Abstract
The mineralocorticoid receptor (MR) is indispensable for survival through its critical role in maintaining blood pressure in response to sodium scarcity or bleeding. Activation of MR by aldosterone in the kidney controls water and electrolyte homeostasis. This review summarizes recent advances in our understanding of MR function, specifically in vascular endothelial and smooth muscle cells. The evolving roles for vascular MR are summarized in the areas of (i) vascular tone regulation, (ii) thrombosis, (iii) inflammation, and (iv) vascular remodeling/fibrosis. Synthesis of the data supports the concept that vascular MR does not contribute substantially to basal homeostasis but rather, MR is poised to be activated when the vasculature is damaged to coordinate blood pressure maintenance and wound healing. Specifically, MR activation in the vascular wall promotes vasoconstriction, inflammation, and exuberant vascular remodeling with fibrosis. A teleological model is proposed in which these functions of vascular MR may have provided a critical evolutionary survival advantage in the face of mechanical vascular injury with bleeding. However, modern lifestyle is characterized by physical inactivity and high fat/high sodium diet resulting in diffuse vascular damage. Under these modern conditions, diffuse, persistent and unregulated activation of vascular MR contributes to post-reproductive cardiovascular disease in growing populations with hypertension, obesity, and advanced age.
Keywords: aldosterone, blood pressure, cardiovascular disease, endothelial cell, hypertension, mineralocorticoid receptor, smooth muscle cell
The transition by vertebrates to terrestrial life outside the high sodium environment of the sea required a closed circulatory system with tight control of water and electrolyte homeostasis and the ability to rapidly heal wounds that threaten vascular integrity. The machinery to produce the mineralocorticoid hormone aldosterone developed at this critical phase of vertebrate evolution.1,2 By activating mineralocorticoid receptors (MR) in the kidney, aldosterone promotes renal sodium reabsorption and potassium excretion thereby conserving sodium in an environment of scarcity. Like all steroid hormone receptors, the MR is an intracellular receptor that is poised to translate systemic hormonal signals into tissue-specific actions. MR activation produces rapid effects via cytoplasmic signaling and long-term genomic effects by acting as a ligand-activated transcription factor.3
The renin–angiotensin–aldosterone system (RAAS) is triggered by a decline in blood pressure sensed by the kidney. This culminates in renal MR activation and function to restore volume and blood pressure homeostasis. The critical role of MR in sodium reabsorption and volume maintenance is evidenced in humans with pseudohypoaldosteronism, a condition caused by MR inactivating variants characterized by elevated plasma aldosterone, sodium wasting, hyperkalemia, and neonatal death if not supplemented with sodium.4 Similarly, mice with global MR deletion have severe dehydration, hyperaldosteronism, hyperkalemia, and death unless rescued with sodium supplementation,5–7 supporting the potential to model aspects of MR function in rodents.
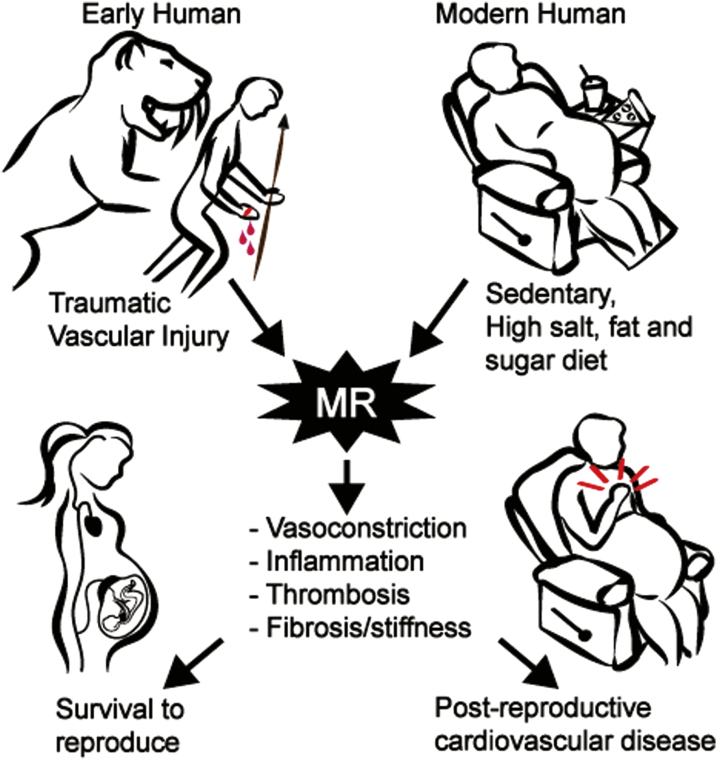
It might be imagined that evolutionary pressure in the face of terrestrial life would also select for mechanisms that can restore vascular integrity in the face of acute injury. Consider the fate of an early human after an unfortunate encounter with a saber-toothed cat resulting in loss of a finger (Figure 1, left). The RAAS would be triggered by hypotension from bleeding and while renal MR activation would contribute to slow volume restoration by sodium avidity, survival would depend on rapid vasoconstriction, blood clotting, infection control, vascular wound healing, and scar formation (fibrosis). Beyond the renal epithelium, the MR is expressed in non-epithelial cells including neurons, immune cells, adipocytes, cardiomyocytes, and vascular endothelial (EC) and smooth muscle cells (SMCs).
Figure 1.
Proposed evolutionary model for the detrimental role of vascular mineralocorticoid receptors with modern lifestyle. Recent studies support the concept that mineralocorticoid receptors (MR) in the vasculature are poised to be activated in response to vascular injury to promote vascular constriction, inflammation, thrombosis, remodeling, and fibrosis. Such effects are seen in animal models and humans in response to mechanical vascular injury, obesity, hypertension, and aging. Such a localized vascular MR response might have benefited early humans by contributing to recovery from traumatic injury and survival to reproduce. In the modern age, vessel damage from a sedentary lifestyle and poor diet promotes diffuse vascular MR activation that contributes to post-reproductive cardiovascular diseases including hypertension, heart attack, stroke, aortic aneurism, and heart and kidney failure.
This review focuses on MR in the vasculature. MR has been found in all vascular beds and vessel sizes tested including the aorta, carotid, coronary, renal, and mesenteric vessels, consistent with a role in global responses to vascular stress via large conduit arteries and small resistance vessels.8,9 In addition to aldosterone, the stress hormone cortisol circulates in high abundance and can compete with aldosterone for binding to MR. Cortisol is inactivated by 11-beta hydroxysteroid dehydrogenase-2 (11βHSD2) in aldosterone-responsive tissues such as the kidney.10 11βHSD2 has also been found in human EC and SMC,11,12 thus vascular MR can respond to aldosterone, although a role for cortisol under conditions of stress has not been ruled out. Substantial progress has recently been made in our understanding of the role of MR in vascular function based on in vitro studies and in vivo models using MR antagonist drugs or mice with MR levels modulated in specific cell types. As the roles of MR in the vasculature were previously summarized,13–17 this review focuses on the most current advances and on contextualizing these data into an evolving model in which vascular MR does not substantially regulate basal vascular homeostasis but rather is poised to maintain blood pressure and activate wound healing when necessary.
This review focuses on the role of vascular MR yet it should be noted that MR signaling in myeloid cells also impacts vascular inflammation and function and has recently been reviewed elsewhere.18–21 Here, we summarize recent advances in our understanding of how MR activation in SMC and EC under conditions of vascular injury/damage contributes to: (i) vascular tone, (ii) thrombosis, (iii) inflammation, and (iv) wound healing with fibrosis. It is concluded that while all of these processes could be lifesaving in the aftermath of an encounter with a saber-toothed cat, they become maladaptive in an environment where our most dangerous predator is our modern lifestyle (Figure 1).
Vascular MR in regulation of vascular tone
MR contributes to vessel tone in response to vascular stress.
Conduit arteries such as the aorta and carotid contribute to vascular pulsatility and stiffness with little impact on blood pressure control. Smaller diameter arteries that contain SMCs, including mesenteric and coronary resistance arteries, can influence systemic arterial pressure and/or regional blood flow by modulating smooth muscle myogenic tone. Ample data in mouse models support a role for vascular MR in promoting a state of increased myogenic tone, either by directly enhancing vessel contraction or impairing vessel dilation. Comparison between published studies reveals differences in the genetic models used, the cardiovascular risk factors to which they are exposed, the investigative methods, and the vascular bed tested. These differences are summarized in Tables 1 and 2. In the text, we attempt to synthesize these data, focusing on the similarities to gain insight into the overall role of MR in vasomotor function. In all cases in which renal function was tested, the influence of vascular MR on vessel function appears to be independent of renal mechanisms as neither SMC nor EC MR deletion affected renal sodium handling or salt sensitivity of blood pressure.8,22–24 In the absence of vascular injury or cardiovascular risk factors, deletion of MR from SMCs or ECs had minimal effect on vasomotor function. However, vascular MR contributes to increased vasomotor tone under conditions of vascular stress.
Table 1.
Summary of studies exploring smooth muscle cell (SMC) mineralocorticoid receptors (MR) in vasomotor function
| Genotype/model | Sex | Cardiovascular risk factor/vascular stress | Vascular bed | Method | Comparison | Impact on vessel dilation | Impact on vessel constriction | Reference |
|---|---|---|---|---|---|---|---|---|
| SMA-Cre ERT2-mediated deletion of MR from smooth muscle cells | Male | No perturbation | 2nd-order mesenteric artery | Wire myograph | Inducible SMC-MR-KO vs. MR intact | KCl↔ PE↔ U46619↔ | McCurley et al.23 | |
| Wild type | Male | Aging (3–4 months old vs. >9 months old) | 2nd-order mesenteric artery | Wire myograph | Young MR intact vs. aged MR intact | AngII↑KCl↑PE↑U46619↑ | ||
| SMA-Cre ERT2-mediated deletion of MR from smooth muscle cells | Male | Aging | 2nd-order mesenteric artery | Wire myograph | Aged inducible SMC-MR-KO vs. aged MR intact | KCl↓PE↔U46619↓BayK-8644↓Myogenic tone↓ | ||
| SMA-Cre ERT2-mediated deletion of MR from smooth muscle cells | Male | Angiotensin II hypertension (2 weeks, 800 ng/kg/min) | 2nd-order mesenteric artery | Wire myograph | Inducible SMC-MR-KO vs. MR intact | AngII↓ | ||
| SMA-Cre ERT2-mediated deletion of MR from smooth muscle cells | Male | Angiotensin II hypertension | 2nd-order mesenteric artery | Wire myograph | Aged inducible SMC-MR-KO vs. aged MR intact | AngII↓ | ||
| SMA-Cre ERT2-mediated deletion of MR from smooth muscle cells | Male | No perterbation | 2nd/3rd-order mesenteric artery | Wire myograph | Inducible SMC-MR-KO vs. MR intact | BayK-8644↔ | DuPont et al.25 | |
| Wild type | Male | Aging (3–4 months old vs. 12 months old) | 2nd/3rd-order mesenteric artery | Wire myograph | Young MR intact vs. aged MR intact | BayK-8644↑AngII ↑ | ||
| SMA-Cre ERT2-mediated deletion of MR from smooth muscle cells | Male | Aging | 2nd/3rd-order mesenteric artery | Wire myograph | Aged inducible SMC-MR-KO vs. aged MR intact | BayK-8644↓ | ||
| microRNA 155 expressing lentivirus in SMC | Male | Aging | 2nd/3rd-order mesenteric artery | Wire myograph | miR155 lentivirus in aged MR intact vs. aged MR intact | BayK-8644↓AngII↓ | ||
| SM22-Cre-mediated deletion of MR from smooth muscle cells | Male | Left coronary artery ligation (2 months post) | Mesenteric artery | Wire myograph | All groups | Ach↔ SNP↔ | Gueret et al.26 | |
| Wild type | Male | Left coronary artery ligation | Interseptal coronary artery | Wire myograph | Myocardial infarction vs. sham | Ach↓SNP↔ | ||
| SM22-Cre-mediated deletion of MR from smooth muscle cells | Male | Left coronary artery ligation | Interseptal coronary artery | Wire myograph | Constitutive SMC-MR-KO vs. MR intact | Ach↑SNP↔ | ||
| Wild type +/– MR inhibition | Male | Left coronary artery ligation | Interseptal coronary artery | Wire myograph | Myocardial infarction and finerenone vs. Sham | Ach↑SNP↔ | ||
| SM22-Cre-mediated deletion of MR from smooth muscle cells | Male | Left coronary artery ligation | Interseptal coronary artery | Wire myograph | MI and finerenone vs. MI and constitutive SMC-MR-KO | Ach↑SNP↔ | ||
| SM22-Cre-mediated deletion of MR from smooth muscle cells | Female | Acute kidney injury with Cyclosporin A | Renal microvessels | Ex vivo isolated perfused kidney | Constitutive SMC-MR-KO vs. MR intact | BayK-8644↓AngII↓KCl↓ | Amador et al.27 |
Abbreviations: Ach, acetylcholine; AngII, angiotensin II; BayK-8644 is a calcium channel agonist; KCl, potassium chloride; MI, myocardial infarction; MR, mineralocorticoid receptor; PE, phenylephrine; SMA, smooth muscle actin; SMA-Cre ERT2 mouse has inducible deletion in SMC; SMC-MR-KO, smooth muscle cell mineralocorticoid receptor knockout mouse; SM22 is a smooth muscle specific protein and the SM22-Cre is constitutively active in SMCs; SNP, sodium nitroprusside; U46619 is a thromboxane A2 receptor agonist.
Table 2.
Summary of studies exploring endothelial cell (EC) mineralocorticoid receptors (MR) in vascular function
| Genotype/model | Sex | Cardiovascular risk factor/vascular stress | Vascular bed | Method | Comparison | Impact on vessel dilation | Impact on vessel constriction | Reference |
|---|---|---|---|---|---|---|---|---|
| Wild type | Male | High fat diet (60% fat, 14 weeks) | Thoracic aorta | Wire myograph | High fat diet vs. normal chow | Ach↓SNP ↔ | Schäfer et al.28 | |
| Wild type +/– MR inhibition (eplerenone 14 weeks, 200 mg/kg/day) | Male | High fat diet | Thoracic aorta | Wire myograph | HFD eplerenone vs. HFD | Ach↑SNP ↔ | ||
| Tie2-Cre-mediated deletion of MR from endothelial cells and leukocytes | Male | High fat diet | Thoracic aorta | Wire myograph | EC/leukocyte-MR-KO vs. MR-intact on HFD | Ach↑ | ||
| Tie2-Cre-mediated deletion of MR from endothelial cells and leukocytes | Male | Aldosterone (2 weeks, 50 µg/kg/day) | Thoracic aorta | Wire myograph | EC/leukocyte-MR-KO vs. MR-intact with aldosterone | Ach↑SNP ↔ | ||
| Wild type | Male | Aldosterone (2 weeks, 0.72 mg/kg/day) | Thoracic aorta | Wire myograph | Aldosterone vs. vehicle | Ach↓ | Rickard et al.29 | |
| Tie2-Cre-mediated deletion of MR from endothelial cells and leukocytes | Male | Aldosterone | Thoracic aorta | Wire myograph | EC/leukocyte-MR-KO aldosterone vs. vehicle | Ach↔ | ||
| Wild type | Male | Aldosterone | 2nd-order mesenteric artery | Pressure myograph | Aldosterone vs. vehicle | Ach↓ | ||
| Tie2-Cre-mediated deletion of MR from endothelial cells and leukocytes | Male | Aldosterone | 2nd-order mesenteric artery | Pressure myograph | EC/leukocyte-MR-KO aldosterone vs. vehicle | Ach↓ | ||
| VECad-Cre-mediated deletion of MR from endothelial cells | Male | No perterbation | 3rd-order mesenteric artery | Wire myograph | EC-MR-KO vs. MR intact | Ach↔ SNP↔ NO relaxation↔EDH relaxation↔ | KCl ↔ET1 ↔U46619 ↓PE ↔AngII↔ | Mueller et al.24 |
| VECad-Cre-mediated deletion of MR from endothelial cells | Male | No perterbation | coronary artery | Wire myograph | EC-MR-KO vs. MR intact | Ach↔SNP↔ | KCl ↔ET1↓U46619↓ | |
| VECad-Cre-mediated deletion of MR from endothelial cells | Male | Angiotensin II hypertension (2 weeks, 800 ng/kg/min) | 3rd-order mesenteric artery | Pressure myograph | EC-MR-KO vs. MR intact | Ach↑SNP↔NO relaxation↑EDH relaxation↑ | ||
| VECad-Cre-mediated deletion of MR from endothelial cells | Male | Angiotensin II | Coronary artery | Wire myograph | EC-MR-KO vs. MR intact | ET1↓U46619↓ | ||
| Inducible overexpression of human MR in endothelial cells | Male | Doxycycline induction of EC MR overexpression | 2nd-order mesenteric artery | Pressure myograph | EC MR overexpression (+Dox) vs. MR-intact (–Dox) | Flow induced dilation ↔ | Myogenic tone ↑ | Nguyen Dinh Cat et al.9 |
| Inducible overexpression of human MR in endothelial cells | Male | Doxycycline induction of EC MR overexpression | 2nd-order mesenteric artery | Wire myograph | EC MR overexpression (+Dox) vs. MR-intact (–Dox) | Ach↔ NO relaxation↔ BK ↔ SNP ↔ | PE↑U46619↑AngII↑ET1 ↑ | |
| VECad-Cre-mediated deletion of MR from endothelial cells | Female | Western diet (16 weeks, 46% fat, 17.5 % sucrose, 17.5% high fructose corn syrup) | Mesenteric artery | Pressure myograph | EC-MR-KO vs. MR intact | Flow-mediated dilation ↑ | Jia et al.30 | |
| Wild type | Female | Western diet | Thoracic aorta | Wire myograph | Western diet vs. normal chow | Ach↓Insulin↓SNP↓ | ||
| VECad-Cre-mediated deletion of MR from endothelial cells | Female | Western diet | Thoracic aorta | Wire myograph | EC-MR-KO vs. MR intact on Western Diet | Ach↑Insulin↑SNP↑ | ||
| Tie2-Cre-mediated deletion of MR from endothelial cells and leukocytes | Male and female equally combined | Angiotensin II hypertension (2 and 4 weeks, 1,000 ng/kg/min) | Renal artery | Wire myograph | EC-MR-KO vs. MR intact | Ach ↔ SNP ↔ | U46619↔ | Laursen et al.8 |
| VECad-Cre-mediated deletion of MR from endothelial cells | Male | No perterbation | 2nd/3rd-order mesenteric artery | Wire myograph | EC-MR-KO vs. MR intact | Ach↔ SNP ↔ | Davel et al.31 | |
| VECad-Cre-mediated deletion of MR from endothelial cells | Female | No perterbation | 2nd/3rd-order mesenteric artery | Wire myograph | EC-MR-KO vs. MR intact | Ach↔ SNP ↔ | ||
| Wild type | Male | High fat diet (40% fat, 12 weeks) | 2nd/3rd-order mesenteric artery | Wire myograph | High fat diet vs. normal chow | Ach↔NO relaxation↓EDH relaxation↑ | ||
| Wild type | Female | High fat diet | 2nd/3rd-order mesenteric artery | Wire myograph | High fat diet vs. normal chow | Ach↓NO relaxation↔EDH relaxation↓ | ||
| VECad-Cre-mediated deletion of MR from endothelial cells | Male | High fat diet | 2nd/3rd-order mesenteric artery | Wire myograph | EC-MR-KO vs. MR intact on HFD | Ach↔ NO relaxation↔ EDH relaxation↔ | ||
| VECad-Cre-mediated deletion of MR from endothelial cells | Female | High fat diet | 2nd/3rd-order mesenteric artery | Wire myograph | EC-MR-KO vs. MR intact on HFD | Ach↑NO relaxation↑EDH relaxation↔ | ||
| Wild type | Male | High fat diet plus hyperlipidemia (PCSK9 mutant adenovirus) | 2nd/3rd-order mesenteric artery | Wire myograph | HFD+hyperlipidemia vs. normal chow | Ach↓NO relaxation↓EDH relaxation↔ | ||
| Wild type | Female | High fat diet plus hyperlipidemia | 2nd/3rd-order mesenteric artery | Wire myograph | HFD+hyperlipidemia vs. normal chow | Ach↓NO relaxation↔EDH relaxation↓ | ||
| VECad-Cre-mediated deletion of MR from endothelial cells | Male | High fat diet plus hyperlipidemia | 2nd/3rd-order mesenteric artery | Wire myograph | EC-MR-KO vs. MR intact on HFD+hyperlipidemia | Ach↔ NO relaxation↔EDH relaxation↔ | ||
| VECad-Cre-mediated deletion of MR from endothelial cells | Female | High fat diet plus hyperlipidemia | 2nd/3rd-order mesenteric artery | Wire myograph | EC-MR-KO versus MR intact on HFD+hyperlipidemia | Ach↑NO relaxation↑EDH relaxation↔ |
Abbreviations: Ach, acetylcholine; AngII, angiotensin II; BK, bradykinin; EC-MR-KO, endothelial cell mineralocorticoid receptor knockout mouse; EDH, endothelial-derived hyperpolarization; HFD, high fat diet; KCl, potassium chloride; MR, mineralocorticoid receptor; NO, nitric oxide; PE, phenylephrine; SNP, sodium nitroprusside; Tie2, angiopoietin-1 receptor and the Tie2-Cre is expressed in EC and leukocytes; U46619 is a thromboxane A2 receptor agonist; VECad, vascular endothelial cadherin and the VECad Cre is EC specific.
SMC-MR contributes to vasoconstriction in hypertension and aging
Young healthy mice with constitutive or inducible knockout of SMC-MR (SMC-MR-KO mice) maintain normal basal blood pressure compared to MR-intact littermates.22,23 However, when hypertension is induced by AngII, SMC-MR-KO mice have lower blood pressure and decreased AngII-induced vasoconstriction in mesenteric arteries.23 Similarly, with aging, SMC-MR-KO mice have lower blood pressure22,23 and decreased mesenteric myogenic tone and vasoconstriction.23 In MR-intact mice, AngII signaling contributes to oxidative stress that is necessary to induce hypertension and is also prevented in the carotid arteries of SMC-MR-KO mice.32 One molecular mechanism by which SMC-MR drives vasoconstriction with aging involves ligand-independent repression of microRNA-155 that increases target gene expression including Cav1.2, the pore forming subunit of the L-type calcium channel, and the angiotensin type-1 receptor.25 Similarly, when renal arteriolar vasoconstriction was induced by cyclosporine, SMC-MR-KO (but not EC-MR-KO) prevented L-type calcium channel-induced vasoconstriction, thereby improving renal function.27 SMC-MR-KO has also been shown to protect from the impairment of coronary vasodilation that occurs after myocardial ischemia.26 To date, published studies comparing vasomotor function in the presence and absence of SMC-MR (Table 1) have been performed only in male mice, with the exception of 1 study exploring renal perfusion only in females.27 Thus, whether SMC-MR controls vasoconstriction by similar mechanisms in females remains to be explored.
EC-MR contributes to endothelial dysfunction in response to cardiovascular risk factors
In addition to enhanced vessel contraction induced by MR in SMC, MR activation in ECs contributes to vascular tone regulation by impairing vasodilation. Vasodilation mediators in ECs include potassium channels that contribute to endothelial-derived hyperpolarization (EDH) of the underlying SMCs and nitric oxide (NO) or prostaglandins that are released from ECs and induce SMC-mediated vasodilation. Studies with EC-MR-KO mice generally reveal no changes in endothelial function under healthy conditions; however, there are diverse effects of EC-MR on vasodilation in response to cardiovascular risk factors (Table 2).
Obesity is a potent contributor to cardiovascular disease risk. One mechanism described in rodent models and humans involves adipose tissue release of factors (including leptin) that increase aldosterone levels to activate MR, a mechanism that is more prominent in females.31,33–36 An early step in the development of cardiovascular disease is impairment of EC function, as measured by a decreased vasodilation to acetylcholine. In mouse models of obesity, acetylcholine-induced vasodilation is impaired in large- and small-diameter arteries, but this is prevented when EC-MR has been knocked out (Table 2).28,30,31 In addition to obesity, hypertension induced by AngII or aldosterone also impairs vasomotor function globally that is ameliorated by EC-MR-KO.8,24,29 Conversely, overexpression of EC-MR induces hypertension with impaired mesenteric vasodilation and enhanced constriction.9 Similarly, changes in coronary vasoconstriction induced by hypertension are attenuated by EC-MR-KO.24 The vasodilatory dysfunction induced by risk factors is EC mediated as evidenced by intact sodium nitroprusside dilation, which depends only on SMC function. Despite these general trends, further research is needed to tease out mechanistic differences in each experimental model and delineate EC-specific functions of MR throughout the vasculature (Table 2).
One important variable to consider is sex. Virtually all studies were performed in only 1 sex, a factor that might also contribute to disparate outcomes. One recent study directly compared the impact of obesity and hyperlipidemia on EC function in males and females. Davel et al.31 demonstrated a sex difference in the mechanism of risk factor-induced mesenteric endothelial dysfunction, with males being more dependent on NO and females on EDH. NO-induced dilation was impaired by obesity and hyperlipidemia in males with no apparent role of EC-MR. In contrast, endothelial dysfunction induced by obesity and hyperlipidemia in females was due to a decline in EDH and the improved dilation in female EC-MR-KO mice was mediated by a compensatory increase in NO.31 EC-MR may also contribute to endothelial dysfunction by upregulating epithelial sodium channel (ENaC) to induce EC stiffness and impair NO production. Aldosterone-infused female mice developed aortic endothelial dysfunction that was prevented in EC-MR-KO or EC-ENaC-KO mice.37 Further exploration of these sex-specific mechanisms is needed, but the data support the potential for MR antagonists to prevent vascular dysfunction induced by cardiometabolic risk factors, particularly in females.
MR in thrombosis
The vasculature allows blood to flow over a large surface area to distribute oxygen while simultaneously supporting rapid thrombus formation to maintain vascular integrity when injured. Damage to the EC monolayer exposes the underlying basement membrane, resulting in clot formation by interaction between the endothelium and platelets to restore homeostasis until vascular repair occurs. Thrombosis can become pathological with dysregulation of any component leading to vascular occlusion that contributes to heart attack, stroke, and limb ischemia.
Although some data support that aldosterone is pro-thrombotic, the concept remains controversial and requires more investigation. It has been hypothesized that MR activation enhances thrombosis based on studies in cultured SMC and EC in which aldosterone upregulated plasminogen activator inhibitor-1 (PAI-1), thereby inhibiting fibrinolysis and promoting thrombosis.38–40 Infusion of aldosterone in rats, albeit at supraphysiological levels (30 µg/kg/h = 720 µg/kg/day), decreased bleeding time and increased ex vivo platelet adhesion to fibrillar collagen, supporting a pro-thrombotic effect.41 If aldosterone indeed promotes coagulation and thrombosis, inhibiting MR should antagonize these effects. However, co-treatment with the MR antagonist eplerenone with aldosterone did not restore bleeding time despite decreasing platelet adhesion.41,42 The human data are also conflicting. Some studies suggest that RAAS activation disturbs the fibrinolytic balance between activators and inhibitors of plasminogen. Specifically, MR inhibition in hypertensives shifted the balance toward fibrinolysis independently of potassium, a key mediator of the coagulation cascade.43 However, a large meta-analysis did not find any impact of MR antagonism on the incidence of bleeding or thrombotic events in human clinical trials.44
Aldosterone may be pro-thrombotic specifically in the context of vascular damage which, like vasoconstriction, could be induced by cardiometabolic risk factors or mechanical damage. Aldosterone application to an intact, non-damaged EC monolayer decreased thrombin formation.45 However, ferric chloride induced-EC injury paradoxically resulted in decreased thrombus formation in EC-MR overexpressing mice in association with increased expression of the anti-thrombotic endothelial protein C receptor.45 One limitation to the interpretation of these data is that the EC-MR overexpressing mice have increased blood pressure,9 which could be a source of vascular damage that complicates the data interpretation. Thus, although it is tempting to conclude that vascular MR promotes thrombosis specifically when the vessel is injured, there are currently insufficient and conflicting data, which limits such a conclusion. In addition, some of the available data are confounded by the use of supraphysiological aldosterone levels and comparison of models with different blood pressures. Moreover, tissue-specific effects of MR on thrombosis have not been explored.
MR and vascular inflammation
The healthy vasculature is an anti-inflammatory barrier. However, in response to invading pathogens, SMC and EC contribute to host defense by releasing chemokines and expressing adhesion molecules to recruit inflammatory cells and by increasing oxidative stress that, together with leukocytes, combats local infection and prevents progression to sepsis and death.46 Although this acute, localized vascular inflammation is critical to survival from an infected wound, chronic diffuse vascular inflammation due to cardiovascular risk factors contributes to atherosclerosis development and triggers plaque rupture, the cause of myocardial infarction and stroke.40,47,48
In patients with cardiovascular risk factors, elevated aldosterone levels are associated with increased risk of myocardial infarction or stroke, independent of blood pressure,49,50 prompting exploration of the role of MR in inflammatory atherosclerosis (reviewed in Moss and Jaffe51 and McGraw et al.52). In vitro studies support a role for SMC-MR and EC-MR in vascular inflammation by promoting leukocyte recruitment and adhesion, respectively. Specifically, conditioned media from aldosterone-treated human coronary SMC, but not HEK293 cells, enhanced monocyte chemotaxis.53 This was inhibited by spironolactone and by anti-vascular endothelial growth factor receptor type-1 (VEGFR1) blocking antibody, implicating MR and VEGF signaling in the mechanism. In human coronary ECs, aldosterone increased transcription and cell surface expression of intracellular adhesion molecule-1 (ICAM1), promoting leukocyte adhesion to EC in an MR-dependent manner.11 Aldosterone-induced ICAM1 transcriptional regulation and leukocyte adhesion were prevented by estrogen,11,54 supporting a potential mechanism for sex differences in coronary disease outcomes that warrants further exploration.
These in vitro mechanisms have recently been tested in atherosclerosis models in vivo. MR inhibition was previously shown to attenuate plaque progression in several atherosclerosis models.55,56 Conversely, in the apolipoprotein-E knockout mouse (ApoE-KO), aldosterone infusion (at a low dose that did not raise blood pressure) significantly increased plaque size and the number of infiltrating monocytes, macrophages, and T cells in aorta, as measured by flow cytometry.53 Aldosterone treatment increased vascular ICAM1 mRNA expression and aldosterone-induced atherosclerosis was prevented in ICAM1-deficient ApoE-KO mice.57 The specific role of EC-MR in atherosclerosis remains to be explored. In the deoxycorticosterone/salt-induced model of cardiac remodeling, EC-MR deletion prevented cardiac inflammation29 yet in the trans-aortic constriction model of cardiac dysfunction, cardiac inflammation required ICAM1 but was independent of EC-MR (despite protection of EC-MR-KO mice from systolic dysfunction in that model).58,59 Thus, further studies are needed to clarify the role of EC-MR regulation of ICAM1 in distinct cardiovascular pathologies.
The specific role of SMC-MR was recently tested in an ApoE-KO model with inducible SMC-MR deletion. Despite in vitro data suggesting a pro-inflammatory effect of SMC-MR activation, extensive studies in male mice showed no effect of SMC-MR deletion on early or late atherogenesis in either the aortic root or the brachiocephalic artery.60 Leukocyte infiltration was also not affected as SMC-MR-KO did not affect the total number of leukocytes or the T-cell or monocyte/macrophage subsets in the vasculature.60 Although MR in SMC has been implicated in vascular calcification in vitro and in kidney disease models,61–63 SMC-MR deletion also did not affect atherosclerotic calcification in this model.60 Overall, the available data support that aldosterone promotes, and MR blockade attenuates, atherosclerosis and vascular inflammation. This does not appear to require SMC-MR, but in vivo testing is needed to determine MR requirements in other cell types including EC (although macrophage MR deletion attenuates atherosclerosis in mouse models64).
Immune and EC production of reactive oxygen species (ROS), particularly superoxide, is an evolutionary line of defense against bacterial and fungal infection. Aldosterone activation of MR contributes to superoxide production by EC in vitro.65,66 The mechanism involves increased transcription and plasma membrane translocation of subunits of the nicotinamide adenine dinucleotide phosphate oxidase including Nox2 and Nox411,30, p22phox28,30 and p47phox.66In vivo, chronic aldosterone infusion increased vascular oxidative stress in the cerebral circulation and this can be prevented by Nox2-KO or EC-MR-KO.67,68 In the ApoE-KO model, MR antagonism similarly decreased vascular oxidative stress and inflammation resulting in improved endothelial function.69 Normal EC metabolism generates oxidative stress that is ameliorated by antioxidant pathways. However, when ROS production exceeds antioxidant defenses in the setting of cardiometabolic risk factors, ROS is no longer beneficial and rather contributes to endothelial dysfunction and atherosclerosis. Thus, EC-MR is strategically poised to respond to vascular damage and invading pathogens by its role in regulating pro-inflammatory and pro-oxidative stress genes.
Vascular MR in wound healing and fibrosis
When physical vascular damage occurs, formerly quiescent SMCs migrate and proliferate to form neointima and produce extracellular matrix proteins that restore arterial integrity whereas re-endothelialization restores barrier function. Systemic MR antagonism with eplerenone attenuated neointima formation in pig models of coronary injury, suggesting a role for MR in vascular remodeling after injury.70,71 A recent study showed a similar benefit of MR inhibition, using the novel nonsteroidal MR antagonist finerenone, with decreased SMC proliferation and neointima formation after mouse femoral wire injury and attenuation of SMC proliferation and decreased EC apoptosis in vitro.72 Conversely, aldosterone enhanced SMC proliferation and vascular fibrosis in a mouse wire carotid injury model.73 Importantly, when the contralateral uninjured carotid was examined, aldosterone had no effect on the uninjured vessel structure, consistent with the notion that MR is primed to respond only when the vessel is damaged. Specifically, SMC-MR deletion did not affect basal vessel structure yet SMC-MR-KO mice were protected from carotid fibrosis after injury and from aldosterone-induced SMC proliferation in injured carotids.74 EC-MR-KO did not affect cerebral artery structure (outer diameter, inner diameter, or wall cross-sectional area) under normotensive conditions but protected from hypertensive remodeling.75 There is a similar lack of effect of EC-MR-KO on basal cardiac structure and function as measured by fractional shortening and ejection fraction.59
Multiple pathways have been implicated in the mechanism by which SMC-MR contributes to vascular healing including Rho-kinase signaling, placental growth factor signaling through VEGFR1, and galectin signaling (reviewed in Koenig and Jaffe16). The absence of SMC-MR did not affect re-endothelialization after wire injury,74 yet re-endothelialization after electrical injury was accelerated by finerenone72 supporting a potential role for EC-MR in that process that remains to be tested.
In the setting of a traumatic injury, aldosterone induction of vascular healing by promoting SMC proliferation and fibrosis may be life-saving, however, when induced by internal wire injury, exuberant neointima is often detrimental. Clinically relevant scenarios include in-stent restenosis and vein graft failure where SMC neointimal proliferation limits patency after vascular procedures. MR antagonism attenuated coronary stent neointimal hyperplasia in swine models, but this benefit has not been confirmed in humans.70,76,77 Bypass surgery exposes the compliant vein to high arterial pressure resulting in venous damage, neointimal hyperplasia, and high rates of vein graft failure. MR is expressed in venous SMC and MR expression is higher in explanted grafted veins compared to normal venous tissue.78 In both a mouse model of aortic interposition vein grafting and a pig model of carotid interposition vein grafting, MR inhibition significantly attenuated vein graft remodeling.76,79 No studies have examined the potential benefit of inhibiting MR in human vein graft patients.
Hypertension causes vascular damage that also induces vascular remodeling. MR inhibition prevents vascular remodeling in response to high blood pressure.80 In the uninephrectomy/aldosterone/salt hypertension model, SMC-MR deletion protected from vascular remodeling and stiffness in association with decreased alpha-5 integrin expression.22 Increased integrin expression and activation contributes to vascular stiffness by enhancing interactions between the SMC cytoskeleton and the extracellular matrix. Eplerenone prevented inward hypotrophic remodeling of both parenchymal arterioles and posterior cerebral arteries in the AngII-induced hypertension model and EC-MR-KO recapitulated these beneficial effects of global MR inhibition.75 MR antagonism also decreases vascular stiffness in hypertensive humans when compared to the same degree of blood pressure lowering with a beta blocker.81 Hypertension is associated with increased risk of aortic aneurism formation and rupture in humans and in a recent mouse study, eplerenone attenuated aortic aneurism progression in association with decreased vascular inflammation and expression of the matrix metalloprotease, MMP2.82
Another stimulus to vascular remodeling is aging. Aortic stiffness measured by pulse wave velocity increases with age in humans and is associated with increased cardiovascular disease risk independent of other risk factors.83,84 Multiple studies indicate that MR expression in SMC increases with age in rodent vessels and cells.25,85 In aging mice, MR antagonism or SMC-MR deletion attenuated the progression of aortic stiffness as measured by pulse wave velocity. The protective mechanism induced by MR antagonism or SMC-MR-KO involved recruitment of an anti-fibrotic vascular gene expression program in aged mice, with decreased MMP2 and connective tissue growth factor expression and decreased vascular fibrosis.86
Overall, the data support that vascular MR becomes activated in response to injury to promote SMC proliferation, neointima formation, remodeling, and increased vascular fibrosis and stiffness. These potentially beneficial effects of vascular MR in the setting of external traumatic injury may contribute to cardiovascular morbidity when injury is induced by revascularization procedures, hypertension, or aging (Figure 1).
How is MR activated to specifically respond to vascular injury?
If indeed increased MR activity provides a selective evolutionary advantage through its rapid response to vascular injury and coordination of EC and SMC-mediated wound healing, it is critical to understand the signals that induce vascular MR activation and how those might be dysregulated in our modern environment. In the setting of hypotension from bleeding or sodium depletion, the RAAS would be activated to increase circulating AngII and aldosterone levels. Aldosterone levels are also elevated in obesity and in heart failure. Obesity is a growing epidemic that preferentially affects women and the obesity-induced rise in aldosterone is greater in females than males.34,36 Similarly in animal models females are more susceptible to obesity-induced microvascular dysfunction that can be rescued by EC-MR deletion.31 Hyperaldosteronism is also associated with resistant hypertension and obstructive sleep apnea87,88 (in part due to obesity) and hence could be a source of SMC-MR activation to induce adverse vascular remodeling leading to vascular stiffness and/or aneurism formation with aging or hypertension.
Enhanced vascular MR activity could also be induced by upregulation of MR expression in response to risk factors or in local areas of vascular injury. Indeed, SMC-MR expression increases in rodent arteries with aging25,85 and in grafted human veins exposed to arterial pressure.78 This could explain the enhanced MR activation even in the absence of changes in hormone levels that may contribute to vasoconstriction, fibrosis, and stiffness with aging and to vein graft failure. Whether MR expression is changed in SMC locally in response to wire or other mechanical injury has not been tested. Similarly, whether MR expression changes in ECs in response to cardiovascular risk factors remains to be explored but this idea would be consistent with the development of hypertension in mice in which MR is overexpressed in ECs.9 Early studies found that MR has 2 alternatively spliced promoter regions that can be regulated by hormone receptors and other transcription factors89; however, the mechanisms-regulating MR expression in vascular cells has never been explored. It is also possible that the cortisol-inactivating 11βHSD2 enzyme could be overwhelmed or dysregulated at sites of injury or in times of stress resulting in MR activation by corticosteroids, although this has not been formally tested.
Finally, MR can be activated in a ligand-independent manner by rapid signaling pathways and posttranslational modifications. AngII directly activates MR in SMC in vitro12 and SMC-MR is necessary for AngII-mediated vasoconstriction and blood pressure elevation in vivo.23,25 Localized changes in vascular AngII signaling induced by injury or aging could thereby contribute to SMC-MR activation in a hormone-independent manner. MR can also be activated by the small GTPase Rac1, a mechanism that contributes to cardiac and renal dysfunction in response to various injury models.90–92 Recently, protection from ischemia-induced renal failure by MR inhibition was shown to be mediated by SMC-MR via Rac1 activation in SMCs.93 Importantly, Rac1 can be activated by oxidative stress or high sodium, potentiators of MR activation in aging, obesity, or hypertension. Because localized vascular damage also enhances vascular oxidative stress, the oxidative stress-Rac1-MR activation mechanism might also contribute to MR activation, specifically in areas of vascular injury. Thus, although further studies are needed to confirm specific mechanisms, MR may be overexpressed or overactivated in EC and SMC in response to conditions that are extremely common in our modern society including obesity, dyslipidemia, hypertension, vascular injury, and aging, thereby contributing to the adverse vascular responses from those risk factors.
CONCLUSION
The threat of attack by a saber-toothed cat has been replaced by an environment in which life expectancy is increased and characterized by physical inactivity and chronic exposure of the vasculature to damage from high salt intake, hyperlipidemia, obesity, and glucose intolerance (Figure 1, right). In this modern environment, one would rarely benefit from the potential survival advantage of a receptor that is poised to retain sodium in the kidney, promote vasoconstriction, cell proliferation, and fibrosis in SMCs, and decrease vasodilation and promote inflammation and thrombosis in the endothelium. Rather, in this modern scenario, vascular MR may contribute to hypertension and vascular stiffness with aging and obesity, inflammatory atherosclerosis, stent and vein graft failure, and cardiac and renal dysfunction. As these disorders are post-reproductive, there is no evolutionary pressure to rectify this detrimental role of vascular MR. Until we succeed at lifestyle modification, enhanced understanding of the mechanisms and consequences of vascular MR activation can assist in developing strategies to block MR activation by environmental factors, inhibit MR function once activated, or block the downstream pathways by which MR contributes to adverse cardiovascular outcomes.
DISCLOSURE
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL119290 to I.Z.J., HL095590 to I.Z.J., 5T32HL007609-31 to L.A.B., and R00HD090198 to M.W. and by American Heart Association EIA 18290005 I.Z.J..
REFERENCES
- 1. Baker ME, Funder JW, Kattoula SR. Evolution of hormone selectivity in glucocorticoid and mineralocorticoid receptors. J Steroid Biochem Mol Biol 2013; 137:57–70. [DOI] [PubMed] [Google Scholar]
- 2. Baker ME, Nelson DR, Studer RA. Origin of the response to adrenal and sex steroids: roles of promiscuity and co-evolution of enzymes and steroid receptors. J Steroid Biochem Mol Biol 2015; 151:12–24. [DOI] [PubMed] [Google Scholar]
- 3. Hermidorff MM, de Assis LV, Isoldi MC. Genomic and rapid effects of aldosterone: what we know and do not know thus far. Heart Fail Rev 2017; 22:65–89. [DOI] [PubMed] [Google Scholar]
- 4. Zennaro MC, Fernandes-Rosa F. 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor mutations. J Endocrinol 2017; 234:T93–T106. [DOI] [PubMed] [Google Scholar]
- 5. Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schütz G. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci U S A 1998; 95:9424–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bleich M, Warth R, Schmidt-Hieber M, Schulz-Baldes A, Hasselblatt P, Fisch D, Berger S, Kunzelmann K, Kriz W, Schütz G, Greger R. Rescue of the mineralocorticoid receptor knock-out mouse. Pflugers Arch 1999; 438:245–254. [DOI] [PubMed] [Google Scholar]
- 7. Hubert C, Gasc JM, Berger S, Schütz G, Corvol P. Effects of mineralocorticoid receptor gene disruption on the components of the renin-angiotensin system in 8-day-old mice. Mol Endocrinol 1999; 13:297–306. [DOI] [PubMed] [Google Scholar]
- 8. Laursen SB, Finsen S, Marcussen N, Quaggin SE, Hansen PBL, Dimke H. Endothelial mineralocorticoid receptor ablation does not alter blood pressure, kidney function or renal vessel contractility. PLoS One. 2018; 13:e0193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, Lacolley P, Henrion D, Jaisser F. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J 2010; 24:2454–2463. [DOI] [PubMed] [Google Scholar]
- 10. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 1988; 242:583–585. [DOI] [PubMed] [Google Scholar]
- 11. Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 2008; 102:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res 2005; 96:643–650. [DOI] [PubMed] [Google Scholar]
- 13. Chrissobolis S. Vascular consequences of aldosterone excess and mineralocorticoid receptor antagonism. Curr Hypertens Rev 2017; 13:46–56. [DOI] [PubMed] [Google Scholar]
- 14. Davel AP, Anwar IJ, Jaffe IZ. The endothelial mineralocorticoid receptor: mediator of the switch from vascular health to disease. Curr Opin Nephrol Hypertens 2017; 26:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DuPont JJ, Jaffe IZ. 30 Years of the mineralocorticoid receptor: the role of the mineralocorticoid receptor in the vasculature. J Endocrinol 2017; 234:T67–T82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koenig JB, Jaffe IZ. Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep 2014; 16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lother A, Hein L. Vascular mineralocorticoid receptors: linking risk factors, hypertension, and heart disease. Hypertension (Dallas, Tex 1979) 2016; 68:6–10. [DOI] [PubMed] [Google Scholar]
- 18. van der Heijden CDCC, Deinum J, Joosten LAB, Netea MG, Riksen NP. The mineralocorticoid receptor as a modulator of innate immunity and atherosclerosis. Cardiovasc Res 2018; 114:944–953. [DOI] [PubMed] [Google Scholar]
- 19. Belden Z, Deiuliis JA, Dobre M, Rajagopalan S. The role of the mineralocorticoid receptor in inflammation: focus on kidney and vasculature. Am J Nephrol 2017; 46:298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marzolla V, Armani A, Feraco A, De Martino MU, Fabbri A, Rosano G, Caprio M. Mineralocorticoid receptor in adipocytes and macrophages: a promising target to fight metabolic syndrome. Steroids 2014; 91:46–53. [DOI] [PubMed] [Google Scholar]
- 21. Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids 2014; 91:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension 2014; 63:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012; 18:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mueller KB, Bender SB, Hong K, et al. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension (Dallas, Tex 1979) 2015; 66:988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K, Yang Y, Yoo JK, Aronovitz M, Baur WE, Christou DD, Hill MA, Jaffe IZ. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 2016; 1:e88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gueret A, Harouki N, Favre J, et al. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension (Dallas, Tex 1979) 2016; 67:717–723. [DOI] [PubMed] [Google Scholar]
- 27. Amador CA, Bertocchio JP, Andre-Gregoire G, Placier S, Duong Van Huyen JP, El Moghrabi S, Berger S, Warnock DG, Chatziantoniou C, Jaffe IZ, Rieu P, Jaisser F. Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int 2016; 89:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Lüscher TF, Verrey F, Matter CM. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 2013; 34:3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rickard AJ, Morgan J, Chrissobolis S, Miller AA, Sobey CG, Young MJ. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension (Dallas, Tex 1979) 2014; 63:1033–1040. [DOI] [PubMed] [Google Scholar]
- 30. Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 2016; 118:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davel AP, Lu Q, Moss ME, et al. Sex-specific mechanisms of resistance vessel endothelial dysfunction induced by cardiometabolic risk factors. J Am Heart Assoc 2018; 7:1–19. doi: 10.1161/JAHA.117.007675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens 2010; 19:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res 1999; 7:355–362. [DOI] [PubMed] [Google Scholar]
- 34. Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015; 132:2134–2145. [DOI] [PubMed] [Google Scholar]
- 35. Huby A-C, Otvos L, Belin de Chantemèle EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension (Dallas, Tex 1979) 2016; 67:1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab 2007; 92:4472–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jia G, Habibi J, Aroor AR, et al. Epithelial sodium channel in aldosterone-induced endothelium stiffness and aortic dysfunction. Hypertension (Dallas, Tex 1979) 2018; 72:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown NJ, Kim KS, Chen YQ, Blevins LS, Nadeau JH, Meranze SG, Vaughan DE. Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab 2000; 85:336–344. [DOI] [PubMed] [Google Scholar]
- 39. Sawathiparnich P, Kumar S, Vaughan DE, Brown NJ. Spironolactone abolishes the relationship between aldosterone and plasminogen activator inhibitor-1 in humans. J Clin Endocrinol Metab 2002; 87:448–452. [DOI] [PubMed] [Google Scholar]
- 40. Campbell LA, Rosenfeld ME. Infection and atherosclerosis development. Arch Med Res 2015; 46:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gromotowicz A, Szemraj J, Stankiewicz A, Zakrzeska A, Mantur M, Jaroszewicz E, Rogowski F, Chabielska E. Study of the mechanisms of aldosterone prothrombotic effect in rats. J Renin Angiotensin Aldosterone Syst 2011; 12:430–439. [DOI] [PubMed] [Google Scholar]
- 42. Stankiewicz A, Gromotowicz A, Szemraj J, Wojewódzka-Zelezniakowicz M, Skrzypkowski P, Chabielska E. Acute aldosterone infusion enhances thrombosis development in normotensive rats. Thromb Haemost 2007; 98:697–699. [PubMed] [Google Scholar]
- 43. Ma J, Albornoz F, Yu C, Byrne DW, Vaughan DE, Brown NJ. Differing effects of mineralocorticoid receptor-dependent and -independent potassium-sparing diuretics on fibrinolytic balance. Hypertension (Dallas, Tex 1979) 2005; 46:313–320. [DOI] [PubMed] [Google Scholar]
- 44. Elbers LP, Sjouke B, Zannad F, Cicoira M, Vizzardi E, Václavík J, Gerdes VE, Squizzato A. Effects of mineralocorticoid receptor antagonists on the risk of thrombosis, bleeding and mortality: a systematic review and meta-analysis of randomized controlled trials. Thromb Res 2016; 144:32–39. [DOI] [PubMed] [Google Scholar]
- 45. Lagrange J, Li Z, Fassot C, Bourhim M, Louis H, Nguyen Dinh Cat A, Parlakian A, Wahl D, Lacolley P, Jaisser F, Regnault V. Endothelial mineralocorticoid receptor activation enhances endothelial protein C receptor and decreases vascular thrombosis in mice. FASEB J 2014; 28:2062–2072. [DOI] [PubMed] [Google Scholar]
- 46. Lubkin A, Torres VJ. Bacteria and endothelial cells: a toxic relationship. Curr Opin Microbiol 2017; 35:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farzaneh-Far A, Rudd J, Weissberg PL. Inflammatory mechanisms. Br Med Bull 2001; 59:55–68. [DOI] [PubMed] [Google Scholar]
- 48. Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 2014; 114:1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautière K, Collet JP, Beygui F, Hennache B, Ennezat PV, Juthier F, Richard F, Dallongeville J, Hillaert MA, Doevendans PA, Jude B, Bertrand M, Montalescot G, Van Belle E. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J 2012; 33:191–202. [DOI] [PubMed] [Google Scholar]
- 50. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005; 45:1243–1248. [DOI] [PubMed] [Google Scholar]
- 51. Moss ME, Jaffe IZ. Mineralocorticoid receptors in the pathophysiology of vascular inflammation and atherosclerosis. Front Endocrinol (Lausanne) 2015; 6:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGraw AP, McCurley A, Preston IR, Jaffe IZ. Mineralocorticoid receptors in vascular disease: connecting molecular pathways to clinical implications. Curr Atheroscler Rep 2013; 15:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McGraw AP, Bagley J, Chen WS, Galayda C, Nickerson H, Armani A, Caprio M, Carmeliet P, Jaffe IZ. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc 2013; 2:e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barrett Mueller K, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, Adler GK, Karas RH, Jaffe IZ. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology 2014; 155:4461–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keidar S, Hayek T, Kaplan M, Pavlotzky E, Hamoud S, Coleman R, Aviram M. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol 2003; 41:955–963. [DOI] [PubMed] [Google Scholar]
- 56. Rajagopalan S, Duquaine D, King S, Pitt B, Patel P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation 2002; 105:2212–2216. [DOI] [PubMed] [Google Scholar]
- 57. Marzolla V, Armani A, Mammi C, Moss ME, Pagliarini V, Pontecorvo L, Antelmi A, Fabbri A, Rosano G, Jaffe IZ, Caprio M. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int J Cardiol 2017; 232:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salvador AM, Nevers T, Velázquez F, Aronovitz M, Wang B, Abadía Molina A, Jaffe IZ, Karas RH, Blanton RM, Alcaide P. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J Am Heart Assoc 2016; 5:e003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salvador AM, Moss ME, Aronovitz M, et al. Endothelial mineralocorticoid receptor contributes to systolic dysfunction induced by pressure overload without modulating cardiac hypertrophy or inflammation. Physiol Rep. 2017; 5:e13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moss ME, DuPont JJ, Iyer SL, McGraw AP, Jaffe IZ. No significant role for smooth muscle cell mineralocorticoid receptors in atherosclerosis in the apolipoprotein-E knockout mouse model. Front Cardiovasc Med 2018; 5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol 2007; 27:799–805. [DOI] [PubMed] [Google Scholar]
- 62. Lang F, Ritz E, Alesutan I, Voelkl J. Impact of aldosterone on osteoinductive signaling and vascular calcification. Nephron Physiol 2014; 128:40–45. [DOI] [PubMed] [Google Scholar]
- 63. Tatsumoto N, Yamada S, Tokumoto M, Eriguchi M, Noguchi H, Torisu K, Tsuruya K, Kitazono T. Spironolactone ameliorates arterial medial calcification in uremic rats: the role of mineralocorticoid receptor signaling in vascular calcification. Am J Physiol Renal Physiol 2015; 309:F967–F979. [DOI] [PubMed] [Google Scholar]
- 64. Shen ZX, Chen XQ, Sun XN, Sun JY, Zhang WC, Zheng XJ, Zhang YY, Shi HJ, Zhang JW, Li C, Wang J, Liu X, Duan SZ. Mineralocorticoid receptor deficiency in macrophages inhibits atherosclerosis by affecting foam cell formation and efferocytosis. J Biol Chem 2017; 292:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 2008; 149:1009–1014. [DOI] [PubMed] [Google Scholar]
- 66. Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension 2006; 48:165–171. [DOI] [PubMed] [Google Scholar]
- 67. Chrissobolis S, Drummond GR, Faraci FM, Sobey CG. Chronic aldosterone administration causes Nox2-mediated increases in reactive oxygen species production and endothelial dysfunction in the cerebral circulation. J Hypertens 2014; 32:1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dinh QN, Young MJ, Evans MA, Drummond GR, Sobey CG, Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res 2016; 1637:146–153. [DOI] [PubMed] [Google Scholar]
- 69. Kratz MT, Schirmer SH, Baumhäkel M, Böhm M. Improvement of endothelial function in a murine model of mild cholesterol-induced atherosclerosis by mineralocorticoid antagonism. Atherosclerosis 2016; 251:291–298. [DOI] [PubMed] [Google Scholar]
- 70. Wakabayashi K, Suzuki H, Sato T, Iso Y, Katagiri T, Takeyama Y. Eplerenone suppresses neointimal formation after coronary stent implantation in swine. Int J Cardiol 2006; 107:260–266. [DOI] [PubMed] [Google Scholar]
- 71. Ward MR, Kanellakis P, Ramsey D, Funder J, Bobik A. Eplerenone suppresses constrictive remodeling and collagen accumulation after angioplasty in porcine coronary arteries. Circulation 2001; 104:467–472. [DOI] [PubMed] [Google Scholar]
- 72. Dutzmann J, Musmann RJ, Haertlé M, et al. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS One 2017; 12:e0184888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Carmeliet P, Ehsan A, Mendelsohn ME. Placental growth factor mediates aldosterone-dependent vascular injury in mice. J Clin Invest 2010; 120:3891–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol 2014; 34:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Diaz-Otero JM, Fisher C, Downs K, et al. Endothelial mineralocorticoid receptor mediates parenchymal arteriole and posterior cerebral artery remodeling during angiotensin II-induced hypertension. Hypertension (Dallas, Tex 1979) 2017; 70:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bacchetta MD, Salemi A, Milla F, Hong MK, Tio F, Zhou Y, Chen R, Southard E, Lee LY, Mack CA, Krieger KH, Isom OW, Ko W, Borer JS, Catanzaro DF. Low-dose spironolactone: effects on artery-to-artery vein grafts and percutaneous coronary intervention sites. Am J Ther 2009; 16:204–214. [DOI] [PubMed] [Google Scholar]
- 77. Dutzmann J, Bauersachs J, Sedding DG. Evidence for the use of mineralocorticoid receptor antagonists in the treatment of coronary artery disease and post-angioplasty restenosis. Vascul Pharmacol 2017; 107:20–26. [DOI] [PubMed] [Google Scholar]
- 78. Bafford R, Sui XX, Park M, Miyahara T, Newfell BG, Jaffe IZ, Romero JR, Adler GK, Williams GH, Khalil RA, Conte MS. Mineralocorticoid receptor expression in human venous smooth muscle cells: a potential role for aldosterone signaling in vein graft arterialization. Am J Physiol Heart Circ Physiol 2011; 301:H41–H47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ehsan A, McGraw AP, Aronovitz MJ, Galayda C, Conte MS, Karas RH, Jaffe IZ. Mineralocorticoid receptor antagonism inhibits vein graft remodeling in mice. J Thorac Cardiovasc Surg 2013; 145:1642–9, 1649.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yan Y, Wang C, Lu Y, Gong H, Wu Z, Ma X, Li H, Wang B, Zhang X. Mineralocorticoid receptor antagonism protects the aorta from vascular smooth muscle cell proliferation and collagen deposition in a rat model of adrenal aldosterone-producing adenoma. J Physiol Biochem 2018; 74:17–24. [DOI] [PubMed] [Google Scholar]
- 81. Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension (Dallas, Tex 1979) 2008; 51:432–439. [DOI] [PubMed] [Google Scholar]
- 82. Kurobe H, Hirata Y, Matsuoka Y, Sugasawa N, Higashida M, Nakayama T, Maxfield MW, Yoshida Y, Shimabukuro M, Kitagawa T, Sata M. Protective effects of selective mineralocorticoid receptor antagonist against aortic aneurysm progression in a novel murine model. J Surg Res 2013; 185:455–462. [DOI] [PubMed] [Google Scholar]
- 83. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension (Dallas, Tex 1979) 2005; 45:652–658. [DOI] [PubMed] [Google Scholar]
- 85. Krug AW, Allenhöfer L, Monticone R, et al. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension (Dallas, Tex 1979) 2010; 55:1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim SK, McCurley AT, DuPont JJ, et al. Smooth muscle cell-mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension (Dallas, Tex 1979) 2018; 71:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Valaiyapathi B, Calhoun DA. Role of mineralocorticoid receptors in obstructive sleep apnea and metabolic syndrome. Curr Hypertens Rep 2018; 20:23. [DOI] [PubMed] [Google Scholar]
- 88. Kline GA, Prebtani APH, Leung AA, Schiffrin EL. Primary aldosteronism: a common cause of resistant hypertension. CMAJ 2017; 189:E773–E778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zennaro MC, Le Menuet D, Lombès M. Characterization of the human mineralocorticoid receptor gene 5’-regulatory region: evidence for differential hormonal regulation of two alternative promoters via nonclassical mechanisms. Mol Endocrinol 1996; 10:1549–1560. [DOI] [PubMed] [Google Scholar]
- 90. Kawarazaki W, Fujita T. Aberrant Rac1-mineralocorticoid receptor pathways in salt-sensitive hypertension. Clin Exp Pharmacol Physiol 2013; 40:929–936. [DOI] [PubMed] [Google Scholar]
- 91. Ayuzawa N, Nagase M, Ueda K, et al. Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension (Dallas, Tex 1979) 2016; 67:99–106. [DOI] [PubMed] [Google Scholar]
- 92. Yoshida S, Ishizawa K, Ayuzawa N, Ueda K, Takeuchi M, Kawarazaki W, Fujita T, Nagase M. Local mineralocorticoid receptor activation and the role of Rac1 in obesity-related diabetic kidney disease. Nephron Exp Nephrol 2014; 126:16–24. [DOI] [PubMed] [Google Scholar]
- 93. Barrera-Chimal J, André-Grégoire G, Nguyen Dinh Cat A, Lechner SM, Cau J, Prince S, Kolkhof P, Loirand G, Sauzeau V, Hauet T, Jaisser F. Benefit of mineralocorticoid receptor antagonism in AKI: role of vascular smooth muscle rac1. J Am Soc Nephrol 2017; 28:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]