Abstract
Lurbinectedin is an inhibitor of active transcription of protein-coding genes, causing DNA-break accumulation, apoptosis and modulation of the tumor microenvironment. Early-phase clinical trials indicate promising activity of lurbinectedin in small-cell lung cancer. Here, we describe the rationale and design of ATLANTIS (NCT02566993), an open-label, randomized, multicenter Phase III study to compare the efficacy of lurbinectedin and doxorubicin combination with standard-of-care chemotherapy, investigator's choice of cyclophosphamide/doxorubicin/vincristine or topotecan, in patients with small-cell lung cancer that has progressed following one line of platinum-based chemotherapy. Patients are randomized in a 1:1 ratio. The primary end point is overall survival and key secondary end points include progression-free survival, best tumor response and duration of response, each assessed by independent review committee.
Keywords: : lurbinectedin, Phase III trial, small-cell lung cancer, targeting transcription
Small-cell lung cancer (SCLC) is a high-grade neuroendocrine malignancy of the lung which accounts for approximately 13–15% of all lung cancers [1]. Outcomes for patients with SCLC have remained virtually unchanged for nearly 30 years, with a median overall survival (OS) of 9–11 months for patient diagnosed with metastatic disease [2–6]. A history of tobacco exposure is the most significant risk factor for developing SCLC and the vast majority of SCLCs occur in patients who are current or former smokers. The SCLC tends to present with bulky mediastinal lymph node involvement and often with distant metastases [7]. The standard-of-care, first-line therapy for metastatic SCLC is combination of platinum (carboplatin or cisplatin) with etoposide or with irinotecan [8]. Unfortunately, despite high-response rates to initial chemotherapy, ranging 50–70% in recent clinical trials [2–5], metastatic SCLC nearly always relapse.
Currently, the topoisomerase-I inhibitor topotecan is the only US FDA- and EMA-approved second-line treatment in SCLC. In recent randomized studies, the median progression-free survival (PFS) for patients treated in the topotecan control arm ranged from 3.0 to 3.5 months and the OS ranged from 7 to 8 months [9–11]. Efficacy of topotecan, as well as other agents used in the second-line setting, varies depending on the degree of platinum sensitivity of the population. Generally, ‘platinum-sensitive’ SCLC refers to disease that relapses >3 months after completion of the first-line therapy, and ‘platinum-resistant’ or ‘refractory’ SCLC refers to disease that relapses ≤ 3 months after completion of the first-line therapy, though the specific terminology and cut-offs defining these categories may vary from study to study. Across 21 studies published between 1984 and 2001, both response rates and OS were superior in platinum-sensitive patients compared with platinum-resistant patients [12]. Notably, topotecan was directly compared with cyclophosphamide/doxorubicin/vincristine (CAV) in a randomized trial of 211 patients and measures of efficacy were statistically equivalent in both groups (overall response rate [ORR] 24.3 vs 18.3%; p = 0.285; time-to-progression 13.1 vs 12.3 weeks; p = 0.552; OS 25.0 vs 24.7 weeks; p = 0.795 in patients treated with topotecan vs CAV, respectively) [13]. Recently, immune-checkpoint inhibitors have shown encouraging activity in SCLC [14,15], although, as of August 1 2018, are not currently FDA- or EMA-approved for use in this disease.
Despite extensive genetic characterization of SCLCs [16–19], no clear targetable alteration has emerged. The common genetic alterations in SCLC are loss of the tumor suppressor genes TP53 and RB1 [18]. A recent preclinical study in SCLC cell lines and genetically engineered mouse models uncovered a dependency on active transcription, with significant vulnerability to inhibition of CDK7 and subsequent reduction of RNA polymerase II-mediated gene transcription [20]. Interestingly, genes associated with highly active so-called ‘super-enhancers’ were involved in regulation of RNA polymerase II-mediated transcription and proposed proto-oncogenic transcription factors. Treatment with the CDK7 inhibitor THZ1 led to preferential down-regulation of super enhancer-associated genes in cell lines and to tumor regressions in genetically engineered mouse models [20]. Collectively, these data suggest that targeting active transcription may be an efficacious therapeutic strategy.
Lurbinectedin
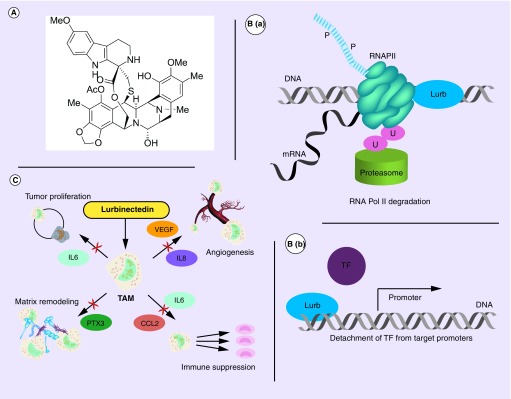
Lurbinectedin (PM01183) is a synthetic analog of the natural marine-based tetrahydroisoquinoline trabectedin. It contains a pentacyclic skeleton composed of two fused tetrahydroisoquinoline rings, with an additional tetrahydro β-carboline moiety (Figure 1A) [21]. Lurbinectedin induces a specific degradation of transcribing RNA Pol II and the subsequent accumulation of DNA breaks (Figure 1B). This degradation occurs when elongating RNA polymerase II is arrested by the drug covalently bound to the CG-rich regions located within the affected gene and blocks the DNA-repair mechanism [22]. This degradation is dependent on active transcription, the presence of functional proteasome machinery and transcription-coupled nucleotide excision repair [22]. Furthermore, in transcriptionally addicted tumor cells (e.g., SCLC), lurbinectedin could cause a detachment of transcription factors (e.g., ASCL1, NeuroD1 and NFIB in SCLC) from their target promoters, inducing the block of its transactivating activity (Figure 1B). Lurbinectedin may also influence the tumor microenvironment via suppression of tumor proliferation, matrix remodeling, angiogenesis and immune suppression (Figure 1C) [23–25]. Lurbinectedin activity on SCLC cell lines was demonstrated in vitro and in vivo [26].
Figure 1. . Chemical structure and schematized actions of lurbinectedin.
(A) Chemical structure of lurbinectedin. (B) Schematic representation of lurbinectedin mechanism of action for binding DNA, inhibiting active transcription and inducing DNA breaks. (C) Schematic representation of lurbinectedin interactions with the tumor microenvironment.
Lurb: Lurbinectedin.
The ATLANTIS study
Here, we describe the design and rationale for the ATLANTIS study (NCT02566993), an open-label, randomized Phase III trial to evaluate efficacy and safety of lurbinectedin and doxorubicin combination versus investigator's choice chemotherapy, either topotecan or CAV, in patients with SCLC who have failed one prior platinum-containing line. The study is funded by Pharma Mar SA, Madrid, Spain.
Background & rationale
In preclinical cell line and xenograft models, lurbinectedin showed activity both alone and in combination with doxorubicin. In xenografted SCLC tumors, the combination demonstrated substantial antitumor activity which appeared to be synergistic [26]. Clinical development of lurbinectedin was started in March 2009. The first-in-human single-agent lurbinectedin study established a recommended Phase II dose of 4.0 mg/m2 or 7.0 mg flat dose (FD) given intravenously, every 21 days [27].
A Phase Ib study assessed the safety and efficacy of lurbinectedin combined with doxorubicin in patients with solid tumors [28]. The primary objective was to determine the recommended dose (RD) for further studies with this combination, and secondary objectives included assessing safety, analyzing possible pharmacokinetic drug–drug interactions and assessing antitumor activity. In the initial cohort (cohort A), doxorubicin was given at a dose of 50 mg/m2 and lurbinectedin was given as 3–5 mg FD, both on day 1 of each 21-day cycle, with continuation of lurbinectedin at a FD of 7 mg after the cumulative dose of doxorubicin reached 450 mg/m2. In this cohort, dose-limiting toxicities of febrile neutropenia were observed in one of ten patients treated without primary granulocyte colony-stimulating factors (G-CSF) prophylaxis. As a result, lurbinectedin 4.0 mg FD and doxorubicin 50 mg/m2 was defined as the RD for the combination and additional patients were enrolled in an expansion cohort treated at the RD. Reversible myelosuppression was the most common toxicity with the lurbinectedin and doxorubicin combination. Episodes of severe neutropenia, thrombocytopenia and febrile neutropenia were transient and successfully managed with cycle delays, dose reductions and G-CSF [28].
The combination-associated myelosuppression raised the question of whether the RD defined in this cohort would be safe and tolerable in a less selected/restricted Phase III population. In addition, the doxorubicin dose used in SCLC patients is usually less than 50 mg/m2 per cycle (either 45 mg/m2 with G-CSF support or 40 mg/m2 without it), as several tobacco-related comorbidities and prior mediastinal irradiation are highly prevalent. As a result, the Phase Ib study was amended to reduce in a second cohort (cohort B) the doxorubicin RD dose by 20% (to 40 mg/m2) to limit the potentially severe myelosuppression and to explore the feasibility of this dose in less selected patients (ECOG PS score = 2 and without any age restriction). This protocol amendment also adjusted the doxorubicin dose to one that is more commonly used in these patients. The lurbinectedin dose was also adapted to a body surface (BSA)-based dose of 2.0 mg/m2. The rationale for this change came from the results of a logistic regression analysis of pooled data from Phase II clinical trials with single-agent lurbinectedin in several solid tumors, which suggested that patients with the lowest BSA values could have a greater possibility of developing grade 3/4 thrombocytopenia and/or neutropenia. Hence, the lurbinectedin 4.0 mg FD was transformed to 2.0 mg/m2 every 21 days, with the dose being capped at a BSA of 2.0 m2 to prevent patients from receiving more lurbinectedin than 4.0 mg FD every 21 days, previously defined as the RD.
Ultimately, 48 patients with SCLC were enrolled into this Phase I study: 21 in the initial cohort (cohort A) and 27 in the second cohort (cohort B). The median age of these patients was 62 and 64, respectively and all had Eastern Cooperative Oncology Group (ECOG) performance status 1–2. In cohorts A and B, 43 and 54% of patients had received prior prophylactic cranial irradiation and the proportions of patients with known brain metastases were 33 and 4%, respectively. The proportion of patients with ‘platinum-sensitive’ disease, defined as a chemotherapy-free interval (CTFI) from completion of the first-line therapy to start of the second-line therapy of more than 90 days, was 52% in cohort A and 64% in cohort B. The proportion of patients with CTFI >30 days was 76 and 78% in cohorts A and B, respectively.
Clinical activity of lurbinectedin and doxorubicin was seen among patients with SCLC in both cohorts. In cohort A, there were two complete responses and 12 partial responses (PRs; ORR = 67%). In cohort B, there was one complete response and nine PRs (ORR = 37%). The disease control rate was 81 and 70% and the median duration of response was 4.5 months and 5.2 months in cohorts A and B, respectively. The median PFS was 4.7 months and 5.3 months among patients with CTFI >30 days and 5.8 months and 6.2 months among patients with platinum-sensitive disease (CTFI >90 days) in cohorts A and B, respectively.
In addition to the lurbinectedin/doxorubicin combination Phase Ib data, data are available from seven SCLC patients treated with lurbinectedin and paclitaxel combination in the third-line setting in a separate Phase Ib study. To date, five of these seven patients had responded to treatment (ORR = 71%) [29].
A multicenter Phase II basket trial to assess the efficacy and safety of single-agent lurbinectedin in several advanced solid tumors, including SCLC, is ongoing. In the SCLC cohort, 50 patients were treated and were evaluable for efficacy. Median age was 60 years and 29 (58%) were males; 45 patients (80%) had an ECOG PS of 0/1, 25 patients (50%) had CTFI ≥90 days. Nineteen patients (38%) had a PR; 52% (13/25) of patients with CTFI ≥90 days had a PR. Twenty patients (40%) had disease stabilization, six of them for >4 months. Median duration of response was 5.3 months and median PFS was 4.2 months (4.7 months for patients with CTFI ≥90 days) [30].
Design
Study design
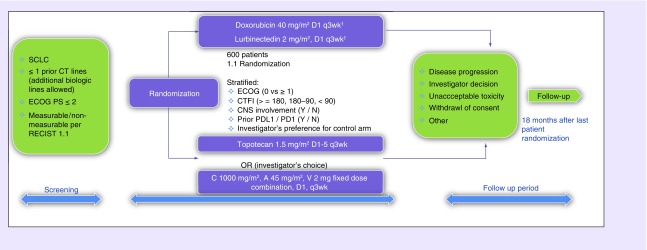
The ATLANTIS study consists of a screening period, a treatment period and a follow-up period (Figure 2). During the screening period, patients provide written informed consent and screening assessments are performed, including medical history and clinical examination, laboratory tests, measurement of left ventricular ejection fraction by echocardiogram or multiple-gated acquisition scan, pregnancy test (for women of childbearing age), electrocardiogram clinical and radiological tumor assessment, CNS tumor assessment, patient-reported outcomes using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and EORTC QLQ-LC13 questionnaires and adverse-event reporting with grading per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.4. Investigators will specify the chemotherapy selected in the control arm prior to randomization. Eligible patients are randomized in a 1:1 ratio using central randomization into one of the following two arms:
Experimental arm: lurbinectedin and doxorubicin;
Control arm: investigator's choice chemotherapy (topotecan or CAV).
Figure 2. . Study design.
†Maximum 10 cycles, lurbinectedin to be continued at 3.2 mg/m2.
CNS: Central nervous system; CT: Computerized tomography; CTFI: Chemotherapy-free interval; ECOG: Eastern Cooperative Oncology Group; N: No; OR: Overall response; SCLC: Small-cell lung cancer; Y: Yes.
Stratification is performed according to the ECOG PS (0 vs 1–2), CTFI after the first-line therapy (≥ 180 days vs 90–179 days vs <90 days), baseline CNS involvement (yes vs no), prior immunotherapy against either PD-1 or PD-L1 therapy (yes vs no) and investigator's preference (best investigator's choice prior to randomization) between topotecan and CAV.
Day 0 is defined as the day of randomization and treatment (cycle 1 day 1) must start within 72 h after randomization. The treatment phase extends from randomization to disease progression, investigator's decision, unacceptable toxicity or withdrawal of consent. During treatment, evaluations include: clinical examination on day 1 of cycle 2 and subsequent cycles; laboratory tests on days 1 and 10 of cycles 1 and 2 and day 1 of subsequent cycles; measurement of left ventricular ejection fraction before cycles 3, 6, 9 and 11 (for experimental and CAV arms and as clinically indicated for topotecan arm); pregnancy test each cycle (for woman of childbearing age); ECG before cycles 6 and 11 (for experimental and CAV arms and as clinically indicated for topotecan arm); pharmacokinetics (for patients in experimental arm only); pharmacogenetics for patients who provide specific written informed consent; clinical and radiological tumor assessment every 6 weeks and CNS radiological assessment as clinically indicated; patient-reported outcomes; survival information and adverse events as per NCI-CTCAE v.4.
An end-of-treatment visit will occur within 30 days of the last treatment infusion or immediately prior to the start of a new therapy, whichever is sooner. After treatment, patients who have withdrawn from the study without progressive disease will continue tumor assessment every 6 weeks until progressive disease (PD), start of a new therapy, death or date of study termination (clinical cut-off), whichever occurs first. After PD is documented or a new antitumor therapy is started, patients will be followed for survival every 3 months during the first 18 months after randomization, and then once every 6 months until death or date of study termination, whichever occurs first.
Primary, secondary & tertiary objectives
The primary objective of the study is to determine whether there is a difference in OS between the lurbinectedin/doxorubicin arm and control arm. Secondary objectives include analyzing difference in OS between the lurbinectedin/doxorubicin and CAV arms, in patients with CAV as best investigator's choice; assessing OS/PFS in patients with and without baseline CNS involvement and subgroup analyses in platinum-sensitive and -resistant populations; assessing PFS by an independent review committee (IRC); assessing antitumor activity by IRC according to RECIST v.1.1 and assessing safety profile. Key tertiary objectives include assessing landmark analyses of OS at 12, 18 and 24 months; safety and efficacy subgroup analyses; PFS, best antitumor response and duration of response by investigator assessment; patient-reported outcomes and pharmacokinetics and pharmacogenetics.
Key eligibility criteria
Key eligibility criteria include age ≥ 18 years, ECOG performance status ≤ 2 and histologically or cytologically confirmed diagnosis of limited or extensive stage SCLC which failed one prior platinum-containing regimen. Patients who had received prior-intervening immune checkpoint inhibitor therapy are eligible. Patients must have adequate hematological, renal, metabolic and hepatic function and a washout of at least 3 weeks since last prior anticancer treatment, at least 4 weeks since completion of whole brain radiation therapy and at least 2 weeks since completion of prophylactic cranial irradiation or palliative radiation. Key exclusion criteria include patients who received more than one prior chemotherapy-containing regimen (including patients re-challenged with the same initial regimen); patients who never received any platinum-containing regimen for SCLC; prior treatment with lurbinectedin, topotecan or anthracyclines; limited-stage patients who are candidates for local or regional therapy and symptomatic or steroid-requiring or progressive CNS disease involvement for at least 4 weeks prior to randomization.
Planned sample size
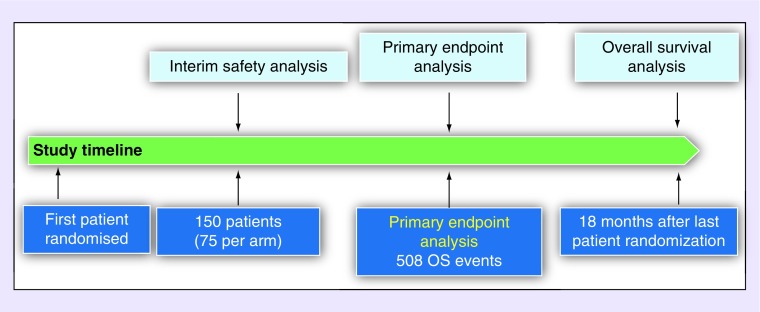
ATLANTIS is a global Phase III trial, with 154 sites in the USA, Canada, Latin America and Europe. Trial enrollment started in September 2016. The prospective assumptions are a one-sided 2.5% significance level with at least 90% power to detect a 25% decrease in the risk of death to be achieved with the experimental arm (HR: 0.75). The OS with either CAV or topotecan is expected to be 7.5 months [10]. To obtain the required 508 death events, approximately 600 patients with SCLC who failed one prior platinum-containing chemotherapy line will be stratified and randomized at a 1:1 ratio. The study reached its target enrollment of 600 randomized patients on 30 July 2018.
Of note, the study had initially been designed to cap the control arm treatments (investigator's choice of topotecan or CAV) each to a maximum of 55% of expected patients, to ensure a sufficiently large number of patients in the CAV group to provide a well-powered doxorubicin-based comparator with the experimental arm. However, as the study has proceeded, the more common investigators’ preference is CAV: as of 24 April 2018, 57% of the patients within the control arm were receiving CAV and 43% were receiving topotecan. Based on this trend, the initially planned percentage of patients treated with CAV would have been capped soon thereafter and the restriction to topotecan may have negatively impacted recruitment and extend the duration of the study. Therefore, the requirement to cap recruitment in the control arm when one of the treatment options reaches 55% of the required number of recruited patients was removed in a protocol amendment dated May 3 2018.
Dose & schedule of therapy
Patients in the experimental arm are treated with doxorubicin 40.0 mg/m2 on day 1, followed by lurbinectedin 2.0 mg/m2 on day 1 of each 21-day cycle. Patients receiving topotecan in the control arm are treated with topotecan at 1.5 mg/m2 daily on days 1–5 of each 21-day cycle, with dose reductions specified for patients with creatinine clearance less than 60 ml/min. Patients receiving CAV in the control arm are treated with cyclophosphamide 1000 mg/m2 on day 1, doxorubicin 45.0 mg/m2 on day 1 and vincristine 2.0 mg total FD on day 1 of each 21-day cycle. In both arms, up to a maximum of ten cycles of doxorubicin-containing regimens are allowed. Then, if applicable, doxorubicin will be discontinued and for patients in the experimental arm, lurbinectedin will be continued as maintenance therapy at a dose of 3.2 mg/m2 (or 2.6 mg/m2 if more than one dose reduction was applied while on combination therapy) on day 1 of each 21-day cycle. All patients in all treatment arms will receive primary G-CSF prophylaxis, with type, dose and scheme according to institutional standard practices and guidelines.
Efficacy evaluations
Antitumor activity will be assessed using RECIST v.1.1 using the appropriate imaging modality (CT or MRI) at baseline and every 6 weeks after randomization until evidence of PD. Imaging studies will be reviewed by an IRC.
Safety evaluations
Patients will be evaluable for safety, if they have received any partial or complete treatment infusion. All adverse events will be graded according to the NCI-CTCAE v.4. Treatment delays, dose omissions, dose reductions and reason for treatment discontinuation will be monitored throughout the study.
An Independent Data Monitoring Committee (IDMC) is overseeing the conduct of the study. Two ad-hoc interim safety analyses, required by the IDMC, have been conducted on the first 50 and 100 patients randomized and treated with two cycles. Both evaluations resulted in the recommendation to continue the trial without modification. The prespecified interim safety analysis, conducted after 150 patients were recruited into each arm (Figure 3), was also conducted with no modifications recommended by the IDMC.
Figure 3. . Analysis plan.
OS: Overall survival.
Statistical analyses
Time-to-event variables (OS, PFS and duration of response) and their set time estimates (such as landmark OS analyses) will be analyzed according to the Kaplan–Meier method. The stratified log-rank test (primary analysis) and the unstratified log-rank test on the intention to treat population will be used to compare the time-to-event variables. Cox regression will be used to calculate the risk reduction (OS, PFS and duration of response) and to evaluate the influence of the stratification variables and other potential prognostic factors on the time-to-event efficacy endpoints. Continuous variables that would have been categorized as discrete variables will also be investigated in the continuum range and if the adjustment is better, then the continuous variable will be kept in the regression model. Counts and percentages, with their corresponding exact 95% confidence intervals, will be calculated for the binomial end points (e.g., response rate). The Fisher's exact test (univariate analyses) and logistic regressions will be used to compare the rates of the experimental arm and the control arm. Waterfall plots will be used to describe the best variation of the sum of target lesions during the treatment.
Conclusion
Lurbinectedin is a novel antitumor agent with encouraging efficacy signals in Phase I studies. The ATLANTIS trial seeks to determine whether the lurbinectedin and doxorubicin combination leads to improved OS compared with standard-of-care chemotherapy in the second-line setting in patients with SCLC. The results of this study will help define the role of lurbinectedin/doxorubicin in SCLC and has potential to redefine standard-of-care therapy in this difficult-to-treat cancer.
Executive summary.
Background
Small-cell lung cancer (SCLC) is a highly recalcitrant cancer for which standard-of-care therapies have remained virtually unchanged for over 20 years.
Topotecan is the only US FDA- and EMA-approved second line therapy, though outcomes are poor.
Lurbinectedin
Lurbinectedin is a novel anticancer marine derivative agent which inhibits active transcription of protein-coding genes and impacts the tumor microenvironment.
Preclinical and early phase studies indicate promising antitumor activity of lurbinectedin both alone and in combination with chemotherapy.
ATLANTIS study
This is an open-label, randomized phase III trial to compare the efficacy and safety of combination lurbinectedin and doxorubicin versus investigator's choice of chemotherapy (topotecan or cyclophosphamide/doxorubicin/vincristine) in patients with SCLC that has progressed following one prior platinum-containing line of therapy.
The study has completed enrollment as of 30 July 2018 and the initial primary end point analysis is anticipated 18 months later.
Conclusion
The results of this pivotal trial will help define the optimal second-line therapy for patients with SCLC following progression after initial platinum-based chemotherapy.
Acknowledgements
The authors thank V Alfaro for review of the manuscript.
Footnotes
Financial & competing interests disclosure
AF Farago received consulting fees from PharmaMar, Bayer, Loxo, Abbvie, Abbvie/Stemcentrx and Genentech. The author further received research funding from PharmaMar, Bayer, Loxo, AbbVie, AbbVie/Stemcentrx, Merck, BMS, AstraZeneca and Novartis. BJ Drapkin received research funding from AstraZeneca, Novartis and AbbVie. JAL-Vilarino de Ramos, CM Golmarini, R Nunez, C Kahatt are employees of PharmaMar. L Paz-Ares gave scientific advice to Astra-Zeneca, BMS, MSD, Roche, Incyte, Takeda, Lilly, Novartis, Pfizer, Pharmamar, Amgen and Genomica. The manuscript arose, in whole or in part, from direct costs funded by NIH (grant number: K12 CA087723). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Pietanza MC, Byers LA, Minna JD, Rudin CM. Small-cell lung cancer: will recent progress lead to improved outcomes? Clin. Cancer Res. 2015;21(10):2244–2255. doi: 10.1158/1078-0432.CCR-14-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalal SI, Lavin P, Lo G, Lebel F, Einhorn L. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a multicenter, adaptive, randomized Phase III study (MATISSE) J. Clin. Oncol. 2017;35(23):2619–2623. doi: 10.1200/JCO.2016.71.7454. [DOI] [PubMed] [Google Scholar]
- 3.Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, Phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J. Clin. Oncol. 2017;35(12):1281–1287. doi: 10.1200/JCO.2016.69.4844. [DOI] [PubMed] [Google Scholar]
- 4.Spigel DR, Townley PM, Waterhouse DM, et al. Randomized Phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J. Clin. Oncol. 2011;29(16):2215–2222. doi: 10.1200/JCO.2010.29.3423. [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J. Clin. Oncol. 2009;27(28):4787–4792. doi: 10.1200/JCO.2009.23.1548. [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 2016;34(31):3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 7.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366(9494):1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 8.Farago AF, Keane FK. Current standards for clinical management of small-cell lung cancer. Transl. Lung Cancer Res. 2018;7(1):69–79. doi: 10.21037/tlcr.2018.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckardt JR, van Powel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J. Clin. Oncol. 2007;25(15):2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 10.von Pawel J, Jotte R, Spigel DR, et al. Randomized Phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J. Clin. Oncol. 2014;32(35):4012–4019. doi: 10.1200/JCO.2013.54.5392. [DOI] [PubMed] [Google Scholar]
- 11.Evans TL, Cho BC, Udud K, et al. Cabazitaxel versus topotecan in patients with small-cell lung cancer with progressive disease during or after first-line platinum-based chemotherapy. J. Thorac. Oncol. 2015;10(8):1221–1228. doi: 10.1097/JTO.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 12.Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J. Thorac. Oncol. 2012;7(5):866–872. doi: 10.1097/JTO.0b013e31824c7f4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Pawel J, Schillar JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin and vincristine for the treatment of recurrent small-cell lung cancer. J. Clin. Oncol. 1999;17(2):658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]; • Randomized trial comparing efficacy of topotecan and cyclophosphamide/doxorubicin/vincristine in relapsed small-cell lung cancer (SCLC).
- 14.Antonia SJ, Lopez-Martin JA, Benderl J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, Phase I/II trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 15.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J. Clin. Oncol. 2017;35(34):3823–3829. doi: 10.1200/JCO.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 16.Rudin CM, Durinck S, Srtawaiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012;44(10):1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012;44(10):1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small-cell lung cancer. Nature. 2015;524(7563):47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drapkin BJ, George J. Genomic and functional fidelity of small-cell lung cancer patient-derived xenografts. Cancer Discov. 2018;8(5):600–615. doi: 10.1158/2159-8290.CD-17-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen CL, Kwiatkowski N, Abrahan BJ, et al. Targeting transcriptional addictions in small-cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell. 2014;26(6):909–922. doi: 10.1016/j.ccell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Preclinical study demonstrating transcriptional dependence of SCLC.
- 21.Leal JF, Martínez-Díez M, García-Hernández V, et al. PM01183: a new DNA minor groove covalent binder with potent in vitro and in vivo antitumour activity. Br. J. Pharmacol. 2010;161(5):1099–1110. doi: 10.1111/j.1476-5381.2010.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santamaria Nunez G, Robles CM, Giraudon C, et al. Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol. Cancer Ther. 2016;15(10):2399–2412. doi: 10.1158/1535-7163.MCT-16-0172. [DOI] [PubMed] [Google Scholar]; • Mechanism of lurbinectedin antitumor activity.
- 23.Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Germano G, Frapolli R, Simone M, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70(6):2235–2244. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 25.Allavena P, Signorelli M, Chieppa M, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65(7):2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 26.Guillen MJ, Cataluna O, Palomares M, et al. AACR Annual Meeting. Philadelphia, PA, USA: 2015. Combination of PM1183 with doxorubicin induces a synergistic antitumor activity in SCLC tumor xenografts. Abstract 2524. [Google Scholar]
- 27.Elez ME, Tabernero J, Geary D, et al. First-in-human Phase I study of lurbinectedin (PM01183) in patients with advanced solid tumors. Clin. Cancer Res. 2014;20(8):2205–2214. doi: 10.1158/1078-0432.CCR-13-1880. [DOI] [PubMed] [Google Scholar]; • The Phase I study reporting safety and preliminary efficacy of lurbinectedin in patients with advanced solid tumors.
- 28.Calvo E, Moreno V, Flynn M, et al. Antitumor activity of lurbinectedin (PM01183) and doxorubicin in relapsed small-cell lung cancer: results from a Phase I study. Ann. Oncol. 2017;28(10):2559–2566. doi: 10.1093/annonc/mdx357. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The Phase I study reporting safety and efficacy of lurbinectedin in patients with relapsed SCLC.
- 29.Olmedo Garcia ME, Forster M, Calvo E, et al. Activity of lurbinectedin as single agent and in combination in patients with advanced small-cell lung cancer (SCLC) Ann. Oncol. 2017;28(Suppl. 5) [Google Scholar]; •• Safety and efficacy of lurbinectedin monotherapy and in combination with doxorubicin or paclitaxel, presented at the ESMO annual meeting 2017.
- 30.Trigo JM, Leary A, Besse B, et al. Efficacy and safety of lurbinectedin (PM1183) in small-cell lung cancer (SCLC): results from a Phase II study. J. Clin. Oncol. 2018;36(Suppl.) [Google Scholar]; •• Phase II safety and efficacy data of single agent lurbinectedin in SCLC, presented at the ASCO annual meeting 2018.