Abstract
In the present study, a qualitative analysis of proanthocyanidins (PAs) from an aqueous-methanolic extract of Salix daphnoides VILL. bark is described. Procyanidin B1 (1), B2 (2), B3 (3), B4 (4), C1 (5), epicatechin-(4β→8)-epicatechin-(4β→8)-catechin (6) and epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin-(4β→8)-catechin (7) have been isolated by a combination of different chromatographic separations on Sephadex® LH-20-, MCI®-, Diol-and RP-18-phases. Mass spectrometry, 1D- and 2D-NMR, circular dichroism and polarimetry were used for their structure elucidation and verification by comparison with the literature. Additionally, two fractions of very polar flavan-3-ols were compared: “regular” polymeric PAs received at the very end of the Sephadex® LH-20 chromatography showing no mobility on silica TLC and “unusual” PAs with the same RF-value but already eluting together with flavonoids in the Sephadex® LH-20 system. These “unusual” PAs were subsequently enriched by centrifugal partition chromatography (CPC). 13C-NMR, polarimetry, thiolysis, acid hydrolysis and phloroglucinol degradation were used to characterize both fractions. Differences in the composition of different flavan-3-ol units and the middle chain length were observed.
Keywords: procyanidins, proanthocyanidins, flavan-3-ols, Salix daphnoides, willow bark, low temperature NMR, thiolysis, polarimetry, polymeric proanthocyanidins
1. Introduction
Willow bark (Salicis cortex, Salix spp., Salicaceae) is an herbal drug monographed in the European Pharmacopoeia (Ph. Eur.) [1]. It is used for its antiphlogistic, antipyretic and analgetic effects and is positively monographed by the European Scientific Cooperative On Phytotherapy (ESCOP) for the treatment of rheumatic diseases, fever and headache [2,3]. Since the monograph of willow bark in the Ph. Eur. [1] does not claim a specific willow species for the drug, but insists on a minimum amount of 1.5% salicylic alcohol derivatives, there is a great variety in willows which can be used for the drug Salicis cortex. Although it is known that these salicylic alcohol derivatives are metabolized to the anti-inflammatory compound salicylic acid in vivo [4,5], there is doubt that only this class of secondary plant ingredients is responsible for the effects described above [2,3] as the resulting in vivo concentration is much too low to explain the overall efficacy of willow bark [5]. As not only anti-inflammatory but also cooperative effects are described for proanthocyanidins (PA) [6,7], which are one of the main groups of Salix ingredients beside the salicylic alcohol derivatives and flavonoids [3], it is necessary to investigate each willow species to broaden the knowledge about the complete phenolic spectrum of these important compounds.
The aim of the present study was to investigate the proanthocyanidin composition of Salix daphnoides VILL., the European violet willow, which is one of the main Salix species used for the drug willow bark due to the European Pharmacopoeia [1]. Pobłocka-Olech and coworkers could identify procyanidin B1 by SPE-HPTLC in S. daphnoides [8], but a systematic investigation of the complete proanthocyanidin pattern is still missing.
2. Results and Discussion
2.1. Screening for Oligomeric Proanthocyanidins
To broaden the knowledge about the composition of oligomeric flavan-3-ols, 100.00 g of a S. daphnoides bark extract were used for their isolation. Compounds 1–4 eluted from Sephadex® LH-20 as stationary phase between 2200 and 2986 mL (fraction S 3.2), compounds 5 and 6 between 2986 and 4154 mL (fraction S3.3), and compound 7 between 4154 and 4634 mL (fraction S3.4) (Figure 1). Each of these fractions were further separated by MCI®-Gel chromatography and finally purified by preparative HPLC (system A: 1, 3; system B: 2, 4; system C: 5, 6; system D: 7).
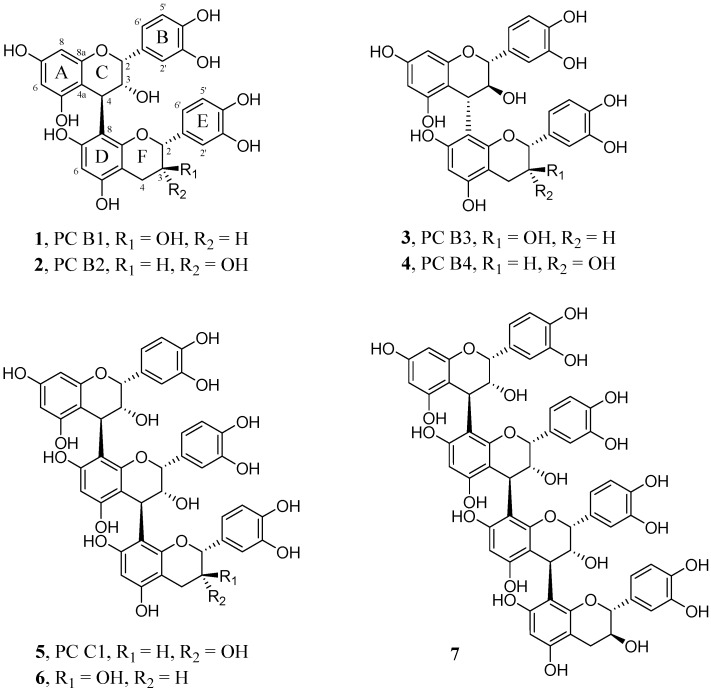
Figure 1.
Structures of the isolated procyanidins (PC) 1–7.
The dimeric procyanidins B1 (1), B2 (2), B3 (3) and B4 (4) were confirmed by comparing the spectroscopic data with results obtained by Shoji et al. [9]: B1 and B2, Kolodziej [10] B1, peracetylated, Saito et al. [11]: B3, [12]: B4, and Mohri et al. [13]: B3 and B4. CD spectroscopy was used to verify the 4α→8 or 4β→8 linkage between the flavan-3-ol units. High-amplitude positive Cotton effects at low wavelengths (220–240 nm) indicate a 4β and negative effects a 4α configuration [14,15,16,17]. The CD-spectra for the isolated compounds were in accordance with the results published by Barrett et al. [15]: B2, B3 and B4. Since there were no CD data available for 1 and 7 as free phenolic compounds in literature (SciFinder®, American Chemical Society, Washington, DC, USA), this general rule was applied to determine the configuration of C-4 in their upper units (Supplementary Figures S1 and S2). As observed by Barrett and coworkers [15], mol. Ellipticity [Θ] of the respective maximum with positive sign rises with an increasing number of monomeric units for these compounds. Thus, the absolute stereochemistry at the interflavan linkages was assigned as 4β→8 in 7.
NMR data sets (1H, 13C) of 3 and 4 (free phenolic) are given in Supplementary Table S1. The 1H-NMR data obtained for compound 5 (procyanidin C1, epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin) at −20 °C were in accordance with Shoji et al. [9], except the coupling constant for H-2′ at δ 7.02 ppm for the middle unit. Whereas Shoji and coworkers found a doublet with J = 8 Hz [9], a typical meta coupling constant of J = 1.6 Hz was found in the present work. The optical rotation and CD-data were in accordance with the literature [15,18,19]. To complete the NMR data, spectra at −40 °C were determined (Supplementary Table S2). The 1H-NMR data of compound 6 (epicatechin-(4β→8)-epicatechin-(4β→8)-catechin) measured at −20 °C are very similar to a trimer identified by Shoji et al. [9]. Whereas Shoji et al., published δ 4.06 ppm (d, J = 8 Hz) for H-3 of ring F and δ 3.99 ppm (d, J = 5 Hz) for the respective C ring proton, δ 4.07 ppm (d, J = 1.3 Hz) and δ 3.99 ppm (d, J = 1.8 Hz) were found in the present study for the same protons. The small coupling constants of both H-3 protons indicate the 2,3-cis relative configuration and thus epicatechin as constituent units. CD data were in agreement with published values [19]. NMR data at −40 °C are given in Supplementary Table S2. Compound 7 was determined as epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin-(4β→8)-catechin by comparing the 1H-NMR data measured at −20 °C with the data of Saito et al. [20]. The 1H-NMR data agree with published data except a 0.08 ppm lower shift for H-2 of the terminal flavan-3-ol unit (δ 5.04 ppm). NMR data sets at −40 °C and −20 °C are presented in Supplementary Table S3.
2.2. Characterization and Comparison of Fractions Enriched with Oligomeric or Polymeric Proanthocyanidins
Sephadex® LH-20 was used as the first stationary phase for column chromatography to separate an extract of S. daphnoides. Using 70% ethanol as mobile phase, the salicylic alcohol derivatives were eluted first (S1), followed by the flavonoids (S2) and the oligomeric proanthocyanidins (PAs) in the last fraction (S3). Polymeric PAs eluted after changing the mobile phase to 70% acetone (S3) [21]. Using vanillin/HCl-spray reagent for the detection of PAs in the TLC control, not only very polar and thus immobile red colored “regular” polymeric PAs were observed at the end of the Sephadex® chromatography with 70% acetone but also similar polar “unusual” proanthocyanidins with an identical chromatographic behavior in the same TLC-system (RF = 0) were eluted already with 70% ethanol together with flavonoids, even before the first monomeric flavan-3-ol. Those Sephadex-fractions were combined and CPC (centrifugal partition chromatography) with a 1-butanol/water system was used to remove most of the flavonoids to enrich the “unusual” PAs in the water phase (S2 W) in order to compare them with the “regular” PAs (S3.8).
Parameters for the comparison of the “unusual” and “regular” PAs were the content of tannins [22,23], the content of procyanidins (PCs) [24] and the determination of the corresponding anthocyanidins yielded by the oxidative cleavage reaction [25]. Further, the mean degree of polymerization (mDP) was estimated by thiolyses [26,27] and spectroscopic data obtained by 13C-NMR [28,29,30], circular dichroism (CD) and polarimetry [21,31] were compared.
The total content of tannins of both fractions was determined by a modified Folin-Ciocalteu reagent assay of the Ph. Eur. [22]. With this functional assay it is possible to determine the content of both, hydrolyzable and condensed tannins. Since it is a convention method, the values generated may not represent the “real” content of tannins, but it is very suitable for a comparison. To get more information about the adsorption properties of the fractions, tannic acid was used as a 100% reference. The fraction with the “unusual” PAs accomplished a content of 66.5% tannins and the “regular” PAs 84.0%. In the monograph “Crataegi fructus” of the Ph. Eur. [24], another convention method is described to determine the content of procyanidins (PCs). An oxidative cleavage reaction with HCl allows the calculation of the procyanidin content as cyanidin chloride by photometry. Using this method, 57.9% PCs were determined in the fraction with the “unusual” PAs and 78.2% with the “regular” PAs. Thus, both methods revealed a slightly higher content of tannins in the fraction with the “regular” PAs.
After the oxidative cleavage reaction, the corresponding anthocyanidins were determined by HPLC using a method of Zhang and coworkers [25] with pelargonidin chloride, cyanidin chloride and delphinidin chloride as reference compounds. To estimate their concentration, the corresponding areas were integrated at 525 nm and set as 100%. Main compound of both fractions was cyanidin (94.3% and 97.8%, respectively) indicating that proanthocyanidins with dihydroxylated B-ring flavan-3-ol constituent units like catechin and epicatechin are dominating. Only small amounts of pelargonidin and delphinidin were identified as cleavage products but the content of delphinidin was slightly higher in the fraction with the “regular” PAs (4.3%) than in the fraction with the “unusual” PAs (0.8%, Table 1) indicating more B-ring trihydroxylated flavan-3-ol constituent units in this fraction. HPLC-chromatograms are shown in Supplementary Figure S3. These results were also confirmed qualitatively by LC-ESI-HRMS.
Table 1.
Estimation of mono-, di-, and trihydroxylated anthocyanidins.
| Fraction | Delphinidin (RT = 5.91 min) | Cyanidin (RT = 10.07 min) | Pelargonidin (RT = 18.28 min) |
|---|---|---|---|
| “regular” PAs | 4.3% | 94.3% | 1.4% |
| “unusual” PAs | 0.8% | 97.8% | 1.4% |
A further estimation of the hydroxylation pattern and the mean degree of polymerization (mDP) of both fractions was determined by thiolysis with benzyl mercaptan and subsequent HPLC analysis [26,27] using the method of Dauer and coworkers [26]. The identification of reaction products was confirmed by LC-ESI-HRMS. The cleaved terminal units are the unsubstituted flavan-3-ols catechin and epicatechin (peaks 1 and 2). The upper units were identified as thioethers (RT at 36.9 to 46.1 min), corresponding to flavan-3-ols and dimeric PAs substituted with benzyl mercaptan in position C-4. Table 2 gives an overview of the detected products yielded from the thiolysis (chromatograms are shown in Supplementary Figure S4). The main reaction products of both fractions are thioethers of dihydroxylated flavan-3-ols (peaks 3 **, 5, 6, 7 **, 8 * and 9). Only in the fraction with the “regular” PAs, peak 4 * could be found which corresponds to a thioether of a trihydroxylated flavan-3-ol (m/z 427.0862). Peak 10 (m/z 395.0963) indicates the presence of flavan-3-ols with monohydroxylated B-rings in both fractions. These results are in accordance with the findings of the determination of anthocyanidins after cleavage reaction with HCl/O2. The difference in the mDP of both fractions is obvious: whereas the mDP in the fraction with the “unusual” PAs was 4.9, a value of 11.8 could be determined for the fraction with the “regular” PAs.
Table 2.
Thiolysis related compounds.
| Peak | RT (min) (LC-HRMS) | RT (min) Anal. HPLC | m/z [M − H]− | Chemical Formula (calcd [M − H]−) |
|---|---|---|---|---|
| 1 | 20.97 | 22.82 | 289.0725 | C15H14O6 (289.0718) |
| 2 | 23.25 | 25.14 | 289.0723 | C15H14O6 (289.0718) |
| 3 ** | 35.06 | 36.92 | 699.1544 | C37H32O12S (699.1542) |
| 4 * | 36.60 | 38.40 | 427.0862 | C22H20O7S (427.0857) |
| 5 | 37.24 | 39.08 | 699.1548 | C37H32O12S (699.1542) |
| 6 | 39.08 | 40.81 | 699.1545 | C37H32O12S (699.1542) |
| 7 ** | 39.33 | 41.71 | 699.1540 | C37H32O12S (699.1542) |
| 8 * | 40.50 | 42.19 | 699.1543 | C37H32O12S (699.1542) |
| 9 | 41.39 | 43.12 | 411.0908 | C22H20O6S (411.0908) |
| 10 | 44.45 | 46.12 | 395.0963 | C22H20O5S (395.0959) |
* Only found in the chromatogram of the “regular” PAs; ** Only found in the chromatogram of “unusual” PAs.
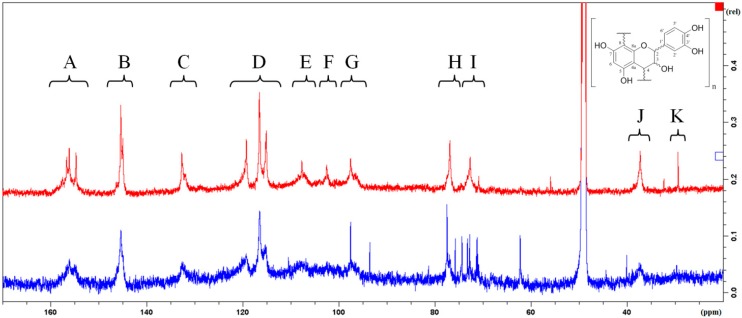
13C-NMR is an established method for the characterization of PA fractions [21,28,29,30]. Figure 2 procures an overview of the 13C-NMR spectra of the “regular” and “unusual” PAs. The ratio of the integrated signals K(C-4 of terminal units) and J (C-4 of extender units) of the “regular” PAs is 1:11 indicating a middle chain length of 12 constituent flavan-3-ol units which is in accordance to the results obtained for the determination of the mDP by thiolysis. However, this result is in contrast to former results obtained with “regular” PAs of Salix purpurea L. with the same method as a mDP of 4–5 flavan-3-ol units could be calculated [30]. Unfortunately, the signal of C-4 of the terminal unit in the 13C-spectrum of the “unusual” PAs is too weak for an exact integration.
Figure 2.
13C-NMR of the “regular” (upper) and the “unusual” (lower) PAs. A (152–160 ppm): C-5, C-7, C-8a; B (144–147 ppm): C-3′, C-4′; C (131–134 ppm): C-1′; D (114–123 ppm): C-2′, C-5′, C-6′; E (106–110 ppm): substituted C-6 and C-8; F (101–104 ppm): C-4a; G (95–99 ppm): unsubstituted C-6 and C-8; H (76–78 ppm): C-2; I (71–74 ppm): C-3; J (36–39 ppm): C-4 of extender units; K (29–30 ppm): C-4 of terminal unit.
The signals between 62.0 and 76.0 ppm in the spectrum of the “unusual” PAs (Figure 2, lower spectrum) seem to be typical sugar signals. But as no flavan-3-ol-glycosides could be found in any HPLC-MS experiment after a cleavage reaction (thiolysis, HCl/O2 and phloroglucinol degradation), they should be due to very polar impurities of S2 W which could not be removed by CPC.
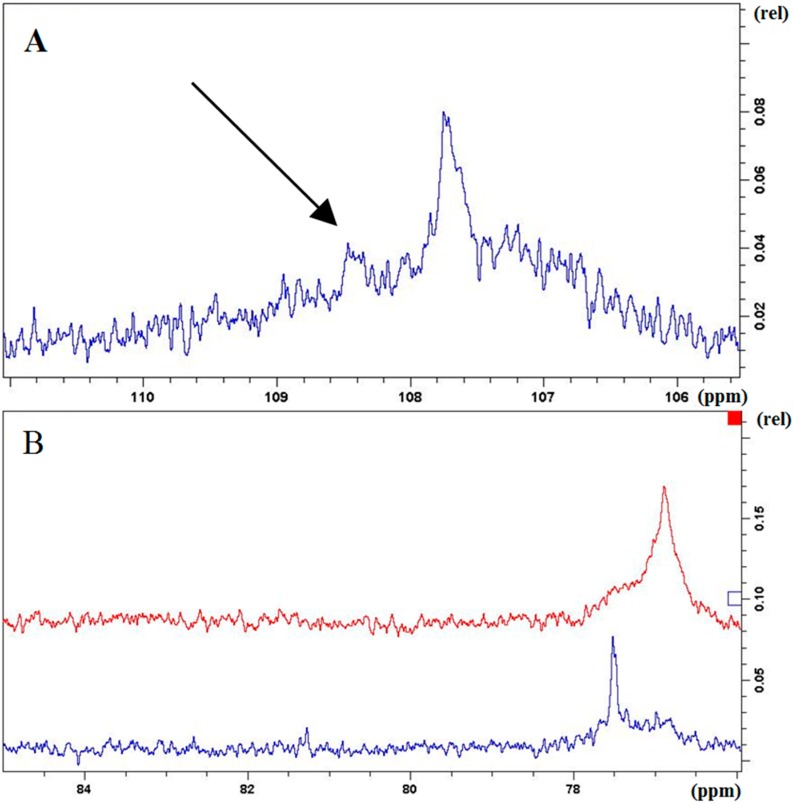
In the spectrum of the “regular” PAs, a small signal at 108.4 ppm could be observed corresponding to C-2ʹ and C-6ʹ of trihydroxylated flavan-3-ols (Figure 3A). This is again in accordance to the former results obtained by the cleavage reactions by HCl/O2 and benzyl mercaptan.
Figure 3.
A: Signal at 108.4 ppm in the 13C-spectrum of the “regular” PAs indicating the presence of monomeric flavan-3-ols with trihydroxylated B-rings; B: 76–85 ppm of the 13C-NMR spectra of the “regular” (upper) and “unusual” (lower) PAs. The area of 76–78 ppm is representative for the C-2 of 2,3-cis configurated flavan-3-ol units. The signals of 2,3-trans configurated units would appear in between 82 and 84 ppm.
Furthermore 13C-NMR enables the possibility to get structural information about the relative configuration of the constituent flavan-3-ol units. The signal cluster from 76 to 78 ppm (Figure 3B) can be assigned to the C-2 carbons of 2,3-cis configurated flavan-3-ols. The absence of signals from 82 to 84 ppm (signals for 2,3-trans flavan-3-ols) indicates that units with 2,3-cis configuration are predominant in both fractions.
This was verified by optical rotation for the fraction with the “regular” PAs (89.9%) [21,31]. As the fraction containing the “unusual” PAs was too colored to get valid data by polarimetry, CD spectroscopy was used as a third tool to determine the C-ring stereochemistry. The positive Cotton effect between 220 and 240 nm indicated the predominance of β-configurated interflavan linkages and thus the predominance of 2,3-cis relative configurated flavan-3-ols [14,15,16,17].
3. Experimental Section
3.1. Reagents and Standards
Methanol, acetonitrile, formic acid, acetic acid, hydrochloric acid, Folin-Ciocalteu’s reagent, dried pyridine, phloroglucinol and acetic anhydride were obtained from Merck Chemicals GmbH (Darmstadt, Germany). Acetone, 1-butanol, benzyl mercaptan, methanol-d4, trifluoroacetic acid, pelargonidin chloride, delphinidin chloride (Fluka, Neu-Ulm, Germany), cyanidin chloride ≥90% (FLUKA), vanillin (Fluka), p-anisaldehyde, and d-(−)-salicin were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany). Tannic acid, sodium carbonate anhydrous, and catechin were acquired from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). Chloroform-d1, and deuterium oxide were from Deutero GmbH (Kastellaun, Germany). Ethyl acetate was from Acros Organics (Geel, Belgium). The water for analysis was generated by an Astacus Reagent bench (MembraPure GmbH, Henningsdorf/Berlin, Germany). Naringenin-7-O-glucoside was obtained from Extrasynthese (Lyon, France). Freiberg hide powder was purchased from the Research Institute for Leather and Plastic Sheeting GmbH (Freiberg, Germany).
3.2. Plant Material
The sliced bark of S. daphnoides (Art. 174702) was a gift from PhytoLab GmbH & Co. KG (Vestenbergsgreuth, Germany). A voucher specimen of this material is deposited at the Institute of Pharmaceutical Biology of the University of Regensburg (Regensburg, Germany).
3.3. Extraction Procedure
The plant material was powdered with a UZM 1 mill (Retsch GmbH, Haan, Germany) with a sieve size of 0.50 (619.8 g) and afterwards mixed with sea sand (1 + 1). Exhaustive percolation with 80% methanol revealed 143.6 g extract after evaporating and drying by lyophylisation (drug-extract-ratio = 4.32:1).
3.4. Sephadex® LH-20
Extract (100.0 g) was separated on Sephadex® LH-20 (265 g, GE Healthcare GmbH, München, Germany; column: 95 × 3 cm, flow = 1 mL/min) with 70% ethanol in three runs. The column chromatography was controlled by TLC on silica gel 60 F254 (Merck Chemicals GmbH; ethyl acetate/formic acid/water 90 + 5 + 5) with vanillin/HCl and anisaldehyde/H2SO4 as detection reagents. Salicin, naringenin-7-O-glucoside and catechin were used as reference compounds in TLC. After elution of catechin, the mobile phase was changed to 70% acetone. A salicylic alcohol derivative enriched (S1, 0–1196 mL, 75.35 g), a flavonoid enriched (S2, 1196–2045 mL, 5.14 g), and a proanthocyanidin enriched fraction (S3, 2045 mL until no more substances were detected in TLC-control, 6.59 g) were yielded.
Finally, S3 was re-chromatographed with Sephadex® LH-20 column with 70% ethanol and TLC-control with ethyl acetate/formic acid/acetic acid/water (100 + 11 + 11 + 26) and vanillin/HCl for detection to yield fractions S3.1 (689.2 mg, 0–2200 mL), S3.2 (643.2 mg, 2200–2986 mL), S3.3 (748.2 mg, 2986–4154 mL), S3.4 (237.8 mg, 4154–4634 mL), S3.5 (194.6 mg, 4634–5104 mL), S3.6 (512.5 mg, 5104–6489 mL), and S3.7 (415.8 mg, 6489–8264 mL). After S3.7 the mobile phase was changed to 70% acetone to yield S3.8 with polymeric proanthocyanidins (3259.8 mg).
3.5. Centrifugal Partition Chromatography (CPC)
A Spot Centrifugal Partition Chromatography machine with a 250 mL rotor (Armen Intrument, Saint-Avé, France; saturated 1-butanol/water system, flow = 5 mL/min, 1500 rpm) and a 510 HPLC pump (Waters GmbH, Eschborn, Germany) was used to enrich “unusual” proanthocyanidins from 1.0 g of S2. TLC-control was the same as in the re-chromatography of S3 (see Section 3.4.). First, the system was run in the ascending mode with the upper phase (saturated 1-butanol) as mobile phase. After 1269 mL, the system was changed to the corresponding descending mode with the lower phase (saturated water) as mobile phase and 678 mL were eluted. The combined fractions of the water-phase yielded in a fraction (S2 W) enriched with the “unusual” PAs (75.1 mg).
3.6. MCI-Gel®
The re-chromatographed fractions from the Sephadex® LH-20 system were further purified by MCI-Gel® CHP20P (170 g, Mitsubishi Chemical Europe GmbH, Düsseldorf, Germany; column: 600 × 25 mm (BESTA-Technik für Chromatographie GmbH, Wilhelmsfeld, Germany)) with a Spot Liquid Chromatography Flash machine (Armen Intrument; flow = 7.5 mL/min, 15.5 mL per fraction). A = 20% methanol, B = 50% methanol; gradient: 0–10 min 0% B, 10–300 min 0% → 100% B, 300–360 min 100% B. Subsequently two isocratic steps for 90 min were applied. The first one used 75% methanol the second one 100% methanol as eluent. TLC-control see Section 3.5.
3.7. Preparative HPLC
System A: binary Varian ProStar preparative HPLC (Varian Deutschland GmbH, Darmstadt, Germany) equipped with a diode array detector and a XDB-C18 PrepHT 21.2 × 250 mm 5-Micron column with guard column (Agilent Technologies Sales & Services GmbH & Co. KG, Waldbronn, Germany) and manual injection. A = water, B = acetonitrile, flow = 17 mL/min, gradient: 0–18 min 10% B isocratic, 18–19 min 10% → 75% B, 19–21 min 75% B isocratic; 21–23 min 75% B → 10% B, 23–28 min 10% B isocratic.
System B: binary Agilent Infinity 1260 HPLC equipped with a 1260 Agilent diode array detector, a 1260 Agilent fraction collector, a 1260 Agilent manual injector and a XDB-C18 PrepHT 21.2 × 250 mm 5-Micron column (Agilent) with guard column. Eluents as in system A, flow = 25.5 mL/min, gradient: 0–13 min 5% → 15% B,13–18 min 15% B isocratic, 18–20 min 15% → 100% B, 20–25 min 100% B isocratic, 25–27 min 100% → 5% B, 27–30 min 5% B isocratic.
System C: HPLC-system as system B, but with an Uptisphere DIOL 6 µm 250 × 21.2 Prep-LC-column with guard column (Interchim, Montluçon Cedex, France). A = acetonitrile, B = 95% methanol, flow = 15 mL/min, gradient:0–5 min 5% B isocratic, 5–25 min 5% → 30% B, 25–26 min 30% → 5% B, 26–30 min 5% B isocratic.
System D: as system C but applying a 10 mL/min flow.
3.8. Quantification of Tannins
To quantify the tannins in PAs rich fractions, the method 2.8.14 of the European Pharmacopeia [22] has been modified. 10.0 mg of a sample or tannic acid as reference were dissolved in 100.0 mL water. This solution contains the total phenols. 10.0 mL of this “total phenols solution” (TPS) is subjected to a flask with 100 mg Freiberg hide powder and stirred for 60 min without light. This preparation was filtered and represented the “not adsorbed phenols solution” (NAPS). Either 2.0 mL TPS or NAPS together with 1.0 mL folin-ciocalteu’s reagent were diluted with a sodium carbonate solution (53.0 g sodium carbonate anhydrous diluted with water to 500 mL) to 25.0 mL. After incubation without light for 15 min, absorbance was measured at 691 nm [23] with a Novaspec II (Pharmacia LKB, Uppsala, Sweden). All experiments were peformed three times and related to the value obtained with tannic acid as 100% reference using Equation (1) to calculate the content of tannins (A = absorbance at 691 nm; m = mass):
| (1) |
3.9. Quantification of Total Amount of Procyanidins and Qualitative Evaluation of Monomeric Constitution
To quantify the procyanidins in fractions, the acid hydrolysis described of the European Pharmacopoeia in the monograph of “Crataegi fructus” [24] has been modified. A sample (10.0 mg) was dissolved in a mixture of 16.0 mL 70% ethanol, 6.0 mL HCl (70.00 g HCl diluted with water to 100 mL) and 4.0 mL water. This preparation was heated under a reflux for 80 min. After filtration and washing, the solution was diluted to 100.0 mL. 50.0 mL were evaporated to approximately 3 mL and transferred to a separating funnel with 15 mL water. After extracting the water phase with 1-butanol (three times with 15 mL), the combined upper phases were diluted to 100 mL. Absorbance of this solution was measured at 545 nm with a Novaspec II (Pharmacia LKB). Each experiment was performed in triplicates. Content of procyanidins was calculated as cyanidin chloride with Equation (2) (A = absorbance at 545 nm; m = mass of examined substances (g)):
| (2) |
Further information about the monomeric constitution of oligo- and polymeric procyanidins were generated via analytical HPLC and LC-ESI-HRMS.
Analytical HPLC: LaChrom Elite (Hitachi High-Technologies Europe GmbH, Krefeld, Germany) equipped with a L-2130 oven (adjusted to 35 °C), a L-2130 pump-cluster, a L-2200 autosampler and a L-2455 diode array detector with a LiChroCART® 250-4, Purospher® STAR RP-18e (5 µm) (Merck Chemicals GmbH), injection volume = 10 µL, absorbance = 525 nm, flow = 0.8 mL/min, mobile phase: 18% acetonitrile containing 0.02% TFA. Retention times of reference compounds: delphinidin 5.91 min, cyanidin 10.07 min and pelargonidin 18.28 min.
LC-ESI-HRMS: 1290 Infinity UHPLC (Agilent) equipped with a G4220A binary pump cluster, a G4226A auto sampler, a G1316C column oven (adjusted to 40.00 °C), a G4212A diode array detector (recorded range: 190–640 nm), Q-TOF 6540 UHD with dual ESI as ion source and acquisition range = 80–1400 m/z and a Zorbax Eclipse Plus C18, 1.8 µm, 50 × 2.1 mm column (Agilent). A = water with 0.1% formic acid, B = acetonitrile with 0.1% formic acid, flow = 0.6 mL/min, gradient: 0.0–4.0 min 5% → 98% B, 4.0–5.0 min 98% B isocratic, 5.0–5.1 min 98% → 5% B, 5.1–6.0 min 5% B isocratic.
3.10. Estimation of the Degree of Polymerization (mDP) via Thiolysis
Sample preparation: around 3.0 mg of a sample were subjected to a microwave-vial, dissolved in 300 µL ethanol, 30 µL benzyl mercaptan and 15 µL acetic acid. Each vial was beaded with an elastic septum and incubated for five days at 95 °C to achieve a complete degradation. Subsequently, the reaction mix was dried under nitrogen and dissolved in 1.00 mL ethanol. This preparation was filtered and analyzed by analytical HPLC: LaChrom Elite (Hitachi High-Technologies Europe GmbH) equipped with a L-2130 oven (adjusted to 25 C), a L-2130 pump-cluster, a L-2200 autosampler and a L-2455 diode array detector with a Hibar® 250-4, Purospher® STAR RP-18e (5 µm) column (Merck Chemicals GmbH); flow = 1 mL/min, absorbance = 280 nm. A = water with 0.02% trifluoroacetic acid, B = methanol/acetonitrile/water (50 + 40 + 10). Gradient: 0–10 min 5% B isocratic, 10–20 min 5% → 30% B, 20–48 min 30% → 70% B, 48–52 min 70% B isocratic, 52–54 min 70% → 100% B, 54–65 min 100% B isocratic, 65–67 min 100% → 5% B, 67–73 min 5% B isocratic.
To calculate the mDP, all peak areas of resulting reaction products were added (terminal and extending units) and divided through the areas belonging to the terminal units. Each reaction was performed in hexaplicates, calculation of the mDP was achieved by Equation (3) after thiolysis (mDP = mean degree of polymerization; AUC = area under the curve):
| (3) |
LC-ESI-HRMS: The equipment was the same like 3.9. with a LiChroCART® 250-4, Purospher® STAR RP-18e (5 µm) (Merck Chemicals GmbH) column, column oven (25 °C), Q-TOF 6540 UHD (Agilent) with dual ESI as ion source and an acquisition range from 80 to 1400 m/z, flow = 1 mL/min, A = water with 0.1% formic acid, B = methanol/acetonitrile/water (50 + 40 + 10); gradient: 0–10 min 5% B isocratic, 10–20 min 5% → 30% B, 20–48 min 30% → 70% B, 48–52 min 70% B isocratic, 52–54 min 70% → 100% B.
3.11. Phloroglucinol Degradation
The method was modified from Bicker et al. [32]. An aliquot of the sample was dissolved in a spillover of acidic ethanolic phloroglucinol solution (1.0 mL hydrochloric acid diluted with ethanol to 100.0 mL, 5.6 mg/mL phloroglucinol). This preparation was incubated without light for 30 min at room temperature. Finally the solution was dried under nitrogen or evaporation. The products of this cleavage were analyzed by LC-ESI-HRMS. The equipment used for the LC-ESI-HRMS was the same as described at 3.9., but the column was a YMC-Triart C18, 1.9 µm, 75 × 2 mm (YMC Europe GmbH, Dinslaken, Germany), column oven (25 °C), flow = 0.6 mL/min; A = water with 0.1% formic acid, B = acetonitrile with 0.1% formic acid; gradient: 0–12 min 0% → 40% B, 12–14 min 40% → 98% B, 14–14.1 min 98% → 0% B, 14.1–16 min 0% B isocratic.
3.12. Peracetylation
To verify and compare some structures by 1H-NMR with literature data, peracetylation was necessary. Samples were dissolved (0.5 mL pyridine and 0.5 mL acetic anhydride) and incubated without light and with moderate shaking for 24 h. Subsequently, the reaction product was precipitated by adding water. For further 12 h, the preparation was incubated in a fridge. To complete the procedure, the compound was washed three times with ice cooled water and finally dissolved in acetone. To dry the substance yielded by this extraction, the acetone was evaporated.
3.13. NMR Spectroscopy
All shift values (δH and δC) are indicated in ppm, coupling constants in Hz. All free phenolic samples were measured in methanol-d4, the peracetylated compounds in chloroform-d1, and the proanthocyanidin enriched fractions in a mixture of methanol-d4/deuterium oxide (7 + 3). All spectra were referenced against residual undeuterated solvent. To get sharp NMR-signals from the samples showing rotary isomerism, low temperature measurements at 233 K and 253 K were necessary. These measurements were performed on a AVANCE III HD NMR (Bruker Corporation, Billerica, MA, USA) operating at 400.13 MHz (1H-NMR) and 100.63 MHz (13C-NMR). The spectra to the structures being accessible to NMR at room temperature were measured with a AVANCE III 600 NMR (Bruker Corporation) equipped with a Bruker 5 mm TCI CryoProbe operating at 600.25 MHz (1H-NMR) and 150.95 MHz (13C-NMR). For structure verification and elucidation, 1D-1H, 1D-13C, [1H-13C]-HSQC, [1H-13C]-HMBC, [1H-1H]-COSY and [1H-1H]-ROESY experiments were performed. The 1D-1H-NMR- and 1D-13C-NMR-spectra of the peracetates were performed on a AVANCE 300 (Bruker Corporation) operating at 300.13 MHz (1H-NMR) and 75.48 MHz (13C-NMR).
3.14. Mass Spectrometry
ESI-HRMS for structure elucidation and verification were performed on a Q-TOF 6540 UHD (Agilent).
3.15. Circular Dichroism
CD-spectra were acquired by a J-715 spectropolarimeter (JASCO Deutschland GmbH, Gross-Umstadt, Germany) operating at 22 °C. Area of measurement was 200–400 nm in 0.5 nm steps with 200 nm/min and 10 cycles. 1 mm Quartz cuvettes were used. Samples were all solved in methanol for spectroscopy, but the fraction containing the “unusual” PAs was solved in a mixture of methanol/water (80 + 20). Compounds 1 to 6 were concentrated at 175.0 µmol/L, compound 7 was concentrated at 36.6 µmol/L. The concentrations of the fractions S2 W and S3.8 were 0.06 mg/mL and 0.12 mg/mL respectively. The spectra were smoothed by the Savitzky-Golay algorithm with a convolution width of 15.
3.16. Polarimetry
Polarimetry of the isolated compounds was achieved by a UniPol L 1000 polarimeter (Schmidt + Haensch GmbH & Co., Berlin, Germany). The samples were solved in methanol for spectroscopy with a concentration of 0.1% and were measured at 589.30 nm in a micro tube (length = 50 mm, volume = 0.55 mL). To estimate the 2,3-cis to 2,3-trans ratio of the oligomeric and polymeric PAs, Equation (4) was used after determinating with a 341 Polarimeter (Perkin Elmer, Inc., Waltham, MA, USA) in a micro tube (length = 100 mm, volume = 1.0 mL; χcis = amount of 2,3-cis configurated flavan-3-ols included in a PA; = specific rotation of a PAs enriched fraction) [21,31]:
| (4) |
3.17. Isolated Compounds
Epicatechin-(4β→8)-catechin, procyanidin B1 (1): 70.9 mg; +21.1; CD (methanol, Θ) 206 (–89460.7), 216 (+99040.8), 233.5 (+36458.0), 279.5 (−1236.81). Negative HR-ESI-MS m/z 577.1370 [M − H]−.
Epicatechin-(4β→8)-epicatechin, procyanidin B2 (2): 11.7 mg; +55.6; CD (methanol, Θ) 205.5 (−88801.9), 216 (+74024.2), 236.5 (+30663.3), 273.5 (+2531.16). Negative HR-ESI-MS m/z 577.1358 [M − H]−.
Catechin-(4α→8)-catechin, procyanidin B3 (3): 45.8 mg; −180.1; CD (methanol, Θ) 213.5 (−256750.0), 236 (−66990.6), 269.5 (+9984.63), 285 (+142.118). Negative HR-ESI-MS m/z 577.1369 [M − H]−.
Catechin-(4α→8)-epicatechin, procyanidin B4 (4): 7.4 mg; −180.1; CD (methanol, Θ) 213 (−173964.0), 236 (−38178.2), 252 (+5365.08), 274 (+7867.0). Negative HR-ESI-MS m/z 577.1364 [M − H]−.
Epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin, procyanidin C1 (5): 8.7 mg; +45.8; CD (methanol, Θ) 205.5 (−161056.0), 217 (+121294.0), 229.5 (+79868.8), 238 (+63979.8), 279 (−8569.24). Negative HR-ESI-MS m/z 865.2001 [M − H]−.
Epicatechin-(4β→8)-epicatechin-(4β→8)-catechin (6): 20.5 mg; +115.3; CD (methanol, Θ) 205.5 (−132845.0), 218 (+112144.0), 229.5 (+67413.4), 237 (+58945.7), 280 (−6730.77). Negative HR-ESI-MS m/z 865.1988 [M − H]−.
Epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin-(4β→8)-catechin (7): 15.3 mg; +74.9; CD (methanol, Θ) 206 (−237987.0), 219.5 (+220751.0), 237.5 (+102010.0), 248 (+25022.5), 279 (−11654.7). Negative HR-ESI-MS m/z 1153.2624 [M − H]−.
Fraction S2 W (“unusual” PAs): CD (methanol/water 80 + 20, Θ) 205.5 (−92820.8), 221.5 (+86787.4), 227 (+95101.3), 280.5 (−8054.69).
Fraction S3.8 (“regular” PAs): +111.1; CD (methanol, Θ) 205.5 (−515604.0), 221.5 (+590460.0), 238 (+429040.0), 279.5 (−30794.0).
4. Conclusions
A systematic investigation of the proanthocyanidin pattern of S. daphnoides revealed the presence of four dimeric, two trimeric as well as a tetrameric procyanidin. In contrast to Pobłocka-Olech et al. [8], who purposed that willow bark does not contain procyanidin B2, this compound could be isolated. Furthermore two kinds of polar and presumably polymeric PA fractions (“regular” PAs and “unusual” PAs) were investigated and compared with different chromatographic and spectroscopic methods. A 2,3-cis configuration predominates in both fractions. The fraction with the “regular” PAs consists of slightly more tannins with more trihydroxylated constituent flavan-3-ol units and longer chains compared to the fraction with the “unusual” PAs. The identification of B-ring vicinal trihydroxylated flavan-3-ol units in polymeric PAs is in accordance with Foo and coworkers [33] who found them in Salix caprea L. However, that these observed slight differences of both fractions are responsible for the different chromatographic behavior on Sephadex® LH-20 seems to be questionable. Further studies are necessary to satisfactorily describe chemical differences of the “unusual” and “regular” PA fractions.
Acknowledgments
Many thanks are due to J. Heilmann for financial support and helpful discussions, to A. Hensel and T. Schmidt (University of Münster) for supporting this scientific collaboration. G. Brunner, M. Untergehrer are acknowledged for their technical support, F. Kastner, A. Schramm and G. Stühler for performing the NMR-experiments, J. Kiermaier for achieving the ESI-MS measurements, Axel Dürkop for generating the CD-spectra (all University of Regensburg), Marcus Lautenschläger (University of Münster) for dividing his experiences in HPLC method development, PhytoLab GmbH & Co. KG (Vestenbergsgreuth, Germany) for donating the plant material and the students for the work during their research practical courses (Wahlpflichtpraktikum at University of Regensburg).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/08/13674/s1.
Author Contributions
S.W. carried out the experimental work, except the polarimetry of the PA enriched fractions was performed by F.P.G.J. and F.P. supervised and designed the work. S.W., G.J. and F.P. prepared the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Europäisches Arzneibuch 8. Ausgabe, 2. Nachtrag. 8th ed. Volume 2. Deutscher Apotheker Verlag; Stuttgart, Germany: 2015. Weidenrinde: Salicis cortex; pp. 2135–2136. [Google Scholar]
- 2.E/S/C/O/P Monographs: The Scientific Foundation for Herbal Medicinal Products. 2nd ed. Thieme; Exeter, UK; Stuttgart, Germany; New York, NY, USA: 2003. Salicis cortex; pp. 445–451. [Google Scholar]
- 3.Meier B., Meier-Liebi M. Salix. In: Blaschek W., Hager H., editors. Hagers Enzyklopädie der Arzneistoffe und Drogen. 6th ed. Volume 14. Wissenschaftliche Verlagsgesellschaft; Stuttgart, Germany: 2007. pp. 81–115. [Google Scholar]
- 4.Knuth S., Abdelsalam R.M., Khayyal M.T., Schweda F., Heilmann J., Kees M.G., Mair G., Kees F., Jürgenliemk G. Catechol conjugates are in vivo metabolites of Salicis cortex. Planta Med. 2013;79:1489–1494. doi: 10.1055/s-0033-1350898. [DOI] [PubMed] [Google Scholar]
- 5.Nahrstedt A., Schmidt M., Jäggi R., Metz J., Khayyal M.T. Willow bark extract: the contribution of polyphenols to the overall effect. Wien. Med. Wochenschr. 2007;157:348–351. doi: 10.1007/s10354-007-0437-3. [DOI] [PubMed] [Google Scholar]
- 6.Butterweck V., Liefländer-Wulf U., Winterhoff H., Nahrstedt A. Plasma levels of hypericin in presence of procyanidin B2 and hyperoside: A pharmacokinetic study in rats. Planta Med. 2003;69:189–192. doi: 10.1055/s-2003-38495. [DOI] [PubMed] [Google Scholar]
- 7.Jürgenliemk G., Nahrstedt A. Dissolution, solubility and cooperativity of phenolic compounds from Hypericum perforatum L. in aqueous systems. Die Pharm. 2003;58:200–203. [PubMed] [Google Scholar]
- 8.Pobłocka-Olech L., Krauze-Baranowska M. SPE-HPTLC of procyanidins from the barks of different species and clones of Salix. J. Pharm. Biomed. Anal. 2008;48:965–968. doi: 10.1016/j.jpba.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Shoji T., Mutsuga M., Nakamura T., Kanda T., Akiyama H., Goda Y. Isolation and structural elucidation of some procyanidins from apple by low-temperature nuclear magnetic resonance. J. Agric. Food Chem. 2003;51:3806–3813. doi: 10.1021/jf0300184. [DOI] [PubMed] [Google Scholar]
- 10.Kolodziej H. Synthesis and characterization of procyanidin dimers as their peracetates and octamethyl ether diacetates. Phytochemistry. 1986;25:1209–1215. doi: 10.1016/S0031-9422(00)81582-4. [DOI] [Google Scholar]
- 11.Saito A., Nakajima N., Tanaka A., Ubukata M. Synthetic studies of proanthocyanidins. Part 2: Stereoselective gram-scale synthesis of procyanidin-B3. Tetrahedron. 2002;58:7829–7837. doi: 10.1016/S0040-4020(02)00936-5. [DOI] [PubMed] [Google Scholar]
- 12.Saito A., Nakajima N., Tanaka A., Ubukata M. Synthetic studies of proanthocyanidins. Part 4. The synthesis of procyanidin B1 and B4: TMSOTf-catalyzed cyclization of catechin and epicatechin condensation. Heterocycles. 2003;61:287. doi: 10.3987/COM-03-S37. [DOI] [Google Scholar]
- 13.Mohri Y., Sagehashi M., Yamada T., Hattori Y., Morimura K., Kamo T., Hirota M., Makabe H. An efficient synthesis of procyanidins. Rare earth metal Lewis acid catalyzed equimolar condensation of catechin and epicatechin. Tetrahedron Lett. 2007;48:5891–5894. doi: 10.1016/j.tetlet.2007.06.047. [DOI] [Google Scholar]
- 14.Thompson R.S., Jacques D., Haslam E., Tanner R.J.N. Plant proanthocyanidins. Part I. Introduction; the isolation, structure, and distribution in nature of plant procyanidins. J. Chem. Soc. Perkin Trans. 1. 1972:1387–1399. doi: 10.1039/p19720001387. [DOI] [Google Scholar]
- 15.Barrett M.W., Klyne W., Scopes P.M., Fletcher A.C., Porter L.J., Haslam E. Plant proanthocyanidins. Part 6. Chiroptical studies. Part 95. Circular dichroism of procyanidins. J. Chem. Soc. Perkin Trans. 1. 1979:2375–2377. doi: 10.1039/p19790002375. [DOI] [Google Scholar]
- 16.Botha J.J., Ferreira D., Roux D.G. Condensed tannins. Circular dichroism method of assessing the absolute configuration at C-4 of 4-arylflavan-3-ols, and stereochemistry of their formation from flavan-3,4-diols. J. Chem. Soc. Chem. Commun. 1978:698–700. doi: 10.1039/c39780000698. [DOI] [Google Scholar]
- 17.Botha J.J., Young D.A., Ferreira D., Roux D.G. Synthesis of condensed tannins. Part 1. Stereoselective and stereospecific syntheses of optically pure 4-arylflavan-3-ols, and assessment of their absolute stereochemistry at C-4 by means of circular dichroism. J. Chem. Soc. Perkin Trans. 1. 1981:1213–1219. doi: 10.1039/p19810001213. [DOI] [Google Scholar]
- 18.Saito A., Tanaka A., Ubukata M., Nakajima N. Efficient stereoselective synthesis of proanthocyanidin trimers with TMSOTf-catalyzed intermolecular condensation. Synlett. 2004:1069–1073. doi: 10.1055/s-2004-822905. [DOI] [Google Scholar]
- 19.Esatbeyoglu T., Jaschok-Kentner B., Wray V., Winterhalter P. Structure elucidation of procyanidin oligomers by low-temperature 1H-NMR spectroscopy. J. Agric. Food Chem. 2011;59:62–69. doi: 10.1021/jf1032334. [DOI] [PubMed] [Google Scholar]
- 20.Saito A., Mizushina Y., Tanaka A., Nakajima N. Versatile synthesis of epicatechin series procyanidin oligomers, and their antioxidant and DNA polymerase inhibitory activity. Tetrahedron. 2009;65:7422–7428. doi: 10.1016/j.tet.2009.07.018. [DOI] [Google Scholar]
- 21.Czochanska Z., Foo L.Y., Newman R.H., Porter L.J. Polymeric proanthocyanidins. Stereochemistry, structural units, and molecular weight. J. Chem. Soc. Perkin Trans. 1. 1980:2278–2286. doi: 10.1039/p19800002278. [DOI] [Google Scholar]
- 22.Europäisches Arzneibuch 8. Ausgabe, 2. Nachtrag. 8th ed. Volume 1. Deutscher Apotheker Verlag; Stuttgart, Germany: 2015. 2.8.14: Gerbstoffe in pflanzlichen Drogen; p. 383. [Google Scholar]
- 23.Glasl H. Zur Photometrie in der Drogenstandardiserung. DAZ. 1983;123:1979–1987. [Google Scholar]
- 24.Europäisches Arzneibuch 7. Ausgabe. 7th ed. Volume 1. Deutscher Apotheker Verlag; Stuttgart, Germany: 2011. Weißdornfrüchte: Crataegi fructus; pp. 1933–1934. [Google Scholar]
- 25.Zhang Z., Kou X., Fugal K., McLaughlin J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J. Agric. Food Chem. 2004;52:688–691. doi: 10.1021/jf034596w. [DOI] [PubMed] [Google Scholar]
- 26.Dauer A., Rimpler H., Hensel A. Polymeric Proanthocyanidins from the bark of Hamamelis virginiana. Planta Med. 2003;69:89–91. doi: 10.1055/s-2003-37022. [DOI] [PubMed] [Google Scholar]
- 27.Matthews S., Mila I., Scalbert A., Pollet B., Lapierre C., Hervé du Penhoat C.L.M., Rolando C., Donelly D.M.X. Method for estimation of proanthocyanidins based on their acid depolymerization in the presence of nucleophiles. J. Agric. Food Chem. 1997;45:1195–1201. doi: 10.1021/jf9607573. [DOI] [Google Scholar]
- 28.Eberhardt T.L., Young R.A. Conifer seed cone proanthocyanidin polymers: Characterization by 13C-NMR spectroscopy and determination of antifungal activities. J. Agric. Food Chem. 1994;42:1704–1708. doi: 10.1021/jf00044a023. [DOI] [Google Scholar]
- 29.Newman R.H., Porter L.J., Foo L.Y., Johns S.R., Willing R.I. High-resolution 13C-NMR studies of proanthocyanidin polymers (condensed tannins) Magn. Reson. Chem. 1987;25:118–124. doi: 10.1002/mrc.1260250207. [DOI] [Google Scholar]
- 30.Jürgenliemk G., Petereit F., Nahrstedt A. Flavan-3-ols and procyanidins from the bark of Salix purpurea L. Pharmazie. 2007;2007:231–234. [PubMed] [Google Scholar]
- 31.Porter L.J. Condensed Tannins. In: Rowe J.W., editor. Natural Products of Woody Plants: Chemicals Extraneous to the Lignocellulosic Cell Wall. Volume 1. Springer Verlag; Berlin, Germany: 1989. pp. 651–690. [Google Scholar]
- 32.Bicker J., Petereit F., Hensel A. Proanthocyanidins and a phloroglucinol derivative from Rumex acetosa L. Fitoterapia. 2009;80:483–495. doi: 10.1016/j.fitote.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Foo L.Y., Porter L.J. Prodelphinidin polymers: Definition of structural units. J. Chem. Soc. Perkin Trans. 1. 1978:1186–1190. doi: 10.1039/p19780001186. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.