Abstract
Objective
We investigated whether an automatic retinal image analysis (ARIA) incorporating machine learning approach can identify asymptomatic older adults harboring high burden of white matter hyperintensities (WMH) using MRI as gold standard.
Methods
In this cross‐sectional study, we evaluated 180 community‐dwelling, stroke‐, and dementia‐free healthy subjects and performed ARIA by acquiring a nonmydriatic retinal fundus image. The primary outcome was the diagnostic performance of ARIA in detecting significant WMH on MRI brain, defined as age‐related white matter changes (ARWMC) grade ≥2. We analyzed both clinical variables and retinal characteristics using logistic regression analysis. We developed a machine learning network model with ARIA to estimate WMH and its classification.
Results
All 180 subjects completed MRI and ARIA. The mean age was 70.3 ± 4.5 years, 70 (39%) were male. Risk factor profiles were: 106 (59%) hypertension, 31 (17%) diabetes, and 47 (26%) hyperlipidemia. Severe WMH (global ARWMC grade ≥2) was found in 56 (31%) subjects. The performance for detecting severe WMH with sensitivity (SN) 0.929 (95% CI from 0.819 to 0.977) and specificity (SP) 0.984 (95% CI from 0.937 to 0.997) was excellent. There was a good correlation between WMH volume (log‐transformed) obtained from MRI versus those estimated from retinal images using ARIA with a correlation coefficient of 0.897 (95% CI from 0.864 to 0.922).
Interpretation
We developed a robust algorithm to automatically evaluate retinal fundus image that can identify subjects with high WMH burden. Further community‐based prospective studies should be performed for early screening of population at risk of cerebral small vessel disease.
Introduction
Stroke and dementia are major diseases in the 21st century. Strategies to maintain and improve brain health can alleviate global healthcare burden. Primary prevention has the greatest impact on society and healthcare.1 To date, however, there is a lack of simple, reliable, and reproducible tool to monitor for brain resilience and cognitive reserve, and to screen for asymptomatic subjects at risk of developing stroke and dementia.2
Cerebral small vessel disease (SVD) is a disease of the small arteries, arterioles, venules, and capillaries of the brain. SVD is a major cause of stroke and cognitive impairment, and often coexists with Alzheimer pathology in mixed‐dementia.3 It is now recognized that white matter hyperintensities (WMH) is an important and inevitable substrate for the development of symptoms in SVD.2 In particular, people with SVD and high burden of WMH are at high risk of developing incident stroke and dementia.2 SVD can further compromise health by an array of disabling symptoms including parkinsonism, gait disturbance, urinary incontinence, mood, and behavioral changes. Over the past decade, community‐based studies using MRI brain to detect WMH and SVD predicted risks of stroke, cognitive decline, dementia, and death.4 Potential screening tools such as detecting white matter hyperintensities (WMH) by MRI, tau or amyloid deposition in brain by positron emission tomography are promising but often limited by cost, expertise, and availability for population‐based application. Moreover, lack of effective dementia treatment options had prompted the identification of biomarker to identify high‐risk individuals for early intervention and recruitment to clinical trials.1
Using retinal imaging to study brain disease, including presymptomatic stroke and dementia, has been increasingly studied in recent years.5, 6, 7 As an extension of the brain, retinal microvasculature features reflect changes of cerebral microvasculature, including vessel caliber, tortuosity, fractal dimensions, and retinopathy.8 This is usually studied by optical coherence tomography (OCT) and retinal fundus imaging, and has been applied to study Alzheimer's disease, vascular dementia, frontotemporal dementia, and dementia with Lewy bodies. Distinct retinal features are commonly associated with stroke and dementia patients. There is great potential and advantage to apply retinal fundus imaging as a diagnostic tool for population‐based screening to identify at‐risk subjects with a high burden of WMH.
An automatic retinal image analysis system incorporating machine learning approach has the potential to become a simple, rapid, and reliable tool for screening population‐at‐risk of cerebrovascular disease and dementia. The method is shown to be able to identify subjects with a high burden of WMH in an asymptomatic population.
Methods
Study subjects
Subjects were prospectively identified from a community‐based cohort called CU‐RISK (The Chinese University of Hong Kong – Risk index for Subclinical Brain Lesions in Hong Kong), which included stroke and dementia‐free healthy adults consecutively recruited between 2013 and 2016 from local community centers and community network by advertisement of the study and word‐of‐mouth in the centers.9, 10 This CU‐RISK study aimed to develop a risk score for screening significant SVD in symptom‐free subjects based on putative clinical correlates and risks factors for SVD. The inclusion criteria for CU‐RISK were (1) aged from 65 years or older; (2) functional independence by a score of 20 on the 20‐point Barthel index and <2 on the Lawton's Instrumental of Daily Living Scale (IADL); (3) Cantonese speaking, (4) sufficient sensorimotor and language competency for cognitive testing; and (5) provided written informed consent. The exclusion criteria were (1) history of clinical stroke or transient ischemic attack ascertained by medical records on the electronic Health Record (Clinical Management System) of the Hospital Authority Hong Kong; (2) history of neurological or psychiatric conditions affecting cognitive functions; (3) dementia determined by medical history; (4) evidence of brain tumors, cerebral infarcts larger than 20 mm in diameter or hydrocephalus on MRI; (5) subjects with medial temporal lobe atrophy (MTLA) as defined by a rating of >2 rated on coronal images on T1‐weighted brain MRI using the Scheltens’ 5‐point (0–4) scale to exclude prodromal Alzheimer's disease. Additional exclusion criteria for retinal‐image acquisition include (6) patients with known retinal disease or disease influencing vessel structure in color retina images, such as mild diabetic retinopathy, age‐related maculopathy, central serous chorioretinopathy, postcataract extraction, and retinal pigment epithelial detachment.
Information on vascular risk factors including hypertension, diabetes mellitus, hyperlipidemia, and heart disease were collected. Hypertension was defined as systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg or current treatment with anti‐hypertensive medications. Diabetes mellitus was defined as fasting plasma glucose ≥ 6.1 mmol/L or HbA1c ≥ 5.8% or current treatment with blood glucose lowering medication. Hyperlipidemia was diagnosed according to established guidelines or current treatment with statins medications. Heart disease was defined as history or current cardiac arrhythmia, atrial fibrillation, left ventricular hypertrophy, congestive heart failure, ischemic heart disease, myocardial infarction, electrocardiographic abnormalities, or possible cardiac embolic source. Trained research assistants administered the Hong Kong version of the Montreal Cognitive Assessment (MoCA) according to standardized procedures in the research clinics at the Prince of Wales Hospital in Hong Kong. All clinical, cognitive, and MRI assessments were performed within a median of 5‐months from each subject after recruitment. Written informed consent was obtained from all subjects, and the project was done according to the guidelines of the Declaration of Helsinki and approved by the Joint CUHK‐NTEC Clinical Research Ethics Committee (CREC Ref. No. 2012.085).
Brain MRI acquisition and analysis
Brain MRI was acquired in all subjects using a 3.0‐T scanner (Achieva 3.0 T TX Series, Philips Medical System, Best, The Netherlands) capable of parallel signal acquisition to reduce scan time. Scanning parameters included 3D FLAIR images in the sagittal plane (TR/TE: 8000/332 msec, TI: 2400 msec, FOV:230–230 mm, contiguous slices, 0.55 mm (RL) thickness, reconstruction matrix: 208–208, NEX 1, time of acquisition = 6 min). Fat suppression was achieved using an inversion delay pulse of 220 msec. This allowed 3D quantitative measurement of T2 WMH. Sequences including T1‐weighted, T2‐weighted, and diffusion tensor imaging was acquired using a single‐shot echo planar imaging technique with the following parameters: actual TR/TE (msec) = 250/16; flip angle = 40; number of repetitive readout pulses = 14; field of view (mm) = 240 × 254; matrix = 68 × 69; voxel size (mm) = 3.5 × 3.6 × 6. All scans were rated according to STRIVE (Standards for Reporting Vascular changes on Neuroimaging). A lacune was defined as a well‐circumscribed lesion equivalent to the signal characteristics of cerebrospinal fluid on T1‐weighted images and measuring 2–20 mm in all dimensions in the subcortical white and gray matter, basal ganglia, cerebellum, and brainstem. On FLAIR, a lacune was hypointense and is commonly surrounded by a hyperintense rim. WMH was determined as ill‐defined hyperintensities ≥5 mm on FLAIR but isointense with normal brain parenchyma on T1‐weighed images. All scans were manually rated by independent raters who are experienced in neuroimaging markers of SVD. WMH severity was visually rated by the age‐related white matter changes (ARWMC) scale.11 The global ARWMC was the ARWMC score of the most severe WMC region among 10 regions, with score 0, 1, 2, and 3 representing nil, focal lesion, early confluent, and confluent lesions respectively.12 Severe WMH was defined as a global ARWMC score of ≥2. ARWMC score was graded by two independent raters (one neurologist and one trained research assistant) using anonymized MRI data. There was high interrater reliability, and the intraclass correlation coefficient between the raters for the ARWMC global score was 0.909. In addition, WMH was measured by a validated semiautomated algorithm segmentation.13 In brief, WMH lesion maps were generated in two steps, including segmentation and registration. The calculation of WMH volume excluded acute infarcts through examining cases with hyperintense regions both on the FLAIR and DWI images. Manual correction of the mapped lesions was performed if necessary. During the registration step, the WMH lesion maps were normalized to adjust for individual brain size differences. Quantification of the WMH volume was performed by multiplying the number of voxels of WMH by the number of voxel spacing.
Retinal imaging acquisition and analysis
Topcon nonmydriatic retinal camera (TRC‐NW6S, Tokyo Optical Co, Tokyo) was used to capture the color retinal image using 45° field of view centered on the fovea. The length and angle measurement tools previously developed as part of the ARIA algorithm were used to estimate the length and angle of vessels. The retinal characteristics measured were described in our prior studies and included: (i) retinal vessel measurements; (ii) arteriole‐venous nicking and arteriole occlusion; (iii) hemorrhages; (iv) tortuosity; (v) bifurcation coefficients; (vi) asymmetry of branches and bifurcation angles.14
Automatic retinal image analysis
We used a fully automatic retinal image analysis (ARIA) method to acquire and analyze retinal images in our study. ARIA was applied and validated in different disease cohorts, including stroke, diabetes, and chronic kidney disease.14, 15, 16 The fully automatic retinal image analysis was developed using R and Matlab computer software. The detailed methods of the automatic retinal imaging analysis method have been reported (US Patent 8787638 B2; http://www.google.com/patents/US8787638). The methods include the use of fractal analysis, high order spectra analysis, and statistical texture analysis. These approaches were used to accomplish the overall estimation of the global ARWMC score.
Statistical analysis and machine learning algorithm
Demographic data (age, gender, years of education) among subjects with low versus high ARWMC score were assessed using two sample t‐test and chi‐square test or Fisher's Exact Test as appropriate. Logistic regression was used to select variables that were different between the two groups controlling for the demographic data. Odd ratios and the corresponding 95% confidence intervals were obtained by logistic regressions.
For the retinal related characteristics with ARIA analysis, we used MathWorks Neural Network Toolbox (www.mathworks.com) and applied two machine learning based methods: (1) the pattern recognition neural network (PRNN) for classification; and (2) the fitting neural network (FNN) for regression analysis. Both technologies are a type of the multilayer feed forward networks which use backpropagation algorithm for training. PRNN can be trained to classify inputs according to target classes. In the study we used PRNN to train the generic feedforward neural network and classify the two global ARWMC score classes (i.e., two categorical classes of global ARWMC score ≥2 and global ARWMC score <2). On the other hand, we used FNN to fit an input‐output relationship (i.e., retinal characteristics and WMH relationship). In such technique, a feed forward network with one hidden layer and enough neurons in the hidden layers can fit any finite input‐output mapping problem.17 In general, the FNN with learning vector quantization NN (LVQNet) consists of two layers. The first layer maps input vectors into clusters that are found by the network during training. The second layer maps merges groups of first layer clusters into the classes defined by the target data. Whereas the total number of first layer clusters is determined by the number of hidden neurons, the larger the hidden layer the more clusters the first layer can learn. In details, our network that is used for function fitting is a two‐layer feedforward network, with a sigmoid transfer function in the hidden layer and a linear transfer function in the output layer. Such network has an advantage of fitting multidimension mapping problems well, given consistent data and enough neurons in its hidden layer. We train the data by using Levenbeg‐Marquardt backpropagation algorithm. Corresponding to the inputs of the above networks, a set of profile patterns are generated using ARIA automatic approach. The goal is to build a classifier that can distinguish between global ARWMC score ≥2 and global ARWMC score <2 patients based from fundus images. Notice that a corresponding reduced set of measurements or ‘features’ that can be used to distinguish patients between two categorical classes of ARWMC score (and to estimate WMH) using trained PRNN (and FNN) classifier. Such features will be obtained from our developed ARIA system, which include retinal vascular related characteristics like central retinal venular equivalent calibre (CRVE), central retinal arteriolar equivalent (CRAE), and so forth.
In order to ensure consistency of the selected network and to avoid overfitting we have separated the samples into training set and testing set. After a model (network) has been trained with good validation results, we then used the test data to check its performance using resampling method by resampling 10 times. We have also carried out 10‐fold cross‐validation analysis to evaluate the performance with comparable results confirming the consistency of the model.
Sample size estimation
In order to obtain a sensitivity and specificity values of 0.85 or higher with a lower bound of the 95% confidence intervals of at least 0.7, we need to have more than 50 subjects for the estimation for sensitivity and specificity.18 In this prospective study, we recruited subjects and assessed WMH until we have more than 50 subjects with global ARWMC grading ≥2. The analysis was carried out once we have achieved the adequate number of cases with more than double for subjects with global ARWMC <2.
Results
Figure 1 showed the study flow diagram. Two hundred and sixty subjects were identified from the CU‐RISK cohort and recruited. All underwent successful MRI and retinal image acquisition. 80 were excluded from analysis due to respective MRI brains were performed more than 6 months from retinal image acquisition. A total of 180 subjects was used in the final analysis. The mean age was 70.3 ± 4.5 years old. 70 (39%) were male. The mean education was 7.9 ± 4.7 years. The commonest vascular risk factor was hypertension (n = 106, 59%), followed by hyperlipidemia (n = 47, 26%) and diabetes mellitus (n = 31, 17%).
Figure 1.
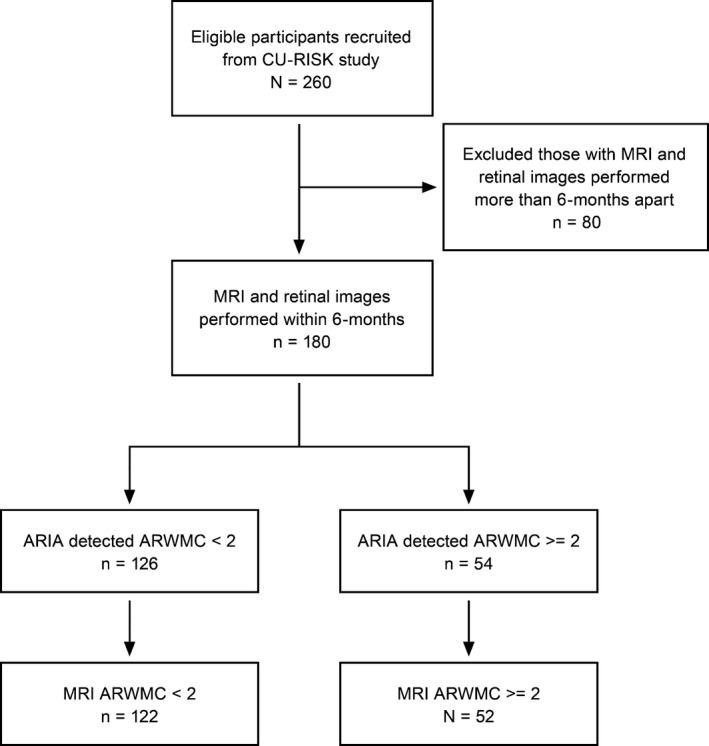
Patient flow diagram. Recruited and analyzed subjects for ARIA and MRI detection of white matter hyperintensities (WMH) assessed by Age‐related White Matter Changes rating (ARWMC). High burden of WMH was defined as ARWMC rating of ≥2 on MRI brain.
The distribution of global ARWMC rating for WMH was 0 (n = 34, 19%), 1 (n = 90, 50%), 2 (n = 43, 24%), and 3 (n = 13, 7%) respectively. Subjects with ARWMC rate 3 has the highest proportion with hypertension (92%). Table 1 shows the comparison between subjects with severe WMH (global ARWMC rating of 2–3) and without severe WMH (global ARWMC rating of 0–1). Severe WMH was found in 56 (31%) subjects. These subjects had a significant higher proportion with hypertension (79% vs. 50%, P < 0.001), but no significant difference between age, education, gender, diabetes mellitus, hyperlipidemia, and ischemic heart disease, as compared to subjects without severe WMH. On MRI, severe WMH subjects had larger mean WMH volume (15.1, 95% CI 10.3–20.0 vs. 3.5, 95% CI 2.9–4.0 mL, P < 0.001) and presence of lacunes (14% vs. 1.6%, P = 0.002). There was no difference for the presence of microbleed (34% vs. 25%, P = 0.28).
Table 1.
Baseline characteristics of 180 Chinese with (global ARWMC score ≥2) and without (global ARWMC score <2) severe white matter hyperintensities on MRI brain
| Global ARWMC <2 N = 124 | Global ARWMC ≥2 N = 56 | P‐value | |
|---|---|---|---|
| Age, median (IQR)a, years | 69.00 (66.25–73.00) | 71.00 (67.00–74.00) | 0.083 |
| Education, median (IQR)a, years | 7.00 (4.00–12.00) | 7.00 (3.25–12.00) | 0.932 |
| Male, N (%)b | 46 (37.1%) | 24 (42.9%) | 0.463 |
| MoCA, median (IQR)a | 23.00 (21.00–26.00) | 22.00 (19.00–25.00) | 0.053 |
| Hypertension, N (%)b | 62 (50.0%) | 44 (78.6%) | <0.001 |
| Diabetes Mellitus, N (%)b | 17 (13.7%) | 14 (25.0%) | 0.063 |
| Hyperlipidemia, N (%)b | 30 (24.2%) | 17 (30.4%) | 0.383 |
| IHD, N (%)c | 5 (4.0%) | 3 (5.4%) | 0.705 |
| Smoker | 0.735 | ||
| Nonsmoker, N (%)c | 105 (84.68%) | 46 (82.14%) | |
| Past smoker, N (%)c | 17 (13.71%) | 8 (14.29%) | |
| Current smoker, N (%)c | 2 (1.61%) | 2 (3.57%) | |
| Alcoholism | |||
| Nondrinker, N (%)c | 105 (84.68%) | 50 (89.29%) | 0.477 |
| Occasional (1 drink/week–month), N (%)c | 8 (6.45%) | 4 (7.14%) | |
| Modest drinker (1–6/week), N (%)c | 11 (8.87%) | 2 (3.57%) | |
| WMH volume, median (IQR)a, mL | 2.53 (1.64–4.34) | 10.01 (4.89–18.17) | <0.001 |
| Log‐transformed WMH volume, mean (IQR)a | 0.93 (0.50–1.47) | 23.00 (1.59–2.90) | <0.001 |
| Presence of Lacunes, median N (IQR)c | 2 (1.6%) | 8 (14.3) | 0.002 |
| Presence of Microbleed, median N (IQR)b | 31 (25.0%) | 19 (33.9%) | 0.281 |
BMI, body‐mass index; WMH, white matter hyperintensities; ARWMC, age‐related white matter change; IQR, interquartile range.
Mann–Whitney U test.
Chi‐square test.
Fisher exact test.
In a univariate logistic regression model for prediction of severe WMH (global ARWMC rating of 2–3), only age (OR 1.07, 95% CI from 1.00 to 1.15, P = 0.047) and hypertension (OR 3.67, 95% CI from 1.77 to 7.60, P < 0.001) were significant predictors. In multivariate model, only hypertension (OR 3.41, 95% CI from 1.63 to 7.12, P = 0.001) remained significant, after adjusting for age, and prespecified variables (education, diabetes mellitus, and hyperlipidemia).
All subjects had successful retinal fundus image acquisition and analysis (Table 2).
Table 2.
Univariate analysis for retinal parameters by severity of WMH according to ARWMC grading
| ARWMC score <2 (n = 124) | ARWMC score ≥2 (n = 56) | ||||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | P‐value | |
| Left eye retinal parameters | |||||
| Zc CRVE (μm) | 204.49 | 203.51–205.46 | 205.82 | 204.45–207.19 | 0.125 |
| sTORTa | 1.0863 | 1.0860–1.0866 | 1.0858 | 1.0854–1.0863 | 0.115 |
| sTORTv | 1.0946 | 1.0943–1.0948 | 1.0943 | 1.0939–1.0946 | 0.160 |
| sTORTt | 1.0897 | 1.0894–1.0899 | 1.0893 | 1.0889–1.0897 | 0.086 |
| LDRt | 11.8925 | 11.7789–12.0060 | 11.6732 | 11.5092–11.8372 | 0.032 |
| Vasymmetry | 0.7149 | 0.7134–0.7163 | 0.7108 | 0.7087–0.7128 | 0.002 |
| Exudates_estp | 0.3402 | 0.3325–0.3480 | 0.3499 | 0.3371–0.3627 | 0.184 |
| Right eye retinal Parameters | |||||
| Zb CRAE (μm) | 140.2900 | 139.86–140.72 | 140.8840 | 140.36–141.40 | 0.081 |
| Zc CRAE (μm) | 142.6592 | 142.19–143.13 | 143.4571 | 142.88–144.04 | 0.035 |
| Zc CRVE (μm) | 204.1346 | 203.63–204.64 | 204.8466 | 204.17–205.52 | 0.094 |
| JEDa | −0.4387 | (−0.4444)–(−0.4331) | −0.4307 | (−0.4396)–(−0.4218) | 0.124 |
| ZbMWt | 75.9177 | 75.7223–76.1131 | 76.1502 | 75.8929–76.4076 | 0.174 |
| LDRt | 10.6449 | 10.5387–10.7511 | 10.8429 | 10.7004–10.9854 | 0.035 |
| BAa | 70.9629 | 70.7520–71.1739 | 71.3946 | 71.0932–71.6960 | 0.067 |
| BAv | 70.8327 | 70.6937–70.9717 | 71.0548 | 70.8764–71.2332 | 0.023 |
| Aocclusion estp | 0.0859 | 0.0805–0.0913 | 0.0773 | 0.0715–0.0831 | 0.032 |
Zb CRVE, Zone B Central retinal venular equivalent caliber; Zc CRVE, Zone C Central retinal venular equivalent caliber; Zb CRAE, Zone B Central retinal arteriolar equivalent caliber; Zc CRAE, Zone C Central retinal arteriolar equivalent caliber; sTORTa, Simple Curvature tortuosity arteriole; sTORTv, Simple Curvature tortuosity venule; sTORTt, Simple Curvature tortuosity total; LDRt, Zone C length to diameter ratio total; Vasymmetry, Asysmmentry ratio of Vennule; JEDa, Zone C Junctional exponent deviation for arterioles; BMWt, Zone B Mean Width total; Exudates estp, estimated probability of exudates; BAa, branching angle arteriole; BAv, branching angle vennule; Aocclusion estp, estimated probability of arterioles occlusion.
In the univariate analysis of the retinal characteristics,19 zone C length to diameter ratio total (LDRt), asymmetry ratio of venule (Vasymmetry) and arteriole occlusion (Aocclusion estp) were significantly smaller (P < 0.05); whereas zone C Central Retinal arteriolar equivalent caliber (Zc CRAE) and bifurcation angle of vennule (BAv) were significantly larger in subjects with severe WMH (P < 0.05).
We also used all retinal characteristics with significant values less than 0.1 as inclusion criteria for a logistic regression analysis for the detecting severe WMH (ARMWC ≥2). The sensitivity and specificity using a conventional logistic regression only reached 0.464 and 0.871 respectively. When ARIA incorporating machine learning approach was used, the sensitivity and specificity for the overall classification becomes 0.929 (95% CI from 0.819 to 0.977) and 0.984 (95% CI from 0.937 to 0.997) respectively. The positive and negative predictive values are 0.963 (95% CI from 0.864 to 0.993) and 0.968 (95% CI from 0.915 to 0.990). We have also estimated the WMH volume using similar approach and obtained a correlation coefficient of 0.897 (95% CI from 0.864 to 0.922) between observed and predicted WMH volume (log‐transformed) (Fig. 2).
Figure 2.
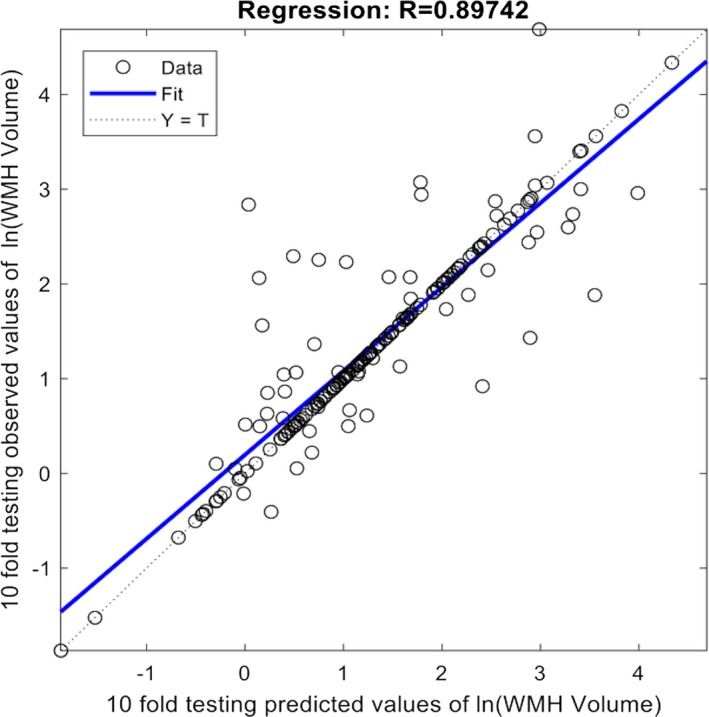
Correlation between observed white matter hyperintensities (WMH) volume measured on brain MRI and predicted by automatic retinal image analysis (ARIA). Good correlation (R = 0.897) was observed.
Discussion
Our study demonstrated that automatic retinal image analysis (ARIA) with a nonmydriatic retinal fundus image can detect stroke and dementia‐free community‐dwelling subjects harboring significant burden of white matter hyperintensities in their brains. ARIA is a quick, noninvasive, and convenient algorithm‐based tool with an overall performance of 93% sensitivity and 98% specificity in detecting subjects with high burden of WMH, and can be further validated as a diagnostic tool for population at risk of cerebrovascular disease and dementia.
Hypertension is an established and significant risk factor for cerebral small vessel disease, yet the causality remains an enigma. In our current study, we have also found a strong association between hypertension and severe WMH; hypertension is the only significant clinical predictor in the multivariate model (OR 3.4, 95% CI from 1.6 to 7.1, P = 0.001) for prediction of WMC ARWMC grade ≥2. However, patients with hypertension may or may not have significant burden of WMH, supported by a global ARWMC grade 0 (44%) and 1 (52%), highlighting that this group of subjects are at risk‐population that warrants more accurate screening and monitoring for development of WMH. On the other hand, early WMH changes (ARWMC grade 1) was found in subjects without hypertension; it would be of interest to explore whether the same mechanism drives the development of SVD and hypertension, and subjects with early WMH changes should actively be monitored and treated for hypertension at a lower threshold of 130/80 mmHg as proposed recently.20 We have found that traditional risk factors for SVD, namely age and hypertension, do not effectively identify subjects harboring high burden of WMH.10 Complementary diagnostic tools are much needed in clinical practice to identify high‐risk group for further clinical evaluations including MRI brain and neuropsychological testing, which are more resource intensive assessments.
This is the first study to apply machine learning, a specific approach in artificial intelligence, and retinal imaging to evaluate presymptomatic community‐dwelling adults harboring high burden of WMH. We are encouraged to see that the US Food and Drug Administration has recently approved using an artificial intelligence‐based device to detect certain diabetes‐related eye problems (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm604357.htm). Artificial intelligence methodologies are now being applied in real life application of eye diseases. The CU‐RISK study contained a well‐defined cohort of stroke‐ and dementia‐free community‐dwelling older adults with detailed clinical, neuropsychological, and MRI profiles. ARIA was setup as a quick and convenient retinal image acquisition on an undilated pupil for easy application in the clinical setting. The findings from fundus retinal images can be compared with those parameters of retinal neuronal structures identified from more sophisticated retinal image, such as OCT and angiogram; these can be correlated with vascular and nonvascular retinal imaging markers of dementia and other neurodegenerative diseases.5, 8 Retinal vessels share important features with cerebral small vessels, including size and pathophysiological characteristics.6 The proposed pathogenesis of SVD, including endothelial damage, impaired cerebral autoregulation, venous collagenosis, and cerebral hypoperfusion, are closely related to changes of the retinal vessel, thereby strengthening the use of retinal imaging for evaluation. The microvascular network changes in fundus images can therefore act as a surrogate marker for diseased cerebral vessels.6
ARIA can be incorporated in future prospective community‐based screening programs for population at risk of cerebrovascular disease and neurodegenerative diseases. Population with risk factors such as hypertension, diabetes, and advancing age can have regular retinal image acquisition and automated analysis, and subjects with high burden of brain lesions detected by ARIA can undergo further evaluation by healthcare specialist and MRI brain imaging,21 ideally also with an automated analysis.22 The cost‐effectiveness of this two‐step screening process with ARIA and MRI should be further explored. Presymptomatic subjects with high burden of WMH can also be recruited for primary prevention clinical trials. More importantly, subjects with high burden of WMH who are at risk of developing cerebral small vessel disease may be identified early with these screening algorithm; this would have tremendous impact on healthcare and society by using technology to prevent and fight against dementia and stroke.23, 24
Summary
We developed a robust algorithm to automatically evaluate retinal fundus image that can identify community subjects with high burden of white matter hyperintensities by MRI brain.
Future community‐based prospective studies should be performed for early screening of population at risk of cerebral small vessel disease.
Author Contributions
Dr. Benny Zee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mok, Fan, Zeng, Zee.
Acquisition, analysis, or interpretation of data: Lau, Mok, Lee, Lam, Wong, Kwok, Lai, Zee.
Drafting of the manuscript: Lau, Mok, Lee, Zee.
Critical revision of the manuscript for important intellectual content: Lau, Mok, Lee, Lam, Wong, Fan, Zeng, Lai, Zee.
Statistical analysis: Lam, Wong, Lee, Kwok, Lai, Zee.
Obtained funding: Mok, Fan, Zeng, Zee.
Administrative, technical, or material support: Kwok.
Study supervision: Mok, Zee.
Conflict of Interest
None.
Acknowledgments
This study was partially supported by the National Key Research and Development Programme of China (2016YFC1300603) and the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No.: CUHK 471911), Technology and Business Development Fund (TBF) of the Chinese University of Hong Kong (No. TBF15MED005), and Innovation and Technology Fund (ITF) (No. MRP/037/17X).
Funding Information
This study was partially supported by the National Key Research and Development Programme of China (2016YFC1300603) and the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No.: CUHK 471911), Technology and Business Development Fund (TBF) of the Chinese University of Hong Kong (No. TBF15MED005), and Innovation and Technology Fund (ITF) (No. MRP/037/17X).
Funding Statement
This work was funded by National Key Research and Development Programme of China grant 2016YFC1300603; Research Grants Council of the Hong Kong Special Administrative Region, China grant CUHK 471911; Technology and Business Development Fund (TBF) of the Chinese University of Hong Kong grant TBF15MED005; Innovation and Technology Fund grant MRP/037/17X.
References
- 1. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 2. Mok VC, Lam BY, Wong A, et al. Early‐onset and delayed‐onset poststroke dementia ‐ revisiting the mechanisms. Nat Rev Neurol 2017;13:148–159. [DOI] [PubMed] [Google Scholar]
- 3. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 4. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snyder PJ, Johnson LN, Lim YY, et al. Nonvascular retinal imaging markers of preclinical Alzheimer's disease. Alzheimer's Dement 2016;4:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGrory S, Cameron JR, Pellegrini E, et al. The application of retinal fundus camera imaging in dementia: a systematic review. Alzheimer's Dement 2017;6:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res 2017;57:89–107. [DOI] [PubMed] [Google Scholar]
- 8. Chan VT, Tso TH, Tang F, Tham C, Mok V, Chen C, Wong TY, Cheung CY et al. Using retinal imaging to study dementia. J Vis Exp 2017;129:e56137 10.3791/56137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hilal S, Mok V, Youn YC, et al. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 2017;88:669–674. [DOI] [PubMed] [Google Scholar]
- 10. Wong A, Law LSN, Liu W, et al. Montreal cognitive assessment one cutoff never fits all. Stroke 2015;46:3547–3550. [DOI] [PubMed] [Google Scholar]
- 11. Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 12. Fan YH, Lam WW, Mok VC, et al. Variability and validity of a simple visual rating scale in grading white matter changes on magnetic resonance imaging. J Neuroimaging 2003;13:255–258. [PubMed] [Google Scholar]
- 13. Shi L, Wang D, Liu S, et al. Automated quantification of white matter lesion in magnetic resonance imaging of patients with acute infarction. J Neurosci Methods 2013;213:138–146. [DOI] [PubMed] [Google Scholar]
- 14. Zee B, Lee J, Li Q, et al. Stroke risk assessment for the community by automatic retinal image analysis using fundus photograph. Qual Primary Care 2016;24:114–124. [Google Scholar]
- 15. Zhuo Y, Yu H, Yang Z, et al. Prediction factors of recurrent stroke among chinese adults using retinal vasculature characteristics. J Stroke Cerebrovasc Dis 2017;26:679–685. [DOI] [PubMed] [Google Scholar]
- 16. Guo VY, Chan JC, Chung H, et al. Retinal information is independently associated with cardiovascular disease in patients with type 2 diabetes. Sci Rep 2016;6:19053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Jesús O, Hagan MT. Backpropagation algorithms for a broad class of dynamic networks. IEEE Trans Neural Netw 2007;18:14–27. [DOI] [PubMed] [Google Scholar]
- 18. Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17:857–872. [DOI] [PubMed] [Google Scholar]
- 19. Frost S, Kanagasingam Y, Sohrabi H, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry 2013;3:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 21. Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol 2018;31:36–43. [DOI] [PubMed] [Google Scholar]
- 22. Shi L, Liang P, Luo Y, et al. Using large‐scale statistical chinese brain template (Chinese 2020) in popular neuroimage analysis toolkits. Front Hum Neurosci 2017;11:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frankish H, Horton R. Prevention and management of dementia: a priority for public health. Lancet 2017;390:2614–2615. [DOI] [PubMed] [Google Scholar]
- 24. Vickrey BG, Brott TG, Koroshetz WJ; Council SRPMSCatNANDaS, Stroke NIoNDa . Research priority setting: a summary of the 2012 NINDS Stroke Planning Meeting Report. Stroke 2013;44:2338–2342. [DOI] [PubMed] [Google Scholar]


