Abstract
In September 2017, the US FDA announced re-approval of gemtuzumab ozogamicin (GO), a CD33-targeting immunoconjugate, for treatment of newly diagnosed and relapsed/refractory acute myeloid leukemia (AML). This is a very significant step toward defining new treatment regimens in AML, as the treatment has essentially stayed unchanged with the ‘7 + 3 induction regimen’ (7 days cytarabine and 3 days of anthracycline) since 1973. GO is the first antibody–drug conjugate to receive FDA approval for treating cancer. This review article discusses the challenges faced and lessons learned during the journey of GO for AML treatment. Selected trials that have made significant contribution in our understanding of the most efficacious and safe use of GO for treating AML patients as well as factors influencing GO response are highlighted in this article.
Keywords: : acute myeloid leukemia, AML, antibody–drug conjugates, calicheamicin, CD33, CD33-directed agents, FDA, fractionated dosing, gemtuzumab ozogamicin, SNP, splicing
Background
Acute myeloid leukemia (AML) is a heterogeneous disease affecting hematopoietic stem and progenitor cells, and is characterized by impairment in the proliferation, differentiation and self-renewal capabilities of these cells. Factors contributing to the heterogeneous nature of this disease include a wide variety of cytogenetic abnormalities such as translocations, deletions, inversions, monosomies and trisomies as well as recurrent molecular mutations and aberrant expression patterns [1,2]. As a result of these factors, treatment options for AML patients are often ineffective for many patient populations [3]. In particular, for younger patients, complete remission (CR) rates of >80% are achievable; however, the 5-year overall survival (OS) still remains relatively low at ∼40% in comparison to other cancers due to high relapse rates [3]. Outcome is even worse for older patients with 5-year OS at <25% [4]. Despite this dismal outcome, the standard of care for AML has essentially remained stagnant since the 1970s as the ‘7 + 3’ regimen (7 days of continuous infusion cytarabine with 3 days of anthracycline) still remains the most common induction regimen. Studies in the last few decades using multiple new technologies and targeted approaches have advanced our understanding of AML disease biology, opening up opportunities for development of new therapeutic agents [2,3,5–7]. In light of these developments, several targeted therapeutics including tyrosine kinase inhibitors, epigenetic-modifying drugs and immunotherapeutic options such as the CD33-directed agent gemtuzumab ozogamicin (GO; known as Mylotarg by trade name) have become popular additions to the mainstay 7 + 3 induction therapy [3,6,7]. After a substantial period of time, several new agents such as midostaurin, enasidenib, CPX351 and CD33-directed GO have received the US FDA approval for treatment of AML thus expanding therapeutic approaches in AML. In this review article, the focus will be on GO, the first FDA-approved antibody–drug conjugate (ADC) for treating cancer.
Gemtuzumab ozogamicin
GO is CD33-directed ADC composed of hP67.6, a CD33-directed monoclonal antibody, covalently linked to the cytotoxic agent N-acetyl γ calicheamicin [8]. The efficacy of GO is rooted in the ubiquitous nature of CD33 as an antigen marker in AML patients thus making the marker a potent target for immunotherapeutic options for AML. Present on AML blasts in 90% of patients, CD33 is a 67 kd transmembrane cell surface glycoprotein and is a prominent member of the sialic acid-binding immunoglobulin-like lectin family with high expression in cells committed to myeloid differentiation [9,10]. While its specific activity is yet to be elucidated, CD33 is believed to inhibit cell signaling through recruitment of the Src homology 2 domain-containing protein tyrosine phosphatases SHP-1 and SHP-2 upon phosphorylation of tyrosine (Y340 and Y358) residues located within the immune-receptor tyrosine-based inhibitory motif domain on the cytoplasmic tail of the protein [9]. Recruitment of these phosphatases cause attenuation of activation signals associated with various cell processes such as calcium mobilization, cytokine release and transcriptional activation [11]. CD33 is internalized when engaged with antibodies thus making it an ideal candidate for development of antibody-based therapies.
Specifically, GO's mechanism of action begins with internalization of the ADC after the monoclonal antibody has successfully bound to the IgV domain of CD33 presented on the surface of leukemic blasts [8,12]. After internalization, the ADC is routed to the lysosome where calicheamicin moiety is cleaved from the antibody–antigen complex and enters the nucleus where it induces apoptosis via DNA single- and double-strand breaks (Figure 1) [8,12,13].
Figure 1. . Mechanism of action of gemtuzumab ozogamicin.
CD33-antibody component of gemtuzumab ozogamicin recognizes the IgV-domain of CD33 antigen. Once engaged, the bound CD33 homodimerizes and is internalized, where within the acidic conditions the lysosome calicheamicin is cleaved and released into the cell where it induces apoptosis via double-strand DNA breaks.
Adapted with permission from PharmGKB © PharmGKB and Stanford University. www.pharmgkb.org/pathway/PA166115250
Calicheamicin is a potent enediyne antitumor antibiotic initially isolated from Micromonospora echinospora and is responsible for the cytotoxic activity of GO [14,15]. Once the GO–CD33 complex is internalized, the acidic atmosphere in lysosomes hydrolyzes the disulfide bond connecting calicheamicin to the acid labile linker of GO, releasing free calicheamicin into the cell [8]. Upon release, calicheamicin is localized to the nucleus where, via its oligosaccharide moiety, it recognizes preferred 3′–5′ sites (AGGA, TCCT and TCCA) on the minor groove of DNA [14,16–19]. At these locations, double-strand breaks are initiated and executed through a series of chemical transformations involving calicheamicin and the recognized DNA site [14]. The overall cytotoxic effect of calicheamicin is potent enough to trigger signals and factors for apoptosis after double-strand break. Currently, mitochondrial-mediated apoptosis and ataxia-telangiectasia mutated/ataxia-telangiectasia related pathway-mediated cell cycle arrest are the two leading proposed DNA damage response pathways that are activated as a result of these breaks leading to apoptosis of leukemic cells [20–22].
Despite the targeted mechanism, the potent apoptotic capabilities of GO and demonstrated efficacy in relapsed patients, it is still subjected to the clinically and molecularly based variability in AML treatment response [23–25]. GO has had a remarkable journey as an immunotherapeutic in the realm of AML. Starting with accelerated approval in 2000-based promising results from Phase II studies, GO was voluntarily withdrawn in 2010 due to increased induction death and no observed survival benefit in the S0106 post approval Phase III study. Despite these setbacks, results from multiple subsequent Phase III clinical trials have allowed recent re-approval of GO by the FDA in September 2017 [26–28]. In this review, we aim to discuss the characteristics of selected clinical trials primarily focusing on de novo adult and pediatric AML. We will also discuss the current state of major contributors associated with intervariation in response to GO.
Methods
We conducted our literature review using a duplexed approach. Using PubMed, we conducted a search using the following string ‘GEMTUZUMAB’[SUPPLEMENTARY CONCEPT] OR ‘GEMTUZUMAB’[ALL FIELDS] OR ‘GEMTUZUMAB OZOGAMICIN’[ALL FIELDS], resulting in 641 entries. In addition to this, we also conducted a search on clinicaltrials.gov using the condition or disease search term ‘ACUTE MYELOID LEUKEMIA’ and other term ‘GEMTUZUMAB OZOGAMICIN’ to cross-check previously identified Phases II and III GO trials, and identify any potentially missed or ongoing trials with no published results. From the total collection of these articles, we selected papers discussing the structure, method and results from Phase II/III GO clinical trials with de novo AML patients (adult and pediatric) and papers discussing lessons learned and factors affecting clinical response of GO including manuscripts from the FDA.
Results
Although GO was initially approved as monotherapy for treatment of elderly patients with relapse AML [28], several studies since then have evaluated it in combination with other drugs. Description of all the GO-containing clinical trials is beyond the scope of this review. In this review, we have selected six clinical trials that investigated various combinatorial treatment strategies and doses of GO in de novo AML (Table 1).
Table 1. . Randomized studies using gemtuzumab ozogamicin for de novo acute myeloid leukemia patients.
| Study name | Dates of recruitment | Patient randomized (n) | Age range (years) | GO dose strategy | Results |
|---|---|---|---|---|---|
| MRC AML 15 [31,32] | 2002–2006 | 1113 | 0–71 | GO dosed at 3 mg/m2 on day 1 with either ADE 10+3+5, DA 3+10, or FLAG-Ida for induction therapy or MACE, or 1.5 or 3 g/m2 of cytarabine for consolidation | No significant difference in OS, RFS and TRM. Significant increase in OS for those favorably stratified by cytogenetics (5 year: 79 vs 51%, p = 0.0003) while 70% of intermediate-risk patients are predicted to experience a 10% improvement in OS. Addition of GO was well tolerated with no significant increase in toxicity |
| GOELAMS AML 2006 IR [41] | 2007–2010 | 238 | 18–60 | GO dosed at 6 mg/m2 on day 4 in addition to standard 3 + 7 induction therapy and in the first course of consolidation with MidAc | No significant difference in 3-year EFS and OS for all patients overall; however, patients who did not undergo ASCT experienced improved EFS (54 vs 27%, p = 0.03). Patients also experienced a significant increase in grade 3/4 liver toxicity (23 vs 13%, p = 0.03) |
| ALFA-0701 [38,39] | 2008–2010 | 278 | 50–70 | GO dosed based on 3-3-3 regiment where patients received GO at 3 mg/m2 on days 1, 4 and 7 during the first course of induction in addition to standard 3 + 7 standard therapy. Consolidation therapy consisted of GO given on day 1 of each course of consolidation in addition to treatment with daunorubicin (60 mg/m2 for 1–2 days) and cytarabine (1000 mg/m2 per 12 h infused over 2 h on days 1–4) | Patients experienced a significant increase in EFS (2-year: 40.8 vs 17.1%; HR: 0.58, 95% CI: 0.43–0.78; p = 0.0003), RFS (2-year: 50.3 vs 22.7%; HR: 0.52, 95% CI: 0.36–0.75; p = 0.0003), and OS (2-year: 53.2 vs 41.9%; HR: 0.69, 95% CI: 0.49–0.98; p = 0.0368). Survival benefit was seen in favorable and intermediate-risk groups |
| NCRI AML 16 and LRF AML 14 [42] | 2006–2010 | 1115 | 51–84 | GO dosed at 3 mg/m2 in addition to DA therapy, DClo therapy or LDAC depending on patient fitness | Intensive therapy: patients experienced significant decrease in rate of relapse (2-year: 61 vs 70%, p = 0.004), leading to better RFS (2-year: 28 vs 23%, p = 0.03). Significant improvement of CR (47 vs 39%, p = 0.02) and OS (2-year: 35 vs 29%, p = 0.04) Nonintensive therapy: significant increase in CR rate (11 vs 21%; OR: 0.46, 95% CI: 0.29–0.75; p = 0.002) as well as overall response rate (17 vs 30%; OR: 0.48, 95% CI: 0.32–0.73; p = 0.006) was observed. No significant improvement in OS. Efficacy mostly experienced by patients with greater WBC. No major increase in toxicity with addition of GO |
| SWOG S0106 [29] | 2004–2009 | 595 | 18–60 | GO dosed 6 mg/m2 on day 4 of induction in DA therapy (daunorubicin given at 45 mg/m2 on days 1–3). Patients not randomized to GO arm received DA therapy with daunorubicin given at 60 mg/m2 on days 1–3 | No difference in CR, OS or RFS overall. Favorable cytogenetic risk experienced improved RFS (5-year: HR: 0.49; p = 0.043) trended toward improved OS (5-year: HR = 0.54; p = 0.12). Increased rate of induction mortality in DA + GO arm (5 vs 1%) |
| EORTC-GIMEMA AML-17 [49] | 2002–2007 | 472 | 60–75 | GO given at 6 mg/m2 on days 1 and 15 of induction therapy and at 3 mg/m2 on day 0 of consolidation therapy | No significant difference in OS and CR. Patients age 70–75 in no GO arm had improved CR + CRp (52 vs 33%; OR: 0.44; 99% CI: 0.19–1.00; p = 0.01). No observed benefit from GO in patients age 61–69. Patients ≥70 had significantly worse OS with GO (HR: 1.64; 99% CI: 1.07–2.51). De novo patients also had significantly shorter OS (HR: 1.37; 99% CI: 1.02–1.85). Increased grade 3/4 liver toxicities (15 vs 10%) and higher 60-day TRM (22 vs 18%) with inclusion of GO |
| COG AAML0531 [48] | 2006–2010 | 1022 | 0.003–29.99 | GO given at 3 mg/m2 on day 6 of induction and day 7 of consolidation in addition to ADE10+3+5 therapy and therapy using mitoxantrone and cytarabine, respectively | Significant improvement of EFS (3-year: 53.1 vs 49.6%; HR: 0.83, 95% CI: 0.70–0.99; p = 0.04) and RR (3-year: 32.8 vs 41.3%; HR: 0.73, 95% CI: 0.58–0.91; p = 0.006) overall. Improved CR (87.4 vs 92.7%, p = 0.03) and RR (3-year: 30.3 vs 19.7%, p = 0.04) seen in intermediate-risk and low-risk cytogenetic groups. Nonsignificant increase in postremission toxic mortality (3-year: 6.6 vs 4.1%; HR: 1.69, 95% CI: 0.93) |
| NCRI AML 17 [35,36] | 2009–2011 | 788 | 0–81 | GO given at 3 or 6 mg/m2 on day 1 of the second course of induction in addition to DA therapy | Significant improvement of CR in patients who used 3 vs 6 mg/m2 (82 vs 76%, odds ratio: 1.46, 95% CI: 1.04–2.06; p = 0.03). Patients in the 6 mg/m2 also experienced increased 30- and 60-day TRM (p = 0.02 and p = 0.01, respectively). |
ADE10+3+5, cytarabine (100 mg/m2 on days 1–10 every 12 h)/daunorubicin (50 mg/m2 on days 1, 3 and 5)/etoposide (100 mg/m2 on days 1–5); DA, daunorubicin (50 mg/m2 on days 1, 3 and 5)/cytarabine (100 mg/m2 on days 1–8 every 12 h); LDAC (20 mg twice daily subcutaneously); DA 3+10, daunorubicin (50 mg/m2 on days 1, 3 and 5)/cytarabine (100 mg/m2 on days 1–10 every 12 h); FLAG-Ida, fludarabine (30 mg/m2 intravenous on days 2–6)/cytosine arabinoside (2 g/m2 over 4 h after fludarabine)/G-CSF (263 μg subcutaneously daily on days 1–7); MACE, amsacrine (100 mg/m2 on days 1–5/cytarabine (200 mg/m2 continuous days 1–5)/etoposide (100 mg/m2 on days 1–5).
ADE: Ara-C + cytarabine + daunorubicin + etoposide; ALFA: Acute Leukemia French Association; AML: Acute myeloid leukemia; ASCT: Autologous stem cell transplant; COG: Children's Oncology Group; CR: Complete remission; CRp: Partial complete remission; DA: Daunorubicin + Ara-C; DClo: Daunorubicin + clofarabine; EFS: Event-free survival; EORTC-GIMEMA: European Organisation for Research and Treatment of Cancer in collaboration with Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto; FLAG-Ida: Fludarabine + IDAC + G-CSF + idarubicin; G-CSF: Granulocyte colony-stimulating factor; GO: Gemtuzumab ozogamicin; GOELAMS: The French Acute Leukemia and Blood Diseases West-East Group; HR: Hazard ratio; IR: Intermediate risk; LDAC: Low-dose Ara-C ; LRF: Leukemia Research Foundation; MACE: Amsacrine + cytarabine + etoposide; MRC: Medical Research Council; NCRI: National Cancer Research Institute; OS: Overall survival; RFS: Relapse-free survival; RR: Relapse rate; SWOG: South-West Oncology Group; TRM: Treatment-related mortality; WBC: White blood cell count.
S0106 study (NCT00085709)
Although GO received accelerated approval in 2000 based on Phase II clinical trials, consistent results showing benefit must be demonstrated in Phase III trials to obtain full regulatory approval. In 2004, the Southwest Oncology Group initiated the first randomized Phase III trial for GO in the USA to fulfill this requirement. Overall, the study enrolled 637 patients who were randomized to receive either standard induction with daunorubicin (60 mg/m2/day on days 1, 2 and 3) and cytarabine (100 mg/m2/day from days 1–7) or a GO containing induction with lower dose of daunorubicin (45 mg/m2/day on days 1, 2 and 3), cytarabine (100 mg/m2/day from days 1–7) and GO (6 mg/m2 on day 4; DA + GO). After completing consolidation therapy, eligible patients were again randomized to receive either additional therapy using GO (5 mg/m2, three doses at least 28 days apart) versus observation. Data from 595 patients were available for evaluation after excluding patients’ secondary AML, acute promyelocytic leukemia and other factors [29].
No difference in efficacy as measured by CR, OS or relapse-free survival (RFS) was observed between the DA + GO and DA arms. Interim analysis was complicated by multiple factors including lower dose of daunorubicin in GO containing arm, and showed significantly higher mortality during induction in GO arm. There was a significantly higher rate of mortality in the DA + GO arm versus the DA arm (5 vs 1%), consequently resulting in the withdrawal of GO from the market in 2010 [29].
Although the results of S0106 did not show improved benefit from GO as previous trials had done, an improvement in RFS in DA + GO arm among the favorable cytogenetic risk group patients was observed (hazard ratio [HR]: 0.49; p = 0.043) [29]. In addition to this, the S0106 trial identified potential contributors to the lack of efficacy of this agent. A higher dose of GO (single 6 mg/m2 intravenous bolus dose of GO) in combination with decreased anthracycline dose may have diminished the potential benefit to be gained from GO [30].
UK studies MRC studies
Motivated by the drive to further optimize patient care, the UK MRC initiated multiple clinical trials with combining GO with induction therapy. The first of these trials, MRC AML15 (ISRCTN17161961), a Phase III trial, enrolled 1113 patients, younger than 60 years, who were randomized to receive lower 3 mg/m2 dose of GO in induction I and in consolidation in addition to standard or other experimental therapies at the time [31]. This rather complex trial design had three different induction arms including ADE, DA and Ida/FLAG, where patients in DA and Ida/FLAG arms were randomized to receive GO (ADE recipients did not receive GO), thus creating five different treatment cohorts. Further, patients who completed induction were re-randomized to receive MACE, cytarabine at 1.5 gm/m2 or cytarabine at 3 gm/m2 with and without GO (six different consolidation cohorts). At the end of second consolidation course, patients were randomized for the third time to observation versus fifth course of therapy with 1.5 gm/m2 of cytarabine. In combination, three different randomizations and eight different induction, consolidation and maintenance therapies generated 60 different therapy cohorts with majority of patients having been exposed to GO either at induction, consolidation or both.
Overall, the addition of GO was well tolerated with no substantial increase in toxicity. However, based on the original GO randomization, addition of GO was not associated with improved outcome. The only patients who benefited from treatment with GO were those with favorable karyotype, with no significant survival benefit in those with intermediate or high-risk features [31–33].
Several follow-up trials to MRC AML15, including the MRC AML14 trial (ISRCTN62207270), the MRC/National Cancer Research Institute (NCRI) AML16 trial (ISRCTN11036523) and the MRC/NCRI AML17 trial (ISRCTN55675535), tested GO in different combinatorial dosing strategies with standard therapy. The MRC/NCRI AML16 trial randomized 1115 slightly older patients aged 51–84 years old to receive either standard chemotherapy with or without addition of 3 mg/m2 GO on day 1 of induction therapy. The results confirmed GO tolerability with no significant increase in toxicity and improvement in survival with reduced relapse risk across groups with no impact between treatment interventions [34]. A subsequent meta-analysis of MRC AML15 and MRC/NCRI AML16 study with 1228 patients randomly assigned to GO further confirmed the benefit from addition of 3 mg/m2 GO to standard therapy [34].
The MRC/NCRI AML17 trial was designed to define the optimal GO dose. The study enrolled 788 patients, majority under 60 years old, who were randomized to receive GO on day 1 of induction I treatment given at either 3 or 6 mg/m2 treatment. Higher CR rates were observed in patients dosed at 3 mg/m2 (82 vs 76%; p = 0.03), although overall response rates were similar across both groups (89 vs 86%; p = 0.17). The 6 mg/m2 group experienced a significant increase in the 30- (7 vs 3%; p = 0.02) and 60-day mortality rates (9 vs 5%; p = 0.01) with higher occurrence of veno-occlusive disease (5.6 vs 0.5%; p < 0.0001). Thus, the study results demonstrated that there was no clinical benefit in using a single dose of 6 mg/m2 GO as compared with 3 mg/m2 in combination with induction therapy [35,36].
French ALFA-0701 trial (NCT00927498)
In contrast to the complex trial design of MRC and SWOG trials, French ALFA-0701 Phase III trial used a simple, single randomization open-label trial that investigated the effect of adding three fractionated doses of 3 mg/m2 to standard induction therapy in de novo adult AML patients. The study included 280 patients, ages 50–70, who were randomized to receive either standard DA therapy or DA + GO therapy. Patients were randomized to receive GO at 3 mg/m2 on days 1, 4 and 7 during the first course of induction. The rationale behind the 3-3-3 dosing strategy was to take advantage of CD33 re-expression and reduce hepatotoxicity and risk of treatment-related mortality while maintaining a cumulative dose high enough to see efficacy [37]. GO was also given to patients on day 1 of each of the two consolidation chemotherapy courses. Remission rates (CR + CRp; 81 vs 75%, p = 0.25) and OS (3-year, 38 vs 36%, p = 0.18) were not significantly improved in GO arm while event-free survival (EFS; 3-year, 31 vs 19%, p = 0.0026) and RFS (2-year, 38 vs 25%, p = 0.006) significantly improved in the GO arm [38,39]. Although higher hematological toxicity was observed in GO arm, the risk of death due to toxicity was not observed [38,40]. The ALFA-0701 study was also unique in that it was the first study to analyze efficacy by mutational profile in addition to risk-stratified analyses with results showing that patients with FLT3-ITD+ AML experienced significant benefit from GO addition as compared with patients without FTL3-ITD+ AML. The positive results of the ALFA-0701 study are in many regards attributable to the fractional dosing strategy employed which established the presence of benefit in favorable and intermediate-risk subgroups as well as the entire cohort. This observed benefit made ALFA-0701 a pivot trial for consideration of GO re-approval by FDA.
AML 2006 IR study
The French Groupe Ouest Est d'Etude des Leucemies Aigues et Autres Maladies du Sang (GOELAMS) conducted a trial using a similar dosing strategy to S0106. In this rather small trial, 238 de novo AML patients aged 18–60 were randomly assigned to receive standard induction therapy with or without the addition of GO (6 mg/m2 on day 4). GO was also added to consolidation therapy in addition to mitoxantrone and cytarabine on the basis of initial randomization. Although the study was unable to identify any significant difference in OS and EFS between the two groups in overall study population, improved EFS was observed in patients with intermediate cytogenetics who were not eligible for allogeneic transplant within the GO arm (53.7 vs 27%, p = 0.0308) [41]. Additionally, this study reported higher rates of veno-occlusive disease and grade 3–4 hepatic toxicities in the GO arm.
In summary, despite early disappointing results from rather complex trials, more recent data have demonstrated clear efficacy of GO AML regardless of age, by reducing risk of relapse and improving survival [32,38,41–43]. Many of these clinical trials have also shown survival benefits in favorable and intermediate-risk groups, but clear response in high-risk group patients remains elusive. A recent meta-analysis using data from MRC AML15, S0106, MRC/NCRI AML 16, GOELAMS AML 2006 IR and ALFA-0701 trials (total n = 3325 patients) confirmed these results and demonstrated that addition of GO significantly reduced the risk of relapse and improved 5-year OS, although no significant improvement in CR was observed [44]. The only caveat of this meta-analysis is that overwhelming majority of the patients in this meta-analysis were from MRC and SWOG trials that showed no benefit to GO, thus potentially diluting the benefit seen in ALFA and GOELAMS trials that demonstrated efficacy in intermediate-risk patients.
Impact of GO in children & young adults
A pilot open-label dose escalation study supported by National Cancer Institute and Children's Oncology Group (COG) was performed to evaluate safety and efficacy of GO in CD33+ pediatric patients with multiple relapsed or primary refractory AML. The study demonstrated that GO was well tolerated at two doses of 6 mg/m2 with a 14-day interval between doses [45]. A follow-up pilot study, AAML03P1, further investigated the safety of GO in 350 younger patients, aged from 1 month to 21 years old, using a dosing strategy adapted from the MRC regimen [46]. All patients received two doses of GO at 3 mg/m2 in combination with the standard chemotherapy, one on day 6 of course 1 and again on day 7 of course 4. CR was observed in 83.1% of patients after course 1 and 87% after course 2 with 3-year EFS and OS being 53 ± 6% and 66 ± 5%, respectively. Overall, the results of this trial demonstrated that GO can be safely used in de novo pediatric AML patients with toxicity in line with other chemotherapeutic regimens and survival rates better than other clinical trials [46,47].
Encouraged by results from AAML03P1 National Cancer Institute/COG conducted AAML0531 the largest randomized pediatric de novo AML trial that enrolled all children and young adults up to age 30. AAML0531 consisted of two arms, one with the addition of two doses of GO at 3 mg/m2, (one in induction and one in intensification 2, GO arm, n = 511) to standard therapy and one with standard therapy alone (No-GO, n = 511). Similar to AAML03P1, incorporation of GO in addition to standard ADE therapy resulted in favorable clinical outcomes. Addition of GO significantly improved EFS (3-year: 53.1 vs 49.6%; HR: 0.83, 95% CI: 0.70–0.99; p = 0.04), but as with previous studies no impact was observed for OS. Although no improvement in remission rates was observed, significant reduction in relapse risk was observed (32.8 vs 41.3%; HR: 0.73, 95% CI: 0.58–0.91; p = 0.006) in GO arm. AAML0531 further validated the results of stratified analyses from previous trials, showing a 7% improvement in EFS and a 10% reduction in relapse risk in favorable risk group patients when treated with GO-containing regimen. Within the intermediate-risk group, addition of GO also improved CR rates (87.4 vs 92.7%, p = 0.03) and trended toward reduced RR and improved EFS and OS. No benefit from adding GO was observed in high-risk group patients consistent with studies conducted in adult AML patients [48].
Lessons learned & application toward re-approval of GO by FDA
The successes and failure of trials using GO have left an abundance of lessons to be learned regarding the use of GO in de novo AML patients. Among the most prevalent of the aforementioned is dosing strategy and sequence of administration (Table 1). Primarily, the results from these studies demonstrated that the dose and sequence of administration of GO is critical to avoid unwanted toxicity and achieve maximum benefit. In September 2017, the FDA re-approved GO for treatment of newly diagnosed CD33+ AML based on the positive results seen in ALFA-0701 and EORTC-GIMEMA AML 19 (NCT00091234) studies which employed fractionated dosing strategies of GO [38,40,49]. The FDA has recently reported their evaluation of these trials using the ALFA-0701 trial as the standard for GO combination therapy and the EORTC-GIMEMA AML19 trial as the standard for GO monotherapy for treatment of newly diagnosed AML [40]. GO also received approval for use in relapse or refractory CD33+ AML as a fractionated low dose of 3 mg/m2 on days 1, 4 and 7 [50]. In their approval, the FDA noted the safety of lower doses and fractionated regimens of GO without loss of clinical benefit from adding GO in adult AML patients as well as the acceptable risk of hepatic veno-occlusive disease and early mortality rates.
Factors affecting response
Given what we know about the mechanism of action of GO, factors influencing crucial steps including ADC internalization, release and activation of calicheamicin, the intracellular levels and DNA binding capabilities of calicheamicin, as well as efficiency of downstream DNA damage repair pathways and apoptotic pathways can play critical role in defining therapeutic efficacy of GO. Two of the most studied factors include expression levels of CD33, the GO target and P-glycoprotein 1 (PgP1/MDR1), a drug transporter implicated in efflux of calicheamicin.
CD33 expression & single nucleotide polymorphisms
Expression levels of CD33 have been evaluated from multiple Phase II and Phase III clinical trials. Previous in vitro data have shown CD33 expression to be associated with greater GO efficacy; however, results from initial clinical trials in adult AML patients have shown conflicting results with CD33 expression with clinical response [51–56]. In a retrospective analysis of specimens from the AAML03P1 clinical trial, high interpatient variability in cell-surface intensities of CD33, measured using mean fluorescence intensity by as much as 2-log fold was observed [57]. Quartile-based classification of patients with respect to CD33 cell-surface intensity identified higher CD33 levels to be associated with FLT3-ITD+ AML and other high-risk group features, supporting results reported from the mutational profile analysis done in the ALFA-0701 trial [57,58]. Similar findings have also been reported from retrospective analyses of MRC AML17 and MRC AML16 studies confirming the association of CD33 expression with the aforementioned disease characteristics [59]. In the MRC studies, after exclusion of core binding factor-AML, no benefit for adding GO was observed in patients with low CD33 expression quartile. Overall this study reported an interaction between GO dose and CD33 expression levels [59]. A follow-up study with classification of patients based on quartiles with respect to CD33 expression in the GO randomized pediatric clinical trial AAML0531 confirmed the association of higher CD33 expression with FLT3-ITD positivity as well as with high-risk group features [60]. Comparative analysis by CD33 expression quartile identified no benefit from addition of GO to patients with low CD33 expression (quartile 1); however, significant reduction in relapse risk (32 vs 49%; p < 0.001) and improvement in EFS (53 vs 41%; p = 0.005) were observed in patients with higher CD33 expression (quartiles 2–4) within GO arm as compared with NO-GO arm [60]. The effect CD33 expression has likewise been evaluated in the ALFA-0701 study likewise, showing that those with higher CD33 expression (≥70% blasts are CD33 positive) had significantly improved outcome compared with patients with <70% blasts CD33 [61]. On the other hand, the results from two published meta-analyses did not reveal any significant association between leukemic blast CD33 expression and clinical effectiveness of GO [62,63].
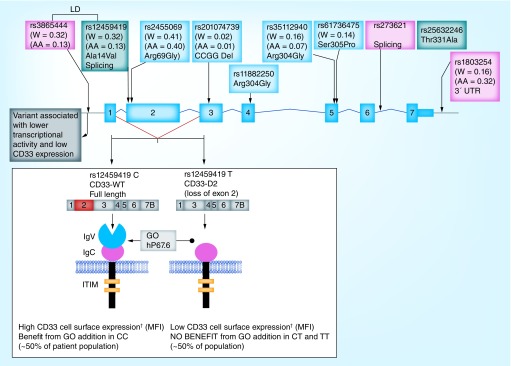
We had previously described genetic polymorphisms in CD33 by sequencing the complete coding region and flanking introns of CD33 in subset of patients from the multicenter St Jude AML02 clinical trial. We reported occurrence of six nonsynonymous single nucleotide polymorphisms (SNPs): rs12459419 (Exon 2, Ala14Val), rs2455069 (Exon 2, Arg69Gly), rs1188250 (Exon 4, Phe243Leu), rs35112940 (Exon 6, Arg304Gly), rs61736475 (Exon 6, Ser305Pro) and rs35632246 (Exon 7, Thr311Ala), an intronic splicing SNP rs273621 (Intron 6) and one 3′UTR SNP rs1803254 with potential clinical relevance [64,65]. Preliminary analysis associated the T allele of rs12459419 with poor response, while a follow-up study in a larger cohort of pediatric AML patients from AAML03P1 clinical trial identified associations of rs12459419, rs2455069 and rs1803254 with cell-surface expression of CD33 [65]. Figure 2 highlights some of the potentially relevant SNPs in CD33.
Figure 2. . CD33 SNPMap showing selected single nucleotide polymorphisms reported previously.
Potential effect of the most significant splicing single nucleotide polymorphism rs12459419 on based on previous reports is also highlighted (Mortland et al., 2011, Lamba et al., 2009, 2017 and 2018).
†Indicated mean fluorescence intensity determined using p67.6 using flow cytometer analysis.
The most interesting of these polymorphisms is CD33 SNP rs12459419 (C<T; Ala14Val) in the exon 2 is present within four base pairs of the intron/exon junction and impacts the exonic splicing enhancer binding site for SRSF2. The T allele for this SNP mediates spliceosome binding and splicing of exon 2 that encodes the GO binding site (CD33 IgV V-set domain). The shorter CD33-isoform (D2-CD33), generated as a result of alternate splicing, lacks the IgV V-set domain (Figure 2) [66–68]. Loss of the V-set antibody binding domain has two significant implications. As it appears that most (perhaps all) available diagnostic antibodies are directed at the V-set domain, thus patients with the rs12459419 T genotype would appear to be CD33 negative, due to the lack of inclusion of the V-set domain. More importantly, loss of the V-set domain would directly impact the binding, internalization and clinical efficacy of GO [69]. Recent data from newly diagnosed AML patients randomized to receive standard-five-course chemotherapy alone (No-GO arm, n = 408) or with addition of two doses of GO one during induction I and one in intensification II (GO arm, n = 408) as per the Children's Oncology group-AAML0531 trial show significant association of the T allele with higher levels of D2-CD33 transcript (p <1.0e-6) and with lower diagnostic leukemic cell surface CD33 intensity (p <1.0e-6) (Figure 2).
Perhaps the most compelling impact of this SNP was its role in mediating response to GO. In COG-AAML0531, which overall showed only a modest 6% improvement in EFS with no survival benefit, patients with CC genotype had a remarkable 23% decrease in RR in the GO arm compared with the NO-GO arm (26 vs 49%; p < 0.001). In contrast, in patients with CT/TT genotypes, addition of GO did not impact RR (39 vs 40%; p = 0.5). Disease-free survival was also significantly higher in CC genotype patients in the GO arm compared with the No-GO arm (65 vs 46%; p = 0.004), and overall the benefit from addition of GO was not seen in patients with the CT/TT genotypes [69]. These results suggest that loss of IgV V-set domain due to presence of the splicing SNP compromises GO efficacy. The rs12459419 splicing SNP occurs in linkage disequilibrium with a promoter SNP, rs3865444. In vitro functional data, obtained through a mini-gene experiment, has confirmed the association between the T allele of rs1245419 and production of CD33-D2. Further the linked promoter SNP was associated with lower transcriptional activity of a CD33 gene construct in in vitro reporter assays [70].
The rs12459419 splicing SNP is among the most significant factors shown to impact GO response with presence of CC genotype reducing the relapse risk by almost 50% in the GO arm of AAML0531. Recent investigation in adult AML through combined analysis of MRC AML15 and NCRI AML17, also observed significant association of TT genotype with lower CD33 expression as measured by mean fluorescence intensity; however, no difference in outcome between genotype groups was observed with similar RFS in GO and No-GO arms [56,71]. One of the significant differences between these two studies is varying schedules and dosing of GO incorporated in the treatment regimen, which might be contributing to the observed inconsistencies. Overall, based on the functional biology of this CD33 SNP, its regulation of the splice isoform has potentially significant scientific and clinical implications. Validation studies for the role of CD33 SNP in mediating response to CD33-directed therapy are forthcoming.
In addition to the splicing SNP, other SNPs in CD33 have been shown to impact CD33 expression and have been recently investigated in COG-AAML0531 clinical trial [65,72]. Given that the splicing SNP is more common in the European patients but is less frequent in other population, it is important to investigate other CD33 SNPs, some of which are more prevalent in African–American patients. Future studies focusing on the comprehensive evaluation of CD33 in multiple cohorts should improve GO therapy in future.
Effect of Pgp1 on antileukemic effects of GO
Given that the antileukemic effects of GO are largely due to calicheamicin-induced DNA damage, intracellular levels of calicheamicin are critical for clinical efficacy of GO. Previous studies have shown that the drug efflux transporter, Pgp1, which is encoded by the ABCB1 gene, influences the cellular accumulation of calicheamicin [73]. Pgp1 activity in leukemic cells has been shown to inversely correlate with GO response in patients with AML. In vitro studies have also shown significant relationship between higher Pgp1 levels and GO chemosensitivity as well as with clinical response of GO in AML patients [74–78]. In vitro cellular sensitivity of unconjugated calicheamicin has been shown to have upwards of 100,000-fold difference between resistant and sensitive AML patient samples [79]. Preliminary evidence suggests genetic variation in ABCB1 to have an influence on GO response future studies are needed to enhance our understanding of its contribution to GO efficacy [80].
Effect of other factors
Studies have also approached understanding the efficacy of GO from the perspective of methylation and other regulators of gene expression and function. In older patients treated with GO, methylation status of the suppressor of cytokine signaling (SOCS3) gene, has been identified as a potential regulator of OS [81,82]. SOCS3 has been implicated in proteasomal degradation of CD33 when bound to the phosphorylated state of CD33 [83,84]. Specifically, patients who had hypermethylation of SOCS3 tended to trend toward better response rates (86 vs 56%; p = 0.17) and longer OS (25.1 vs 10.3 months; p = 0.09) in comparison to those with hypomethylated SOCS3 [81].
Among other factors, overexpression of antiapoptotic proteins BCL2 and BCL21, which are known to inhibit mitochondrial-mediated apoptotic pathways through downregulation of mitochondrial permeability transition activation as well as cytochrome C release, has also been implicated in the development of resistance to GO [20–22].
Other CD33-targeted agents
Given the significance of CD33 in AML, several other agents targeting CD33 have been developed and are in different stages of clinical trial. Although the detailed information on these agents is beyond the scope of this review, there are a few worth mentioning.
Vadastuximab talirine (SGN33A) is an ADC containing the lintuzumab antibody and a pyrrolobenzodiazepine dimer. Early clinical trials in relapsed AML have shown encouraging results; unfortunately, due to a higher rate of deaths in a Phase III clinical trial, all SGN33A studies have been placed on hold. At this time, the cause of these early deaths is not clear, and further work will be required before the potential of SGN33A can be re-evaluated for treatment of AML [85]. IMGN779, another CD33-directed conjugate which uses a DNA alkylator as the warhead, is currently being investigated in clinical trials (NCT02674763) [86].
225Ac-lintuzumab is a CD33-directed α-particle therapeutic, which carries the radioisotope 225Ac. Once released inside the cell, 225Ac induces a cytotoxic dose of α radiation, causing cell death [87,88]. Safety studies have shown encouraging results and Phase I trials are underway (NCT02575963).
CD33/CD3 BiTE (bispecific T-cell engaging antibodies) have shown efficacy in ex vivo studies. Development is ongoing with Phase I trials underway (NCT02520427). In addition, CD33 is also currently being explored as a target for therapeutic strategies surrounding chimeric antigen receptor T-cell therapy [89–91].
Future perspective
Recent re-approval of GO AML is a major breakthrough in AML treatment. The new knowledge regarding safe and efficient use of fractionated lower dose of GO in combination with standard therapy has changed the paradigm of treatment in AML. Identification of key factors such as CD33 splicing SNP and interpatient variation in Pgp 1 may assist in personalizing GO treatment leading to patients deriving the maximum benefit from treatment with GO.
In addition, GO in combination with epigenetic modifiers such as the hypomethylating agent azacytidine, the histone deacetylating agent vorinostat and decitabine is being investigated [92–95]. Preliminary data from these studies show that epigenetic modifiers are capable of inducing an increased sensitivity to GO in AML blast [93,96,97]. Other CD33-directed agents are being designed with modifications to overcome the limitations associated with GO and once shown safe and efficacious in clinical trials, CD33-directed therapy might be mainline in AML chemotherapy. Overall enhanced understanding of CD33 as a therapeutic drug target for AML and the lessons learned during development, approval, withdrawal and re-approval of GO are critical in changing the treatment paradigms in AML.
Executive summary.
Acute myeloid leukemia
Acute myeloid leukemia (AML) is a heterogeneous disease characterized with wide variety of cytogenetic abnormalities and genomic lesions.
For the last 40 years, treatment of AML has essentially remained unchanged.
Although the majority of patients achieve complete remission, a significant proportion of patients experience relapse.
Outcome is worse for older patients with 5-year overall survival at <25%.
Antibody-directed therapies in AML
CD33, a cell-surface antigen is expressed highly in myeloid cells including AML blasts in 90% of patients, making it an attractive therapeutic target for AML.
Upon engaging with antibodies, CD33 is internalized which is leveraged in development of antibody-based drug conjugates.
Gemtuzumab in AML
Gemtuzumab ozogamicin (GO), a CD33-directed monoclonal antibody linked to cytotoxic calicheamicin is a re-emerging agent with great potential in AML treatment.
Met with initial success, followed by a failure in a Phase III clinical trial, resulting in GO withdrawal in 2010.
Results from several subsequent large randomized clinical trials have shown that dose and sequence of GO administration are critical to avoid toxicity and achieve maximum benefit from addition of GO to chemotherapy.
In light of new results in September 2017, the US FDA and the EU approved GO for treating both de novo and relapse/refractory CD33+ AML as a fractionated low dose of 3 mg/m2.
Factors influencing GO response in AML
Although validation in bigger cohorts is required, the top factors that impact GO response are CD33 genetic, its cell-surface expression and levels of drug transporter PgP1.
Recently reported exon 2 CD33 splicing SNP (rs12459419 C>T) results in loss of IgV domain.
Since IgV domain is recognized by GO, patients with genotype resulting in this loss do not benefit from addition of GO to chemotherapy.
Conclusion
Success and re-approval of GO is a major breakthrough for AML treatment.
Identification of CD33 splicing single nucleotide polymorphism is significant factor for personalizing GO treatment.
Other anti-CD33–drug conjugates such as IMGN779, SGN33A, 225Ac-lintuzumab as well as CD33/CD3 (BiTE; bispecific T-cell engaging antibodies) or trispecific killer engagers are in different stages of testing in various clinical trials.
Footnotes
Financial & competing interests disclosure
JK Lamba is funded through NIH – R01-CA132946 and R21-CA155524 and Children's Oncology Group – The Hematologic Malignancies Translational Pilot Studies Program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Company review
In addition to the peer-review process, with the author's consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made by the authors at their discretion and based on scientific or editorial merit only. The author maintained full control over the manuscript, including content, wording and conclusion.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Grimwade D, Ivey A, Huntly BJP. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood. 2015;127(1):29–42. doi: 10.1182/blood-2015-07-604496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner K, Döhner H. Molecular characterization of acute myeloid leukemia. Haematologica. 2008;93(7):2–8. doi: 10.3324/haematol.13345. [DOI] [PubMed] [Google Scholar]
- 3.Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–62. doi: 10.1182/blood-2015-08-604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013;119(15):2720–2727. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kihara R, Nagata Y, Kiyoi H, et al. Comprehensive analysis of genetic alterations and their prognostic impacts in adult acute myeloid leukemia patients. Leukemia. 2014;28(8):1586–1595. doi: 10.1038/leu.2014.55. [DOI] [PubMed] [Google Scholar]
- 6.Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2015;127(1):42–53. doi: 10.1182/blood-2015-07-604512. [DOI] [PubMed] [Google Scholar]
- 7.Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood. 2015;127(1):71–78. doi: 10.1182/blood-2015-07-604538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann PR, Hinman LM, Hollander I, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody – calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 2002;13(1):47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 9.Paul SP, Taylor LS, Stansbury EK, McVicar DW. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood. 2000;96(2):483–490. [PubMed] [Google Scholar]
- 10.Crocker PR, Varki A. Siglecs, sialic acids and innate immunity. Trends Immunol. 2001;22(6):337–342. doi: 10.1016/s1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]
- 11.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130(22):2373–2376. doi: 10.1182/blood-2017-09-797712. [DOI] [PubMed] [Google Scholar]
- 13.Cowan AJ, Laszlo GS, Estey EH, Walter RB. Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin. Front. Biosci. (Landmark Ed.) 2013;18:1311–1334. doi: 10.2741/4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zein N, Sinha A, McGahren W, Ellestad G. Calicheamicin γ 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988;240(20):198–201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]
- 15.Sievers EL, Appelbaum FR, Spielberger RT, et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a Phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93(11):3678–3684. [PubMed] [Google Scholar]
- 16.Drak J, Iwasawa N, Danishefsky S, Crothers DM. The carbohydrate domain of calicheamicin γ I1 determines its sequence specificity for DNA cleavage. Proc. Natl Acad. Sci. USA. 1991;88(17):7464–7468. doi: 10.1073/pnas.88.17.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellestad GA. Structural and conformational features relevant to the anti-tumor activity of calicheamicin γ 1I. Chirality. 2011;23(8):660–671. doi: 10.1002/chir.20990. [DOI] [PubMed] [Google Scholar]
- 18.Smith AL, Nicolaou KC. The enediyne antibiotics. J. Med. Chem. 1996;39(11):2103–2117. doi: 10.1021/jm9600398. [DOI] [PubMed] [Google Scholar]
- 19.Prokop A, Wrasidlo W, Lode H, et al. Induction of apoptosis by enediyne antibiotic calicheamicin θII proceeds through a caspase-mediated mitochondrial amplification loop in an entirely Bax-dependent manner. Oncogene. 2003;22(57):9107–9120. doi: 10.1038/sj.onc.1207196. [DOI] [PubMed] [Google Scholar]
- 20.Rosen DB, Harrington KH, Cordeiro JA, et al. AKT signaling as a novel factor associated with in vitro resistance of human AML to gemtuzumab ozogamicin. PLoS ONE. 2013;8(1):e53518. doi: 10.1371/journal.pone.0053518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amico D, Barbui AM, Erba E, Rambaldi A, Introna M. Differential response of human acute myeloid leukemia cells to gemtuzumab ozogamicin in vitro: role of Chk1 and Chk2 phosphorylation and caspase 3. Blood. 2003;101(11):4589–4597. doi: 10.1182/blood-2002-07-2311. [DOI] [PubMed] [Google Scholar]
- 22.Linenberger ML. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19(2):176–182. doi: 10.1038/sj.leu.2403598. [DOI] [PubMed] [Google Scholar]
- 23.Parigger J, Zwaan CM, Reinhardt D, Kaspers GJL. Dose-related efficacy and toxicity of gemtuzumab ozogamicin in pediatric acute myeloid leukemia. Expert Rev. Anticancer Ther. 2016;16(2):137–146. doi: 10.1586/14737140.2016.1129903. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita A. Efficacy and resistance of gemtuzumab ozogamicin for acute myeloid leukemia. Int. J. Hematol. 2013;97(6):703–716. doi: 10.1007/s12185-013-1365-1. [DOI] [PubMed] [Google Scholar]
- 25.Gottardi M, Mosna F, De Angeli S, et al. Clinical and experimental efficacy of gemtuzumab ozogamicin in core binding factor acute myeloid leukemia. Hematol. Rep. 2017;9(3):87–90. doi: 10.4081/hr.2017.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabhan C, Tallman MS. Early Phase I/II trials with gemtuzumab ozogamicin (Mylotarg®) in acute myeloid leukemia. Clin. Lymphoma. 2002;2(March):S19–S23. doi: 10.3816/clm.2002.s.004. [DOI] [PubMed] [Google Scholar]
- 27.Nabhan C, Rundhaugen LM, Riley MB, et al. Phase II pilot trial of gemtuzumab ozogamicin (GO) as first line therapy in acute myeloid leukemia patients age 65 or older. Leuk. Res. 2005;29(1):53–57. doi: 10.1016/j.leukres.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Bross PF, Beitz J, Chen G, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001;7(6):1490–1496. [PubMed] [Google Scholar]
- 29.Petersdorf SH, Kopecky KJ, Slovak M, et al. A Phase III study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The results of S0106 study resulted in withdrawal of gemtuzumab ozogamicin (GO).
- 30.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009;361(13):1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J. Clin. Oncol. 2013;31(27):3360–3368. doi: 10.1200/JCO.2012.47.4874. [DOI] [PubMed] [Google Scholar]; • One of the few randomized studies of critical significance in demonstrating the benefit of GO.
- 32.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J. Clin. Oncol. 2011;29(4):369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]; • One of the few randomized study of critical significance in demonstrating the benefit of GO.
- 33.Rowe JM, Lowenberg B. Gemtuzumab ozogamicin in acute myeloid leukemia: a remarkable saga about an active drug. Blood. 2013;121(24):4838–4841. doi: 10.1182/blood-2013-03-490482. [DOI] [PubMed] [Google Scholar]
- 34.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J. Clin. Oncol. 2012;30(32):3924–3931. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 35.Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m][\ in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2016;125(25):3878–3886. doi: 10.1182/blood-2015-01-623447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnett A, Cavenagh J, Russell N, et al. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: a comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 trial. Haematologica. 2016;101(6):724–731. doi: 10.3324/haematol.2016.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical trial of significant interest demonstrating comparision of 3 versus 6 mg/m2 dose of GO in adult acute myeloid leukemia (AML).
- 37.Van Der Velden VHJ, Te Marvelde JG, Hoogeveen PG, et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97(10):3197–3204. doi: 10.1182/blood.v97.10.3197. [DOI] [PubMed] [Google Scholar]
- 38.Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, Phase III study. Lancet. 2012;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]; •• Reports the clinical outcome from the ALFA0701 study, which demonstrated that low fractionated dose of GO when added to standard therapy is safe and improves efficacy. This study along with other trials was a significant contributor in the US FDA's re-approval of GO.
- 39.Castaigne S, Pautas C, Terré C, et al. Final analysis of the ALFA 0701 study. Blood. 2014;124(21) [Google Scholar]; •• Reports the clinical outcome from the ALFA0701 study, which demonstrated that low fractionated dose of GO when added to standard therapy is safe and improves efficacy. This study along with other trials was a significant contributor in FDA's re-approval of GO.
- 40.Jen EY, Ko C-W, Lee JE, et al. FDA approval: gemtuzumab ozogamicin for the treatment of adults with newly-diagnosed CD33-positive acute myeloid leukemia. Clin. Cancer Res. 2018;24(14):3242–3246. doi: 10.1158/1078-0432.CCR-17-3179. [DOI] [PubMed] [Google Scholar]; •• Report from FDA based on results from ALFA0701 study announcing GO approval in newly diagnosed CD33+ AML.
- 41.Delaunay J, Recher C, Pigneux A, et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR study. Blood. 2011;118(79):37–38. [Google Scholar]
- 42.Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27(1):75–81. doi: 10.1038/leu.2012.229. [DOI] [PubMed] [Google Scholar]
- 43.Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the α group. Leukemia. 2007;21(1):66–71. doi: 10.1038/sj.leu.2404434. [DOI] [PubMed] [Google Scholar]
- 44.Hills RK, Castaigne S, Appelbaum FR, et al. The addition of gemtuzumab ozogamicin to induction chemotherapy in acute myeloid leukaemia: an individual patient data meta-analysis of randomised trials in adults. Lancet. Oncol. 2014;15(9):986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arceci RJ, Sande J, Lange B, Shannon K, Sievers EL, Al E. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood. 2005;106(4):1183–1188. doi: 10.1182/blood-2004-10-3821. [DOI] [PubMed] [Google Scholar]
- 46.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. 2012;118(3):761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 47.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children's Oncology Group Phase III trial for untreated pediatric acute myeloid leukemia: a report from the Children's Oncology Group. Blood. 2008;111(3):1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized Phase III Children's Oncology Group trial AAML0531. J. Clin. Oncol. 2014;32(27):3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Results from the largest pediatric randomized study showing the benefit of adding two doses of GO to standard therapy in pediatric AML patients.
- 49.Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized Phase III EORTC-GIMEMA AML-19 trial. J. Clin. Oncol. 2016;34(9):972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 50.Norsworthy KJ, Ko C, Lee JE, et al. FDA approval summary: Mylotarg for treatment of patients with relapsed or refractory CD33-positive acute myeloid leukemia. Oncologist. 2018;23(9):1103–1108. doi: 10.1634/theoncologist.2017-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jawad M, Seedhouse C, Mony U, Grundy M, Russell NH, Pallis M. Analysis of factors that affect in vitro chemosensitivity of leukaemic stem and progenitor cells to gemtuzumab ozogamicin (Mylotarg®) in acute myeloid leukaemia. Leukemia. 2010;24(1):74–80. doi: 10.1038/leu.2009.199. [DOI] [PubMed] [Google Scholar]
- 52.Sievers BEL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 2001;19(13):3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 53.Jilani I, Estey E, Huh Y, et al. Differences in CD33 intensity between various myeloid neoplasms. Am. J. Clin. Pathol. 2002;118(4):560–566. doi: 10.1309/1WMW-CMXX-4WN4-T55U. [DOI] [PubMed] [Google Scholar]
- 54.Van der Velden VHJ, Boeckx N, Jedema I, et al. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg®) treatment in acute myeloid leukemia patients. Leukemia. 2004;18(5):983–988. doi: 10.1038/sj.leu.2403350. [DOI] [PubMed] [Google Scholar]
- 55.Walter RB, Raden BW, Kamikura DM, et al. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin – induced cytotoxicity . Blood. 2004;105(3):1295–1302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 56.Godwin CD, Gale RP, Walter RB. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017;31(9):1855–1868. doi: 10.1038/leu.2017.187. [DOI] [PubMed] [Google Scholar]
- 57.Pollard JA, Alonzo TA, Loken M, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119(16):3705–3711. doi: 10.1182/blood-2011-12-398370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renneville A, Abdelali R Ben, Chevret S, et al. Clinical impact of gene mutations and lesions detected by SNP-array karyotyping in acute myeloid leukemia patients in the context of gemtuzumab ozogamicin treatment: results of the ALFA-0701 trial. Oncotarget. 2014;5(4):916–932. doi: 10.18632/oncotarget.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan N, Hills RK, Virgo P, et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia. 2017;31(5):1059–1068. doi: 10.1038/leu.2016.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollard JA, Loken M, Gerbing RB, et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized Phase III Children's Oncology Group trial AAML0531. J. Clin. Oncol. 2016;34(7):747–755. doi: 10.1200/JCO.2015.62.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports significant association of CD33 expression with disease risk group features as well as clinical outcome in GO-containing chemotherapeutic regimen.
- 61.Guillaume O, Estelle G, Jullien G, et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Blood. 2016;127(17):2155–2157. doi: 10.1182/blood-2016-01-689976. [DOI] [PubMed] [Google Scholar]
- 62.Loke J, Khan JN, Wilson JS, Craddock C, Wheatley K. Mylotarg has potent anti-leukaemic effect: a systematic review and meta-analysis of anti-CD33 antibody treatment in acute myeloid leukaemia. Ann. Hematol. 2014;94(3):361–373. doi: 10.1007/s00277-014-2218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Xu SN, Qin DB, Tan Y, Gong Q, Chen JP. Effect of adding gemtuzumab ozogamicin to induction chemotherapy for newly diagnosed acute myeloid leukemia: a meta-analysis of prospective randomized Phase III trials. Ann. Oncol. 2014;25(2):455–461. doi: 10.1093/annonc/mdt566. [DOI] [PubMed] [Google Scholar]
- 64.Lamba JK, Pounds S, Cao X, et al. Coding polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML. Leukemia. 2009;23(2):402–404. doi: 10.1038/leu.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mortland L, Alonzo TA, Walter RB, et al. Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clin. Cancer Res. 2013;19(6):1620–1627. doi: 10.1158/1078-0432.CCR-12-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malik M, Simpson JF, Parikh I, et al. CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing. J. Neurosci. 2013;33(33):13320–13325. doi: 10.1523/JNEUROSCI.1224-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malik M, Chiles J, Xi HS, et al. Genetics of CD33 in Alzheimer's disease and acute myeloid leukemia. Hum. Mol. Genet. 2015;24(12):3557–3570. doi: 10.1093/hmg/ddv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raj T, Ryan KJ, Replogle JM, et al. CD33: increased inclusion of exon 2 implicates the Ig V-set domain in Alzheimer's disease susceptibility. Hum. Mol. Genet. 2014;23(10):2729–2736. doi: 10.1093/hmg/ddt666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamba JK, Chauhan L, Shin M, et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: report from randomized Phase III Children's Oncology Group trial AAML0531. J. Clin. Oncol. 2017;35(23):2674–2682. doi: 10.1200/JCO.2016.71.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports CD33 splicing single nucleotide polymorphism as the most promising predictor of GO response in pediatric AML patients.
- 70.Lamba JK, Voigt AP, Chauhan L, et al. CD33 splicing SNP regulates expression levels of CD33 in normal regenerating monocytes in AML patients. Leuk. Lymphoma. 2018;59(9):2250–2253. doi: 10.1080/10428194.2017.1421756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gale RE, Popa T, Wright M, et al. No evidence that CD33 splicing SNP impacts the response to GO in younger adults with AML treated on UK MRC/NCRI trials. Blood. 2018;131(4):468–471. doi: 10.1182/blood-2017-08-802157. [DOI] [PubMed] [Google Scholar]; •• In adult AML receving GO at differnet schedules and dosing, CD33 splicing single nucleotide polymorphism did not predict response to GO.
- 72.Lamba J, Chauhan L, Shin M, et al. CD33-single nucleotide polymorphism (CD33-SNP) score predicts gemtuzumab ozogamicin response in childhood acute myeloid leukemia: report from Children's Oncology Group AAML0531 trial. Blood. 2017;130(Suppl. 1):3826. [Google Scholar]
- 73.Cianfriglia M, Mallano A, Ascione A, Dupuis ML. Multidrug transporter proteins and cellular factors involved in free and mAb linked calicheamicin-γ1 (gentuzumab ozogamicin, GO) resistance and in the selection of GO resistant variants of the HL60 AML cell line. Int. J. Oncol. 2010;36(6):1513–1520. doi: 10.3892/ijo_00000638. [DOI] [PubMed] [Google Scholar]
- 74.Walter RB, Gooley TA, Van Der Velden VHJ, et al. Brief report CD33 expression and P-glycoprotein – mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109(10):4168–4170. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris KL, Adams JA, Liu Yin JA. Effect of gemtuzumab ozogamicin on acute myeloid leukaemia blast cells in vitro, as a single agent and combined with other cytotoxic agents. Br. J. Haematol. 2006;135(4):509–512. doi: 10.1111/j.1365-2141.2006.06326.x. [DOI] [PubMed] [Google Scholar]
- 76.Walter RB, Raden BW, Cronk MR, et al. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize AML cells to gemtuzumab ozogamicin. Blood. 2004;103(11):4276–4284. doi: 10.1182/blood-2003-11-3825. [DOI] [PubMed] [Google Scholar]
- 77.Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML. Multidrug resistance protein attenuates gemtuzumab ozogamicin – induced cytotoxicity in acute myeloid leukemia cells. Blood. 2003;102(4):1466–1473. doi: 10.1182/blood-2003-02-0396. [DOI] [PubMed] [Google Scholar]; • PgP1 drug transporter-involved calicheamicin efflux shows association with in vitro GO response.
- 78.Linenberger ML, Hong T, Flowers D, et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood. 2001;98(4):988–994. doi: 10.1182/blood.v98.4.988. [DOI] [PubMed] [Google Scholar]; •• PgP1 drug transporter implicated in calicheamicin efflux shows association with GO clinical response.
- 79.Goemans BF, Zwaan CM, Vijverberg SJH, et al. Large interindividual differences in cellular sensitivity to calicheamicin may influence gemtuzumab ozogamicin response in acute myeloid leukemia. Leukemia. 2008;22(12):2284–2285. doi: 10.1038/leu.2008.147. [DOI] [PubMed] [Google Scholar]
- 80.Chauhan L, Alonzo TA, Wang J, et al. Drug transporter ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with gemtuzumab ozagamicin: a report from Children's Oncology Group AAML0531 trial. Blood. 2017;130(Suppl. 1):2526. doi: 10.1038/s41408-019-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Middeldorf I, Galm O, Osieka R, Jost E, Herman JG, Wilop S. Sequence of administration and methylation of SOCS3 may govern response to gemtuzumab ozogamicin in combination with conventional chemotherapy in patients with refractory or relapsed acute myelogenous leukemia (AML) Am. J. Hematol. 2010;85(7):477–481. doi: 10.1002/ajh.21723. [DOI] [PubMed] [Google Scholar]
- 82.Liu YX, Wang L, Liu WJ, et al. MIR-124-3p/B4GALT1 axis plays an important role in SOCS3-regulated growth and chemo-sensitivity of CML. J. Hematol. Oncol. 2016;9(1):1–12. doi: 10.1186/s13045-016-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ball ED. Pairing SOCS with CD33. Blood. 2006;109(3):852–852. [Google Scholar]
- 84.Orr SJ, Morgan NM, Elliott J, et al. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood. 2007;109(3):1061–1068. doi: 10.1182/blood-2006-05-023556. [DOI] [PubMed] [Google Scholar]
- 85.Sutherland MSK, Walter RB, Jeffrey SC, et al. SGN-CD33A: a novel CD33-targeting antibody–drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122(8):1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- 86.Kovtun Y, Noordhuis P, Whiteman KR, et al. IMGN779, a novel CD33-targeting antibody–drug conjugate with DNA alkylating activity, exhibits potent antitumor activity in models of AML. Mol. Cancer Ther. 2018;17(6):1271–1279. doi: 10.1158/1535-7163.MCT-17-1077. [DOI] [PubMed] [Google Scholar]
- 87.Jurcic JG, Rosenblat TL, McDevitt MR, et al. Phase I trial of the targeted α-particle nano-generator actinium-225 (225Ac)-lintuzumab (Anti-CD33; HuM195) in acute myeloid leukemia (AML) Blood. 2011;118(21):768. [Google Scholar]
- 88.Jurcic JG, Levy MY, Park JH, et al. Phase I trial of targeted α-particle therapy with actinium-225-lintuzumab and low-dose cytarabine (LDAC) in patients age 60 or older with untreated acute myeloid leukemia (AML) Blood. 2016;128(22):4050. [Google Scholar]
- 89.Aigner M, Feulner J, Schaffer S, et al. T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia. 2013;27(5):1107–1115. doi: 10.1038/leu.2012.341. [DOI] [PubMed] [Google Scholar]
- 90.Laszlo GS, Gudgeon CJ, Harrington KH, et al. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood. 2014;123(4):554–561. doi: 10.1182/blood-2013-09-527044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kenderian SS, Ruella M, Shestova O, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walter RB, Medeiros BC, Powell BL, Schiffer CA, Appelbaum FR, Estey EH. Phase II trial of vorinostat and gemtuzumab ozogamicin as induction and post-remission therapy in older adults with previously untreated acute myeloid leukemia. Haematologica. 2012;97(5):739–742. doi: 10.3324/haematol.2011.055822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Medeiros BC, Gardner KM, Orlowski KF, et al. Gemtuzumab ozogamicin in combination with vorinostat and azacitidine in older patients with relapsed or refractory acute myeloid leukemia (AML): final results from a Phase I/II study. Blood. 2013;122(21):3936. doi: 10.3324/haematol.2013.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nand S, Othus M, Godwin JE, et al. A Phase II trial of azacitidine and gemtuzumab ozogamicin therapy in older patients with acute myeloid leukemia. Blood. 2013;122(20):3432–3439. doi: 10.1182/blood-2013-06-506592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Daver N, Kantarjian H, Ravandi F, et al. A Phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30(2):268–273. doi: 10.1038/leu.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bots M, Johnstone RW. Rational combinations using HDAC inhibitors. Clin. Cancer Res. 2009;15(12):3970–3977. doi: 10.1158/1078-0432.CCR-08-2786. [DOI] [PubMed] [Google Scholar]
- 97.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]