Abstract
Seven phenolic compounds were isolated from the fruits of Viburnum sargentii Koehne by silica gel column chromatography and preparative HPLC. On the grounds of chemical and spectroscopic methods, their structures were identified as (−)-Epicatechin (1), 5,7,4′-trihydroxy-flavonoid-8-C-β-d-glucopyranoside (2), 1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-α-l-rhamnopyranoxypropyl)-2-methoxyphenoxy]-1,3-propane-diol (erythro) (3), 1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-α-l-rhamnopyranoxypropyl)-2-methoxyphenoxy]-1,3-propanediol (threo) (4), (R)-4-hydroxylphenol O-(6-O-oleuropeoyl)-β-d-glucopyranoside (5), (R)-3-methoxy-4-hydroxylphenol O-(6-O-oleuropeoyl)-β-d-glucopyranoside (6), quercetin-3-O-rutinoside (7). Compounds 5 and 6 are new monoterpene phenolic glycosides, compounds 1, 3 and 4 were isolated from the Viburnum genus for the first time, and compounds 2 and 7 from the Viburnum sargentii Koehne for the first time. Compounds 1–7 were also assayed for their antioxidant activities with DPPH free radicals.
Keywords: Viburnum sargentii, phenolic glycoside, monoterpene, epicatechin, quercetin
1. Introduction
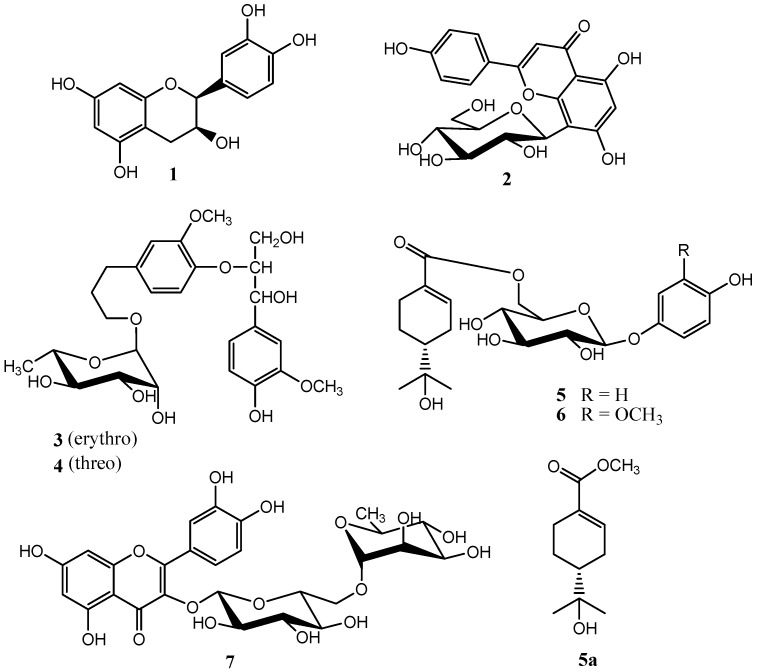
Viburnum sargentii koehne, a deciduous shrub of Caprifoliaceae, is widely distributed in the northeast and northwest regions of China. As traditional Chinese medicines, the branches are used for rheumatoid arthritis and traumatic injuries, the leaves for boils, ringworm and skin itching, and the fruits for cough [1]. Although there are a number of the pharmacological studies of the fruits of Viburnum sargentii [2,3], the isolation and structure identification have not been investigated in detail. As one part of our Caprifoliaceae studies, the isolation and structure identification of chemical constituents from the fruits of Viburnum sargentii Koehne have been carried out, and we report the isolation and identification of two new monoterpene phenolic glycosides (5 and 6), together with five phenolic compounds 1, 2, 3, 4 and 7 in the present study (Figure 1).
Figure 1.
Chemical structures of compounds 1–7.
2. Results and Discussion
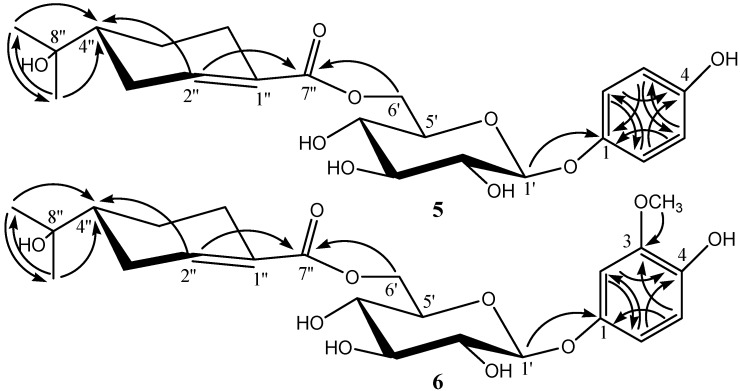
Compound 5 was obtained as colorless needles. Its HRESIMS displayed a [M + H]+ ion peak at m/z 439.1957 (calcd for C22H31O9, 439.1968), indicating the molecular formula C22H30O9. Acidic hydrolysis of 5 yielded d-glucose as a sugar residue. The 1H- and 13C-NMR spectra showed two 2H doublets (δH 6.83 (2H, d, J = 8.7 Hz), 7.03 (2H, d, J = 8.7 Hz)) and signals of six symmetric aromatic C-atoms at δC 154.3 (C), 152.1(C), 119.5 (2 CH), and 117.4 (2 CH), arising from a symmetrically 1,4-O-disubstituted benzene ring moiety, a set of signals characteristic of β-d-glucopyranosyl moiety (anomeric H-atom signal at δH 4.88 (d, J = 7.5 Hz)). The 13C-NMR and DEPT spectra showed ten carbon signals comprising one carboxylic (δC 168.2), one trisubstituted double bond (δC 142.2, 131.5), two methyl (δC 27.1, 26.6), three methylene (δC 29.1, 26.9, 24.9), one methine (δC 45.7), and one oxygen-bearing quaternary carbon (δC 72.2). These observations suggested the presence of an oleuropeic acid unit [4,5]. The positions of the oleuropeoyl ester and glycosidic linkages in 5 were established by 2D-NMR experiments. In the HMBC spectrum of 5, the glucosyl CH2 (6′) (δH 4.60, 4.22) correlated with the oleuropeoyl carboxylic C-atom C (7″) (δC 168.2), and the glucosyl H-C (1′) (δH 4.88) with and C (1) (δC 152.1) of 1,4-O-disubstituted benzene ring moiety. The full assignments of all protons and carbons were preformed through the correlations in 2D-NMR spectra (1H-1H COSY, HMQC and HMBC) of 5. All the data of 1H-, 13C-, and HMBC-NMR of compound 5 see Table 1, and key correlations and the structure of compound 5 see Figure 2. Methanolysis of 5 with MeONa in MeOH afforded 5a which was identified as (R)-oleuropeic acid methyl ester by comparing and 1H- and 13C-NMR spectra data with the reported [4,5]. Based on the above evidence, the structure of 5 was determined to be (R)-4-hydroxylphenol O-(6-O-oleuropeoyl)-β-d-glucopyranoside.
Table 1.
1H-NMR (methanol-d4, 400 MHz), 13C-NMR (methanol-d4, 100 MHz) and HMBC data of compound 5 and 6 (TMS as the internal standard, δ in ppm J in Hz).
| No. | 5 | 6 | ||||
|---|---|---|---|---|---|---|
| δC | δH J (Hz) | HMBC (H→C) | δC | δH J (Hz) | HMBC (H→C) | |
| 1 | 152.1 | 153.0 | ||||
| 2 | 119.5 | 7.03 d (8.7) | 154.3, 119.5 | 104.5 | 6.80 br. s | 143.4, 110.4 |
| 3 | 117.4 | 6.83 d (8.7) | 152.1, 117.4 | 149.2 | ||
| 3-OCH3 | 56.8 | 3.90 s | 149.2 | |||
| 4 | 154.3 | 143.4 | ||||
| 5 | 117.4 | 6.83 d (8.7) | 152.1, 117.4 | 116.2 | 6.75 d (8.2) | 153.0, 149.2 |
| 6 | 119.5 | 7.03 d (8.7) | 119.5, 154.3 | 110.4 | 6.65 d (8.2) | 104.5, 143.4 |
| 1′ | 103.3 | 4.88 d (7.5) | 168.2 | 103.6 | 4.83 d (7.8) | 153.0 |
| 2′ | 75.2 | 3.29 m | 75.2 | 3.51 m | ||
| 3′ | 78.4 | 3.46 m | 78.2 | 3.53 m | ||
| 4′ | 72.2 | 3.36 m | 72.3 | 3.43 m | ||
| 5′ | 75.6 | 3.75 m | 75.8 | 3.71 m | ||
| 6′α | 65.6 | 4.60 d (11.5) | 168.2 | 65.2 | 4.59 d (11.5) | 168.2 |
| 6′β | 4.22 dd (11.5, 7.7) | 168.2 | 4.32 dd (11.5, 7.3) | 168.2 | ||
| 1″ | 131.5 | 131.5 | ||||
| 2″ | 142.2 | 7.04 br. s | 168.2, 29.1, 45.7 | 141.8 | 7.09 br. s | 168.2, 45.4 |
| 3″α | 29.1 | 2.48 d (16.5) | 28.9 | 2.42 d (17.5) | ||
| 3″β | 2.20 m | 2.12 m | ||||
| 4″ | 45.7 | 1.63 m | 45.4 | 1.65 m | ||
| 5″α | 24.9 | 2.16 m | 24.8 | 2.12 m | ||
| 5″β | 1.32 m | 1.32 m | ||||
| 6″α | 26.9 | 2.59 d (16.0) | 26.7 | 2.51 d (16.4) | ||
| 6″β | 2.26 m | 2.21 m | ||||
| 7″ | 168.2 | 169.0 | ||||
| 8″ | 72.2 | 73.2 | ||||
| 9″ | 27.1 | 1.28 s | 45.7, 72.2, 26.6 | 27.3 | 1.38 s | 45.4, 73.2, 26.8 |
| 10″ | 26.6 | 1.28 s | 45.7, 72.2, 27.1 | 26.8 | 1.28 s | 45.4, 73.2, 27.3 |
Note: The assignments were based on DEPT, HMQC, 1H-1H COSY, and HMBC experiments.
Figure 2.
The Key HMBC Correlation of compound 5 and 6 (arrows point from proton to carbon).
Compound 6 was obtained as colorless needles. Its molecular formula C23H32O10 was elucidated from the HRESIMS m/z 469.2086 [M + H]+ (calcd for C23H33O10, 469.2074). Acidic hydrolysis of 6 also yielded d-glucose as a sugar residue. The 1H- and 13C-NMR spectra of 6 were similar to those of 5, except for the signals of benzene ring moiety. The 1H- and 13C-NMR spectra showed a typical three proton ABX aromatic spin system at δH 6.75 (1H, d, J = 8.2 Hz), 6.65 (1H, d, J = 8.2 Hz), 6.80 (1H, br. s) and six aromatic C-atom signals at δC 153.0 (C), 149.2 (C), 143.4 (C), 116.2 (CH), 110.4 (CH) and 104.5 (CH), as well as one signal from a MeO group [δH 3.90 (s, 3H), δC 56.8) ], suggesting that there is a 1,3,4-O-trisubstituted benzene ring moiety in compound 6 in stead of a 1,4-O-disubstituted benzene ring moiety, and one additional methoxy group. The positions of the oleuropeoyl ester, glycosidic linkages and MeO group in 6 were also established by 2D-NMR experiments. In the HMBC spectrum of 6, the glucosyl CH2 (6′) (δH 4.59, 4.32) correlated with the oleuropeoyl carboxylic C-atom C (7″) (δC 169.0), the glucosyl H-C (1′) (δH 4.83) with C (1) (δC 153.0) of the 1,3,4-O-trisubstituted benzene ring moiety, and the MeO group δH 3.90 correlated with C (3) (δC 149.2) of 1,3,4-O-trisubstituted benzene ring moiety. The full assignments of all protons and carbons were preformed through the correlations in 2D-NMR spectra (1H-1H COSY, HMQC and HMBC) of 6. All the data of 1H-, 13C-, and HMBC-NMR of compound 6 see Table 1, and key correlations and the structure of compound 6 see Figure 2. Methanolysis of 6 also afforded 5a. Thus, the structure of 6 was determined to be (R)-3-methoxy-4-hydroxylphenol O-(6-O-oleuropeoyl)-β-d-glucopyranoside.
Using similar methods as described above, compounds 1–4 and 7 were identified as (−)-Epicatechin (1) [6], 5,7,4′-trihydroxy-flavonoid-8-C-β-d-glucopyranoside (2) [7], 1-(4-hydroxy-3-methoxy-phenyl)-2-[4-(3-α-l-rhamnopyranoxypropyl)-2-methoxyphenoxy]-1,3-propane-diol (erythro) (3) [8], 1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-α-l-rhamnopyranoxypropyl)-2-methoxyphenoxy]-1,3-propanediol (threo) (4) [8], quercetin-3-O-rutinoside (7) [9].
Compounds 1–7 were next assayed for their antioxidant activity with DPPH free radicals, and the results are shown in Table 2. The data proved that (−)-Epicatechin showed strongest antioxidant activity.
Table 2.
IC50 values of compounds 1–7.
| Compound | IC50 (µg·mL−1) |
|---|---|
| 1 | 9.85 ± 0.5 |
| 2 | 357.1 ± 6.2 |
| 3 | 520.9 ± 7.6 |
| 4 | 522.3 ± 8.1 |
| 5 | 610.8 ± 6.1 |
| 6 | 620.1 ± 7.3 |
| 7 | 37.5 ± 0.3 |
Note: All values are averages of at least three runs in Table 2.
3. Experimental Section
3.1. General Information
NMR spectra were recorded on a Bruker AV-400 spectrometer (Bruke Corporation, Faellanden, Switzerland). UV Spectra were recorded on a Shimadzu UV-2401A spectrometer (Shimadzu Corporation, Kyoto, Japan). HR-ESI-MS were recorded on a Bruker microOTOF-Q II mass spectrometer (Bruke Corporation, Bremen, Germany). Optical rotations were measured with a HORIBA SEPA-300 high-sensitive polarimeter (Horiba Ltd, Kyoto, Japan). Melting points (m.p.) were measured with a X-4 Microscopic melting point apparatus (Shanghai Hui Tong Optical Instrument Co., Ltd, Shanghai, China). HPLC was performed Shimadzu LC-10A with a SPD-10A detector (Shimadzu Corporation, Kyoto, Japan) and Gemini 5μ C18 110A column (250 mm × 10.00 mm, 5 μm, flow rate: 3.0 mL/min, Phenomenex, Torrance, CA, USA). GC was performed an Agilent 7820A gas chromatograph with a quartz capillary column (30 mm × 0.32 mm × 0.25 μm, Agilent Technolgies Inc., Santa Clara, CA, USA); detection, FID. Column chromatography was performed on silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), D101 polyporous resin (Tianjin Pesticide Co., LTD., Resin Branch, Tianjin, China), polyamide (80–120 mesh, Taizhou Luqiao Sijia Biochemical Plastic Factory, Taizhou, China) and MCI-gel CHP-20P (75–150 μm; Mitsubishi Chemical Co., Tokyo, Japan). TLC was performed on glass precoated silica gel GF254 plates (Qingdao Haiyang Chemical Co., Ltd, Qingdao, China), detection under UV light or by spraying with 10% H2SO4 in 95% EtOH followed by heating. The bioactivities were measured on a DNM-9602 Enzyme immunoassay spectrophotometer (Beijing, China), using 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH) (Sigma-Aldrich, Shanghai, China). The fruits of Viburnum sargentii Koehne were collected in Changchun District of Jilin Province, China. They were identified by Jing-min Zhang of the School of Pharmaceutical Sciences, Jilin University.
3.2. Extraction and Isolation
The fresh fruits of V. sargentii (8 kg) were extracted with 70% aqueous ethanol at room temperature (3 L × 10 L, weekly). The extracts were concentrated under reduced pressure and then subjected to D101 polyporous resin column chromatography eluated with H2O, 10% aqueous ethanol, 30% aqueous ethanol, 60% aqueous ethanol and 95% aqueous ethanol. The eluate of 30% ethanol was chromatographed over silica gel, eluting with CHCl3–EtOAc–MeOH–H2O (3.5:1.2:4:1.2, v/v, lower layer), to afford four fractions, Frs. 1–4. Compound 1 (521 mg) was recrystallized from Fr. 1, Fr. 2 and 4 were further chromatographicly separated by gradient elution with MeOH–H2O (from 0% to 65%, 5% a time, v/v), and recrystallization of compound 2 (45 mg) were obtained from Fr. 2 and compound 7 (100 mg) from Fr. 4. Fr. 3 was further subjected to silica gel column chromatography eluting with CHCl3–EtOAc–MeOH–H2O (3.0:1.2:4:1.2, v/v, lower layer) to afford three subfractions, Frs. 3a–c. They were further isolated by semi-preparative RP-HPLC using acetonitrile–H2O as the mobile phase. Compound 3 (47 mg) and 4 (101 mg) were obtained from Fr. 3a with gradient elution (10%–15% acetonitrile from 0.00–10.00 min, 15%–18% acetonitrile from 10.00–20.0 0 min, 18% acetonitrile from 20.00–50.00 min), and by using 18% acetonitrile as the mobile phase compound 5 (62 mg) from Fr. 3b and compound 6 (57 mg) from Fr. 3c.
Compound 1: Pale amorphous powder, yielded a positive reaction to FeCl3 reagent. mp 234–236 °C. −56.8 (c 1.0, MeOH). UV (MeOH), λmax 217, 280 nm. HRESIMS m/z 291.0877 [M + H]+ (calcd for C15H15O6, 291.0869). 1H-NMR (DMSO-d6, 400 MHz) δ: 2.48 (1H, dd, J = 16.0, 3.6 Hz, H-4a), 2.68 (1H, dd, J = 16.0, 4.8 Hz, H-4b), 4.01(1H, m, H-3), 4.65 (1H, d, J = 4.8 Hz, H-2), 5.89 (1H, d, J = 2.0 Hz, H-8), 5.72 (1H, d, J = 2.0 Hz, H-6), 6.64 (1H, dd, J = 8.0, 1.6 Hz, H-6′), 6.67 (1H, d, J = 8.0 Hz, H-5′), 6.89 (1H, d, J = 1.6 Hz, H-2′). 13C-NMR (DMSO-d6,100 MHz) δ: 78.0 (C-2), 64.9 (C-3), 28.2 (C-4), 156.5 (C-5), 94.1 (C-6), 156.2 (C-7), 95.1 (C-8), 155.8 (C-9), 98.5 (C-10), 130.6 (C-1′), 114.9 (C-2′), 144.5 (C-3′), 144.4 (C-4′), 114.7 (C-5′), 117.9 (C-6′).
Compound 2: Yellow amorphous powder, yielded a positive reaction to FeCl3 reagent. mp 238–240 °C. UV (MeOH), λmax 268, 339. HRESIMS m/z 433.1126 [M + H]+ (calcd for C21H21O10, 433.1135). 1H-NMR (DMSO-d6, 400 MHz) δ: 8.02 (2H, d, J = 8.0 Hz, H-2′,6′), 6.89 (2H, d, J = 8.0 Hz, H-3′,5′), 6.77 (1H, s, H-3), 6.28 (1H, s, H-6), 4.69 (1H, d, J = 8.0Hz, Glu-H-1). 13C-NMR (DMSO-d6, 100 MHz) δ: (C-7), 104.6 (C-8), 156.0 (C-9), 104.0 (C-10), 121.6 (C-1′), 128.9 (C-2′), 115.8 (C-3′), 160.3 (C-4′), 115.8 (C-5′), 128.9 (C-6′), 73.3 (Glu-1), 70.8 (Glu-2), 78.6 (Glu-3), 70.5 (Glu-4), 81.8 (Glu-5), 61.3 (Glu-6).
Compound 3: Pale amorphous powder, yielded a positive reaction to FeCl3 reagent. mp 190–192 °C. −26.9 (c 0.30, MeOH). UV (MeOH), λmax 228, 280. HRESIMS m/z 525.2328 [M + H]+ (calcd for C26H37O11, 525.2336). 1H-NMR (methanol-d4, 400 MHz) δ: 7.10 (1H, s, H-2′), 6.92 (2H, d, J = 8.0 Hz, H-6′,5), 6.88 (1H, s, H-2), 6.83 (1H, d, J = 8.0 Hz, H-5′), 6.75 (1H, d, J = 8.0 Hz, H-6), 4.92 (1H, d, J = 7.6 Hz, H-7′), 4.73 (1H, s, Rha-H-1), 4.38 (1H, m, H-8′), 3.94 (1H, m, H-9′a), 3.83 (1H, m, H-9′b), 3.74 (1H, m, H-9a), 3.46 (1H, m, H-9b), 2.72 (2H, m, H-7), 1.96 (2H, m, H-8), 3.90 (3H, s, 3′-OCH3), 3.88 (3H, s, 3-OCH3), 1.32 (3H, d, J = 6.4 Hz, Rha-H-6). 13C-NMR (methanol-d4, 100 MHz) δ: 138.1 (C-1), 114.3 (C-2), 152.2 (C-3), 147.5 (C-4), 119.9 (C-5), 122.2 (C-6), 33.3 (C-7), 32.7 (C-8), 68.0 (C-9), 134.4 (C-1′), 112.1 (C-2′), 149.0 (C-3′), 147.3 (C-4′), 116.0 (C-5′), 121.2 (C-6′), 74.4 (C-7′), 86.9 (C-8′), 62.4 (C-9′), 56.8 (3′-OCH3), 56.7 (3′-OCH3), 102.0 (Rha-1), 72.8 (Rha-2), 72.6 (Rha-3), 74.3 (Rha-4), 70.1 (Rha-5), 18.3 (Rha-6).
Compound 4: Pale amorphous powder, yielded a positive reaction to FeCl3 reagent. mp 186–188 °C. −29.5 (c 0.30, MeOH). UV (MeOH), λmax 230, 280. HRESIMS m/z 525.2325 [M + H]+ (calcd for C26H37O11, 525.2336). 1H-NMR (methanol-d4, 400 MHz) δ: 7.11 (1H, s, H-2′), 7.07 (H, d, J = 8.0 Hz, H-5), 6.95 (1H, d, J = 8.0Hz, H-6′), 6.94 (1H, s, H-2), 6.85 (1H, d, J = 8.0 Hz, H-5′), 6.80 (1H, d, J = 8.0 Hz, H-6), 4.96 (1H, d, J = 6.8 Hz, H-7′), 4.73(1H, s, Rha-H-1), 4.30 (1H, m, H-8′), 3.80 (1H, m, H-9′a), 3.74 (1H, m, H-9′b), 3.54 (1H, m, H-9a), 3.45 (1H, m, H-9b), 2.74 (2H, m, H-7), 1.97 (2H, m, H-8), 3.95 (3H, s, 3′-OCH3), 3.91 (3H, s, 3-OCH3), 1.32 (3H, d, J = 6.0 Hz, Rha-H-6). 13C-NMR (methanol-d4, 100 MHz) δ: 138.2 (C-1), 114.2 (C-2), 152.0 (C-3), 148.0 (C-4), 119.9 (C-5), 122.4 (C-6), 33.3 (C-7), 32.7 (C-8), 68.0 (C-9), 134.1 (C-1′), 112.0 (C-2′), 149.1 (C-3′), 147.5 (C-4′), 116.2 (C-5′), 121.1 (C-6′), 74.5 (C-7′), 88.0 (C-8′), 62.2 (C-9′), 56.9 (3′-OCH3), 56.7 (3′-OCH3), 102.0 (Rha-1), 72.8 (Rha-2), 72.7 (Rha-3), 74.3 (Rha-4), 70.1 (Rha-5), 18.3 (Rha-6).
Compound 5: colorless needles (MeOH), yielded a positive reaction to FeCl3 reagent. mp 198–200 °C, −17.5 (c 1.0, MeOH). UV (MeOH), λmax 210, 261nm. HRESIMS m/z 439.1957 [M + H]+ (calcd for C22H31O9, 439.1968). 1H-NMR (methanol-d4, 400 MHz), see Table 1; 13C-NMR (methanol-d4, 100 MHz), see Table 1.
Compound 6: colorless needles (MeOH), yielded a positive reaction to FeCl3 reagent. mp 190–192 °C, −24.5 (c 0.8, MeOH). UV (MeOH), λmax 215, 269 nm. HRESIMS m/z 469.2086 [M + H]+ (calcd for C23H33O10, 469.2074). 1H-NMR (methanol-d4, 400 MHz), see Table 1; 13C-NMR (methanol-d4, 100 MHz), see Table 1.
Compound 7: Yellow amorphous powder, yielded a positive reaction to FeCl3 reagent. mp 185–187 °C. UV (MeOH), λmax 259, 359. HRESIMS m/z 611.1620 [M + H]+ (calcd for C27H31O16, 611.1612). 1H-NMR (DMSO-d6, 400MHz) δ: 6.19 (1H, s, H-6), 6.38 (1H, s, H-8), 7.53 (1H, d, J = 1.6 Hz, H-2′), 6.84 (1H, d, J = 8.0 Hz, H-5′), 7.54 (1H, dd, J = 8.0, 1.6 Hz, H-6′), 5.34 (1H, d, J = 6.8 Hz, Glc-H-1), 4.39 (1H, s, Rha-H-1), 1.00 (3H, d, J = 6.0 Hz, Rha-H-6). 13C-NMR (DMSO-d6, 100 MHz) δ: 156.4 (C-2), 133.3 (C-3), 177.3 (C-4), 161.2 (C-5), 98.6 (C-6), 164.1 (C-7), 93.5 (C-8), 156.5 (C-9), 103.9 (C-10), 121.1 (C-1′), 115.2 (C-2′), 144.7 (C-3′), 148.4 (C-4′), 116.2 (C-5′), 121.5 (C-6′), 101.2 (Glc-1), 74.0 (Glc-2), 76.4 (Glc-3), 70.0 (Glc-4), 75.9 (Glc-5), 66.9 (Glc-6), 100.7 (Rha-1), 70.5 (Rha-2), 70.3 (Rha-3), 71.8 (Rha-4), 68.2 (Rha-5), 17.7 (Rha-6).
3.3. Acid Hydrolysis of 5 and 6
Compounds 5 and 6 (each 6 mg) were hydrolyzed with 1.5 N HCl (2 mL) at 80 °C for 5 h. The mixture was then neutralized with NaOH (1 N). The mixture was passed through MCI-gel CHP-20P, developing with H2O. The H2O eluate was evaporated to dryness. The dry powders were dissolved in pyridine (2 mL), l-cysteine methyl ester hydrochloride (1.5 mg) was added, and the mixture was heated at 60 °C for 1 h. Trimethylsilylimidazole (1.5 mL) was added, and the mixture was heated at 60 °C for another 0.5 h. An aliquot (4 μL) of the supernatant was subjected to GC analysis under the following conditions: column temp 180–280 °C at 3 deg/min, carrier gas N2 (1 mL/min), injector and detector temp 250 °C, split ratio 1:50. The configurations of d-gluose for compounds 5 and 6 were determined by comparison of the retentions times of the corresponding derivatives with standard d-glucose (retention time: 19.208 min), respectively.
3.4. Methanolysis of 5 and 6
A solution of 5 (12 mg) in 0.02 M NaOMe–MeOH (2 mL) was kept standing at room temperature for 12 h. The solution was then subjected to MCI-gel CHP-20P column chromatography, eluting with H2O, 60% and 100% MeOH to give (R)-methyl oleuropeic acid methyl ester (5a) (3.0 mg): colorless oil; +65.5 (c 0.2, CHCl3); 1H-NMR (CDCl3, 400MHz) δ 7.06 (1H, m, H-2), 2.30, 2.00 (m, H-3), 1.53 (m, H-4), 2.01, 1.22 (m, H-5), 2.51, 2.12 (m, H-6), 1.20 (3H, s, H-9), 1.21 (3H, s, H-10), 3.72 (3H, s, OCH3); 13C-NMR (CDCl3, 100MHz) δ 130.2 (C-1), 139.9 (C-2), 27.4 (C-3), 44.5 (C-4), 23.4 (C-5), 25.1 (C-6), 167.8 (C-7), 72.2 (C-8), 27.4 (C-9), 26.6 (C-10), 51.6 (OCH3). Similar methanolysis of 6 also gave 5a ( +62.7 (c 0.18, CHCl3)).
3.5. Bioactivity Assay
The antioxidant activities of compounds 1–7 were assessed according to their DPPH (1,1-diphenyl-2-picrylhydrazyl free radical, Sigma-Aldrich, Shanghai, China) scavenging ability. Reaction mixtures, containing 0.5 mL of the relevant compound (dissolved in EtOH) and 2.5 mL of a 100 μM DPPH ethanolic solution, were added to 96-well microtiter plates and incubated at 37 °C for 30 min. Absorbances were measured at 515 nm. Percent inhibition was determined by comparison with an EtOH-treated control group. IC50 values denote the concentration of samples required to scavenge 50% of the DPPH free radicals.
4. Conclusions
Compounds 5 and 6 are new monoterpene phenolic glycosides. Compounds 1, 3 and 4 were isolated from the Viburnum genus for the first time, and compounds 2 and 7 from the Viburnum sargentii Koehne for the first time. Compounds 1–7 were also assayed for their antioxidant activities with DPPH free radicals, and the data proved that (−)-Epicatechin showed the strongest antioxidant activity.
Acknowledgments
The authors gratefully thank Jing-Min Zhang (School of Pharmaceutical Sciences, Jilin University) for the identification of plants.
Author Contributions
Guang-Shu Wang designed research, analyzed the data and wrote the paper; Yang Xie, Jing Wang, Yan-Mei Geng, Zhi Zhang and Yan-Fei Qu performed research. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1, 2, 5 and 7 are available from the authors.
References
- 1.The Editorial Board of Zhong Hua Ben Cao of State Administration of Traditional Chinese Medicine of the People’s Republic of China . Zhong Hua Ben Cao. 1st ed. Volume 7. Scientific and Technical Publishers; Shanghai, China: 1999. p. 599. [Google Scholar]
- 2.Li Y.B., Zhang L., Yan J. Study on the cough effect of Viburnum sargentii Koehne fruit. Inf. Tradit. Chin. Med. 2002;19:60. [Google Scholar]
- 3.Zhang C.X., Chen H., Ren Y.L., Zhu J. Study on the antibacterial effect of Viburnum sargentii Koehne fruit extract. J. Anhui Agric. Sci. 2010;38:11767–11768, 11782. [Google Scholar]
- 4.Manns D., Hartmann R. Monoterpene glucosides from Cunila Spicata. Planta Med. 1994;60:467–469. doi: 10.1055/s-2006-959534. [DOI] [PubMed] [Google Scholar]
- 5.Ito H., Koreishi M., Tokuda H., Nishino H., Yoshida T. Cypellocarpins A–C, phenol glycosides esterified with oleuropeic acid, from Eucalyptus Cypellocarpa. J. Nat. Prod. 2000;63:1253–1257. doi: 10.1021/np0001981. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q.H., Hubisihalatu, Wu J.S., Rong J., Narenchaoketu, Dai N.Y.T. Chemical constituents of Helianthemum ordosicum. Chin. Tradit. Herb. Drugs. 2014;45:2607–2610. [Google Scholar]
- 7.Li H.Y., Sun J.Y., Dai S.W. Stduies on chemical constituents from Phyllostachys pubescens. J. Chin. Med. Mater. 2003;26:563–564. [PubMed] [Google Scholar]
- 8.Miyase T., Ueno A., Takizawa N., Kobayashi H., Oguchi H. Studies on the glycosides of Epimedium grandiflorum MORR. var. thunbergianum (MIQ.) NAKAI. II. Chem. Pharm. Bull. 1987;35:3713–3719. doi: 10.1248/cpb.35.3713. [DOI] [Google Scholar]
- 9.Chen L.S., Liang X.X., Cai Z.Y., Li L.H. Chemical constituents from Tamarix Chinensis. Chin. Tradit. Herb. Drug. 2014;45:1829–1833. [Google Scholar]