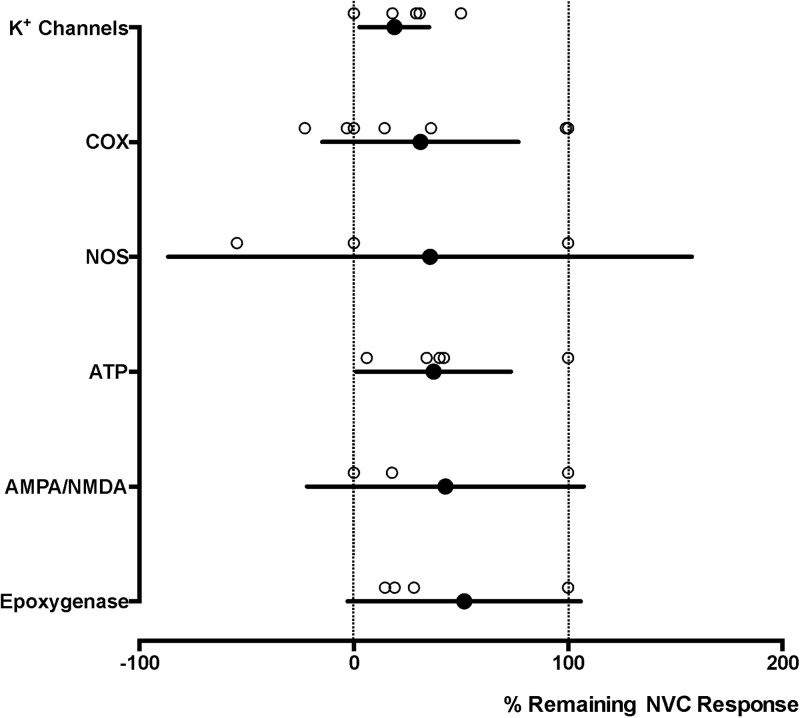
Fig. 3.
The effects of blocking hypothesized signalling mechanisms of neurovascular coupling in vitro. Summary plot illustrating the percentage means (with 95% confidence intervals) of neurovascular coupling (NVC) responses that remain in conditions of pharmacological or genetic blockade of hypothesized signalling pathways in vitro. Individual data points illustrate the magnitude of the effects of the 36 experimental treatments reported in the publications referenced: K+ Channels; (Filosa et al., 2006; Longden et al., 2014, 2017; Longden et al., 2011; Zaritsky et al., 2000), COX; (Filosa et al., 2006; Gordon et al., 2008; Mishra et al., 2016), NOS; (Mapelli et al., 2017; Mishra et al., 2016; Zonta et al., 2003), ATP; (Mishra et al., 2016), AMPA/NMDA; (Hall et al., 2014; Mapelli et al., 2017; Mishra et al., 2016; Zonta et al., 2003), Epoxygenase; (Hall et al., 2014; Mishra et al., 2016).
A systematic review constrained by these criteria returned 61 reports, from which we analysed the results of 79 experimental conditions in vivo and 36 experimental conditions in vitro. Upon aggregation, blockade of neuronal NOS (nNOS) was found to have the largest effect of blocking any individual target, reducing the magnitude of the neurovascular response by 64% (average of the results of 11 in vivo studies) (Fig. 2). Attempts to inhibit multiple targets simultaneously, all of which included nNOS blockade, resulted in a similar reduction of the response magnitude (by 61%; average of the results of 6 studies) (Fig. 2). A potent vasodilator, NO is produced by three NOS isoforms: endothelial NOS (eNOS), nNOS and inducible NOS (Alderton et al., 2001). The temporal profile of inducible NOS activation and deactivation is incompatible with fast control of cerebral blood flow in accordance with neuronal activity. Both eNOS and nNOS are expressed by various cellular components of the neurovascular unit. There is evidence that neurovascular response to somatosensory stimulation is reduced in eNOS deficient mice (Toth et al., 2015). However, other studies reported that blockade of all NOS isoforms with Nω-nitro-l-arginine (L-NNA) attenuated neurovascular response in both the wild type and eNOS-deficient mice (Ayata et al., 1996) but had no effect in conditions of nNOS deficiency (Ma et al., 1996). Furthermore, the use of non-selective NOS inhibitors was found to have a similar effect on the magnitude of the neurovascular response (reduction by 58%, 14 in vivo studies; Fig. 2) to that of a pharmacological or genetic nNOS blockade. It is important to note that NOS blockade is associated with a significant reduction of resting cerebral blood flow (by 28% on average; data retrieved from 6 studies that reported baseline changes). Reduction in basal flow per se would be expected to increase the relative magnitude of the evoked neurovascular response (as observed, for example, after caffeine administration (Mulderink et al., 2002)). Depending on the experimental design and particularities of data analysis this may potentially result in overestimation of the neurovascular response in these conditions. However, Iadecola et al. (1993) applied hypocapnia to induce a comparable reduction in cerebral blood flow as observed following systemic NOS blockade and found no effect of this experimental manoeuvre on the magnitude of the neurovascular response in anesthetised rats.
Thus, NO production by nNOS appears to be critically important for the development of the neurovascular response. Yet, a significant proportion (∼1/3) of the response remains after pharmacological blockade of nNOS. This assumes complete blockade, which is likely as genetic nNOS deletion was found to have an effect of similar magnitude as pharmacological intervention (Yang et al., 2003). Almost complete enzyme inhibition is possible with pharmacology: for example, Nitro-l-arginine was reported to inhibit 95 ± 4% of NOS activity when superfused on the cortical surface of anesthetised rats. However, this treatment was associated with a mere 37 ± 4% inhibition of the neurovascular response in the area (Zhang et al., 1995). Another study in anesthetised rats did find a positive correlation between the efficacy of NOS inhibition and the degree reduction of the neurovascular response magnitude in the barrel cortex with r2 = 0.63 (Irikura et al., 1994). It is important to note that the relative proportion of nNOS expressing neurones varies between different brain areas. In the cerebellar cortex, where the granular layer has the highest level of nNOS expression of all brain areas, blockade of the enzyme was found to completely abolish activity-dependent dilations in slices (Mapelli et al., 2017). In the rodent cerebral cortex, the proportion of nNOS expressing neurones is relatively low (0.5–2%), and all these cells are GABAergic interneurons unevenly distributed between different cortical layers (Valtschanoff et al., 1993). One study conducted in anaesthetized rats, reported that addition of a NO donor can restore the full magnitude of the neurovascular response triggered by whisker simulation in conditions of NOS blockade by superfusion of the brain surface with l-NNA (Lindauer et al., 1999). This suggested that NO might be essential for other signalling pathway(s) to operate by having something what one may call a “permissive” role. One report investigated the effect of systemic NOS blockade with l-NMMA on the neurovascular response in 10 human subjects (White et al., 1999). In contrast to the results obtained in the vast majority of animal studies, NOS blockade had no effect on neurovascular response when the participants performed a finger-tap task.
Several studies targeted another notable hypothesised pathway of neurovascular coupling which involves the release and vasodilator effects (on smooth muscle cells and capillary pericytes) of certain products of arachidonic acid metabolism including prostaglandins (PGs) and epoxyeicosatrienoic acids (EETs). All the enzymes required for the Ca2+-dependent production of arachidonic acid and PGs from membrane phospholipids are expressed in astrocytes (Mishra et al., 2016), cells which are often placed at the centre of the neurovascular unit (Fig. 1). PGs (PGE2 and PGI2 in particular) are potent vasodilators. Recent evidence has implicated PGE2 as the dominant prostaglandin mediating neurovascular coupling in the rodent cerebral cortex (Lacroix et al., 2015). However, there is also evidence obtained in vitro that PGE2 may, under certain conditions, constrict (rather than dilate) brain arterioles of rats and mice (Dabertrand et al., 2013). Systematic review (Fig. 2) has revealed that interfering with this pathway by either genetic deletion or pharmacological blockade of epoxygenase, inhibition of cyclooxygenases (COX) with non-specific agents or blockade of COX-2 specifically, results in a 55, 44 or 47% reduction in the magnitude of the neurovascular response, respectively. In humans, systemic non-specific COX inhibition was also reported to reduce the magnitude of the BOLD response induced by visual stimulation by 47% (Bruhn et al., 2001). It is important to note that indomethacin, used in all these studies as a non-specific COX inhibitor, is also a potent inhibitor of cyclic AMP-dependant protein kinase activity (Goueli and Ahmed, 1980), which is integral to any vasomotor response. Therefore, conclusions drawn from the results of the experiments using indomethacin as a COX inhibitor may somewhat overestimate the importance of this pathway. For example, in humans indomethacin was found to reduce cerebrovascular CO2 reactivity, while the other non-specific COX inhibitors (naproxen, ketorolac) had no effect on CO2-induced dilations (Hoiland et al., 2016). On the other hand, the effects of specific inhibition or genetic elimination of COX-2 on neurovascular response were found to be similar to that of indomethacin, suggesting that off-target activity of this drug may not be relevant in this context. Specific pharmacological or genetic blockade of COX-1 activity was reported to reduce the neurovascular response by 24% on aggregate (Fig. 2). Based on this analysis it would be logical to suggest that the actions of PGs and EETs mediate the proportion of the neurovascular response which remains after nNOS blockade. However, combined blockade of NOS and COX or NOS, COX and epoxygenase activity reduces the magnitude of the neurovascular response on average by 68% (Golanov and Reis, 1994; Leithner et al., 2010; Tarantini et al., 2015). This falls within the range of the effects observed following inhibition of nNOS activity alone (Fig. 2).
NO, PGE2 and EETs dilate cerebral blood vessels by their actions on the arterial smooth muscle cells and/or capillary pericytes, and, therefore, act as the “effector” molecules of the neurovascular response. Studies of the mechanisms underlying the initiation of the response focused on the signalling molecules released by active neurones. Previously favoured models of the neurovascular coupling mechanism were centred on synaptic glutamate triggering Ca2+-dependent release of vasoactive substances by astrocytes (Attwell et al., 2010). However, this model has been challenged by the evidence showing that mature astrocytes may not express appropriate glutamate receptors (Sun et al., 2013; but also see Nizar et al., 2013) and that neuronal activity-induced changes in flow are not affected in the absence of astroglial IP3 receptors (Jego et al., 2014; Nizar et al., 2013; Takata et al., 2013). We proposed in 2015 that (instead of glutamate) the release of purine nucleotide ATP may mediate signalling between neurones and astrocytes (Wells et al., 2015), a conclusion supported by the results of another study involving our laboratory (Mishra et al., 2016). Astroglial IP3 receptors would not be essential for the operation of this mechanism as ATP may trigger Ca2+ responses in astrocytes via activation of ionotropic (P2X) receptors (Mishra et al., 2016). Blockade of ATP actions (by facilitated enzymatic degradation) or application of purinoceptor antagonists was found to reduce the magnitude of the neurovascular response by 57% on average (Fig. 2). Neurovascular response was also reported to be affected following blockade of other key neurotransmitter signalling pathways, including AMPA and NMDA receptors (inhibition by 44%), metabotropic glutamate receptor 5 (inhibition by 37%), muscarinic acetylcholine receptors (inhibition by 54%) or destruction of GABAergic neurones/pharmacological blockade of GABA receptors (inhibition by 24%) (Fig. 2).
Released ATP is rapidly broken down to adenosine by the activities of extracellular nucleotidases (Fig. 1). Seven studies targeted adenosine receptors (some are expressed by vascular smooth muscle cells) and reported (on average) a 53% reduction in the magnitude of the neurovascular response (Fig. 2). One study involving combined nNOS and adenosine receptor blockade showed that 42% of the neurovascular response remains in these conditions (Dirnagl et al., 1994), which is in the range of the effects elicited by NOS blockade alone (Fig. 2), indicating no additive effect of adenosine receptor inhibition. In contrast, human studies demonstrated that non-selective adenosine receptor antagonist caffeine increases the magnitude of BOLD response to visual stimulation by as much as 37%, an effect which was suggested to be due to a reduction in the baseline flow (Mulderink et al., 2002). It is important to note that neurones express both the inhibitory (A1) and excitatory (e.g. A2A,2B,3) adenosine receptors, all sensitive to blockade by caffeine, which is also a weak inhibitor of phosphodiesterase activity, important for the maintenance of vascular tone and reactivity (Pelligrino and Wang, 1998).
Another potential player which has been proposed to mediate the neurovascular response is potassium. Original data suggested that Ca2+-activated K+ (BK) channels on astroglial end-feet release K+ which in turn activates inward-rectifying K+ channels (KIR) expressed by smooth muscle cells, causing hyperpolarisation and relaxation (Filosa et al., 2006). However, deletion of BK channels was found to have no effect on the neurovascular response (Girouard et al., 2010). A modified hypothesis suggested that enhanced neuronal activity increases extracellular K+ sufficiently to trigger smooth muscle relaxation (Longden et al., 2017). Knock-out of a specific subunit, KIR2.1, was found to reduce the neurovascular response by ∼50% (Longden et al., 2017), while deletion of KIR6.1 had no effect (Hosford et al., 2018). On aggregate, attempts to interfere with K+-mediated mechanism(s) resulted in 40% inhibition of the neurovascular response (Fig. 2). The effects of these manipulations in combination with nNOS blockade remain to be determined.
While the data obtained using the in vivo preparations are in general agreement between the studies (Fig. 2), the results obtained in vitro are largely inconsistent and represented by the extremes (Fig. 3). For example, after NOS or COX inhibition the neurovascular response is either unaffected or completely inhibited (Fig. 3). Accepted major caveats of the in vitro approach is that the integrity of the neurovascular unit is compromised and the vessels are not studied at optimal physiological tension, - the limitation which can be partially addressed by application of constricting agents (Filosa, 2010). Studies of the vascular responses using the in vitro preparations also necessitate electrical stimulation to be applied in a close proximity to the site from which the neurovascular response is being recorded and could result in a pattern of neuronal connections and/or network recruitment that may not accurately reflect the activities of physiological neuronal pathways. This highlights the importance of studying the mechanisms of neurovascular coupling both in vitro and in conditions of intact synaptic connectivity, normal myogenic tone and blood flow. In the opinion of the authors of this analysis, in vivo animal models remain to be most useful for studies of the neurovascular coupling mechanisms as experiments involving human participants are also constrained by limitations imposed by the use of generally safe pharmacological agents and non-invasive methods of assessing changes in brain blood flow.
4. Regional segmentation
There is emerging evidence of functional regional segmentation of the cerebrovascular tree. Using vascular single-cell transcriptomics Vanlandewijck et al. (2018) demonstrated a clear gradual phenotypic change (zonation) along the continuum of the arteriovenous network in the mouse brain. Recent work has also suggested that the signalling mechanisms responsible for arteriolar vs capillary dilations are distinct and involve the release and actions of NO and PGE2, respectively (Mishra et al., 2016). However, the majority of studies included in this analysis targeted specific hypothesised signalling mechanisms of neurovascular coupling without taking into the consideration the potential functional regional segmentation of the cerebral vessels capable of active dilation. In the opinion of the authors, the mechanism which controls the vascular tone at the point of most resistance to flow will play the key role in shaping the neurovascular response. According to the recent data reported by Charpak’s group (Rungta et al., 2018) this point (which the authors called “primary functional unit”) could be around the first branching of the penetrating arteriole, as it dilates first in response to increases in local neuronal activity (in the mouse olfactory bulb).
We also do not discount the possibility that the mechanisms of the neurovascular response could vary by the brain region. This analysis included studies of neurovascular coupling in several brain regions, including olfactory bulb, cerebellum, visual, barrel and somatosensory cortical regions with the majority of the experiments carried out in the latter two. Only two studies included in the analysis reported complete abolition of the neurovascular response in vivo. In anesthetised rats, the neurovascular responses were blocked in the somatosensory forepaw region of the cortex after nNOS inhibition (Stefanovic et al., 2007) and in the visual cortex, also in conditions of non-specific NOS inhibition (Ido et al., 2004). There is no in vivo evidence that the neurovascular response has been effectively blocked in any other brain region. In the absence of a clear consensus on key cell types and vascular segments mediating the neurovascular response in different brain regions, results of all the relevant studies were considered together, pooled and analysed by the targeted signalling pathway.
5. Conclusions
This analysis points to the existence of as yet an unidentified mechanism (or mechanisms) of neurovascular coupling which accounts for approximately a third of the neurovascular response. There are other precedents in the history of physiology illustrating the fact that the signalling mechanisms underlying activity-dependent changes in blood flow are sometimes difficult to identify. For example, the nature of the mediator(s) responsible for exercise-induced coronary vasodilation and increases in coronary blood flow (critically important to support myocardial oxygen demands) remains unclear despite similar research interest and significant effort. The involvement of common pathways was experimentally tested, but prostaglandins and K+ were found to play no role, while studies involving simultaneous blockade of KATP channels, NOS, and adenosine receptors demonstrated no effect of the combined treatment on exercise-induced changes in coronary blood flow (Goodwill et al., 2017). On the other hand, if functional hyperaemia is mediated by a single signalling molecule, then the underlying mechanism(s) can be rapidly and unequivocally identified (Burnett et al., 1992).
Considering the importance of maintaining brain energy balance it is likely that there are several parallel signalling pathways evolved to work in concert to shape the neurovascular response and in conditions when one pathway is blocked experimentally, recruitment of the other pathway(s) compensates. However, this raises an obvious question of how the alternative pathways are engaged to maintain (at least partially) the vascular response when the targeted pathway is no longer functional. Some form of a feedback mechanism which can recruit these redundant pathway(s) when the need arises would be required. Alternatively, if the effects of parallel pathways are not simply additive, then a feedback mechanism may not be needed. It is not inconceivable that some form of negative synergy limits the effects of one signalling pathway when it operates in conjunction with the others in the intact system. Finally, in the opinion of the authors, full understanding of the complex mechanisms underlying the development and maintenance of the neurovascular response may emerge if some of the original ideas of metabolic feedback hypothesis proposed by Roy and Sherrington (1890) are re-examined using contemporary experimental approaches.
Disclosure
The authors declare that there is no conflict of interest.
Acknowledgments
Results of the authors’ experimental studies mentioned in this essay were obtained with generous support of The Wellcome Trust (Refs: 095064 and 200893) and the British Heart Foundation (Ref: RG/14/4/30736). A.V.G is a Wellcome Trust Senior Research Fellow. We thank Professor David Attwell for helpful discussions and critical review of the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2018.11.011.
Contributor Information
Patrick S. Hosford, Email: p.hosford@qmul.ac.uk.
Alexander V. Gourine, Email: a.gourine@ucl.ac.uk.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Adachi T., Inanami O., Sato A. Nitric oxide (NO) is involved in increased cerebral cortical blood flow following stimulation of the nucleus basalis of Meynert in anesthetized rats. Neurosci. Lett. 1992;139:201–204. doi: 10.1016/0304-3940(92)90552-i. [DOI] [PubMed] [Google Scholar]
- Akgoren N., Mathiesen C., Rubin I., Lauritzen M. Laminar analysis of activity-dependent increases of CBF in rat cerebellar cortex: dependence on synaptic strength. Am. J. Physiol. 1997;273:H1166–1176. doi: 10.1152/ajpheart.1997.273.3.H1166. [DOI] [PubMed] [Google Scholar]
- Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova P.R., Kasymov V., Christie I., Sheikhbahaei S., Turovsky E., Marina N., Korsak A., Zwicker J., Teschemacher A.G., Ackland G.L., Funk G.D., Kasparov S., Abramov A.Y., Gourine A.V. Functional oxygen sensitivity of astrocytes. J. Neurosci. 2015;35:10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneric S.P., Iadecola C., Underwood M.D., Reis D.J. Local cholinergic mechanisms participate in the increase in cortical cerebral blood flow elicited by electrical stimulation of the fastigial nucleus in rat. Brain Res. 1987;411:212–225. doi: 10.1016/0006-8993(87)91072-9. [DOI] [PubMed] [Google Scholar]
- Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., Newman E.A. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Ayata C., Ma J., Meng W., Huang P., Moskowitz M.A. L-NA-sensitive rCBF augmentation during vibrissal stimulation in type III nitric oxide synthase mutant mice. J. Cereb. Blood Flow Metab. 1996;16:539–541. doi: 10.1097/00004647-199607000-00002. [DOI] [PubMed] [Google Scholar]
- Bakalova R., Matsuura T., Kanno I. The cyclooxygenase inhibitors indomethacin and Rofecoxib reduce regional cerebral blood flow evoked by somatosensory stimulation in rats. Exp. Biol. Med. (Maywood) 2002;227:465–473. doi: 10.1177/153537020222700710. [DOI] [PubMed] [Google Scholar]
- Biesold D., Inanami O., Sato A., Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci. Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- Bonvento G., Cholet N., Seylaz J. Sustained attenuation of the cerebrovascular response to a 10 min whisker stimulation following neuronal nitric oxide synthase inhibition. Neurosci. Res. 2000;37:163–166. doi: 10.1016/s0168-0102(00)00109-7. [DOI] [PubMed] [Google Scholar]
- Bruhn H., Fransson P., Frahm J. Modulation of cerebral blood oxygenation by indomethacin: MRI at rest and functional brain activation. J. Magn. Reson. Imaging. 2001;13:325–334. doi: 10.1002/jmri.1047. [DOI] [PubMed] [Google Scholar]
- Burke M., Buhrle C. BOLD response during uncoupling of neuronal activity and CBF. Neuroimage. 2006;32:1–8. doi: 10.1016/j.neuroimage.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Burnett A.L., Lowenstein C.J., Bredt D.S., Chang T.S., Snyder S.H. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Busija D.W., Bari F., Domoki F., Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res. Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R.B., Frank L.R. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J. Cereb. Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Calcinaghi N., Jolivet R., Wyss M.T., Ametamey S.M., Gasparini F., Buck A., Weber B. Metabotropic glutamate receptor mGluR5 is not involved in the early hemodynamic response. J. Cereb. Blood Flow Metab. 2011;31:e1–10. doi: 10.1038/jcbfm.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.C., Chesler M. pH transients evoked by excitatory synaptic transmission are increased by inhibition of extracellular carbonic anhydrase. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7786–7790. doi: 10.1073/pnas.89.16.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholet N., Seylaz J., Lacombe P., Bonvento G. Local uncoupling of the cerebrovascular and metabolic responses to somatosensory stimulation after neuronal nitric oxide synthase inhibition. J. Cereb. Blood Flow Metab. 1997;17:1191–1201. doi: 10.1097/00004647-199711000-00008. [DOI] [PubMed] [Google Scholar]
- Dabertrand F., Hannah R.M., Pearson J.M., Hill-Eubanks D.C., Brayden J.E., Nelson M.T. Prostaglandin E2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. J. Cereb. Blood Flow Metab. 2013;33:479–482. doi: 10.1038/jcbfm.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U., Lindauer U., Villringer A. Role of nitric oxide in the coupling of cerebral blood flow to neuronal activation in rats. Neurosci. Lett. 1993;149:43–46. doi: 10.1016/0304-3940(93)90343-j. [DOI] [PubMed] [Google Scholar]
- Dirnagl U., Niwa K., Lindauer U., Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am. J. Physiol. 1994;267:H296–301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- Fernandez-Moncada I., Ruminot I., Robles-Maldonado D., Alegria K., Deitmer J.W., Barros L.F. Neuronal control of astrocytic respiration through a variant of the Crabtree effect. Proc. Natl. Acad. Sci. U. S. A. 2018;115:1623–1628. doi: 10.1073/pnas.1716469115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa J.A. Vascular tone and neurovascular coupling: considerations toward an improved in vitro model. Front. Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa J.A., Bonev A.D., Straub S.V., Meredith A.L., Wilkerson M.K., Aldrich R.W., Nelson M.T. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Freeman R.D., Li B. Neural-metabolic coupling in the central visual pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H., Bonev A.D., Hannah R.M., Meredith A., Aldrich R.W., Nelson M.T. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanov E.V., Reis D.J. Nitric oxide and prostanoids participate in cerebral vasodilation elicited by electrical stimulation of the rostral ventrolateral medulla. J. Cereb. Blood Flow Metab. 1994;14:492–502. doi: 10.1038/jcbfm.1994.61. [DOI] [PubMed] [Google Scholar]
- Goodwill A.G., Dick G.M., Kiel A.M., Tune J.D. Regulation of coronary blood flow. Compr. Physiol. 2017;7:321–382. doi: 10.1002/cphy.c160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G.R., Choi H.B., Rungta R.L., Ellis-Davies G.C., MacVicar B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goueli S.A., Ahmed K. Indomethacin and inhibition of protein kinase reactions. Nature. 1980;287:171–172. doi: 10.1038/287171a0. [DOI] [PubMed] [Google Scholar]
- Gourine A.V., Kasymov V., Marina N., Tang F., Figueiredo M.F., Lane S., Teschemacher A.G., Spyer K.M., Deisseroth K., Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., O’Farrell F.M., Buchan A.M., Lauritzen M., Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiland R.L., Tymko M.M., Bain A.R., Wildfong K.W., Monteleone B., Ainslie P.N. Carbon dioxide-mediated vasomotion of extra-cranial cerebral arteries in humans: a role for prostaglandins? J. Physiol. 2016;594:3463–3481. doi: 10.1113/JP272012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford P.S., Christie I.N., Niranjan A., Aziz Q., Anderson N., Ang R., Lythgoe M.F., Wells J.A., Tinker A., Gourine A.V. A critical role for the ATP-sensitive potassium channel subunit KIR6.1 in the control of cerebral blood flow. J. Cereb. Blood Flow Metab. 2018 doi: 10.1177/0271678X18780602. 271678X18780602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C., Gleeson P., Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C., Sutherland B., Choi H.B., Martin C., Lind B.L., Khennouf L., LeDue J.M., Pakan J.M., Ko R.W., Ellis-Davies G., Lauritzen M., Sibson N.R., Buchan A.M., MacVicar B.A. A critical role for astrocytes in hypercapnic vasodilation in brain. J. Neurosci. 2017;37:2403–2414. doi: 10.1523/JNEUROSCI.0005-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Zhang F., Xu X. Role of nitric oxide synthase-containing vascular nerves in cerebrovasodilation elicited from cerebellum. Am. J. Physiol. 1993;264:R738–746. doi: 10.1152/ajpregu.1993.264.4.R738. [DOI] [PubMed] [Google Scholar]
- Ido Y., Chang K., Williamson J.R. NADH augments blood flow in physiologically activated retina and visual cortex. Proc. Natl. Acad. Sci. U. S. A. 2004;101:653–658. doi: 10.1073/pnas.0307458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irikura K., Maynard K.I., Moskowitz M.A. Importance of nitric oxide synthase inhibition to the attenuated vascular responses induced by topical L-nitroarginine during vibrissal stimulation. J. Cereb. Blood Flow Metab. 1994;14:45–48. doi: 10.1038/jcbfm.1994.7. [DOI] [PubMed] [Google Scholar]
- Jego P., Pacheco-Torres J., Araque A., Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J. Cereb. Blood Flow Metab. 2014;34:1599–1603. doi: 10.1038/jcbfm.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis A., Sylantyev S., Hadjihambi A., Hosford P.S., Kasparov S., Gourine A.V. Hemichannel-mediated release of lactate. J. Cereb. Blood Flow Metab. 2016;36:1202–1211. doi: 10.1177/0271678X15611912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S.S. Circulation and metabolism of the human brain in health and disease. Am. J. Med. 1950;8:205–217. doi: 10.1016/0002-9343(50)90363-9. [DOI] [PubMed] [Google Scholar]
- Kitaura H., Uozumi N., Tohmi M., Yamazaki M., Sakimura K., Kudoh M., Shimizu T., Shibuki K. Roles of nitric oxide as a vasodilator in neurovascular coupling of mouse somatosensory cortex. Neurosci. Res. 2007;59:160–171. doi: 10.1016/j.neures.2007.06.1469. [DOI] [PubMed] [Google Scholar]
- Ko K.R., Ngai A.C., Winn H.R. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am. J. Physiol. 1990;259:H1703–1708. doi: 10.1152/ajpheart.1990.259.6.H1703. [DOI] [PubMed] [Google Scholar]
- Kocharyan A., Fernandes P., Tong X.K., Vaucher E., Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J. Cereb. Blood Flow Metab. 2008;28:221–231. doi: 10.1038/sj.jcbfm.9600558. [DOI] [PubMed] [Google Scholar]
- Lacroix A., Toussay X., Anenberg E., Lecrux C., Ferreiros N., Karagiannis A., Plaisier F., Chausson P., Jarlier F., Burgess S.A., Hillman E.M., Tegeder I., Murphy T.H., Hamel E., Cauli B. COX-2-derived prostaglandin E2 produced by pyramidal neurons contributes to neurovascular coupling in the rodent cerebral cortex. J. Neurosci. 2015;35:11791–11810. doi: 10.1523/JNEUROSCI.0651-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C., Toussay X., Kocharyan A., Fernandes P., Neupane S., Levesque M., Plaisier F., Shmuel A., Cauli B., Hamel E. Pyramidal neurons are "neurogenic hubs" in the neurovascular coupling response to whisker stimulation. J. Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithner C., Royl G. The oxygen paradox of neurovascular coupling. J. Cereb. Blood Flow Metab. 2014;34:19–29. doi: 10.1038/jcbfm.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithner C., Royl G., Offenhauser N., Fuchtemeier M., Kohl-Bareis M., Villringer A., Dirnagl U., Lindauer U. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J. Cereb. Blood Flow Metab. 2010;30:311–322. doi: 10.1038/jcbfm.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U., Leithner C., Kaasch H., Rohrer B., Foddis M., Fuchtemeier M., Offenhauser N., Steinbrink J., Royl G., Kohl-Bareis M., Dirnagl U. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J. Cereb. Blood Flow Metab. 2010;30:757–768. doi: 10.1038/jcbfm.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U., Megow D., Matsuda H., Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am. J. Physiol. 1999;277:H799–811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- Longden T.A., Dabertrand F., Hill-Eubanks D.C., Hammack S.E., Nelson M.T. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7462–7467. doi: 10.1073/pnas.1401811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden T.A., Dabertrand F., Koide M., Gonzales A.L., Tykocki N.R., Brayden J.E., Hill-Eubanks D., Nelson M.T. Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 2017;20:717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden T.A., Dunn K.M., Draheim H.J., Nelson M.T., Weston A.H., Edwards G. Intermediate-conductance calcium-activated potassium channels participate in neurovascular coupling. Br. J. Pharmacol. 2011;164:922–933. doi: 10.1111/j.1476-5381.2011.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ayata C., Huang P.L., Fishman M.C., Moskowitz M.A. Regional cerebral blood flow response to vibrissal stimulation in mice lacking type I NOS gene expression. Am. J. Physiol. 1996;270:H1085–1090. doi: 10.1152/ajpheart.1996.270.3.H1085. [DOI] [PubMed] [Google Scholar]
- Makani S., Chesler M. Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J. Neurophysiol. 2010;103:667–676. doi: 10.1152/jn.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D., Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Mapelli L., Gagliano G., Soda T., Laforenza U., Moccia F., D’Angelo E.U. Granular layer neurons control cerebellar neurovascular coupling through an NMDA receptor/NO-dependent system. J. Neurosci. 2017;37:1340–1351. doi: 10.1523/JNEUROSCI.2025-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno J.R., Nguyen T.S., Jensen E.M., Alexander West G., Groysman L., Kung D.K., Ngai A.C., Britz G.W., Winn H.R. Effect of caffeine on cerebral blood flow response to somatosensory stimulation. J. Cereb. Blood Flow Metab. 2005;25:775–784. doi: 10.1038/sj.jcbfm.9600075. [DOI] [PubMed] [Google Scholar]
- Mishra A., Reynolds J.P., Chen Y., Gourine A.V., Rusakov D.A., Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016;19:1619–1627. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulderink T.A., Gitelman D.R., Mesulam M.M., Parrish T.B. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage. 2002;15:37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- Ngai A.C., Meno J.R., Winn H.R. L-NNA suppresses cerebrovascular response and evoked potentials during somatosensory stimulation in rats. Am. J. Physiol. 1995;269:H1803–1810. doi: 10.1152/ajpheart.1995.269.5.H1803. [DOI] [PubMed] [Google Scholar]
- Niwa K., Araki E., Morham S.G., Ross M.E., Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J. Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K., Haensel C., Ross M.E., Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ. Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- Nizar K., Uhlirova H., Tian P., Saisan P.A., Cheng Q., Reznichenko L., Weldy K.L., Steed T.C., Sridhar V.B., MacDonald C.L., Cui J., Gratiy S.L., Sakadzic S., Boas D.A., Beka T.I., Einevoll G.T., Chen J., Masliah E., Dale A.M., Silva G.A., Devor A. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhauser N., Thomsen K., Caesar K., Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J. Physiol. 2005;565:279–294. doi: 10.1113/jphysiol.2005.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L., Dreier J.P., Hadjikhani N., Jespersen S.N., Dirnagl U., Dalkara T. Neurovascular coupling during cortical spreading depolarization and -depression. Stroke. 2015;46:1392–1401. doi: 10.1161/STROKEAHA.114.008077. [DOI] [PubMed] [Google Scholar]
- Parpaleix A., Goulam Houssen Y., Charpak S. Imaging local neuronal activity by monitoring PO(2) transients in capillaries. Nat. Med. 2013;19:241–246. doi: 10.1038/nm.3059. [DOI] [PubMed] [Google Scholar]
- Pelligrino D.A., Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog. Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Peng X., Carhuapoma J.R., Bhardwaj A., Alkayed N.J., Falck J.R., Harder D.R., Traystman R.J., Koehler R.C. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2029–2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- Peng X., Zhang C., Alkayed N.J., Harder D.R., Koehler R.C. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J. Cereb. Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Petzold G.C., Albeanu D.F., Sato T.F., Murthy V.N. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raszkiewicz J.L., Linville D.G., Kerwin J.F., Jr., Wagenaar F., Arneric S.P. Nitric oxide synthase is critical in mediating basal forebrain regulation of cortical cerebral circulation. J. Neurosci. Res. 1992;33:129–135. doi: 10.1002/jnr.490330116. [DOI] [PubMed] [Google Scholar]
- Roy C.S., Sherrington C.S. On the regulation of the blood-supply of the brain. J. Physiol. 1890;11(85-158):117. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungta R.L., Chaigneau E., Osmanski B.F., Charpak S. Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron. 2018;99(362–375):e364. doi: 10.1016/j.neuron.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan H.L., Austin V.C., Blamire A.M., Schnupp J.W., Lowe A.S., Allers K.A., Matthews P.M., Sibson N.R. Regional differences in neurovascular coupling in rat brain as determined by fMRI and electrophysiology. Neuroimage. 2010;53:399–411. doi: 10.1016/j.neuroimage.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Sotelo-Hitschfeld T., Niemeyer M.I., Machler P., Ruminot I., Lerchundi R., Wyss M.T., Stobart J., Fernandez-Moncada I., Valdebenito R., Garrido-Gerter P., Contreras-Baeza Y., Schneider B.L., Aebischer P., Lengacher S., San Martin A., Le Douce J., Bonvento G., Magistretti P.J., Sepulveda F.V., Weber B., Barros L.F. Channel-mediated lactate release by K(+)-stimulated astrocytes. J. Neurosci. 2015;35:4168–4178. doi: 10.1523/JNEUROSCI.5036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Lawrence K.S., Ye F.Q., Lewis B.K., Frank J.A., McLaughlin A.C. Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn. Reson. Med. 2003;50:99–106. doi: 10.1002/mrm.10502. [DOI] [PubMed] [Google Scholar]
- Stefanovic B., Schwindt W., Hoehn M., Silva A.C. Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase. J. Cereb. Blood Flow Metab. 2007;27:741–754. doi: 10.1038/sj.jcbfm.9600377. [DOI] [PubMed] [Google Scholar]
- Sun W., McConnell E., Pare J.F., Xu Q., Chen M., Peng W., Lovatt D., Han X., Smith Y., Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N., Nagai T., Ozawa K., Oe Y., Mikoshiba K., Hirase H. Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S., Hertelendy P., Tucsek Z., Valcarcel-Ares M.N., Smith N., Menyhart A., Farkas E., Hodges E.L., Towner R., Deak F., Sonntag W.E., Csiszar A., Ungvari Z., Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J. Cereb. Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.K., Peterson M.R., Freeman R.D. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science. 2003;299:1070–1072. doi: 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- Toth P., Tarantini S., Davila A., Valcarcel-Ares M.N., Tucsek Z., Varamini B., Ballabh P., Sonntag W.E., Baur J.A., Csiszar A., Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1837–1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki M., Linn F., Hossmann K.A. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J. Cereb. Blood Flow Metab. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- Valtschanoff J.G., Weinberg R.J., Kharazia V.N., Schmidt H.H., Nakane M., Rustioni A. Neurons in rat cerebral cortex that synthesize nitric oxide: NADPH diaphorase histochemistry, NOS immunocytochemistry, and colocalization with GABA. Neurosci. Lett. 1993;157:157–161. doi: 10.1016/0304-3940(93)90726-2. [DOI] [PubMed] [Google Scholar]
- Vanlandewijck M., He L., Mae M.A., Andrae J., Ando K., Del Gaudio F., Nahar K., Lebouvier T., Lavina B., Gouveia L., Sun Y., Raschperger E., Rasanen M., Zarb Y., Mochizuki N., Keller A., Lendahl U., Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- Wei H.S., Kang H., Rasheed I.D., Zhou S., Lou N., Gershteyn A., McConnell E.D., Wang Y., Richardson K.E., Palmer A.F., Xu C., Wan J., Nedergaard M. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron. 2016;91:851–862. doi: 10.1016/j.neuron.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J.A., Christie I.N., Hosford P.S., Huckstepp R.T., Angelova P.R., Vihko P., Cork S.C., Abramov A.Y., Teschemacher A.G., Kasparov S., Lythgoe M.F., Gourine A.V. A critical role for purinergic signalling in the mechanisms underlying generation of BOLD fMRI responses. J. Neurosci. 2015;35:5284–5292. doi: 10.1523/JNEUROSCI.3787-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.P., Hindley C., Bloomfield P.M., Cunningham V.J., Vallance P., Brooks D.J., Markus H.S. The effect of the nitric oxide synthase inhibitor L-NMMA on basal CBF and vasoneuronal coupling in man: a PET study. J. Cereb. Blood Flow Metab. 1999;19:673–678. doi: 10.1097/00004647-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Willie C.K., Tzeng Y.C., Fisher J.A., Ainslie P.N. Integrative regulation of human brain blood flow. J. Physiol. 2014;592:841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf T., Lindauer U., Villringer A., Dirnagl U. Excessive oxygen or glucose supply does not alter the blood flow response to somatosensory stimulation or spreading depression in rats. Brain Res. 1997;761:290–299. doi: 10.1016/s0006-8993(97)00354-5. [DOI] [PubMed] [Google Scholar]
- Yang G., Chen G., Ebner T.J., Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am. J. Physiol. 1999;277:R1760–1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- Yang G., Iadecola C. Obligatory role of NO in glutamate-dependent hyperemia evoked from cerebellar parallel fibers. Am. J. Physiol. 1997;272:R1155–1161. doi: 10.1152/ajpregu.1997.272.4.R1155. [DOI] [PubMed] [Google Scholar]
- Yang G., Zhang Y., Ross M.E., Iadecola C. Attenuation of activity-induced increases in cerebellar blood flow in mice lacking neuronal nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H298–304. doi: 10.1152/ajpheart.00043.2003. [DOI] [PubMed] [Google Scholar]
- Zaritsky J.J., Eckman D.M., Wellman G.C., Nelson M.T., Schwarz T.L. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ. Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- Zhang F., Xu S., Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience. 1995;69:1195–1204. doi: 10.1016/0306-4522(95)00302-y. [DOI] [PubMed] [Google Scholar]
- Zonta M., Angulo M.C., Gobbo S., Rosengarten B., Hossmann K.A., Pozzan T., Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.