Abstract
Dihydro-5,6-dehydrokavain (DDK) is the major and most promising component of the tropical plant Alpinia zerumbet (shell ginger), a species of the ginger family Zingiberaceae. Alpinia zerumbet is known for its human use as a traditional herbal medicine, food, and dietary supplement. With its α-lactone ring, DDK belongs to the large chemical group of kavalactones, which are also found in kava (Piper methysticum), another herbal medicine; DDK is characterized by a double-bond linkage at positions 5,6 and the absence of a double-bond linkage at positions 7,8. This dissociates DDK from other kavalactones with their linkages at positions 7,8 and 5,6 that are both either completely saturated or unsaturated, or may have an unsaturated bond at the position 7,8 as well as a saturated bond at the position 5,6. DDK is easily identified and quantified by HPLC and GC. DDK contents in fresh leaves, stems and rhizomes range from 80 to 410 mg/g, requiring solvent extraction procedures to ensure high DDK yield. This is best achieved by hexane extraction from fresh rhizomes that were previously boiled in water, allowing DDK yields of up to 424 mg/g. Successful synthesis of DDK can be achieved by asymmetric pathways, whereas its simple chemical structure facilitates the synthesis of DDK derivatives by HCl hydrolysis. Thus, all synthesized products may be used for various commercial purposes, including the potential development of promising antiobesity pharmaceutical drugs, preparation of specific and safe dietary supplements, and use as effective natural herbicides or fungicides.
Keywords: DDK; dihydro-5,6-dehydrokavain; dehydrokavain; kavalactones; shell ginger; Alpinia zerumbet; Piper methysticum
1. Introduction
Dihydro-5,6-dehydrokavain (DDK) is the major constituent of Alpinia zerumbet, a plant widely growing in the tropics [1] that represents a species of the Alpinia group [2]. Alpinia is the largest genus of the family Zingiberaceae, classified by Charles Plumier, and is named after Prospero Alpino, the well-known Italian botanist of the sixteenth century. Plants of the genus Alpinia and their constituents have numerous positive effects, including antimicrobial, antiparasitic, insecticidal, anticancer, antiproliferative, antiinflammatory, analgesic, antiallergic, neuroprotective, and antioxidant properties [2]. Some plants of the Alpinia genus also may exert specific beneficial effects, related to osteoarthritis [3], aging [4], gastric cancer [5], and diabetes [6]. Similar to these positive features [2,3,4,5,6] are the promising properties specifically described for Alpinia zerumbet and its anti-obesity effects elicited by DDK and 5,6-dehydrokawain (DK) [6,7] including its derivative hispidin that can be obtained by hydrolysis in stomach acid and subsequent metabolism by hepatic microsomal CYP2C9 [8]. All three chemicals increased lipolysis when incubated with differentiated 3T3-L1 adipocytes, using glycerol release as parameter. Compared to controls, hispidin, DDK, or DK significantly increased glycerol release by 276%, 225%, or 137%. All these compounds also reduced intracellular triglycerides in a dose-dependent manner [7]. Therefore, Alpinia zerumbet with its ingredients and hispidin may well establish a new therapeutic principle for anti-obesity with lipocytes as the primary organ target rather than liver or intestinal cells.
Most of these properties have been reported in experimental studies and not yet in humans [1,2,3,4,5,6]. Nevertheless, it appears that plants of the Alpinia genus have some potential of further research and development. Others have even called Alpinia the gold mine of future therapies [2]. Whether or not this perspective also applies to Alpinia zerumbet syn. Alpinia speciosa remains to be established by further research and clinical trials, proving therapeutic efficacy, lack of major adverse reactions, and establishing a positive benefit/risk profile. These next steps will be challenging and require the expertise of clinicians familiar with the criteria of evidence based medicine, focusing on those diseases that best profit from a tentative treatment by Alpinia zerumbet.
The present review article provides detailed information of current developments and trends in research of Alpinia zerumbet with focus on DDK, its extraction, properties, and synthesis. For reasons of comparison, DDK merits additional but only brief attention, as this chemical is a component also of another plant, namely kava (Piper methysticum). Water-based herbal extracts derived from kava rhizomes exert anxiolytic properties [9,10,11,12,13] and are known for their therapeutic use after proven efficacy by evidence based medicine criteria [13]. Questions of therapeutic efficacy in selected ailments also pertain to Alpinia zerumbet, the key issue will be how to achieve clinical trials to establish efficacy in few selected ailments. Based on present knowledge, a systematic approach should also be feasible to further characterize A. zerumbet with its DDK.
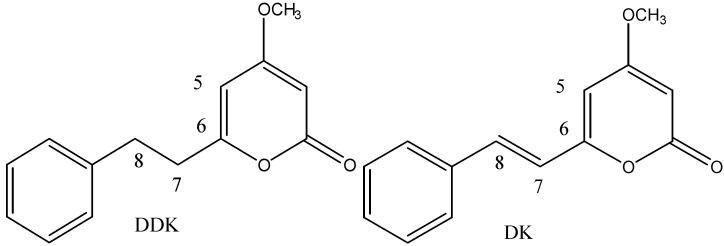
2. Chemical Structure of DDK
According to their pyrone moieties, kavalactones can be classified as types A–D through the presence or absence of double-bond linkages at positions 5,6 and 7,8. Type A is characterized by the absence of double-bond linkages in both positions 5,6 and 7,8. Conversely, type B has an unsaturated bond at position 7,8, and type C is completely unsaturated at both positions [9]. Type D has a saturated bond at position 7,8 but position 5,6 is unsaturated. Therefore, DDK belongs to the type D, its derivative DK to type C (Figure 1). Structurally, the biological activities of kavalactones depend on differences of linkages at positions 5,6 and 7,8 [9]; this likely explains the various biological activities of the individual kavalactones including DDK and DK present in Alpinia zerumbet and kava, but further studies are needed.
Figure 1.
Chemical structure of the kavalactones DDK and its derivative DK isolated from Alpinia zerumbet (shell ginger) and from Piper methysticum (kava).
3. Isolation and Identification of DDK
3.1. DDK Isolation from Alpinia zerumbet
DDK can be isolated from fresh rhizomes of Alpinia zerumbet by extraction with methanol [14]. This methanol extract is shaken with petrol and then with chloroform. From the chloroform soluble fraction, DDK may be separated by silica gel chromatography. As an alternative applied to Alpinia zerumbet leaves, extraction with acetone requiring one month was described [15]. An essential oil fraction is obtained by steam distillation of the extract. Its non-volatile fraction is extracted with n-hexane, benzene, ethyl acetate, and then n-butanol. The n-hexane and benzene extracts were fractionated by silica gel column chromatography to yield DDK.
Although these methods lead to a successful isolation of DDK, the overall isolation procedures remain complex and time-consuming. Subsequently, a more practicable and simplified method to isolate DDK was described by soaking leaves of Alpinia zerumbet in boiling water for 2–3 h [15]. These water based crude extracts can be further processed by extraction using chloroform as solvent to yield DDK, but traces of impurities consisting of essential oils and DK commonly remain. A yellowish solid material is obtained by evaporating the chloroform layer, and the resulting product again is dissolved in boiling water, followed by rapidly filtering out the insoluble matter [15]. The resulting filtrate is crystallized at 8 °C to yield DDK. This isolation method for DDK is superior to other reported methods because column chromatography is not any more necessary, but it is hampered by the use of chloroform with its known toxicity risk. In our laboratory, hexane replaces now the chloroform as solvent to reduce the inhalative risk of chloroform toxicity. This is associated with a higher yield of DDK compared to chloroform [9].
3.2. DDK Isolation from Kava
Kava contains at least 18 kavalactones and many other compounds, rendering the isolation of DDK from its extracts much more complex [9] than its simple isolation from the hexane layer of Alpinia zerumbet [16,17,18]. Column chromatography with acetone, chloroform, and water is effective to separate DDK and major kavalactones from kava roots [9]. Other solvents such as methanol, ethanol, and hexane are less effective [14]. Protocols for identification and isolation of DDK, using HPLC, GC-MS, LC-MS, TLC, and CC, were established [15,16,17,18,19]. The effectiveness of different extracting solvents regarding DDK yields was also examined [9].
3.3. Identification
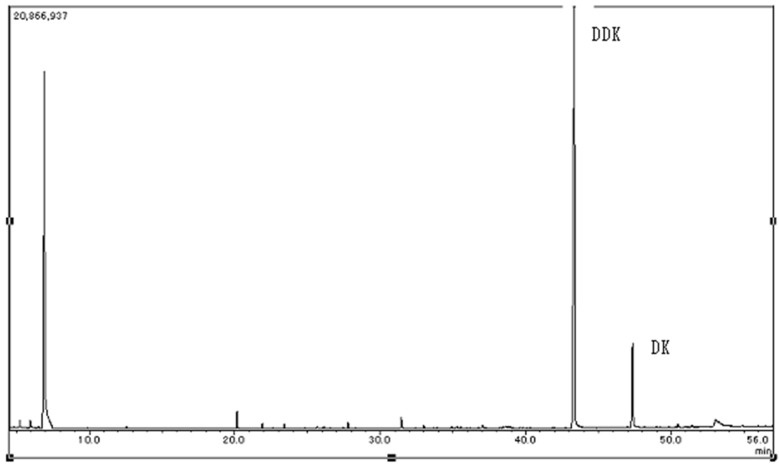
DDK can be identified by GC-MS (QP-2010, Shimadzu Co., Kyoto, Japan) [17,18]. DDK and DK were detected at 43.3 and 47.3 min, respectively. Other peaks were unknown (Figure 2).
Figure 2.
Detection of DDK and DK by GC-MS, obtained from hexane extract derived from leaves of Alpinia zerumbet [17,18].
The isolation of DDK was first reported 40 years ago from Alpinia zerumbet rhizomes [20], and its structure of dihydro-5,6-dehydrokavain was assessed by spectroscopic methods of IR, NMR, and mass spectrum [8,14,20]. The presence of DDK in Alpinia zerumbet leaves was later confirmed, using 1H-NMR, 13C-NMR, IR, FDMS and EIMS [9]. DDK was also found in other plant parts of Alpinia zerumbet [15,16,17,18,21]. LC-MS is also efficient to determine DDK but GC-MS is much convenient as DDK is not completely dissolved in LC solvents such as water and methanol.
4. Efficacy of Extraction Solvents
As the structure of DDK looks hydrophobic, several organic extracting solvents such as methanol and acetone have been applied to isolate DDK from Alpinia zerumbet [8,14,20], but the yields were low and did in no way reflect the actual DDK amounts present in the plant. To increase the yield and efficacy of isolation, the leaves or rhizomes of A. zerumbet were boiled in water for 2–3 h, and after cooling and filtering, the water extract was then subjected to various organic solvents as the preferred method [15]. Actually, the melting point of DDK is 96–97 °C, enhancing the release of large amounts of DDK from Alpinia plant parts and reaching DDK yields of up to 424 mg/g and 148 mg/g from fresh rhizomes and leaves, respectively [18]. These figures were higher compared to previous research when fresh plant parts of Alpinia zerumbet were cut and soaked by ethanol for one week, which resulted in lower DDK amounts of 350 mg/g in fresh rhizomes but with a high purity (>95%) of DDK [15]. Among the boiled solvents, chloroform, hexane, methanol, ethanol, and acetone had been examined [9]. Chloroform and hexane are the most effective solvents to yield large amounts of DDK; under toxicity aspects, hexane is now the preferred solvent over chloroform.
5. Quantification of DDK
5.1. Approaches
DDK can be quantified by HPLC as described earlier [15], with some modifications such as the use of gradient and column as described below to improve the accuracy of DDK quantification [17,18]. DDK was detected at 35 min by comparing the retention time of the authentic compound.
5.2. Content of DDK in Alpinia zerumbet
DDK and DK as well as multiple other chemicals are found in Alpinia zerumbet (Table 1). Generally, all plant parts of Alpinia zerumbet including leaves, stems, rhizomes, flowers, and seeds contain both DDK [15,19] and its derivative 5,6-dehydrokavain (DK, Figure 1) [14,18,19]. Other compounds of relevance include phenolics, essential oils, labdadienes, catechins, kaempferol, zerumins, chalcones, flavanones, chalcones, cardamonin, alpinetin, and rutin (Table 1) [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Major bioactive chemicals are in the leaves, but some are found in rhizomes and seeds (Table 1). Essential oils from leaves and rhizome have main components of 1,8-cineol, camphor and methyl cinnamate [17,18,19]. At an industrial level, essential oils of Alpinia zerumbet are obtained by steam distillation of its leaves. On a quantitative basis, the yields of essential oils as the preferred products are low. From about 100 kg fresh leaves of Alpinia zerumbet, 100 L of distillate can be obtained, from which at best 100 mL of essential oils can be produced. In Japan with preference of Okinawa, oils of Alpinia zerumbet are used as cosmetics, perfumes, and soaps [19]. Isothymol, thymol, and eugenol in the essential oils of Alpinia zerumbet possess strong antifungal activity against plant pathogenic fungi [19]. Antioxidant and antibacterial activities of DDK, essential oils, and phenolics in alpinia were investigated, which were also sprayed with copper sulphate [17]. After stressed by copper sulphate, DDK increased but essential oils decreased, suggesting that DDK plays some role in plant defense system of alpinia [17].
Table 1.
DDK, DK, and other chemicals in various plant parts of Alpinia zerumbet.
| Chemical Name | Plant Part | References |
|---|---|---|
| Dihydro-5,6-dehydrokawain (DDK) | Leaves, stems, rhizomes | [15] |
| 5,6-Dehydrokawain (DK) | Leaves, stems, rhizomes | [15] |
| Essential oils | Leaves, roots | [17,18,19,20] |
| (E)-Labda-8(17)-12-diene-15-ol-16-al | Rhizomes | [21] |
| (E)-15,16-Bisnorlabda-8(17)-11-diene-13-one | Rhizomes | [21] |
| Phenolic acids | Leaves, stems, rhizomes | [14,17,18] |
| 12-Labdaiene-15,16-dial (labdadiene) | Rhizomes | [22] |
| Rutin | Leaves | [23] |
| Kaempferol-3-o-glucuronide | Leaves | [23] |
| (+) Catechin | Leaves | [23] |
| (−) Epicatechin | Leaves | [23] |
| p-Hydroxycinnamalehyde | Rhizomes | [24] |
| [Di-(p-hydroxy-cis-styryl)] methane | Rhizomes | [24] |
| 1,7-Diarylheptanoid | Rhizomes | [25] |
| Zerumin A | Seeds | [26] |
| Zerumin B | Seeds | [26] |
| Kaempferol-3-o-rutinoside | Leaves | [27] |
| Coronarin E | Seeds | [26,27] |
| (E)-15,16-Bisnorlabda-(8(17)-11-diene-13-one | Seeds | [28] |
| Dihydroflavokavain B | Rhizomes | [14] |
| Cardamonin (2′,4′-Dihydroxy-6′-methoxy chalcone) | Seeds | [28] |
| Alpinetin (7-hydroxy-5-methoxy flavanone) | Seeds | [28] |
| Trans-1-(4′-Hydroxy-3′-methoxyphenyl-7-phenylhept-1-en-3-one (yakuchinone-B) | Pericarps | [29] |
| 1-(4′-hydroxy-3′-methoxyphenyl)-7-phenyl-3-heptanone | Fruits | [30] |
| Labda-8(17),12-diene-15,16-dial | Rhizomes | [31] |
| Bisabolanes | Leaves | [31] |
| Steroids | Seeds | [32] |
On a quantitative basis, DDK and DK show differences in amounts in fresh leaves, stems, and rhizomes of Alpinia zerumbet, with higher amounts of DDK compared to DK in all plant parts (Table 2) [15]. Contents of DDK and essential oils in alpinia are varied among varieties, locations, and time within a year (unpublished laboratory data). The reason why alpinia accumulates such a large amount of DDK and DK in leaves and rhizomes is unknown. It can be proposed that DDK and DK may play an important role in allelopathy of alpinia to suppress growth of other plants in its vicinity and expands its population in the plant ecosystem.
Table 2.
DDK and DK contents in plant parts of Alpinia zerumbet [15].
| Compounds | Quantity (mg/g of Fresh Weight) | ||
|---|---|---|---|
| Leaves | Stems | Rhizomes | |
| Dihydro-5,6-dehydrokawain (DDK) | 410.0 | 80.0 | 350.0 |
| 5,6-Dehydrokawain (DK) | 10.0 | 20.0 | 100.0 |
5.3. Content of DDK in Kava
In addition to Alpinia zerumbet (Table 1 and Table 2), DDK is also found in kava (Piper methysticum) rhizomes (Table 3) as one of a total of 18 kavalactones [9]. Compounds other than DDK, DK, and kavalactones are flavokavains, phenolics, chalcones, pipermethystine, flavanones, and glutathione (Table 3) [9,33,34,35,36]. Except for DDK, DK, and other kavalactones, phenolic acids, and glutathione that were quantified, contents of other compounds such as chalcones, flavanones, cinnamic acid bornyl ester, 2,5,8-trimethyl-1-naphthol, 5-methyl-1-phenylhexen-3-yn-5-ol have not been determined in kava [9,33,34,35,36]. All compounds detected in kava were from rhizomes (Table 3), but their presence in leaves and stems have not been studied.
Table 3.
DDK, DK, and other chemicals in various plant parts of kava.
| Chemical Name | Plant Part | References |
|---|---|---|
| Phenolic acids | Rhizomes | [33] |
| Dihydro-5,6-dehydrokavain (DDK) | Rhizomes | [9] |
| 5,6-Dehydrokavain (DK) | Rhizomes | [9] |
| Other kavalactones: dihydromethysticin, 7,8-dihydrokavain, kavain, methysticin, desmethoxyyangonin, yangonin, dihydro-5,6-dehydrokavain, methoxyyangonin, hydroxykavain, 7,8-dihydrokavain, 11-hydroxy-12-methoxydihydrokavain, 5,6,7,8-tetrahydroyangonin, 5,6-dehydromethysticin 11,12-dimethoxydihydrokavain, 11-methoxy-12-hydroxydehydrokavain, 11-hydroxyyangonin | Rhizomes | [9] |
| Flavokavain A | Rhizomes | [9] |
| Flavokavain B | Rhizomes | [9,34,35] |
| Flavokavain C | Rhizomes | [9] |
| Cinnamic acid bornyl ester | Rhizomes | [9] |
| 5,7-Dimethoxyflavanone | Rhizomes | [9,35] |
| 2,5,8-Trimethyl-1-naphthol | Rhizomes | [9] |
| 5-Methyl-1-phenylhexen-3-yn-5-ol | Rhizomes | [9] |
| 8,11-Octadecadienoic acid-methyl ester | Rhizomes | [9] |
| Pinostrobin chalcone | Rhizomes | [9,35] |
| 5-hydroxy-4′-7-Dimethoxyflavanone | Rhizomes | [9] |
| 5,7(OH)2-4′-one-6,8-dimethylflavone | Rhizomes | [9] |
| Pipermethystine | Rhizomes | [9,36] |
| Glutathione | Rhizomes | [9] |
The yields of DDK show a wide range, depending on the type of the extracting medium: hexane, ethanol, methanol, chloroform, acetone, or water (Table 4) [9]. Among the seven major kavalactones, there are four different categories, types A-D [9]: to type A belong the kavalactones dihydromethysticin and 7,8-dihydrokavain; to type B kavain and methysticin; to type C desmethoxyyangonin and yangonin; and to type D dihdydro-5,6-dehydrokavain (Table 4). In kava roots and on a quantitative basis, kavalactone type A predominates with variable results for the other types; among the examined extracting solvents, acetone is the most effective one, followed by water and chloroform (Table 4). The extraction by water is preferred as it is safer than chloroform.
Table 4.
Content of DDK and other major kavalactones in different extracting solvents prepared from kava roots (mg/g extract) [9]. * Structure of kavalactones divided by types A–D moieties.
| Kavalactones | Moieties * | Quantity (mg/g Extracting Solvents) | |||||
|---|---|---|---|---|---|---|---|
| Water | Acetone | Chloroform | Methanol | Ethanol | Hexane | ||
| Dihydromethysticin | A | 31.5 | 51.9 | 18.9 | 5.4 | 3.2 | 3.6 |
| 7,8-Dihydrokavain | A | 3.8 | 55.1 | 23.0 | 18.6 | 9.4 | 10.1 |
| Kavain | B | 36.9 | 41.5 | 14.7 | 6.9 | 3.3 | 4.7 |
| Methysticin | B | 0.0 | 5.5 | 14.4 | 0.0 | 0.0 | 1.2 |
| Desmethoxyyangonin | C | 6.7 | 21.0 | 7.6 | 4.3 | 2.1 | 2.7 |
| Yangonin | C | 6.8 | 84.1 | 22.9 | 5.7 | 2.4 | 2.1 |
| Dihydro-5,6-dehydrokavain | D | 22.9 | 27.1 | 4.7 | 4.7 | 2.1 | 1.9 |
| Total kavalactones | 108.6 | 286.2 | 106.2 | 45.6 | 22.5 | 26.3 | |
6. DDK in Other Alpinia Species
Kavalactones have been identified in other Alpinia species such as Alpinia kumatake [37], Alpinia galanga [38,39], and Alpinia oxyphyllae [38,39,40,41]. However, DDK and DK coexist only in Alpinia zerumbet [18,19] and Alpinia kumatake [37], and not in Alpinia galangal and Alpinia oxyphyllae [39,40]; DDK is also present in A. blepharocalyx but it was not quantified [41]. Interestingly, P. methysticum, A. zerumbet, and A. belepharocalyx all contain DDK as major biological constituent, although they belong to two different plant families, namely the Piperaceae and the Zingiberaceae family, respectively.
To date, there is to our knowledge not a single report investigating whether kavalactones other than DDK and DK are present in Alpinia zerumbet and other Alpinia species. Therefore, further studies are required to evaluate whether other kavalactones are found at least in minimal amounts just above the detection limit or whether there is evidence of complete lack of these additional kavalactone compounds. Overall, this may be an interesting detail to further elucidate the biosynthesis of other kavalactones; additional experimental studies should focus on the various enzymatic pathways leading to the synthesis of kavalactones, comparing Piper methysticum and Alpinia and studying the genetic relevance of variable kavalactone synthesis of these two plant species. The high number of different kavalactones synthesized by kava is a specific phenomenon.
7. Synthesis of DDK and DDK Derivatives
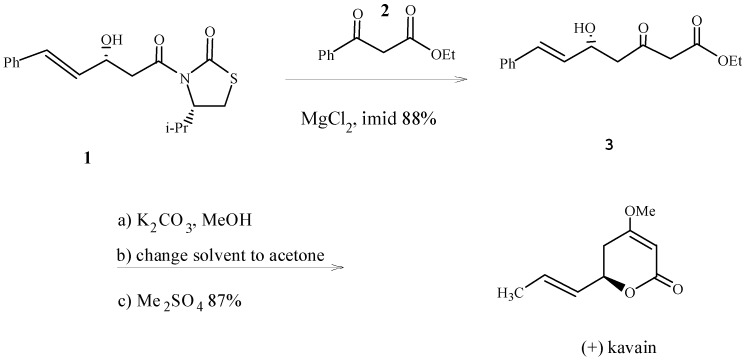
Three asymmetric pathways to synthesize kavalactones are described [42], which also represented the first synthesis of kavain. The first method is chiral auxiliary-based and utilizes aldol reactions of N-acetyl thiazolidinethiones, followed by a malonate displacement/decarboxylation reaction. The second approach uses the asymmetric catalytic additions [43] of dienolate nucleophile equivalents developed earlier [44,45]. The third approach uses tin-substituted intermediates, as advanced general precursors of kavalactone derivatives via Pd(0)-catalyzed Stille couplings [46] with aryl halides (Scheme 1). These are the three most simple and efficient approaches to the asymmetric synthesis of the kavalactones, which is acknowledged as the first enantioselective synthesis of (+)-kavain [42].
Scheme 1.
Three asymmetric pathways to synthesize kavalactones [42]. 1: N-acetyl- thiazolidinethione; 2: Potassium salt of monoethyl malonate; 3: Phenyl-δ-hydroxy-β-ketoester.
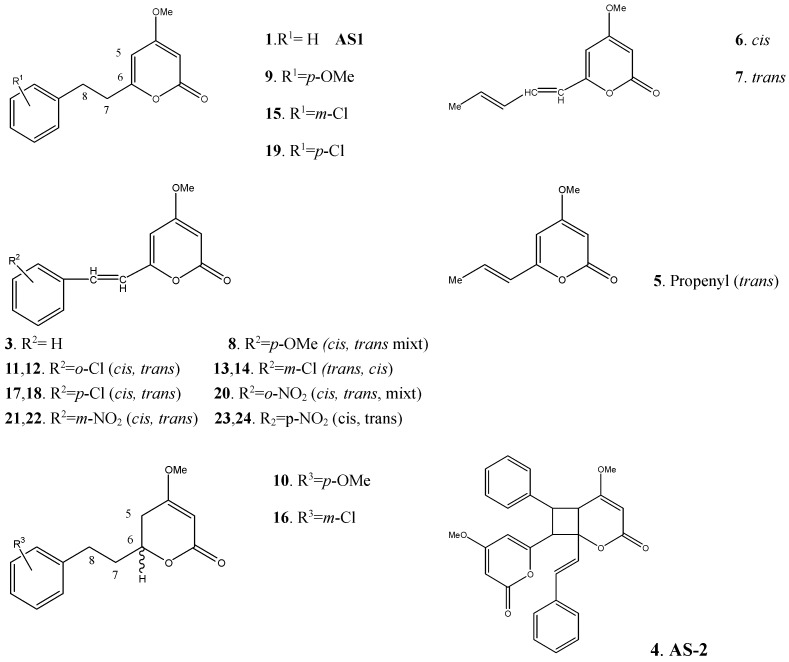
DDK and several DDK derivatives have been synthesized (Figure 3) [16]. The compounds AS-1 and AS-2 were separated from Alpinia zerumbet leaves, extracted with Me2CO for a month and extracted with n-hexane and benzene to isolate the AS-1 as a plant growth inhibitor, AS-2 was a minor compound. The DDK was synthesized by conversion of 4-methoxy-6-methyl-2H-pyran-2-one (2) to 4-methoxy-6-styryl-2H-pyran-2-one (3). Then this was converted to the 6-styryl derivative. The preparation of DDK derivatives was carried out in a similar manner to that of DDK, using the corresponding triphenylphosphonium and PtO2 as a catalyst, hydrogenating the 6-(p-methoxystyryl) and 6-(m-chlorostyryl) derivatives to yield DDK derivatives (Figure 3).
Figure 3.
Some DDK derivatives synthesized by Fujita et al. [16].
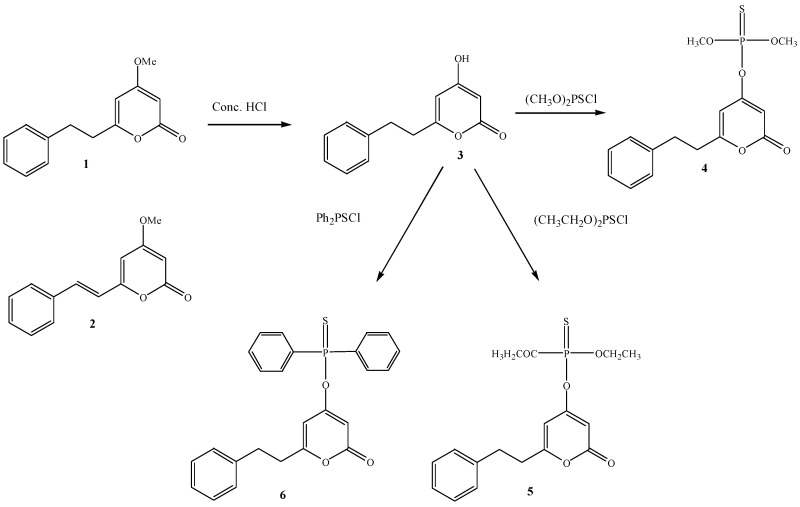
Tawata et al. [15] synthesized some DDK derivatives as shown in Scheme 2. DDK was hydrolyzed by HCl to yield hydroxy-6-(2-phenylethyl)-2H-pyran-2-one which has a hydroxyl group instead of a methoxy group at position 4 of DDK. Compound 3 was reacted with dimethyl chlorothiophophate, diethyl chlorothiophosphate, and diphenylphosphinothioyl chloride by using trimethylamine as a base to yield three derivatives 4, 5 and 6. The IR spectra of the compounds showed the existence of P=S, P-O, and P-O-C groups. 1H-NMR and 13C-NMR data showed similar chemical shifts in the values for the 6-(2-phenylethyl)-2-oxo-1H-pyran-4-yl group.
Scheme 2.
Structures and synthesis route of DDK derivatives [15]. 1: DDK; 2: DK; 3: 4-Hydroxy-6-(2-phenylethyl)-2H-pyran-2-one; 4: Dimethyl-[6-2-phenyethyl]-2-oxo-2H-pyran-4-yl]-phosphorothionate; 5: Diethyl-[6-(2-phenylethyl)-2-oxo-2H-pyran-4-yl] phosphorothionate; 6: [6-(2-Phenylethyl)-2-oxo-2H-pyran-4-yl]-diphenylphosphorothionate.
8. Perspectives
Molecular aspects of DDK and its derivatives have thoroughly been examined, awaiting pragmatic approaches of their further usage as drugs, dietary supplements, and agriculture products. Based on DDK, its derivatives, and other ingredients, Alpinia zerumbet is a promising plant in analogy of other alpinia species with known positive properties, which all belong to the well researched Zingiberaceae family. These plants are edible and may provide little risks for human use, characteristics which merit further studies of possible applications.
9. Conclusions
DDK can easily be obtained by synthesis or isolation from Alpinia zerumbet. It is synthetized very conveniently by asymmetric pathways, whereas its simple chemical structure facilitates the synthesis of DDK derivatives by HCl hydrolysis. In addition, DDK and its derivatives are easily isolated by solvents such as chloroform and hexane, preferentially using rhizomes of Alpinia zerumbet to obtain a high yield of the compounds. All synthesized products as well as Alpinia zerumbet itself appear promising, awaiting further proof of efficacy and safety. They may be used for various commercial purposes, including the potential development of future pharmaceutical drugs, preparation of specific and safe dietary supplements, and use as effective natural herbicides or pesticides. It appears that the successful exploitation of this ginger plant also may help to improve the rural development in the tropics.
Acknowledgments
This paper is dedicated to Shinkichi Tawata, Emeritus Professor of the University of the Ryukyus, who is a pioneer in alpinia research. The authors also thank Jhonamie Mabuhay for helpful advice to the manuscript.
Author Contributions
Tran Dang Xuan and Rolf Teschke contributed equally to this manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interests regarding this paper.
References
- 1.Yob N.Y., Jofrry S.M., Meor M.M.R., Teh L.K., Salleh M.Z., Zakaria Z.A. Zingiber zerumbet (L.) Smith: A review of its ethnomedicinal, chemical, and pharmacological uses. J. Evid. Based Complement. Altern. Med. 2011:543216. doi: 10.1155/2011/543216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh S., Rangan L. Alpinia: The gold mine of future therapeutics. 3 Biotech. 2013;3:173–185. doi: 10.1007/s13205-012-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman R.D., Marcussen K.C. Effects of ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheumat. 2001;11:2531–2538. doi: 10.1002/1529-0131(200111)44:11<2531::AID-ART433>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Shi G.F., Liu X.Z., An L.J., Guan S. Anti-ageing effects of protocatechuic acid from Alpinia on spleen and liver antioxidative system of senescent mice. Cell Biochem. Funct. 2011;29:342–347. doi: 10.1002/cbf.1757. [DOI] [PubMed] [Google Scholar]
- 5.Hadjzadeh M.A.R., Ghanbari H., Keshavarzi Z., Tavakol-Afshari J. The effects of aqueous extract of Alpinia galanga on gastric cancer cells (AGS) and L929 cells in vitro. Iran. J. Cancer Prev. 2014;7:142–146. [PMC free article] [PubMed] [Google Scholar]
- 6.Rajasekar R., Manokaran K., Rajasekaran N., Duraisamy G., Kanakasabapathi D. Effect of Alpinia calcarata on glucose uptake in diabetic rats—An in vitro and in vivo model. J. Diab. Meta Dis. 2014;13:33. doi: 10.1186/2251-6581-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu P.T.B., Tawata S. Anti-obesity effects of hispidin and Alpinia zerumbet bioactives in 3T3-adipocytes. Molecules. 2014;19:16656–16671. doi: 10.3390/molecules191016656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao Y.K., Shih H.N., Lee Y.C., Cheng W.T., Hung H.C., Wang H.C., Chen C.J., Tzeng Y.M., Lee M.J. Purification of kavalactones from Alpinia zerumbet and their protective actions against hydrogen peroxide-induced cytotoxicity in PC12 cells. J. Biosci. Bioeng. 2014;118:679–688. doi: 10.1016/j.jbiosc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Xuan T.D., Fukuta M., Ao C.W., Elzaawely A.A., Khanh T.D., Tawata S. Efficacy of extracting solvents to chemical components of kava (Piper methysticum) roots. J. Nat. Med. 2008;62:188–194. doi: 10.1007/s11418-007-0203-2. [DOI] [PubMed] [Google Scholar]
- 10.Teschke R., Lebot V. Proposal for a kava quality standardization code. Food Chem. Toxicol. 2011;49:2503–2516. doi: 10.1016/j.fct.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 11.Teschke R., Qiu S.X., Xuan T.D., Lebot V. Kava and kava hepatotoxicity: Requirements for novel experimental, ethnobotanical, and clinical studies based on a review of the evidence. Phytother. Res. 2011;25:1262–1274. doi: 10.1002/ptr.3464. [DOI] [PubMed] [Google Scholar]
- 12.Vandenbroucke H., Mournet P., Glaszmann J.C., Chair H., Malapa R., Lebot V. Comparative analysis of genetic variation in kava (Piper methysticum) assessed by SSR and DArT reveals zygotic foundation and clonal diversification. Genome. 2015;58:1–11. doi: 10.1139/gen-2014-0166. [DOI] [PubMed] [Google Scholar]
- 13.Billia A.R., Gallori S., Vincieri F.F. Kava-kava and anxiety: Growing knowledge about the efficacy and safety. Life Sci. 2002;70:2581–2597. doi: 10.1016/S0024-3205(02)01555-2. [DOI] [PubMed] [Google Scholar]
- 14.Itokawa H., Morita M., Mihashi S. Phenolic compounds from the rhizomes of Alpinia speciosa. Phytochemistry. 1981;20:2503–2506. doi: 10.1016/0031-9422(81)83082-8. [DOI] [Google Scholar]
- 15.Tawata S., Taira S., Kobamoto N., Ishihara M., Toyama S. Syntheses and biological activities of dihydro-5,6-dehydrokawain derivatives. Biosci. Biotech. Biochem. 1996;60:1643–1645. doi: 10.1271/bbb.60.1643. [DOI] [PubMed] [Google Scholar]
- 16.Fujita T., Nishimura H., Kaburagi K., Mizutani J. Plant growth inhibiting α-pyrones from Alpinia speciosa. Phytochemistry. 1994;36:23–27. doi: 10.1016/S0031-9422(00)97005-5. [DOI] [Google Scholar]
- 17.Elzaawely A.A., Xuan T.D., Tawata S. Changes in essential oils, kava pyrones and total phenolics of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. leaves exposed to copper sulphate. Environ. Exp. Bot. 2007;59:347–353. [Google Scholar]
- 18.Elzaawely A.A., Xuan T.D., Tawata S. Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. and their antioxidant activity. Food Chem. 2007;103:486–494. [Google Scholar]
- 19.Tawata S., Fukuta M., Xuan T.D., Deba F. Total utilization of tropical plants Leucaena leucocephala and Alpinia zerumbet. J. Pestic. Sci. 2008;33:40–43. doi: 10.1584/jpestics.R07-10. [DOI] [Google Scholar]
- 20.Pooter H.L., Omar M.N., Coolsaet B.A., Schamp N.M. The essential oil of greater galanga (Alpinia galanga) from Malaysia. Phytochemistry. 1985;24:93–96. doi: 10.1016/S0031-9422(00)80814-6. [DOI] [Google Scholar]
- 21.Itokawa H., Yoshimoto S., Morita H. Diterpenes from the rhizome of Alpinia formosana. Phytochemistry. 1988;27:435–438. doi: 10.1016/0031-9422(88)83115-7. [DOI] [Google Scholar]
- 22.Chompoo J., Upadhyay A., Kishimoto W., Makise T., Tawata S. Advanced glycation end product inhibitors from Alpinia zerumbet rhizomes. Food Chem. 2011;128:709–715. doi: 10.1016/j.foodchem.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Mpalantinos M.A., Moura R.S., Parente J.P., Kuster R.M. Biological active flavonoids and kava pyrones from the aqueous extract of Alpinia zerumbet. Phytother. Res. 1998;12:442–444. doi: 10.1002/(SICI)1099-1573(199809)12:6<442::AID-PTR320>3.0.CO;2-Y. [DOI] [Google Scholar]
- 24.Barik B.R., Kundu A.B., Dey A.K. Two phenolic constituents from Alpinia galanga rhizhomes. Phytochemistry. 1987;26:2126–2127. doi: 10.1016/S0031-9422(00)81779-3. [DOI] [Google Scholar]
- 25.Athamaprasangsa S., Buntrarongroi U., Dampawan P., Ongkavoranan N., Rukachaisikirui V., Sethuinda S., Sornarintra M., Sriwub P., Taylor W.C. A 1,7-diarylheptanoid from Alpinia conchigera. Phytochemistry. 1994;37:371–373. doi: 10.1016/S0031-9422(00)90374-1. [DOI] [Google Scholar]
- 26.Xu H.X., Dong H., Sim K.Y. Labdane diterpenes from Alpinia zerumbet. Phytochemistry. 1996;42:149–151. [Google Scholar]
- 27.Sirat H.M., Marsi D., Rahman A.A. The distribution of labdane diterpenes in the Zingiberaceae of Malaysia. Phytochemistry. 1994;36:699–701. doi: 10.1016/S0031-9422(00)89800-3. [DOI] [Google Scholar]
- 28.Krishna M.B., Chaganty R.B. Cardamonin and alpinetin from the seeds of Alpinia speciosa. Phytochemistry. 1973;12:238. doi: 10.1016/S0031-9422(00)84672-5. [DOI] [Google Scholar]
- 29.Itokawa H., Aiyama R., Ikuta A. A pungent principle from Alpinia oxyphylla. Phytochemistry. 1982;21:241–243. doi: 10.1016/0031-9422(82)80061-7. [DOI] [Google Scholar]
- 30.Itokawa H., Aiyama R., Ikuta A. A pungent diarylheptanoid from Alpinia oxyphylla. Phytochemistry. 1981;20:769–771. doi: 10.1016/0031-9422(81)85171-0. [DOI] [Google Scholar]
- 31.Sy L.K., Brown G.D. Oxygenated bisabolanes from Alpinia densibracteata. Phytochemistry. 1997;45:537–544. [Google Scholar]
- 32.Chompoo J., Upadhyay A., Gima S., Fukuta M., Tawata S. Antiatherogenic properties of acetone extract of Alpinia zerumbet seeds. Molecules. 2012;17:6237–6248. doi: 10.3390/molecules17066237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xuan T.D., Tsuzuki E., Terao H., Matsuo M., Khanh T.D. Identification of potential allelochemicals from kava (Piper methysticum L.) root. Allelopathy J. 2003;12:197–204. [Google Scholar]
- 34.Wu D., Muraleedharan G.N., Dewitt D.L. Novel compound from Piper methysticum Forst (Kava Kava) roots and their effect on cyclooxygenase enzyme. J. Agric. Food Chem. 2002;50:701–705. doi: 10.1021/jf010963x. [DOI] [PubMed] [Google Scholar]
- 35.Zhou P., Gross S., Liu J.H., Yu B.Y., Feng L.L., Nolta J., Sharma V., Piwnica-Worms D., Qiu S.X. Flavokawain, B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress trough modulation of IKK/NF-κB and MAPK signaling pathways. FASEB J. 2010;24:4722–4732. doi: 10.1096/fj.10-163311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragull K., Yoshida W.Y., Tang C.S. Piperidine alkaloids from Piper methysticum. Phytochemistry. 2003;63:193–198. doi: 10.1016/S0031-9422(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 37.Kimura Y., Takido M., Nakano K., Takishita S. Studies on the constituents of Alpinia. X. On the constituents of the rhizomata of Alpinia speciosa K. Schumann and A. kumatake Makino (A. formosana K. Schumann) Yakugaku Zasshi. 1966;86:1184–1187. doi: 10.1248/yakushi1947.86.12_1184. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 38.Chauhan V.S., Swapna M., Singh A. Phytochemical investigation and cytotoxic activity of methanolic extract of Alpinia galanga. Intel. J. Appl. Biol. Pharm. 2014;5:186–189. [Google Scholar]
- 39.Chen F., Li H.L., Li I.H., Tan Y.F., Zhang J.Q. Quantitative analysis of the major constituents in Chinese medicinal preparation SuoQuan formulae by ultrafast high performance liquid chromatography/quadrupole tandem mass spectrometry. Chem. Cent. J. 2013;7:131. doi: 10.1186/1752-153X-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qing Z.J., Yong W., Hui L.Y., Yong L.W., Long L.H., Ao D.J., Xia P.L. Two new natural products from the fruits of Alpinia oxyphylla with inhibitory effects on nitric oxide production in lipopolysaccharide activated RAW264.7 macrophage cells. Arch. Pharm. Res. 1982;35:2143–2146. doi: 10.1007/s12272-012-1211-7. [DOI] [PubMed] [Google Scholar]
- 41.Ali M.S., Banskota A.H., Tezuka Y., Saiki I., Kadota S. Antiproliferative activity of diarylheptanoids from the seeds of Alpinia blepharocalyx. Biol. Pharm. Bull. 2001;24:525–528. doi: 10.1248/bpb.24.525. [DOI] [PubMed] [Google Scholar]
- 42.Smith T.E., Djiang M., Velander A.J., Downey C.W., Carrol K.A., Alphen S.V. Versatile asymmetric synthesis of the kavalactones: First synthesis of (+)-kavain. Org. Lett. 2004;6:2317–2320. doi: 10.1021/ol0493960. [DOI] [PubMed] [Google Scholar]
- 43.Izawa T., Mukaiyama T. A convenient method for the preparation of δ-hydroxy-β-ketoesters and 6-akyl-5,6-dihydro-4-hydroxy-2-pyrones. Application to the syntheses of kawain and dihydrokawain. Chem. Lett. 1975;2:161–164. doi: 10.1246/cl.1975.161. [DOI] [Google Scholar]
- 44.Singer R.A., Carreira E.M.J. Catalytic, enantioselective dienolate additions to aldehydes: Preparation of optically active acetoacetate aldol adducts. Am. Chem. Soc. 1995;117:12360–12361. doi: 10.1021/ja00154a049. [DOI] [Google Scholar]
- 45.Sato M., Sunami S., Sugita Y., Kaneko C. Use of 1,3-dioxin-4-ones and related compounds in synthesis. XLIV. Asymmetric aldol reaction of 4-trimethylsiloxy-6-methylene-1,3-dioxines: Use of tartaric acid-derived (acyloxy) borane complex as the catalyst. Chem. Pharm. Bull. 1994;42:839–845. doi: 10.1248/cpb.42.839. [DOI] [Google Scholar]
- 46.Farina V., Krishnamurthy V., Scott W.J. The Stille Reaction. John Wiley & Sons; New York, NY, USA: 1998. [Google Scholar]