Abstract
Two new mexicanolide-type limonoids, carapanolides T–U (1–2), and three new phragmalin-type limonoids, carapanolides V–X (3–5), were isolated from the seeds of Carapa guianensis (andiroba). Their structures were determined on the basis of 1D- and 2D-NMR spectroscopy.
Keywords: Carapa guianensis, Meliaceae, andiroba, seeds, limonoid, mexicanolide, phragmalin, NO production
1. Introduction
Limonoids, a series of structurally-diverse and highly-oxygenated tetranortriterpenes, are mainly found in the Meliaceae family and have been attracting attention from biogenetic and synthetic perspectives [1,2,3]. Carapa guianensis Aublet (Meliaceae), known locally as andiroba, is a tall tropical tree that is widely distributed in the Amazonas State of Brazil, and its wood is extensively used as commercial timber. The towering tree of andiroba grows up to 40 m in height. Extracts from its bark, flowers and seeds have been used for centuries by the Amazonian people and exhibit various repellent [4], analgesic [5], anti-bacterial [6], anti-inflammatory [7], wound healing [8], anti-malarial [9], anti-allergic [10] and anti-plasmoidal [11] activities, in addition to acute and subacute toxicities [12]. Our previous study on the components of the seed oil of Carapa guianasis revealed the structures of two new unusual 9,10-seco-mexicanolide-type limonoids, carapanolides A and B [13], two novel carbon skeletal limonoids, guianolides A and B [14], and carapanolides C–I [15], carapanolides J–L [16] and carapanolides M–S [17]. We herein describe the isolation and structural determination of five novel limonoids, carapanolides T–X (1–5). The structures of 1–5 were determined on the basis of NMR spectroscopy, including 1D and 2D (1H, 1H-COSY, NOESY, HSQC, HMBC) NMR and HRFABMS.
2. Results and Discussion
Carapanolide T (1) (Figure 1) was obtained as a colorless amorphous solid, and its molecular formula was established as C31H40O10 ([M + H]+; m/z 573.2704, calcd. for 573.2697) by HRFABMS, implying 12 on the degrees of unsaturation. IR and UV spectra showed the presence of hydroxylgroups at νmax 3462 cm−1, ester groups at νmax 1727 cm−1 and αβ-unsaturated δ-lactone at λmax 230 nm (log ε 3.85). 1H- and 13C-NMR data indicated that eight out of the 12 units of unsaturation came from three carbon-carbon double bonds and three ester carbonyls, including a lactone carbonyl and ketone.
Figure 1.
Chemical structures of Compounds 1–5.
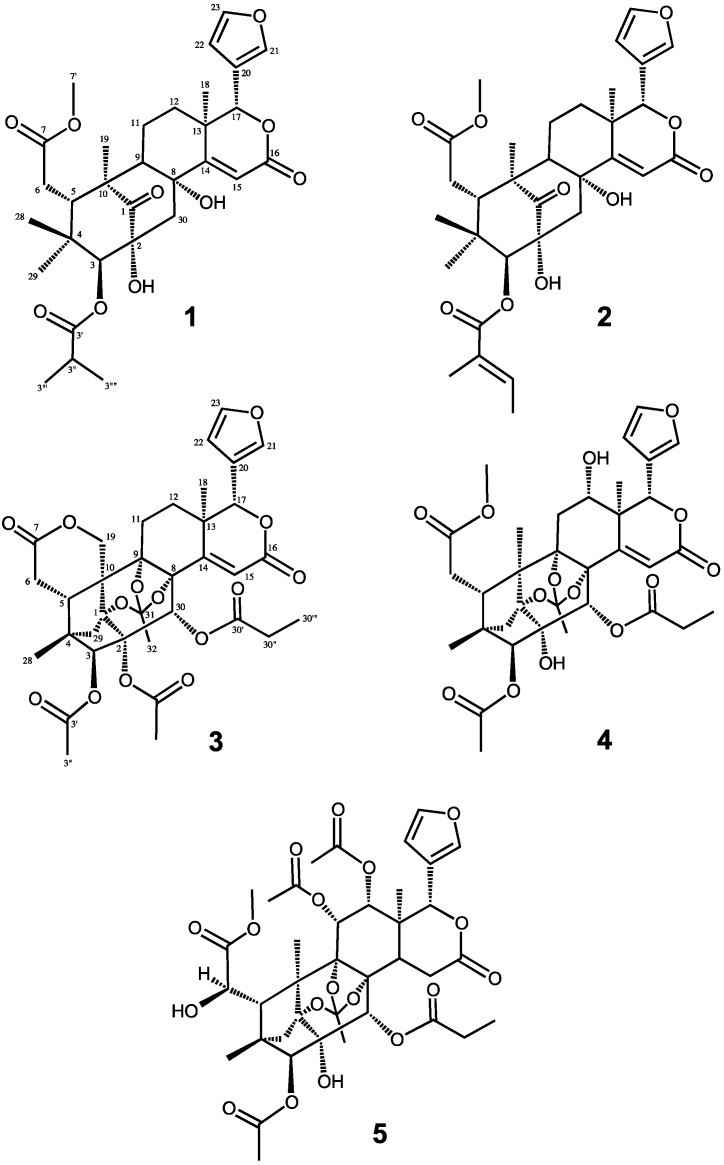
Therefore, the remaining degrees of unsaturation required 1 to be pentacyclic. The 1H- and 13C-NMR spectra of 1 (Table 1) indicated the presence of four tertiary methyls (δH 0.69, 0.86, 1.23, 1.27 (each s)), a 2-methyl propanoyl (δH 1.25 and 1.27 (each 3H, d), 2.71 (1H, sept); δC 19.1 and 19.2 (each q), 34.3 (d), 176.2 (s)), methyl ester (δH 3.71 (s); δC 52.2 (q), 173.6 (s)), four methylenes (δC 20.7 (t), 32.9 (t), 33.7 (t), 45.0 (t)), four sp3 methines, including two oxymethines (δH 4.80 (s), 5.16 (s)), a furan ring (δH 6.49 (dd), 7.44 (t), and 7.51 (brs)), an α,β-unsaturated δ-lactone (δH 5.16 (s), 6.18 (s); δC 78.8 (d), 116.3 (d), 167.5 (s)), five sp3 quaternary carbons, including two oxycarbons (δC 72.8 (s), 76.7 (s)), and a ketone (δC 216.8 (s)). An analysis of the 1H-1H COSY spectrum of 1 revealed the partial structures shown in bold face in Figure 2.
Table 1.
1H- (600 MHz) and 13C- (150 MHz) NMR spectroscopic data of compounds 1 and 2.
| Position | 1 | 2 | |||||
|---|---|---|---|---|---|---|---|
| 1H a (J, Hz) | 13C b | 1H a (J, Hz) | 13C b | ||||
| 1 | 216.8 | 216.7 | |||||
| 2 | 76.7 | 77.0 | |||||
| 3 | 4.80 | s | 85.5 | 4.88 | s | 85.8 | |
| 4 | 39.7 | 40.0 | |||||
| 5 | 3.36 | dd (9.4, 1.5) | 41.8 | 3.39 | dd (7.9, 1.1) | 41.8 | |
| 6 | α | 2.34 | dd (17.3, 1.5) | 32.9 | 2.37 | m | 32.9 |
| β | 2.38 | dd (17.3, 9.4) | 2.34 | m | |||
| 7 | 173.6 | 173.6 | |||||
| 8 | 72.8 | 72.9 | |||||
| 9 | 1.85 | dd (13.3, 6.0) | 60.2 | 1.87 | t (5.6) | 60.1 | |
| 10 | 47.9 | 47.8 | |||||
| 11 | α | 1.48 | m | 20.7 | 1.67 | m | 20.7 |
| β | 1.52 | m | 1.53 | m | |||
| 12 | α | 1.28 | m | 33.7 | 1.34 | m | 33.8 |
| β | 2.00 | ddd (14.1, 6.8, 3.6) | 2.02 | m | |||
| 13 | 38.5 | 38.5 | |||||
| 14 | 164.7 | 164.6 | |||||
| 15 | 6.18 | s | 116.3 | 6.16 | s | 116.3 | |
| 16 | 167.5 | 167.6 | |||||
| 17 | 5.16 | s | 78.8 | 5.18 | s | 79.8 | |
| 18 | 1.27 | s | 23.2 | 1.28 | s | 23.2 | |
| 19 | 1.23 | s | 18.3 | 1.24 | s | 18.4 | |
| 20 | 119.8 | 119.8 | |||||
| 21 | 7.51 | brs | 141.7 | 7.51 | br s | 141.7 | |
| 22 | 6.49 | dd (2.1, 0.9) | 110.4 | 6.49 | m | 110.4 | |
| 23 | 7.44 | t (2.1) | 143.1 | 7.44 | t (1.4) | 143.1 | |
| 28 | 0.69 | s | 22.3 | 0.70 | s | 22.4 | |
| 29 | 0.86 | s | 22.6 | 0.88 | s | 22.6 | |
| 30 | α | 2.51 | d (16.0) | 45.0 | 2.52 | dd (14.9, 1.2) | 45.0 |
| β | 3.55 | d (16.0) | 3.58 | d (14.9) | |||
| 3′ | 176.2 | 167.2 | |||||
| 3′′ | 2.71 | sept (6.7) | 34.3 | 128.1 | |||
| 3′′′ | 1.25 | d (6.7) | 19.1 | 6.96 | q (7.1) | 138.8 | |
| 3′′′′ | 1.27 | d (6,7) | 19.2 | 1.88 | d (7.1) | 14.7 | |
| 3′′′′′ | 1.92 | s | 12.4 | ||||
| 7' | 3.71 | s | 52.2 | 3.70 | s | 52.1 | |
| 2-OH | 4.05 | s | |||||
| 8-OH | 2.81 | s | |||||
Figure 2.
Key HMBC, COSY and NOESY correlations for carapanolide T (1).
In the HMBC spectrum (Figure 2), cross-peaks were observed from Me-18 (δH 1.27 (s)) to C-12, C-13, C-14 (δC 164.7 (s)) and C-17 (δC 78.8 (d)), from Me-19 (δH 1.23 (s)) to C-1 (δC 216.8 (s)), C-5, C-9, and C-10, from H-5 (δH 3.36 (dd)) to C-4, C-6, C-7 (δC 173.6 (s)) and C-10, from H2-30 (δH 2.51 and 3.55 (each d)) to C-1, C-2 (δC 76.7 (s)), C-3 (δC 85.5 (d)), C-8 (δC 72.8 (s)) and C-9 and from H-15 (δH 6.18 (s)) to C-8, C-13, C-14 (δC 164.7 (s)) and C-17. In the 1H-1H COSY spectrum, (H-5–H2-6; H-9–H2-11–H2-12; H-22–H-23; H-3′′–H-3′′′ and H-3′′′′) revealed the partial structure shown in Figure 2. Significant NOE interactions (Figure 2) were observed from H-3 (δH 4.80 (s))/Me-28, Me-29; H-5 (δH 3.36 (dd))/H-11β, Me-28, H-30β; 2-OH (δH 4.05 (s))/H-12α, Me-19; 8-OH (δH 2.81 (s))/H-9, H-12α, Me-18; H-17 (δH 5.16 (s))/H-5, H-11β, H-12β and H-30β, which indicated the α-orientation of H-3, H-9, Me-18, Me-19, 2-OH and 8-OH and the β-orientation of H-5 and H-17. Therefore, the relative structure of 1 was established as shown in Figure 1.
Carapanolide U (2) was isolated as colorless needles and shown to have the molecular formula C32H40O10 (m/z: 585.2701 [M + H]+ (calcd for 585.2700) by HRFABMS. IR and UV spectra revealed the presence of hydroxy and ester groups and an αβ-unsaturated δ-lactone at νmax 3537, 1748 and 1719 cm−1, and λmax at 230 nm (log ε 4.11). The 1H- and 13C-NMR spectra of 2 (Table 1) were very similar to those of 1, except for the absence of the 2-methylpropanoyl group at C-3 and the presence of the tigroyl group at C-3. The relative structure of 2 was determined as shown in Figure 1.
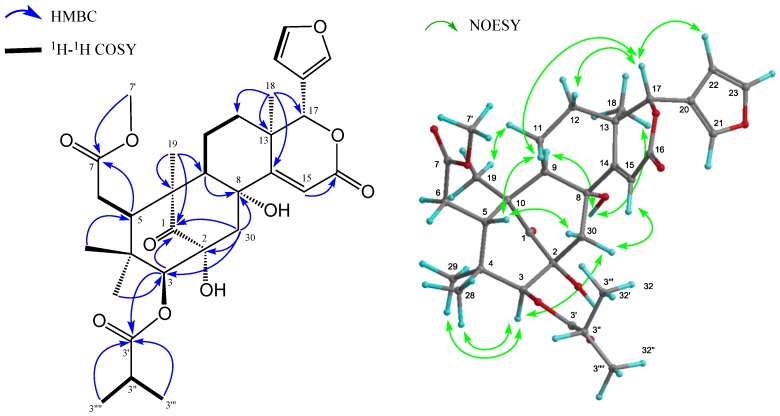
Carapanolide L (3) was obtained as colorless crystals, and its molecular formula was established as C35H38O14 ([M + H]+; m/z 683.2335, calcd. for 683.2340) by HRFABMS, implying 17 on the index of hydrogen deficiency. IR and UV spectra revealed the presence of ester groups and an αβ-unsaturated δ-lactone at νmax 1748 and 1719 cm−1 and λmax at 226 nm (log ε 3.73). 1H- and 13C-NMR data indicated that eight out of the 17 units of unsaturation came from three carbon-carbon double bonds and five ester carbonyls, including two lactone carbonyls. Therefore, the remaining degrees of unsaturation required 3 to be non-acyclic. The 1H- and 13C-NMR spectra of 3 (Table 2) indicated the presence of two tertiary methyls (δH 1.02, 1.14 (each s)), two acetyls (δH 2.04 (s), 2.17 (s); δC 20.8 (q), 21.8 (q), 169.1 (s), 170.1 (s)), an n-propanoyl group (δH 1.07 (3H, t), 2.25 (1H, m), 2.29 (1H, m); δC 8.5 (q), 27.4 (t), 173.3 (s)), an orthoacetyl group (δH 1.68 (3H, s); δC 20.9 (q), 120.1 (s)), four methylenes, including an oxymethylene (δH 4.34 and 4.86 (each 1H, d), four sp3 methines, including three oxymethines (δH 5.10 (s), 5.27 (s) and 5.78 (s)), a furan ring (δH 6.44 (dd), .44 (t) and 7.52 (brs)), seven sp3 quaternary carbons, including four oxycarbons (δC 82.6, 83.9, 84.2, 84.6 (each s)), and two lactone carbonyl groups (δC 163.0 and 171.0 (each s)). An analysis of the 1H-1H COSY spectrum of 3 revealed the partial structures shown in bold face in Figure 3. In the HMBC spectrum (Figure 3), cross-peaks were observed from Me-18 (δH 1.14 (s)) to C-12, C-13, C-14 (δC 159.6) and C-17 (δC 80.4), from Me-28 (δH 1.02 (s)) to C-3 (δC 81.3), C-4, C-5 and C-29, from H-30 (δH 5.78 (s)) to C-1 (δC 84.6 (s)), C-2 (δC 84.2 (s)), C-3, C-8 (δC 82.6 (s)) and C-9 (δC 83.9 (s)) and from H-15 (δH 6.05 (s)) to C-8, C-13, C-14 and C-16 (δC 163.0 (s)). Therefore, the planar structure of 3 was established as phragmalin-1,8,9-orthoacetate [13], and the positions of the two acetyls and an n-propyl group were located at C-2, C-3 and C-30, respectively, by detailed 1H-1H COSY and HMBC correlations (Figure 3). In the NOESY spectrum, significant NOEs (Figure 3) were observed between H-3 and H-29 pro-S, H-30 and Me-28, between H-5β (δH 2.72 (dd)) and H-12β, Me-28 and H-30 between H-15 (δH 6.05 (s)) and H-17β and H-30, between H-17β (δH 5.10 (s)) and H-12β, H-15, H-22 and H-30β and between Me-18 (δH 1.14 (s)) and H-11α, H-12α and Me-32. Therefore, the relative structure of 3 was established as shown in Figure 1.
Table 2.
1H- (600 MHz) and 13C- (150 MHz) NMR spectroscopic data of compounds 3–5.
| Position | 3 | 4 | 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1H a (J, Hz) | 13C b | 1H a (J, Hz) | 13C b | 1H a (J, Hz) a | δC b | |||||
| 1 | 84.6 | 84.2 | 85.1 | |||||||
| 2 | 84.2 | 83.5 | 79.6 | |||||||
| 3 | 5.27 | s | 81.3 | 5.22 | s | 85.0 | 4.59 | s | 83.7 | |
| 4 | 46.4 | 44.6 | 45.5 | |||||||
| 5 | 2.72 | dd (4.7, 3.2) | 33.8 | 2.11 | m | 39.9 | 3.30 | brs | 40.1 | |
| 6 | α | 2.64 | dd (17.0, 3.2) | 68.6 | 2.34 | m | 33.7 | 5.98 | brs | 71.5 |
| β | 2.59 | dd (17.0, 4.7) | 2.36 | m | ||||||
| 7 | 171.0 | 173.9 | 169.4 | |||||||
| 8 | 82.6 | 83.7 | 84.8 | |||||||
| 9 | 83.9 | 86.1 | 86.0 | |||||||
| 10 | 46.9 | 48.1 | 46.2 | |||||||
| 11 | α | 2.19 | m | 25.6 | 1.97 | m | 34.6 | 4.49 | d (2.3) | 69.4 |
| β | 2.33 | m | 2.21 | dd (14.7, 4.1) | ||||||
| 12 | α | 1.50 | m | 26.5 | 3.86 | dd (13.5, 4.1) | 66.6 | 4.48 | d (2.3) | 71.7 |
| β | 1.64 | m | ||||||||
| 13 | 37.6 | 44.8 | 38.4 | |||||||
| 14 | 159.6 | 153.8 | 2.79 | dd (10.4, 0.6) | 42.3 | |||||
| 15 | 6.05 | s | 121.0 | 6.62 | s | 123.7 | 2.90 | dd (18.7, 10.4) | 26.9 | |
| 3.22 | dd (18.7, 0.6) | |||||||||
| 16 | 163.0 | 163.5 | 170.8 | |||||||
| 17 | 5.10 | s | 80.4 | 5.90 | s | 78.8 | 5.98 | s | 76.9 | |
| 18 | 1.14 | s | 18.7 | 1.48 | s | 13.0 | 1.43 | s | 15.8 | |
| 19 | α | 4.86 | d (14.0) | 31.4 | 1.32 | s | 15.5 | 1.23 | s | 13.8 |
| β | 4.34 | d (14.0) | ||||||||
| 20 | 119.4 | 121.4 | 120.9 | |||||||
| 21 | 7.52 | br s | 141.4 | 7.53 | br s | 144.8 | 7.48 | brs | 141.0 | |
| 22 | 6.44 | dd (1.7, 0.6) | 109.7 | 6.61 | dd (1.7, 0.9) | 109.6 | 6.46 | dd (1.8, 1.5) | 110.9 | |
| 23 | 7.44 | t (1.7) | 143.3 | 7.64 | t (1.7) | 142.4 | 7.00 | t (1.8) | 143.1 | |
| 28 | 1.02 | s | 14.2 | 0.74 | s | 14.5 | 1.10 | s | 15.4 | |
| 29 | pro-R | 1.78 | d (11.6) | 39.2 | 1.72 | d (11.5) | 39.8 | 1.81 | d (10.9)) | 39.9 |
| pro-S | 2.38 | d (11.6) | 1.96 | d (11.5) | 2.06 | d (10.9) | ||||
| 30 | 5.78 | s | 68.1 | 5.35 | s | 74.3 | 6.01 | s | 69.8 | |
| 31 | 120.1 | 119.7 | 119.3 | |||||||
| 32 | 1.68 | s | 20.9 | 1.70 | s | 16.5 | 1.76 | s | 21.1 | |
| 2′ | 170.1 | |||||||||
| 2′′ | 2.17 | s | 21.8 | |||||||
| 3′ | 169.1 | 169.1 | 169.6 | |||||||
| 3′′ | 2.04 | s | 20.8 | 2.09 | s | 21.7 | 2.18 | s | 21.2 | |
| 7′ | 3.71 | s | 52.3 | 3.69 | s | 53.1 | ||||
| 11′ | 169.7 | |||||||||
| 11′′ | 2.22 | s | 21.4 | |||||||
| 12′ | 169.6 | |||||||||
| 12′′ | 1.70 | s | 20.1 | |||||||
| 30′ | 173.3 | 173.8 | 172.5 | |||||||
| 30′′ | A | 2.25 | m | 27.4 | 2.45 | m | 28.1 | 2.38 | dq (11.2, 7.3) | 27.9 |
| B | 2.29 | m | 2.38 | dq (11.2, 7.3) | ||||||
| 30′′′ | 1.07 | t (7.6) | 8.5 | 1.16 | t (7.7) | 8.9 | 1.09 | t (7.3) | 8.6 | |
a Measured at 600 MHz in CDCl3. b Measured at 150 MHz in CDCl3.
Figure 3.
Key HMBC, COSY and NOESY correlations for carapanolide V (3).
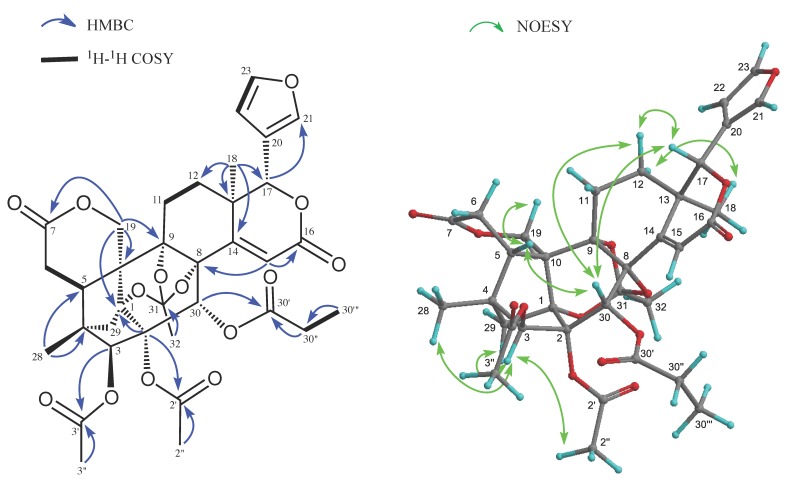
Carapanolide W (4) was obtained as colorless crystals, and its molecular formula was established as C34H40O14 ([M + H]+; m/z 673.2492, calcd. for 673.2496) by HRFABMS. IR and UV spectra revealed the presence of hydroxy groups and ester groups and an αβ-unsaturated δ-lactone at νmax 3657, 1728 and 1698 cm−1 and λmax at 230 nm (log ε 3.73). The 1H- and 13C-NMR spectra of 4 (Table 2) indicated the presence of three tertiary methyl groups (δH 0.74, 1.32, 1.48 (each s)), an acetyl (δH 2.09 (s); δC 21.7 (q), 169.1 (s)), an n-propanoyl group (δH 1.16 (3H, t), 2.45 (2H, m); δC 8.9 (q), 28.1 (t), 173.8 (s)), methyl ester (δH 3.71 (3H, s); δC 52.3 (q), 173.9 (s)), three methylene groups, five sp3 methine groups, including four oxymethine groups (δH 3.86 (dd), 5.22 (s), 5.35 (s), 5.90 (s)), a furan ring (δH 6.61 (dd), 7.53 (br s), 7.64 (t)), seven sp3 quaternary carbons, including four oxycarbons (δC 83.5 (s), 83.7 (s), 84.2 (s), 86.1 (s)), three ester carbonyls (δC 169.1 (s), 173.8 (s), 173.9 (s)) and an αβ-unsaturated δ lactone (δH 6.62 (s), δC 123.7 (d), 163.5 (s)). Cross-peaks were observed in the HMBC spectrum, from Me-18 (δH 1.48 (s)) to C-12 (δC 66.6 (d)), C-13, C-14 (δC 153.8 (s)) and C-17 (δC 78.8 (d)), from Me-19 (δH 1.32 (s)) to C-1 (δC 84.2 (s)), C-5, C-9 (δC 86.1(s)) and C-10, from H-15 (δH 6.62 (s)) to C-8 (δC 83.7 (s)), C-13, C-14 and C-16 (δC 163.5 (s)) and from H-30 (δH 5.35 (s)) to C-1 (δC 84.2 (s)), C-2 (δC 83.5 (s)), C-3 (δC 85.0 (d)), C-8 and C-9. The singlet oxymethine proton at δH 5.22 was assigned to C-3 through HMBC correlations to C-1, C-2, C-5, C-28, C-29, C-30 (δC 74.3 (d)) and C-3′ (δC 169.1 (s)), while another singlet proton at δH 5.35, showing HMBC correlations to C-1, C-2, C-8, C-9, C-14 (δC 153.8 (s)) and C-30′ (δC 173.8 (s)), was assigned to C-30. An oxymethine proton at δH 5.22 (dd) was assigned to C-12 through HMBC correlations to C-9, C-11, C-13, C-14, C-17 (δC 78.8 (d)) and C-18. In an analysis of the 1H-1H COSY spectrum of 4, H-5–H2-6; H2-11–H-12; H-22–H-23; H-30′′–H-30′′′) revealed the partial structure. The relative configuration of 4 was established on the basis of NOE interactions. Significant NOE interactions were observed from H-5 (δH 2.11 (m))/H-11β, H-30, Me-28; H-30 (δH 5.35 (s))/H-3, H-5, H-15; H-17 (δH 5.90 (s))/H-5, H-12β, H-15, H-21, H-22, which indicated the α-orientation of H-3, Me-18 and Me-19 and the β-orientation of H-5, H-12, H-17 and H-30. Therefore, the relative structure of 4 was established as shown in Figure 1.
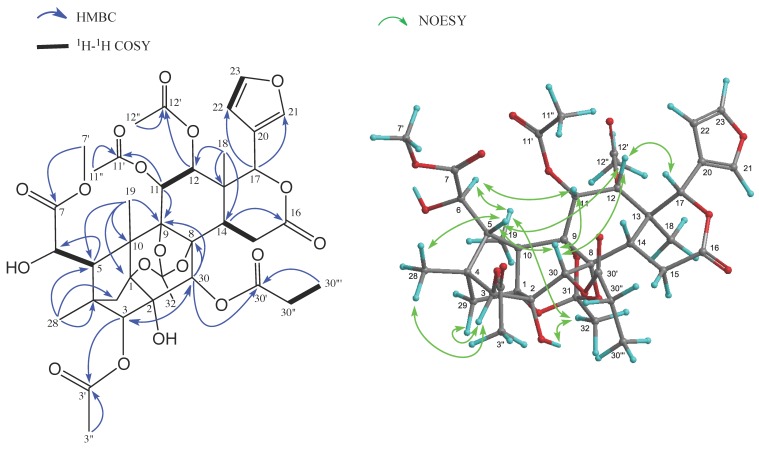
Carapanolide X (5) was isolated as a colorless amorphous solid and had the molecular formula C38H46O18 ([M + H]+; m/z 791.2765, calcd. for 791.2763) as determined by HRFABMS. The IR spectrum showed the presence of a hydroxyl at νmax 3352 cm−1 and ester groups at νmax 1742 cm−1. 1H- and 13C-NMR spectra (Table 2) indicated the presence of three methyls (δH 1.10, 1.23, 1.43 (each 3H, s)), three acetyl groups (δH 1.70, 2.18, 2.22 (each 3H, s)), a propanoyl group (δH 1.09 (3H, t), 2.38 (2H, dq), δC 172.5 (s)), a methoxycarbonyl group ((δH 3.69 (3H, s), δC 53.1 (q), 169.4 (s)), sp3 methylene ((δC 26.9 (t)), δ-lactone (δH 5.98 (1H, s), δC 76.9 (d), 170.8 (s)), a tertiary hydroxyl group (δC 79.6 (s)), seven sp3 methines, including five oxymethines (δH 4.48 (d), 4.49 (d), 4.59 (s), 5.98 (s)), seven sp3 quaternary carbons, including four oxycarbons (δC 79.6 (s), 84.8 (s), 85.1 (s), 86.0 (s)), and a furan ring (δH 6.46 (dd), 7.00 (t), 7.48 (brs)). In the HMBC spectrum, cross-peaks were observed between Me-18 (δH 1.43 (s)) and C-12 (δC 71.7 (d)), C-13, C-14 and C-17 (δC 76.9 (d)), between Me-19 (δH 1.23 (s)) and C-1 (δC 85.1 (s)), C-5, C-9 (δC 86.0 (s)) and C-10, between Me-28 (δH 1.10 (s)) and C-3 (δC 83.7 (d)), C-4, C-5 and C-29 and between H-3 (δH 4.59 (s)) and C-1 and C-2 (δC 79.6 (s)), C-4, C-5, C-28, C-29, C-30 (δC 69.8 (d)) and C-3′ (δC 169.6 (s)), between H-5 (δH 3.30 (brs)) and C-1, C-3, C-4, C-6 (δC 71.5 (d)), C-7 (δC 169.4 (s)), C-10, C-19, C-28 and C-29, between H-6 (δH 5.98 (brs)) and C-4, C-5, C-7 and C-10 and between H-30 (δH 6.01 (s)) and C-1, C-2, C-3, C-8 (δC 84.8 (s)), C-9 and C-30′ (δC 172.5 (s)). In the 1H-1H COSY spectrum, (H-5–H-6; H-11–H-12; H-14–H2-15; H-22–H-23 and H-30′′–H-30′′′) was observed. Therefore, the positions of two hydroxyls, a propanoyl and a methoxycarbonyl were located at C-2, C-6, C-30 and C-7, while three acetyl groups were located at C-3, C-11 and C-12, respectively (Figure 4). In the NOESY experiments, cross peaks (Figure 4) were observed between H-3 (δH 4.59 (s)) and H-29 pro-S, H-30 and Me-28, between H-5 (δH 3.30 (brs)) and Me-28 and H-30, between H-6 (δH 5.98 (brs)) and H-5β and H-11, between H-11 (δH 4.49 (d)) and H-5β and H-12, between H-12 (δH 4.48 (d)) and H-5β, H-11 and H-17β, between H-30β (δH 6.01 (s)) and H-11 and H-12 and between Me-19 and H-6 and Me-32. Therefore, the propanoyl group at C-30 and acetoxy groups at C-11 and C-12 were all α, while the acetoxy group at C-3 had a β orientation. The configuration of C-6 was presumed to be R, which was the same as that of carapanolide N [17].
Figure 4.
Key HMBC, COSY and NOESY correlations for carapanolide X (5).
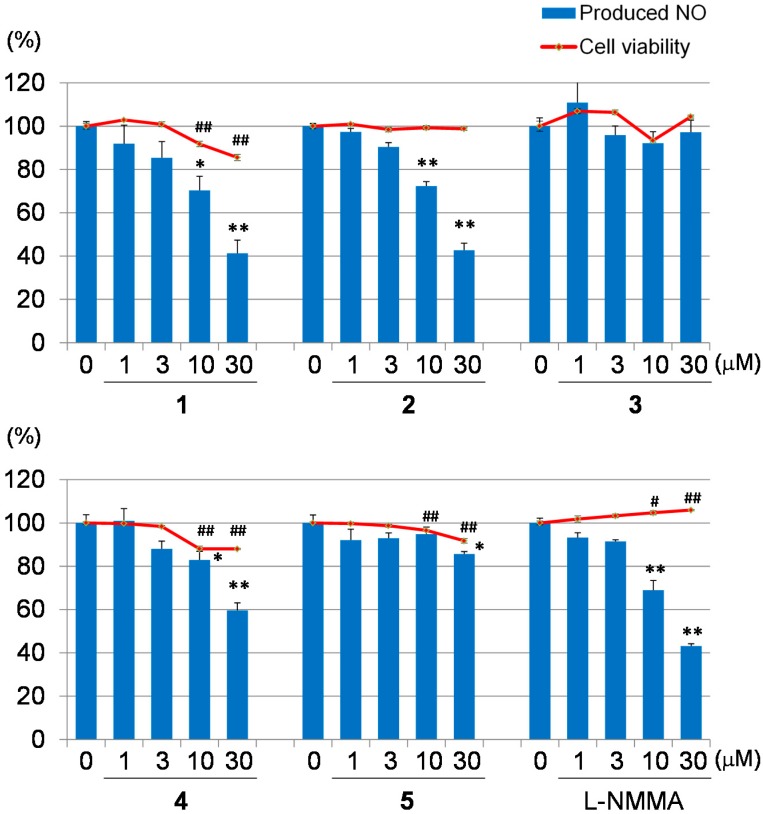
Macrophages may be a potential therapeutic target for inflammatory diseases [18]. Activated macrophages release pro-inflammatory mediators, such as NO, reactive oxygen, interleukin-1 beta, tumor necrosis factor-alpha and other inflammatory mediators, which play important roles in biological defense. However, the overexpression of these mediators had been implicated in diseases, such as osteoarthritis, rheumatoid arthritis and diabetes, because the increased production of pro-inflammatory mediators has been shown to induce severe or chronic inflammation [18]. In the present study, four limonoids and NG-monomethyl-l-arginine acetate (l-NMMA), an inducible nitric oxide synthase (iNOS) inhibitor, were evaluated for their inhibitory effects on NO production in LPS-stimulated RAW264.7 cells (Figure 5). To determine safe concentrations, the cytotoxicities of these limonoids against RAW 264.7 were assessed by the MTT assay. In the NO inhibitory assay, Compounds 1 and 2 exhibited comparable NO inhibitory activities (IC50 1: 22.0 μM; 2: 23.3 μM) to l-NMMA (IC50 23.9 μM). Of these, 2 did not show cytotoxicities at 1–30 μM. Compound 1 exhibited low cytotoxicity at 30 μM, but not at the effective concentration, namely 10 μM. Compounds 3–5 did not exhibit inhibitory effects on macrophage activation (IC50 >30 μM). These results suggested that Compounds 1 and 2 have potential as anti-inflammatory disease agents. In a previous study, we revealed inhibitory activities on NO production of a phragmalin-type limonoid, such as carapanolide J (IC50 37.4 μM) [16], and gedunin-type limonoids, such as 7-deacetoxy-7-oxogedunin (IC50 12.8 μM), 6α-acetoxygedunin (IC50 7.9 μM), 6α-hydroxygedunin (IC50 19.1 μM), 6α-acetoxy-7α-deacetoxy-7α-hydroxygedunin (IC50 9.4 μM), gedunin (IC50 4.6 μM) and 7-deacetoxy-7-hydroxygedunin (IC50 8.4 μM), [19]. Compounds 1 and 2 exhibited stronger inhibitory activities on NO production than carapanolide J, however weaker than the gedunin-type limonoids. These data suggest that gedunin-type limonoids are more effective for the inhibition of NO production than phragmalin-type ones in general.
Figure 5.
Inhibitory activities on NO production and cytotoxicities of Compounds 1–5 and l-NMMA. Each value represents the mean ± the standard error (S.E.) of four determinations. Significant differences from the vehicle control (0 μM) group shown as * p < 0.05 and ** p < 0.01 in the NO inhibitory assay and # p < 0.05 and ## p < 0.01 in the cytotoxicity assay.
3. Experimental Section
3.1. General Procedures
Melting points were determined on a Yanagimoto micro-melting point apparatus and were uncorrected. Optical rotations were measured using a JASCO DIP-1000 digital polarimeter. IR spectra were recorded using a Perkin-Elmer 1720X FTIR spectrophotometer (Perkin-Elmer Inc., Wellesley, MA, USA). 1H- and 13C-NMR spectra were obtained on an Agilent vnmrs 600 spectrometer (Agilent Technologies, Santa Clara, CA, USA) with standard pulse sequences, operating at 600 and 150 MHz, respectively. CDCl3 was used as the solvent and TMS as the internal standard. FABMS were recorded on a JEOL-7000 mass spectrometer (JEOL, Tokyo, Japan). Column chromatography was performed over silica gel (70–230 mesh, Merck, Darmstadt, Germany), while medium pressure liquid chromatography (MPLC) was conducted with silica gel (230–400 mesh, Merck). HPLC was run on a JASCO PU-1586 instrument (JASCO, Tokyo, Japan) equipped with a differential refractometer (RI 1531). Fractions obtained from column chromatography were monitored by TLC (silica gel 60 F254, Merck).
3.2. Plant Material
The oil of (2.03 kg) Carapa guianensis AUBLET (Meliaceae) was collected in the Amazon, Brazil, in March 2013, and was kindly provided by Mr. Akira Yoshino (who is a representative of the “NGO Green Heart Love Amazon Project”). A voucher specimen (CGS-01-2) was deposited in the Herbarium of the Laboratory of Medicinal Chemistry, Osaka University of Pharmaceutical Sciences.
3.3. Isolation of Compounds 1–5
The seed oil of Carapa guianensis AUBLET (Meliaceae) (2.03 kg) was dissolved in CHCl3, and the CHCl3 solution was subjected to CC (silica gel 14 kg), affording 7 fractions: Fraction A (fraction (Fr.) No. 1–85, 1.512 kg) was eluted with n-hexane-CHCl3 = 1:1; B (Fr. No. 86–179, 229.1 g) was eluted with CHCl3; C (Fr. No. 180–20, 29.3 g) was eluted with CHCl3–EtOAc = 5:1; D (Fr. No. 221–225, 13.2 g) was eluted with CHCl3–EtOAc = 2:1; E (Fr. No. 226–265, 84.5 g) was eluted with CHCl3-EtOAc = 2:1; F (Fr. No. 266–290, 25.3 g) was eluted with EtOAc; G (Fr. No. 291–315, 72.8 g) was eluted with EtOAc:MeOH = 5:1; and H (Fr. No. 316–333, 45.4 g) was eluted with MeOH. Residue D was rechromatographed over a silica gel column (CC) (230–400 mesh, 300 g) eluted with n-hexane–EtOAc (1:1) to give 13 fractions: D1 (Fr. No. 1–35, 1.52 g), D2 (Fr. No. 36–49, 0.81 g), D(3) (Fr. No. 50–88, 0.70 g), D(4) (Fr. No. 89–115, 0.53 g), D(5) (Fr. No. 116–130, 0.60 g), D(6) (Fr. No. 131–140, 0.52 g), D(7) (Fr. No. 141–205, 0.47 g), D(8) (Fr. No. 206–215, 0.51 g), D(9) (Fr. No. 216–220, 0.42 g), D(10) (Fr. No. 221–240, 0.40 g), D(11) (Fr. No. 241–250, 1.11 g) and D(12) (Fr. No. 251–313, 1.36 g). Fraction D(6) was subjected to CC (230–400 mesh, 40 g) eluted with n-hexane–EtOAc (3:1) to give an amorphous solid (24.1 mg) that was separated by HPLC (ODS, 75% MeOH, at 25 °C, flow rate 4.0 mL min−1, UV = 220 nm, column 250 × 20 mm i.d., 5 μm) to give Compounds 2 (6.2 mg) and 3 (1.8 mg). Fraction D(8) was subjected to CC (230–400 mesh, 40 g) eluted with n-hexane-EtOAc (3:1) to give an amorphous solid (34.0 mg), which was subsequently subjected to CC (230–400 mesh, 40 g) eluted with n-hexane–EtOAc (3:1) to give an amorphous solid that was purified by HPLC (ODS, 75% MeOH, at 25 °C, flow rate 4.0 mL min−1, UV = 220 nm, column 250 × 20 mm i.d., 5 μm) to give Compounds 1 (7.50 mg) and 4 (3.8 mg). Fraction D(9) was subjected to CC (230–400 mesh, 30 g) eluted with n-hexane-EtOAc (3:1) to give an amorphous solid (25.5 mg), which was subsequently separated by HPLC (ODS, 70% MeOH, at 25 °C, flow rate 4.0 mL min−1, UV = 220 nm, column 250 × 20 mm i.d., 5 μm) to give Compound 5 (6.3 mg).
3.4. Analytical Data
Compound 1: Colorless crystals; mp 83–85 °C; +16.6° (c 0.1, CHCl3); HRFABMS m/z: 573.2719 [M + H]+ (C31H41O10, calcd for 573.2720); UV (EtOH) λmax nm (log ε): 230 (3.85); IR (KBr) νmax cm−1; 3462 (OH), 2970, 1727 (O-C=O), 1649 (C=C-C=O), 1461; 1H- and 13C-NMR, see Table 1. FABMS m/z (relative intensity (rel. int.)): 573 ([M + H]+, 100), 555 (11), 485 (20).
Compound 2: Colorless crystals; mp 133–136 °C; −18.1° (c 0.1, EtOH); HRFABMS m/z: 585.2701 [M + H]+ (C32H41O10, calcd for 585.2700); UV λmax (EtOH) nm (log ε): 230 (4.11); IR (KBr) νmax cm−1: 3537 (OH), 1748, 1719; 1H- and 13C-NMR, see Table 1. FABMS m/z (rel. int.): 607 (3) [M + Na]+, 585 (6), [M + H]+, 567 (6), 485 (7), 83 (100).
Compound 3: Colorless crystals; mp 181–184 °C; −74° (c 0.4, EtOH); HRFABMS m/z: 683.2335 [M + H]+ (C35H38O14, calcd for 683.2340); UV λmax (EtOH) nm (log ε): 226 (3.73); IR (KBr) νmax cm−1: 1748, 1719; 1H- and 13C-NMR, see Table 1. FABMS m/z (rel. int.): 683 (100) [M + H]+, 641 (5), 586 (7), 507 (7), 95 (21).
Compound 4: Colorless crystals; mp 144–146 °C; +43.3° (c 0.1, CHCl3); HRFABMS m/z: 673.2492 [M + H]+ (C34H41O14, calcd for 673.2496); UV λmax (EtOH) nm (log ε): 230 (3.73); IR (KBr) νmax cm−1: 3657 (OH), 1728, 1698; 1H- and 13C-NMR, see Table 1. FABMS m/z (rel. int.): 695 (6) [M + Na]+, 673 (47) [M + H]+, 613 (10), 599 (100).
Compound 5: Colorless amorphous; −46.8° (c 0.1, CHCl3); HRFABMS m/z: 791.2765 [M + H]+ (C38H47O18, calcd for 791.2763); UV λmax (EtOH) nm (log ε): 208 (1.26), IR (KBr) νmax cm−1: 3352 (OH), 1742 (O-C=O); 1H- and 13C-NMR, see Table 2. FABMS m/z (rel. int.): 791 [M + H]+ (55), 735 (5), 95 (54), 43 (100), 329 (14), 176 (47).
3.5. Determination of RAW264.7 Cell Proliferation
RAW264.7 cell proliferation was examined according to a method reported previously [20] with few modifications. Briefly, RAW264.7 cells (5 × 104 cells in 100 μL) were seeded onto 96-well microplates and incubated for 24 h. D-MEM (100 μL) containing test samples (final concentration of 100, 30, 10 or 3 μM) dissolved in DMSO (final concentration 0.2%) was added. After the cells had been treated for 24 h, the MTT solution was added. After 3 h of incubation, 20% sodium dodecyl sulfate (SDS) in 0.1 M HCl was added to dissolve the formazan produced by the cells. The absorbance of each well was read at 570 nm using a microplate reader. The optical density of vehicle control cells was assumed to be 100%.
3.6. Inhibitory Assay of NO Production
An inhibitory assay of nitric oxide production was performed according to a method reported previously [16] with slight modifications. Briefly, RAW264.7 cells (5 × 104 cells in 100 μL) were seeded onto 96-well microplates and incubated for 24 h. D-MEM (100 μL) containing test samples (final concentration of 100, 30, 10 or 3 μM) dissolved in DMSO (final concentration 0.2%) and LPS (final concentration of 5 μg/mL) were added. After cells had been treated for 24 h, 50 μL of 0.1% N-(1-naphtyl)ethylenediamine in H2O and 50 μL of 1% sulfanilamide in 5% phosphoric acid were added. After being incubated for 30 min, the absorbance of each well was read at 570 nm using a microplate reader. The optical density of vehicle control cells was assumed to be 100%.
4. Conclusions
Two novel mexicanolide-type limonoids, carapanolides T–U (1–2), as well as three novel phragmalin-type limonoids, carapanolides V–X (3–5), were isolated from the seeds of Carapa guianensis (andiroba). Their structures were determined by spectroscopic analyses. Compounds 1 and 2 were mexicanolide-type limonoids that had OH in C-2 and C-8. Compounds 3–5 were phragmalin-type limonoids that were highly oxidized.
In the NO inhibitory assay, Compounds 1 and 2 exhibited similar NO inhibitory activities (IC50 1: 22.0 μM; 2: 23.3 μM) against L-NMMA (IC50 23.9 μM). Compound 1 did not exhibit cytotoxicity at 1–30 μM, while Compound 2 exhibited low cytotoxicity at 30 μM, but not at an effective concentration at 10 μM. These results suggest that Compounds 1 and 2 have potential as anti-inflammatory disease agents.
Acknowledgments
We thank Mihoyo Fujitake (this university) for MS measurements.
Author Contributions
R. Tanaka prepared the manuscript. T. Miyake, S. Ishimoto, N. Ishimatsu and K. Higuchi contributed to the isolation and structure elucidation. K. Minoura conducted NMR measurement. T. Kikuchi performed evaluation of bioactivities. T. Yamada and O. Muraoka supervised whole research project.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Tan Q.G., Luo X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011;111:7437–7522. doi: 10.1021/cr9004023. [DOI] [PubMed] [Google Scholar]
- 2.Fang X., Di Y.T., Hao X.J. The advaces in the limonoid chemistry of the Meliaceae family. Curr. Org. Chem. 2011;15:1363–1391. doi: 10.2174/138527211795378254. [DOI] [Google Scholar]
- 3.Liao S.G., Chen H.D., Yue J.M. Plant orthoesters. Chem. Rev. 2009;109:1092–1140. doi: 10.1021/cr0782832. [DOI] [PubMed] [Google Scholar]
- 4.Prophiro J.S., da Silva Mario A.N., Kanis L.A., da Rocha L.C.B.P., Duque-Luna J.E., da Silva O.S. First report on susceptibility of wild Aedes aegypty (Diptera: Culicidae) using Carapa guianensis (Meliaceae) and Copaifera sp. (Leguminosae) Parasitol. Res. 2012;110:699–705. doi: 10.1007/s00436-011-2545-7. [DOI] [PubMed] [Google Scholar]
- 5.Penido C., Costa K.A., Pennaforte R.J., Costa M.F.S., Pereira J.F.G., Siani A.C., Henriques M.G.M.O. Anti-allergic effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet onallergen-induced vascular permeability and hyperalgesia. Inflamm. Res. 2005;54:295–303. doi: 10.1007/s00011-005-1357-6. [DOI] [PubMed] [Google Scholar]
- 6.Nayak B.S., Kanhai J., Milne D.M., Pereira L.P., Swanston W.H. Experimental Evaluation of Ethanolic Extract of Carapa guianensis L. Leaf for Its Wound Healing Activity Using Three Wound Models. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1093/ecam/nep160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penido C., Conte F.P., Chagas M.S.S., Rodrigue C.A.B., Pereira J.F.G., Henriques M.G.M.O. Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice. Inflamm. Res. 2006;55:457–464. doi: 10.1007/s00011-006-5161-8. [DOI] [PubMed] [Google Scholar]
- 8.Nayak B.S., Kanhai J., Milne D.M., Swanston W.H., Mayers S., Eversley M., Rao A.V. Investigation of the wound healing activity of Carapa guianensis L. (Meliaceae) bark extract in rats using excision, incision, and dead space wound models. J. Med. Food. 2010;13:141–146. doi: 10.1089/jmf.2009.0214. [DOI] [PubMed] [Google Scholar]
- 9.Bickii J., Njifutie N., Foyere J.A., Basco L.K., Ringwald P.J. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae) J. Ethnopharmacol. 2000;69:27–33. doi: 10.1016/S0378-8741(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 10.Ferraris F.K., Rodrigues R., da Silva V.P., Figueiredo R., Penido C., Henriques M.G.M.O. Modulation of T lymphocyte and eosinophil functions in vitro by natural tetranortriterpenoids isolated from Carapa guianensis Aublet. Int. Immunopharmacol. 2011;11 doi: 10.1016/j.intimp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Miranda J.R.N.C., Dolabela M.F., da Silva M.N., Povoa M.M., Maia J.G.S. Antiplasmoidal activity of the andiroba (Carapa guianensis Aublet., Meliaceae) oil and its limonoid-rich fraction. J. Ethnopharmacol. 2012;142:679–683. doi: 10.1016/j.jep.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Costa-Silva H., Lima C.R., Silva E.J.R., Araujo A.V., Fraga M.C.C.R., Ribeiro E., Ribwiro A., Arruda A.C., Lafayette S.S.L., Wanderley J. Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J. Ethnopharmacol. 2008;116:495–500. doi: 10.1016/j.jep.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T., Nagai Y., Mitooka A., Ujike R., Muraoka O., Yamada T., Tanaka R. Carapanolides A and B: Unusual 9,10-seco-mexicanolides having a 2R,9S-oxygen bridge From the seeds of Carapa guianensis. Tetrahedron Lett. 2012;53:6685–6688. doi: 10.1016/j.tetlet.2012.09.108. [DOI] [Google Scholar]
- 14.Inoue T., Matsui Y., Kikuchi T., In Y., Yamada T., Muraoka O., Matsunaga S., Tanaka R. Guianolides A and B, New Carbon Skeletal Limonoids from the seeds of Carapa guianensis. Org. Lett. 2013;15:3018–3021. doi: 10.1021/ol400924u. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T., Matsui Y., Kikuchi T., In Y., Muraoka O., Yamada T., Tanaka R. Carapanolides C–I from the seeds of andiroba (Carapa guianensis, Meliaceae) Fitoterapia. 2014;96:56–64. doi: 10.1016/j.fitote.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Matsui Y., Kikuchi T., Inoue T., Muraoka O., Yamada T., Tanaka R. Carapanolides J–L from the Seeds of Carapa guianensis (Andiroba) and Their Effects on LPS-Activated NO Production. Molecules. 2014;19:17130–17140. doi: 10.3390/molecules191117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue T., Matsui Y., Kikuchi T., Yamada T., In Y., Muraoka O., Sakai C., Ninomiya K., Morikawa T., Tanaka R. Carapanolides M–S from seeds of andiroba (Carapa guianensis, Meliaceae) and triglyceride metabolism-promoting activity in high glucose-pretreated HepG2 cells. Tetrahedron. 2015;71:2753–2760. doi: 10.1016/j.tet.2015.03.017. [DOI] [Google Scholar]
- 18.Zhang Y., Liu C., Dong B., Ma X., Hou L., Cao X., Wang C. Anti-inflammatory Activity and Mechanism of Surfactin in Lipopolysaccharide-Activated Macrophages. Inflammation. 2015;38:756–764. doi: 10.1007/s10753-014-9986-y. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto A., Tanaka Y., Yamada T., Kikuchi T., Muraoka O., Ninomiya K., Morikawa T., Tanaka R. Andirolides W-Y from the Flower Oil of Andiroba (Carapa guianensis, Meliaceae) Fitoterapia. 2015;100:81–87. doi: 10.1016/j.fitote.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Yamada T., Muroga Y., Jinno M., Kajimoto T., Usami Y., Numata A., Tanaka R. New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities. Bioorg. Med. Chem. 2011;19:4106–4113. doi: 10.1016/j.bmc.2011.05.008. [DOI] [PubMed] [Google Scholar]