Abstract
We conducted comprehensive analyses to assess the diagnostic ability of miRNA-451 in cancers. A systematic online search was conducted in PubMed, Web of Science, China’s national knowledge infrastructure, and VIP databases from inception to July 31, 2017. The bivariate random effect model was used for calculating sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and area under cure (AUC). The whole pooled sensitivity and specificity were 0.85 (0.77–0.90) and 0.85 (0.78–0.90) with their 95% confidence interval (95%CI), respectively. The pooled AUC was 0.91 (95%CI: 0.89–0.94). Positive likelihood ratio was 5.57 (95%CI: 3.74–8.31), negative likelihood ratio was 0.18 (95%CI: 0.11–0.28), and diagnostic odds ratio was 31.33 (95%CI: 15.19–64.61). Among Asian population, the sensitivity and specificity were 0.85 (95%CI: 0.77–0.91) and 0.86 (95%CI: 0.78–0.91), respectively. The positive likelihood ratio and negative likelihood ratio were 5.87 (95%CI: 3.78–9.12) and 0.17 (95%CI: 0.11–0.28). The diagnostic odds ratio and AUC were 34.31 (15.51–75.91) and 0.92 (0.89–0.94). The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and AUC for digestive system cancer were 0.83, 0.88, 6.87, 0.20, 35.13, and 0.92, respectively. The other cancers were 0.87, 0.81, 4.55, 0.16, 28.51, and 0.90, respectively. For sample source, the results still remain consistent. Our results indicated miRNA-451 has a moderate diagnostic ability for cancers, and could be a potential early screening biomarker, and considered as an adjuvant diagnostic index when being combined with other clinical examinations.
Keywords: Cancer, MicroRNA-451, Meta-analysis, Tumor marker
Introduction
Cancer is a major public health issue all over the world, and incidence rates have increased in developing and developed countries. It was estimated that cancers had caused more than 8,000,000 deaths worldwide only in 2013 and became the second leading death cause in this year [1]. The incidence of cancer is higher in developing countries. However, higher mortality is observed in developing countries. Although great work has been made for cancer prevention and treatment, disease burden of cancer is still increasing because of the trend of global aging [2]. It was reported that the 5-year overall survival rate of patients with cancer was approximately 50% [3]. In fact, the prevention and treatment of cancer largely depend on early screening and detection. It is extremely difficult to achieve curable treatment effects for patients with end-stage cancer. Therefore, it is of great importance for patients to conduct early screening, and find early tumor biomarkers appear so significant.
The microRNA (miRNA) is a family of mature non-coding small RNAs. As a member of miRNA family, binding to the 3’-untranslated regions (3’UTR) of the target mRNA induces translational repression or miRNA degradation of many genes. Many studies had reported miRNA can regulate many cancer cell proliferation, growth, and development via different signaling pathways [4]. The miRNAs are highly stable and abundant in plasma, serum, and other body fluids. Moreover, miRNA signatures in blood are similar in men and women, as well as individuals of different ages and other factors. The miR-451 is one of the most conservative miRNAs and has important clinical application value. Significant expression difference of miRAN-451 was observed in a variety of cancers such as esophageal cancer [5], gastric cancer [6], renal cell carcinoma [7], hepatocellular carcinoma [8], colorectal cancer [9], and breast cancer [10]. These results indicate miRNA-451 is an important regulating factor in the occurrence and development of cancer and could be a potential tumor biomarker of early-stage screening. Currently, many studies have reported that the miRNA-451 expression was different in different cancers. The accuracy diagnostic ability still remained unclear because single study has some limitations such as sample size, cancer type, and population. We systematically conducted online searches and collected data from different cancers, with the aim of giving a comprehensive assessment for clinical diagnostic value of miRNA-451.
Methods
Search strategy
We performed a systematic search in PubMed, Web of Science, China’s national knowledge infrastructure, and VIP databases from inception to April 15, 2018. Both Medical Subject Headings (MeSHs) and key words were used to obtain potential studies. The following search words were adopted in combinations: (‘microRNA-451’ OR ‘miRNA-451’ OR ‘miR-451’ OR ‘has-mir-451’) AND (‘cancer’ OR ‘tumor’ OR ‘neoplasms’ OR ‘carcinoma’). We also retrieved the lists of articles and reviews for potentially eligible literatures.
Criterion for inclusion and exclusion
Inclusion criteria: (1) study must be conducted among human, and type of tumor was confirmed by the pathology standard; (2) studies focused on the microRNA-451 in patients with tumor, and evaluated the diagnostic ability of microRNA-451; (3) study provided sufficient data to allow the calculation of diagnostic index, including true positive (TP), false positive (FP), false negative (FN), and true negative (TN). (4) Studies performed in vitro, vivo, and animals were excluded; the latest publication was used for duplicates; comments, letter, cases, and reviews were excluded as well as those without effective information.
Data extraction
A standardized excel sheet was used for data extraction. Two investigators independently conducted data extraction, and disputes were solved by the third author (S.L.F.). For each included study, the following information was extracted: the first author, year of publication, region, type of cancer, source of sample, methods of examination, gold standard method, sample size (case/control), sensitivity, specificity, and all four fold values (TP, FP, FN, and TN).
Assessment of quality
We used the updated quality assessment of diagnostic accuracy studies 2 to assess the quality of included studies [11]. This scale tool includes five key items: patient’s selection, index test, reference standard, and flow patients and timing of the index tests and reference standard. Each item consists of two subitems: risk of bias and applicability. The signaling questions are answered as ‘yes’, ‘no’, or ‘unclear’ and are phrased such that ‘no’ means high risk of bias, ‘yes’ indicates low risk of bias, and ‘unclear’ indicates unclear risk of bias.
Statistical analysis
We firstly examined whether there was threshold value effect by Spearman correlation or not. If threshold value effect exists, we will conduct our analyses through hierarchical summary receive operating characteristic [12,13]. The bivariate mixed effects were used without threshold value effect [14]. Heterogeneity within studies was evaluated by the Chi-square test and I2 statistics, I2 > 50% or P<0.05 indicated significant heterogeneity [15]. We used random effects to combine the following parameters: sensitivity, specificity, positive likelihood ratios (PLRs) and negative likelihood ratios (NLRs) (PLR > 10, NLR < 0.1: exclusion and confirmation; PLR > 10, NLR > 0.1: confirmation only; PLR < 10, NLR < 0.1 exclusion only; PLR < 10, NLR > 0.1: no exclusion or confirmation), and diagnostic odds ratios (DORs) with 95% confidence intervals (CIs). We also conducted subgroup analysis in different settings: population, cancer types (digestive system vs others), and sample sources (serum or plasma). We also calculated the area under the summary receiver operator characteristic cure (SROC) and Fagan plots to evaluate the diagnostic ability of miR-451. The area under cure (AUC) of 1.0 means perfect diagnostic ability, while an AUC close to 0.5 indicates a poor diagnostic ability [16]. We used linear regression test for funnel plot asymmetry [17]. All analyses were conducted on Stata 14.0 software (StataCorp LP, College Station, TX, U.S.A.). P<0.05 indicated statistical significance.
Results
Study selection
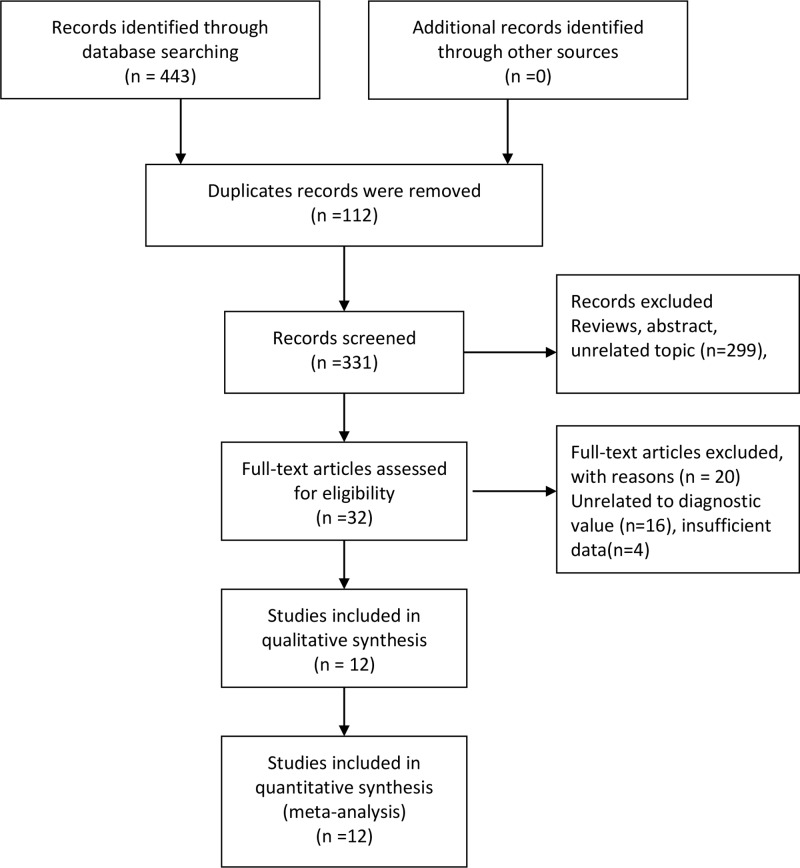
As presented in the flow Figure 1, our initial search returned 443 records, and we did not obtain additional records through other sources. After removing 112 duplicates records, we got 331 records for further screening. A total of 229 records were excluded because of some reviews and unrelated topic records. We prepared 32 full-text articles for potential eligibility. A total of 16 studies with unrelated to diagnostic value and 4 records with insufficient data were excluded. Finally, 10 articles with 12 studies data entered into qualitative and quantitative synthesis [5–10,18–21].
Figure 1. Flow diagram of studies selection.
General characteristics of included studies
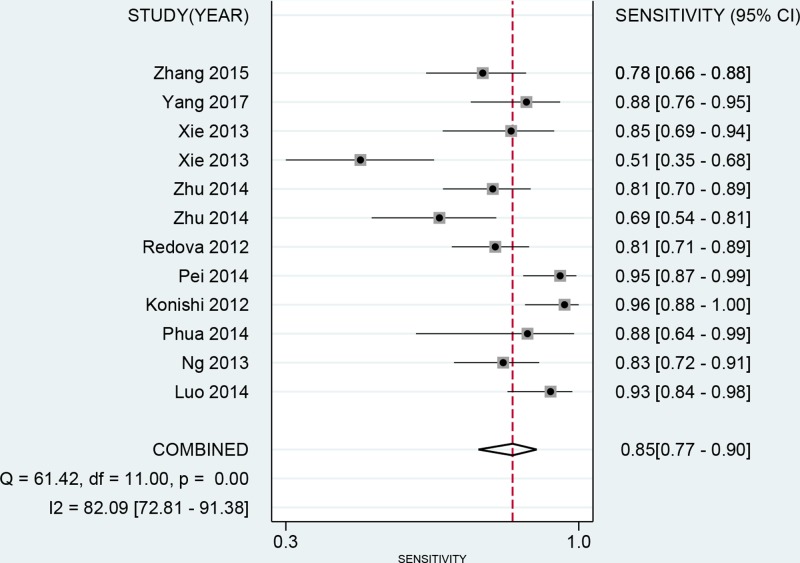
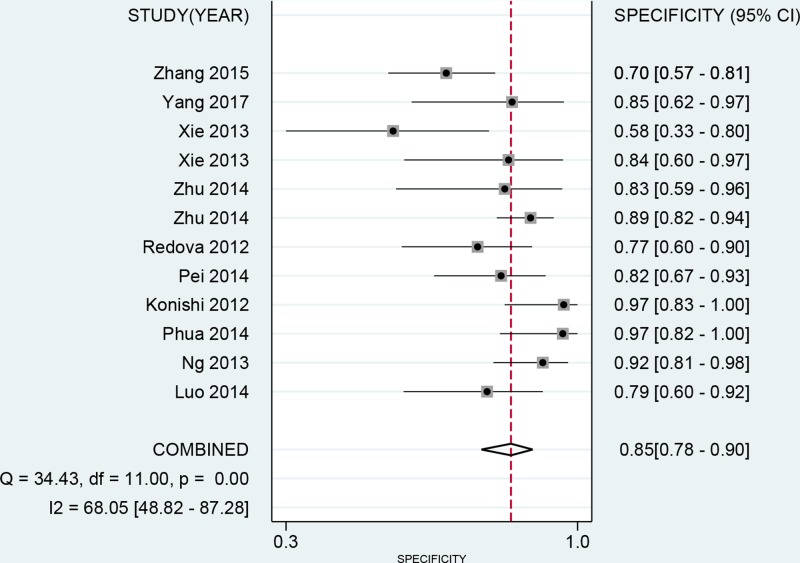
Tables 1 and 2 presented the general characteristics of included studies. These studies were published from 2012 to 2017. Nine of ten studies were from Asian population, and only one study was conducted among European. Of ten studies, three studies were for esophageal cancer, three for gastric cancer, two for breast cancer, one for thyroid carcinoma, one for renal cell carcinoma, and one for hepatocellular carcinoma. The tissue samples were mainly from serum and plasma. But two samples were from saliva and one from feces. Two studies detected miRNA expression via Real-time PCR, and the rest used qRT-PCR. All cancers were confirmed through tissue pathology. The sample size ranged from 45 to 150, with total number of 1177. The sensitivity of included studies was from 51 to 96%, and the specificity was from 58 to 97%. The Tables 1 and 2 presented the specific details.
Table 1. General characteristics of included studies in the meta-analysis.
| Author | Year of publication | Region | Type of cancer | Source of sample | Methods of examination | Gold standard |
|---|---|---|---|---|---|---|
| Zhang | 2015 | Asia | Thyroid carcinoma | Serum | qRT-PCR | Tissue pathology |
| Yang | 2017 | Asia | Esophageal cancer | Serum | qRT-PCR | Tissue pathology |
| Xie | 2013 | Asia | Esophageal cancer | Saliva | qRT-PCR | Tissue pathology |
| Xie | 2013 | Asia | Esophageal cancer | Saliva | qRT-PCR | Tissue pathology |
| Zhu | 2014 | Asia | Gastric cancer | Plasma | qRT-PCR | Tissue pathology |
| Zhu | 2014 | Asia | Gastric cancer | Plasma | qRT-PCR | Tissue pathology |
| Redova | 2012 | Europe | Renal cell carcinoma | Serum | qRT-PCR | Tissue pathology |
| Pei | 2014 | Asia | Hepatocellular carcinoma | Serum | Real-time PCR | Tissue pathology |
| Konishi | 2012 | Asia | Gastric cancer | Plasma | qRT-PCR | Tissue pathology |
| Phua | 2014 | Asia | Colorectal cancer | Feces | Real-time PCR | Tissue pathology |
| Ng | 2013 | Asia | Breast cancer | Plasma | qRT-PCR | Tissue pathology |
| Luo | 2014 | Asia | Breast cancer | Serum | qRT-PCR | Tissue pathology |
Table 2. Parameters of included studies in the meta-analysis.
| Author | Year | Sample size (case/control) | Total | TP | FP | FN | TN | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|---|
| Zhang | 2015 | 60/60 | 120 | 47 | 18 | 13 | 42 | 78 | 70 |
| Yang | 2017 | 50/20 | 70 | 44 | 3 | 6 | 17 | 88 | 85 |
| Xie | 2013 | 39/19 | 58 | 20 | 3 | 19 | 16 | 85 | 58 |
| Xie | 2013 | 39/19 | 58 | 33 | 8 | 6 | 11 | 51 | 84 |
| Zhu | 2014 | 48/102 | 150 | 33 | 11 | 15 | 91 | 81 | 83 |
| Zhu | 2014 | 72/18 | 90 | 58 | 3 | 14 | 15 | 69 | 89 |
| Redova | 2012 | 90/35 | 125 | 73 | 8 | 17 | 27 | 81 | 77 |
| Pei | 2014 | 66/40 | 106 | 63 | 7 | 3 | 33 | 95 | 82 |
| Konishi | 2012 | 56/30 | 86 | 54 | 0 | 2 | 30 | 96 | 97 |
| Phua | 2014 | 17/28 | 45 | 15 | 0 | 2 | 28 | 88 | 97 |
| Ng | 2013 | 70/50 | 120 | 58 | 4 | 12 | 46 | 83 | 92 |
| Luo | 2014 | 60/29 | 89 | 56 | 6 | 4 | 23 | 93 | 79 |
Assessment of quality
The Supplementary Figure S1A,B gives details about quality assessment of each study. All included studies received moderately high scores from quality scale. One study reported high risk of patient’s selection and one for flow and timing. Two studies gave high risk scores in index test, and one for reference standard. The studies with high risk bias were less than 10%; the possible bias was from the index test and flow and timing. The unclear risks were less than 30% in Risk of Bias and were almost 50% in Applicability Concerns. The rest of studies give moderate quality scores.
Pooled results
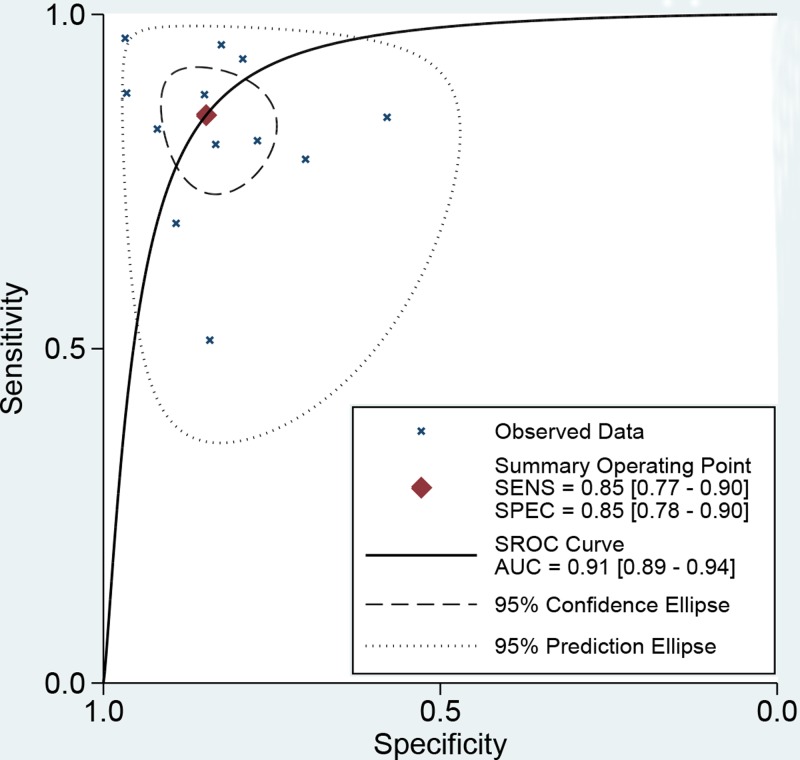
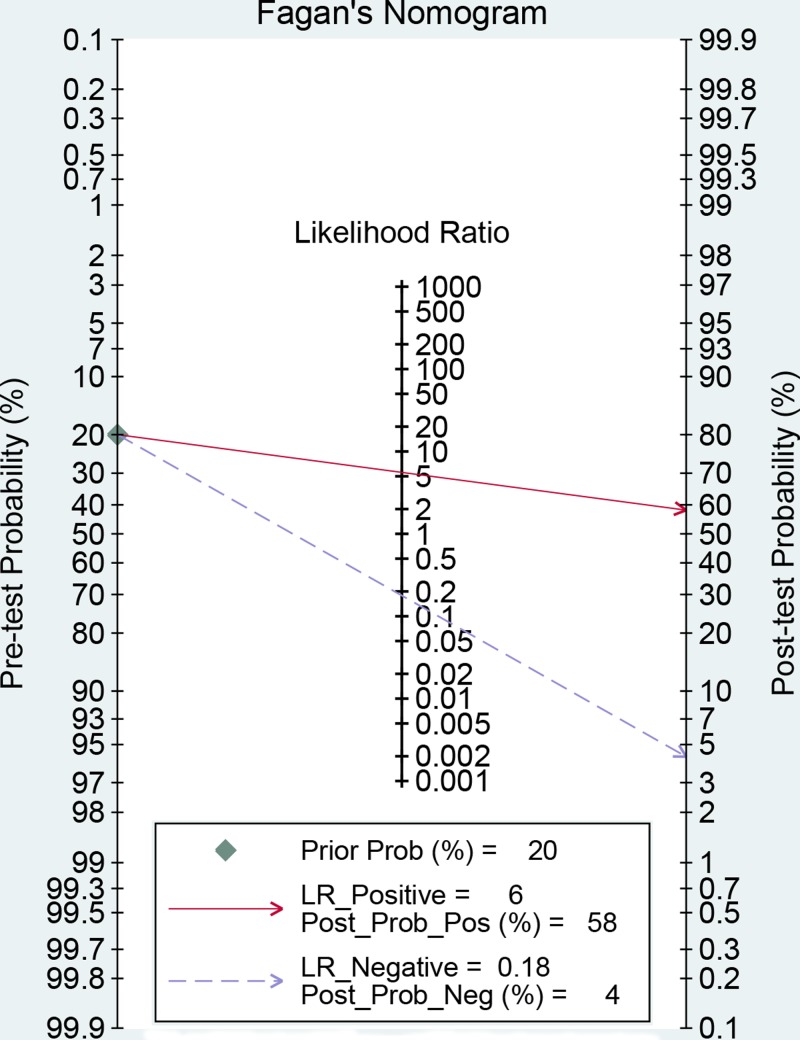
No threshold value effect was found for the present studies (r = −0.203, P=0.527). Besides, as the I2 value for meta-analysis, we used bivariate random effect model to conduct all analyses. The Table 3 presented the diagnostic ability of miR-451 for human cancers. The whole pooled sensitivity and specificity were 0.85 (0.77–0.90, Figure 2) and 0.85 (0.78–0.90, Figure 3) with their 95% CI, respectively. The pooled area under the cure was 0.91 (95%CI: 0.89–0.94, Figure 4). This index indicates the diagnostic ability of miR-451 was high. We also calculated other parameters as follows: the pooled PLR was 5.57 (95%CI: 3.74–8.31), the NLR was 0.18 (95%CI: 0.11–0.28), and the DOR was 31.33 (95%CI: 15.19–64.61). The Figure 5 gives the prediction of pre-test probability and post-test probability. If the pre-test probability is 20% for a patient, the post-test probability will be 58% with a PLR of 6 and 4% with a NLR of 0.18. All parameters indicated the miR-451 had a high diagnostic ability in detecting cancers.
Table 3. Summary estimated of diagnostic performance of miR-451 for cancer detection.
| Category | SEN (95%CI) | SPE (95%CI) | PLR (95%CI) | NLR (95%CI) | DOR (95%CI) | AUC (95%CI) |
|---|---|---|---|---|---|---|
| Overall | 0.85 [0.77–0.90] | 0.85 [0.78–0.90] | 5.57 [3.74–8.31] | 0.18 [0.11–0.28] | 31.33 [15.19–64.61] | 0.91 [0.89–0.94] |
| Population | ||||||
| Asian | 0.85 [0.77–0.91] | 0.86 [0.78–0.91] | 5.87 [3.78–9.12] | 0.17 [0.11–0.28] | 34.31 [15.51–75.91] | 0.92 [0.89–0.94] |
| Cancer type | ||||||
| Digestive system | 0.83 [0.70–0.91] | 0.88 [0.78–0.94] | 6.87 [3.40–13.90] | 0.20 [0.10–0.37] | 35.13 [10.65–115.93] | 0.92 [0.90–0.94] |
| Other types | 0.87 [0.79–0.92] | 0.81 [0.73–0.87] | 4.55 [3.04–6.80] | 0.16 [0.10–0.27] | 28.51 [12.66–64.20] | 0.90 [0.87–0.92] |
| Sample source | ||||||
| Serum-based | 0.87 [0.83–0.90] | 0.77 [0.70–0.83] | 3.75 [2.69–5.24] | 0.16 [0.09–0.28] | 27.03 [10.78–67.75] | 0.82 [0.66–0.98] |
| Plasma-based | 0.83 [0.74–0.92] | 0.91 [0.88–0.95] | 8.48 [4.79–15.01] | 0.18 [0.11–0.39] | 55.18 [17.70–172.07] | 0.96 [0.899–1.00] |
Figure 2. Forest plot of pooled and each study’s sensitivity of miRNA-451 for cancer.
Figure 3. Forest plot of pooled and each study’s specificity of miRNA-451 for cancer.
Figure 4. The symmetric receiver operating characteristic curve of miRNA-451 for cancer.
Figure 5. Fagan diagram evaluating the overall diagnostic value of miRNA-451 for cancer (If the pre-test probability is 20% for a patient, the post-test probability will be 59% with a PLR of 6).
Subgroup analyses
We also conducted subgroup analyses among population, cancer type, and sample source to test the stability of results. The pooled sensitivity, specificity, PLR, NLR, DOR, and AUC for subgroup were presented in Table 3. Because nine of ten studies were from Asian, we only give the pooled results among Asian population. The sensitivity and specificity were 0.85 (95%CI: 0.77–0.91) and 0.86 (95%CI: 0.78–0.91), respectively. The PLR and NLR were 5.87 (95%CI: 3.78–9.12) and 0.17 (95%CI: 0.11–0.28), respectively. The DOR and AUC were 34.31 (15.51–75.91) and 0.92 (0.89–0.94), respectively. The pooled sensitivity, specificity, PLR, NLR, DOR, and AUC for digestive system cancer were 0.83, 0.88, 6.87, 0.20, 35.13, and 0.92, respectively. The other cancers were 0.87, 0.81, 4.55, 0.16, 28.51, and 0.90, respectively. For sample source, the results still remained consistent. The corresponding parameters were 0.87, 0.77, 3.75, 0.16, 27.03, and 0.82 and 0.83, 0.91, 8.48, 0.18, 55.18, and 0.97.
Publication bias
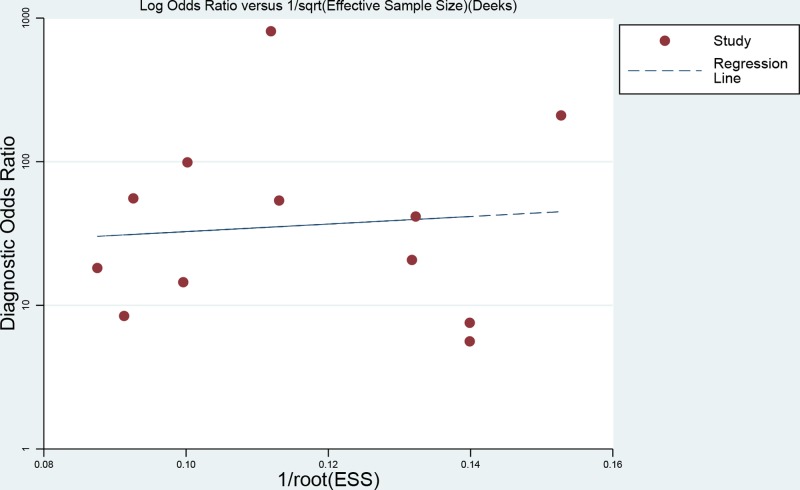
We used the linear regression test of funnel plot asymmetry to assess the publication bias in this meta-analysis. As presented in Figure 6, the P value for bias test was 0.781, indicating there was no publication bias for the present meta-analysis.
Figure 6. Line regression plot of publication bias (The closer to 0 degree the angel between X-ray gets, the lesser the publication bias gets).
Discussion
Our results found that the diagnostic ability of miRNA-451 was moderately high with the pooled sensitivity of 0.85 (95%CI: 0.77–0.90) and specificity of 0.85 (95%CI: 0.78–0.90). The AUC of miRNA-451 was 0.91 (95%CI: 0.89–0.94). This diagnostic accuracy was higher than some biomarkers such as carcinoma-embryonic antigen and squamous-cell carcinoma (with sensitivity of 47.5 and 49 for detecting lung cancer) [22,23]. The miRNA-451 could be a potential tumor biomarker of early-stage cancer detection.
Cancers have been becoming important public health issues around the world. It is urgent to find effective and simple diagnostic methods. In the past several decades, researchers had found many non-invasive methods for cancer detection and screening. MicroRNAs (miRNAs) are short non-coding RNAs that regulate gene expression at the post-transcription level and maintain a dynamic balance of body by regulating cell proliferation, differentiation, and apoptosis. Recently, a lot of studies have revealed that some miRNAs were involved in the tumorigenesis and tumor progression as oncogenes or tumor suppressors [24]. Furthermore, many studies also reported that some miRNAs could be a diagnostic biomarker for cancers [25,26]. The miRNA-451 is located on section of chromosome 17q11.2, which reverses transcription of some proteins with miRNA-144 [27]. Recently, a series of studies reported that there were differences in expression of miRNA-451 in different tumor tissue cells, which indicated the potential diagnostic ability in tumor. Xie found the expression of miRNA-451 was up-regulated in saliva, with the sensitivity of 84.6% and specificity of 57.9% in diagnosing esophagus cancer [18]. Ng also found the expression of serum miRNA-451 was up-regulated in breast cancer patients. The combined positive and negative rates were 88% and 92%, respectively, and the positive rate was 96% in diagnosing early-stage ductal carcinoma in situ [9]. Besides, Redova found down-regulated miRNA-451 was observed in the renal carcinoma patients. A combination of miR-378 and miR-451 enables identification of renal cell carcinoma (RCC) serum with the sensitivity of 81% and specificity of 83% [7]. These results indicated that miRNA-451 could be a potential diagnostic marker in tumor. We conducted a comprehensive analysis due to study limitation of individual study. Our results reported higher sensitivity and specificity (85% vs 85%). We also calculated other parameters. The DOR reflects the combination of sensitivity and specificity, ranging from 0 to infinity. The higher the value, the better the diagnostic ability. Our results found that the DOR was 31.33 (95%CI: 15.91–64.61), which means the diagnostic accuracy was high. Otherwise, we also calculated the PLR and NLR. Two indices can assess the clinical application value. The combined PLR of 5.57 means that cancer patients have higher chance by 5.57-fold than those without cancer when miRNA-451 was positive in examination. The combined PLR was 0.18, indicating the probability of patients with cancer was 18% when detection results were negative. However, according to the criteria, only if PLR > 10 and NLR < 0.1, the diagnostic ability was the best and had better ability of confirmation and exclusion [28]. Our results did not conform to the requirements. Previous studies found combination of multi-miRNA can achieve higher diagnostic ability. The higher clinical value could be achieved with combination applications [29,30]. We also conducted subgroup analyses in different populations, cancer types, and sample sources. Similar results were found, indicating the current results were stable and reliable.
Our study still has several limitations. First, we have tried our best to perform systematical searches. Some data may be still ignored such as unpublished study. Second, the included studies were from different cancer types and population settings, which may have some impacts on pooled results because miRNA-451 was low expression in some cancers, and high expression in other cancers. The present results still remained stable in spite of these differences. Third, miRNA-451 had higher diagnostic ability. However, the single miRNA still has some limitations. Combination of multi-miRNA could achieve better utility [31]. Finally, for clinical purpose, it requires more studies and analyses to investigate the mechanism of miRNA-451 in specific cancers, which promoted the application of miRNA-451 in cancer treatment.
In conclusion, our results found that the miRNA-451 has a moderately high diagnostic ability for cancers, and could be a potential early screening biomarker, and considered as an adjuvant diagnostic index when being combined with others clinical examinations. The diagnostic utility did not depend on population, cancer types, and sample sources. The miRNA-451 was of importance in digestive system cancers. Further studies are needed for illustrating mechanism.
Supporting information
Supplementary Figure.
Acknowledgments
We thank the colleagues of Department of Oncology, Xiangya Hospital, Central South University.
Abbreviation
- AUC
area under cure
- CI
confidence interval
- CNKI
China National Knowledge Infrastructure
- DOR
diagnostic odds ratio
- FN
false negative
- FP
false positive
- MeSH
Medical Subject Headings
- NLR
negative likelihood ratio
- PLR
positive likelihood ratio
- PRISM
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ROC
Receiver Operating Characteristic
- SROC
summary receiver operator characteristic
- TN
true negative
- TP
true positive
- qRT-PCR
quantitative real time polymerase chain reaction
- RCC
renal cell carcinoma
Author Contribution
L.Z.Z. and L.F.S. conceived and designed the research; L.Y.Y. and L.Z.Z. analyzed the data; L.N. and L.Z.Z. created all tables and figures; F.J. drafted the manuscript; L.Z.Z. and L.N. made critical revision of the manuscript; All authors read and approved the final manuscript.
Funding
This manuscript was supported by China Postdoctoral Science Foundation [2017M612597] and National Key Clinical Program (Department of Oncology, Xiangya Hospital Central South). The project was supported by National Natural Science and Technology Major Foundation of China [Grant no. 2017ZX090361], National Natural Science Foundation of China [Grant no. 81602683], and Hunan Department of Science and Technology Foundation [Grant no. 2016SK2007].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Fitzmaurice C., Dicker D., Pain A., Hamavid H., Moradi-Lakeh M., MacIntyre M.F.. et al. (2015) The Global Burden of Cancer 2013. JAMA Oncol. 1, 505–527 10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards B.K., Noone A.M., Mariotto A.B., Simard E.P., Boscoe F.P., Henley S.J.. et al. (2014) Annual Report to the Nation on the status of cancer, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. ACS 120, 1975–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fossa S.D., Nilssen Y., Kvale R., Hernes E., Axcrona K. and Moller B. (2014) Treatment and 5-year survival in patients with nonmetastatic prostate cancer: the Norwegian experience. Urology 83, 146–152 10.1016/j.urology.2013.08.081 [DOI] [PubMed] [Google Scholar]
- 4.Tran N. (2016) Cancer Exosomes as miRNA Factories. Trends Cancer 2, 329–331 10.1016/j.trecan.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 5.Zhang M.F., Wu K.Q., Chen D.W., Fei Z.W., Gao M. and Wu Y. (2015) The expression of miR-451 in thyroid papillary carcinoma and its value of the clinical diagnosis. Modern Oncol. 23, 1971–1974 10.3969/j.issn.1672-4992.2015.14.09 [DOI] [Google Scholar]
- 6.Konishi H., Ichikawa D., Komatsu S., Shiozaki A., Tsujiura M., Takeshita H.. et al. (2012) Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre- and post-operative plasma. Br. J. Cancer 106, 740–747 10.1038/bjc.2011.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redova M., Poprach A., Nekvindova J., Iliev R., Radova L., Lakomy R.. et al. (2012) Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J. Transl. Med. 10, 55 10.1186/1479-5876-10-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei L.L., Ren W.H., Li J.S., Xu G.L., Lai W.D. and Ma J.L. (2014) Expression and significance of 4 miRNAs in hepatocellular carcinoma. Acta Univ. Med. Anhui 49, 1287–1291 10.19405/j.cnki.issn1000-1492.2014.09.23 [DOI] [Google Scholar]
- 9.Ng E.K., Chong W.W., Jin H., Lam E.K., Shin V.Y., Yu J.. et al. (2009) Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 58, 1375–1381 10.1136/gut.2008.167817 [DOI] [PubMed] [Google Scholar]
- 10.Luo J., Zhao Q., Zhang W., Zhang Z., Gao J., Zhang C.. et al. (2014) A novel panel of microRNAs provides a sensitive and specific tool for the diagnosis of breast cancer. Mol. Med. Rep. 10, 785–791 10.3892/mmr.2014.2274 [DOI] [PubMed] [Google Scholar]
- 11.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B.. et al. (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 12.Li Z.Z., Shen L.F., Li Y.Y., Chen P. and Chen L.Z. (2016) Clinical utility of microRNA-378 as early diagnostic biomarker of human cancers: a meta-analysis of diagnostic test. Oncotarget 7, 58569–58578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Zhou Q., Li Y., Yan S., Fu J., Huang X.. et al. (2017) Mean cerebral blood volume is an effective diagnostic index of recurrent and radiation injury in glioma patients: A meta-analysis of diagnostic test. Oncotarget 8, 15642–15650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikoloulopoulos A.K. (2015) A vine copula mixed effect model for trivariate meta-analysis of diagnostic test accuracy studies accounting for disease prevalence. Stat. Methods Med. Res. 26, 2270–2286 10.1002/sim.6595 [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.P., Thompson S.G., Deeks J.J. and Altman D.G. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamza T.H., Arends L.R., van Houwelingen H.C. and Stijnen T. (2009) Multivariate random effects meta-analysis of diagnostic tests with multiple thresholds. BMC Med. Res. Methodol. 9, 73 10.1186/1471-2288-9-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks J.J., Macaskill P. and Irwig L. (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 58, 882–893 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 18.Xie Z., Chen G., Zhang X., Li D., Huang J., Yang C.. et al. (2013) Salivary microRNAs as promising biomarkers for detection of esophageal cancer. PLoS One 8, e57502 10.1371/journal.pone.0057502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu C., Ren C., Han J., Ding Y., Du J., Dai N.. et al. (2014) A five-microRNA panel in plasma was identified as potential biomarker for early detection of gastric cancer. Br. J. Cancer 110, 2291–2299 10.1038/bjc.2014.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phua L.C., Chue X.P., Koh P.K., Cheah P.Y., Chan E.C. and Ho H.K. (2014) Global fecal microRNA profiling in the identification of biomarkers for colorectal cancer screening among Asians. Oncol. Rep. 32, 97–104 10.3892/or.2014.3193 [DOI] [PubMed] [Google Scholar]
- 21.Y Y., Du Y.X., Zhang C., Wei S.Z. and Li Q.W. (2017) The expression level of microRNA-451 and the role of curative effect evaluation in serum of patients with esophageal squamous cell carcinoma. J. Xinjiang Med. Univ. 40, 779–782 [Google Scholar]
- 22.Yu D.H., Li J.H., Wang Y.C., Xu J.G., Pan P.T. and Wang L. (2011) Serum anti-p53 antibody detection in carcinomas and the predictive values of serum p53 antibodies, carcino-embryonic antigen and carbohydrate antigen 12-5 in the neoadjuvant chemotherapy treatment for III stage non-small cell lung cancer patients. Clin. Chim. Acta 412, 930–935 10.1016/j.cca.2011.01.028 [DOI] [PubMed] [Google Scholar]
- 23.Chandanwale S.S. and Pal S.S. (2014) Metastatic soft tissue squamous cell carcinoma: unusual presentation of lung cancer. J. Cancer Res. Ther. 10, 216–217 10.4103/0973-1482.131443 [DOI] [PubMed] [Google Scholar]
- 24.Papadavid E., Braoudaki M., Bourdakou M., Lykoudi A., Nikolaou V., Tounta G.. et al. (2016) Aberrant microRNA expression in tumor mycosis fungoides. Tumor Biol. 37, 14667–14675 10.1007/s13277-016-5325-2 [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Li Y. and Xue J. (2017) Clinical significance of miRNA-433 expression in hepatocellular carcinoma. Biomed. Res.-India 28, 7661–7664 [Google Scholar]
- 26.Pan Y., Zhang J., Fu H. and Shen L. (2016) miR-144 functions as a tumor suppressor in breast cancer through inhibiting ZEB1/2-mediated epithelial mesenchymal transition process. Oncotargets Ther. 9, 6247–6255 10.2147/OTT.S103650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dore L.C., Amigo J.D., Dos S.C., Zhang Z., Gai X., Tobias J.W.. et al. (2008) A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc. Natl. Acad. Sci. U. S. A. 105, 3333–3338 10.1073/pnas.0712312105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen L., Wan Z., Ma Y., Wu L., Liu F., Zang H.. et al. (2015) The clinical utility of microRNA-21 as novel biomarker for diagnosing human cancers. Tumor Biol. 36, 1993–2005 10.1007/s13277-014-2806-z [DOI] [PubMed] [Google Scholar]
- 29.Xiong D.D., Lv J., Wei K.L., Feng Z.B., Chen J.T., Liu K.C.. et al. (2017) A nine-miRNA signature as a potential diagnostic marker for breast carcinoma: An integrated study of 1,110 cases. Oncol. Rep. 37, 3297–3304 10.3892/or.2017.5600 [DOI] [PubMed] [Google Scholar]
- 30.Shin V.Y., Ng E.K., Chan V.W., Kwang A. and Chu K.M. (2015) A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol. Cancer 14, 202 10.1186/s12943-015-0473-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Zhang G., Li H., Han L., Fu A., Zhang N.. et al. (2015) Serum microRNA-365 in combination with its target gene TTF-1 as a non-invasive prognostic marker for non-small cell lung cancer. Biomed. Pharmacother. 75, 185–190 10.1016/j.biopha.2015.07.026 [DOI] [PubMed] [Google Scholar]