Abstract
Carbazoles represent an important class of heterocycles. These have been reported to exhibit diverse biological activities such as antimicrobial, antitumor, antiepileptic, antihistaminic, antioxidative, anti-inflammatory, antidiarrhoeal, analgesic, neuroprotective and pancreatic lipase inhibition properties. A series of carbazole derivatives such as N-substituted carbazoles, benzocarbazoles, furocarbazoles, pyrrolocarbazoles, indolocarbazoles, imidazocarbazoles, etc. have been synthesized. The N-substituted derivatives have gained the attention of researchers due to their therapeutic potential against neurological disorders and cell proliferation. Herein an attempt is made to review the medicinal importance of recently synthesized N-substituted carbazoles.
Keywords: N-substituted carbazoles, antimicrobial, anticancer, neuroprotective
1. Introduction
Heterocycles are inextricably woven into the life processes [1]. The importance of heterocycles in drug discovery is one of the major areas in medicinal chemistry [2]. There are a vast number of pharmacologically active heterocyclic compounds, many of which are being used clinically, such as vincristine, morphine, chloroquine, meperidine, sulphadiazine, etc. Sulphur- and nitrogen- containing heterocyclic compounds have maintained the interest of researchers through decades of historical development of organic synthesis [3,4].
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six membered benzene ring fused on either side with a five membered nitrogen-containing ring. Carbazole and its derivatives are an important type of nitrogen containing heterocyclic compounds that are widespread in nature [5]. Various classes of carbazoles are given in Figure 1. The Carbazole ring is present in a variety of naturally occurring medicinally active substances [6] e.g., carbazomycins [7,8] and murrayafoline A [9]. Series of carbazole derivatives including oxazinocarbazoles, isoxazolocarbazolequinone, pyrido-carbazolequinone [10], tetrahydrocarbazoles [11], benzocarbazoles [12], furo-carbazoles [13], pyridocarbazoles [14], pyrrolo-carbazoles [15,16], indolocarbazoles [17], oxazolinyl carbazoles [18], thienocarbazoles [19], imidazocarbazoles [20], thiazolocarbazoles [21], benzopyrano-carbazoles [22], benzofurano-carbazoles [23] and N-substituted carbazoles have been synthesized and are well known for their pharmacological activities [24] such as antioxidant [25], anti-inflammatory [26], antibacterial [27], antitumor [28,29], anticonvulsant [30], antipsychotic [31], antidiabetic [32], larvicidal [33] properties, etc. Keeping in view the so vast therapeutical potential of carbazoles, this review will summarize the biological activities so far reported for the N-substituted carbazoles.
Figure 1.
Structures of various classes of carbazoles.
2. Biological Activities of N-Substituted Carbazoles
2.1. Antimicrobial Activity
The Spread of drug resistant bacteria has badly affected the efficiency of many known antibacterial agents [34], while the emergence of fungal infections in the immuno-compromised population has also significantly increased over past few decades [35,36]. Carbazoles are considered to be one of the important classes of antimicrobial agents [37,38].
Zhang et al. [39] reported the antibacterial and antifungal activities of series of N-substituted carbazoles. It has been observed that introduction of 1,2,4-triazole moiety in carbazoles (compound 1) resulted in an increase of antifungal activity against C. albicans, with a minimum inhibitory concentration (MIC) of 2–4 µg/mL. The introduction of an imidazole moiety (compound 2) seems to be favourable for antibacterial efficacy against S. aureus, B. subtilis, E. coli, methicillin resistant S. aureus (MRSA), P. aeruginosa and B. proteus (MIC 1–8 µg/mL). The carbazole triazolium compound, a quaternization product of triazole 3, displayed excellent antibacterial and antifungal activities against all strains with MIC values ranging from 1.0 to 64 µg/mL (Figure 2).
Figure 2.
Structures of imidazole and triazole carbazoles 1–3.
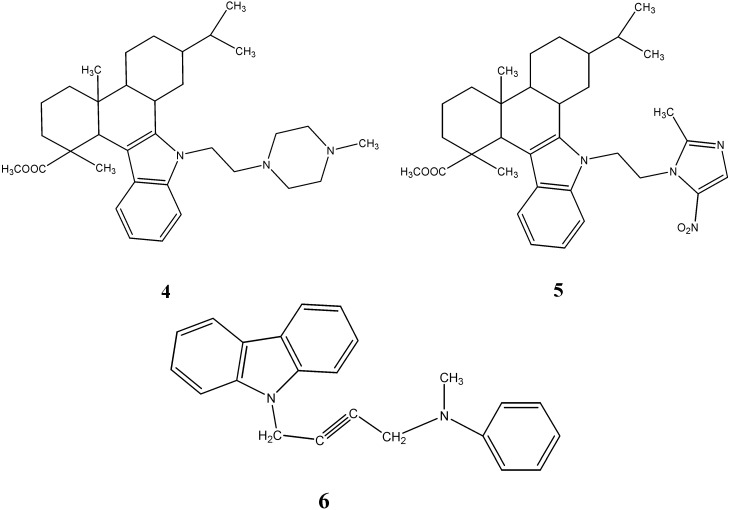
A series of some novel N-substituted derivatives of 2,3,4,4a,9,13c-hexahydro-7-isopropyl-1,4a-dimethyl-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl esters were synthesized by Gu et al. [40]. These newly synthesized compounds have been evaluated for their antimicrobial activity. The N-ethyl-[N-methyl-piperazinyl] derivative 4 showed antibacterial activity against B. subtilis, S. aureus, E. coli, P. fluorescens and antifungal activity against C. albicans, A. niger with MIC values ranging from 1.9 to 7.8 µg/mL. The N-ethyl-[2-methyl-5-nitro imidazole] derivative 5 exhibited antimicrobial activity against B. subtilis (MIC 0.9 µg/mL) comparable to that of the reference drug (amikacin).
Kaissy et al. [41] introduced an efficient procedure for the synthesis of N-acetylenic aminocarbazole derivatives. These have been subjected to bioassay against B. subtilis, S. aureus, E. coli and P. aeruginosa. The compound N-[1-buto-2y-nyl-4(NʹNʹ-methyl-phenyl]carbazole (6) exhibited very highly specific activity against E. coli, a Gram negative bacterium, with a zone of inhibition of 25 mm in diameter at a concentration of 800 µg/mL (Figure 3).
Figure 3.
Structures of acetylenic amine and dibenzo-carbazoles 4–6.
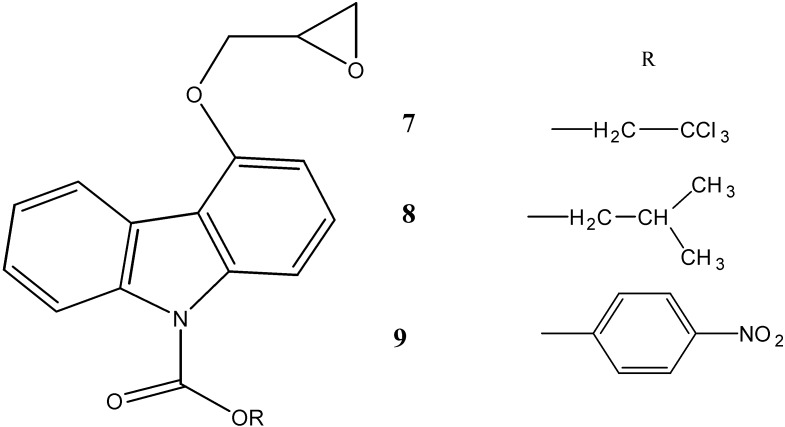
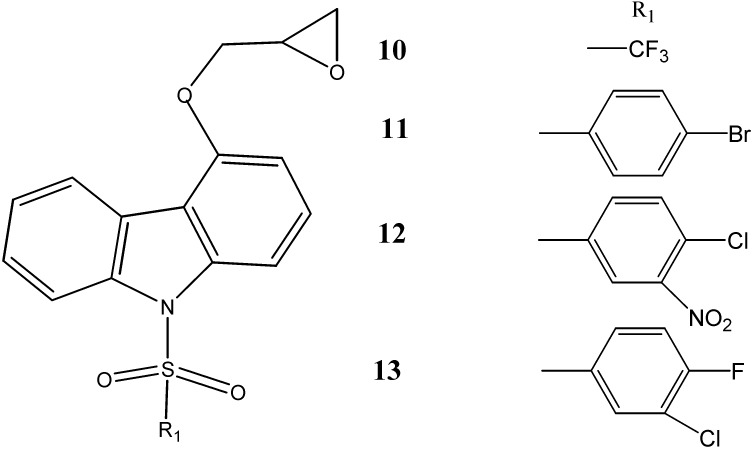
N-substituted carbamates [(substituted phenyl/aliphatic-4-oxiran-2yl-methoxy)-9H-carbazole-9-carboxylate] and sulphonamides [9-(substituted phenyl-sulphonyl)-4-(oxiran-2yl-methoxy)-9H-carbazole] 7–13 have been synthesized by Reddy et al. [42] and evaluated for their antimicrobial activities. The compounds 7, 9, 10 and 13 showed excellent antibacterial activities against S. aureus, B. subtilis, E. coli and antifungal activities against A. niger, C. albicans and F. oxysporium. The zones of inhibition were in the range of 12.6–22.3 mm in diameter at a concentration of 100 µg/mL (Figure 4).
Figure 4.
Structures of carbamate carbazoles 7–9 and sulphonamide carbazoles 11–13.
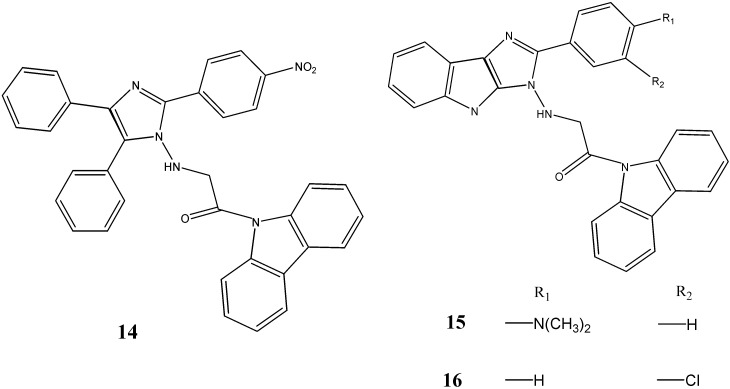
Kumar et al. [43] reported the synthesis of 9N-(hydrazinoacetyl)-carbazoles which have been evaluated for the potential antimicrobial activity. The carbazole derivatives containing imidazole and indole-imidazole moieties such as 1-carbazole-9-yl-2-(4-nitro-phenyl)-4,5-diphenyl-1H-1-yl-amino)-ethanone (14) and 1-carbazole-9-yl-2-(substituted phenyl)-1,4-dihydroimidazo[4,5-b] indol-1-yl-amino)-ethanones 15 and 16 were found to be the most potent against B. subtilis, S. aureus, E. coli and K. pneumoniae with zones of inhibition of 10.3–15.4 mm in diameter at MIC values ranging from 6.2 to 50 µg/mL (Figure 5).
Figure 5.
Structures of hydrazinoacetyl carbazoles 14–16.
The 1-carbazole-9-yl-2-(substituted phenyl)-1,4-dihydroimidazo-[4,5]-indole-1-yl-amino-ethanones 17–21 synthesized by Kaushik et al. [44] were subjected to bioassays for antibacterial activity against S. aureus, B. subtilis, P. aeruginosa, E. coli and antifungal activity against C. albicans, A. niger by the disc diffusion method. The compounds 18 and 20 showed potent antibacterial activity against all bacterial strains, with zones of inhibition of 16.82–26.08 mm in diameter at a concentration of 50 µg/mL, whereas the compounds 17–21 showed significant antifungal activity against C. albicans and A. niger with zones of inhibition of 7.91–16.8 mm in diameter at a concentration of 50 µg/mL (Figure 6).
Figure 6.
Structures of imidazo-indole carbazoles 17–19.
Segall and coworkers [45] reported a potent antifungal activity of N-alkylated carbazoles namely 6,11-dihydro-2-methoxy-11-[2-(1-piperidinyl)]ethyl-5H-benzo[a]carbazole (22) and wiskostatin (23) against C. albicans with MIC < 11 µM and 100 µM, respectively (Figure 7). Wiskostatin, an inhibitor of neuronal Wiskott-Aldrich syndrome protein (N-WASP)-mediated actin polymerization in vitro in relation to WASP, was identified as antifungal agent [46].
Figure 7.
Structures of piperidinyl carbazole 22 and wiskostatin 23.
Two N-substituted carbazoles, 5-[3-(9H-carbazol-9-ylacetyl)triazanylidene]-4,6-dimethylpyrimidin-2(5H)-one (24) and 4-[3-(9H-carbazol-9-yl acetyl)-triazanylidene]-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (25) have been synthesized by Salih et al. [47] (Figure 8).
Figure 8.
Structures of pyrimidine carbazole 24 and pyrazole carbazole 25.
They showed promising antifungal activity against C. albicans and A. fumigatus with MIC values ranging from 8.7 to 10.8 µg/mL and antibacterial activity against S. aureus, B. subtilis, E. coli with MIC values ranging from 1.1 to 10.3 µg/mL. The lipophilic character of these compounds may facilitate the crossing through biological membranes of microorganism and thereby inhibit their growth.
A series of N-substituted-carbazoles, synthesized by Sharma et al. [48], have been screened for their antimicrobial activities. The compounds 5-[(9H-carbazol-9-yl)methyl]-N-[(substituted phenyl)(piperazin-1-yl)methyl]-1,3,4-oxadiazol-2-amines 26, 27 and 28 have displayed antimicrobial activity against bacterial strains (S. aureus, B. subtilis, E. coli, P. aeruginosa) and fungal strains (C. albicans and A. niger) with zones of inhibition of 11.1–24.0 mm in diameter at a concentration of 50 µg/mL (Figure 9).
Figure 9.
Structures of piperazinyl-oxadiazole carbazoles 26–28.
2.2. Anti-Cancer Activity
Cancer has emerged as one of the most alarming disease in the last few decades throughout the world. It is a multifactorial disease contributing towards uncontrolled growth and invasion of abnormal cells leading to the formation of tumors [49]. Pim-kinases control various proteins involved in significant biological processes such as cell cycle progression and apoptosis. Over expression of pim-kinases have been observed in human leukaemia and lymphoma, prostate, pancreatic and colon cancer contributed to tumorigenesis. For these reasons, pim-kinases are considered as important targets for the development of new anticancer drugs.
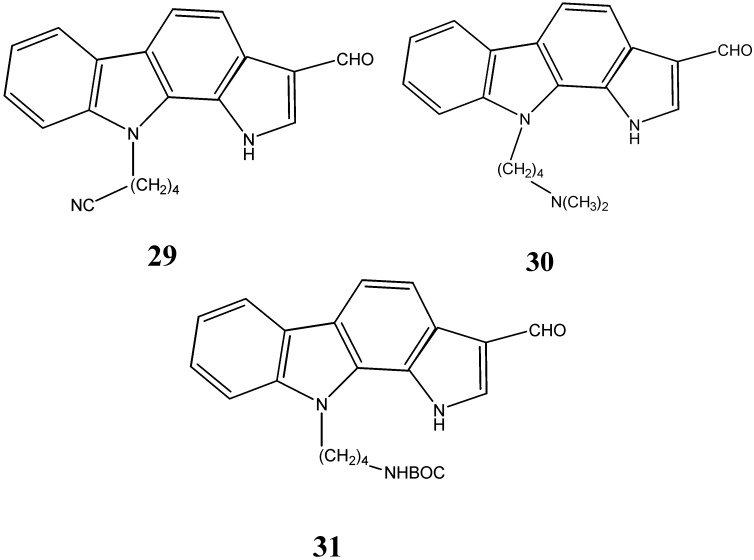
A series of N-substituted carbazoles synthesized by Akue-Gedu et al. [16] have been studied for their antiproliferative activity. These N-substituted pyrrolocarbazoles 29, 30 and 31 were found to be most potent inhibitors for pim-kinase activity with IC50 in the nanomolar range (46–75 nM) and have demonstrated antiproliferative activities against three human cancer cell lines, PA1 (ovarian carcinoma) PC3 and DU145 (prostatic carcinoma) with MIC values in the range of 8–20 µM (Figure 10).
Figure 10.
Structures of pyrrolo-carbazoles 29–31.
Giraud et al. [50] have synthesized N1-N10-bridged pyrrolo [2,3-a] carbazoles. The ability of these compounds to inhibit pim-kinases has been evaluated. The compounds 5,6-dihydro-4H-indolo[1,2,3-ef] pyrrolo[3,2,1-jk][1,5] benzodiazepine-1-carbaldehyde (32) and 5,6,7,8-tetrahydro-4H-indolo[1,2,3-gh] pyrrolo[3,2,1-lm][1,5] benzodiazonine-1-carbaldehyde (33) showed potent activity with nanomolar inhibitory potencies against the acute myeloid leukemia IPC-81 cell line, which is a good predictor of leukaemia therapy (Figure 11).
Figure 11.
Structures of N1-N10 bridged pyrrolo-carbazoles 32 and 33.
The 1-carbazole-9-yl-2-(substituted phenyl)-1,4-dihydroimidazo [4,5] indole-1-yl-amino-ethanones synthesized by Kumar et al. [43] have been evaluated for antitumor potential for laryngeal carcinoma cell lines (HEP2) and Ehrlich’s Ascites Carcinoma (EAC) cells. The compounds 15, 34 and 35 were found to be active against tumor cell lines (Figure 12). The activity may be attributed due to the presence of electron donating group which may increase the basicity of the compound.
Figure 12.
Structures of hydrazinoacetyl carbazoles 34 and 35.
A study was carried out by Kaushik et al. [44] on A549 cell lines in order to explore the anti-tumor potential of N-substituted carbazoles and the compounds 17, 18, 19 and 36 were found to be active (Figure 13). A fluoro group at para-position in compound 18 makes its anticancer activity more significant.
Figure 13.
Structure of imidazo-indole carbazole 36.
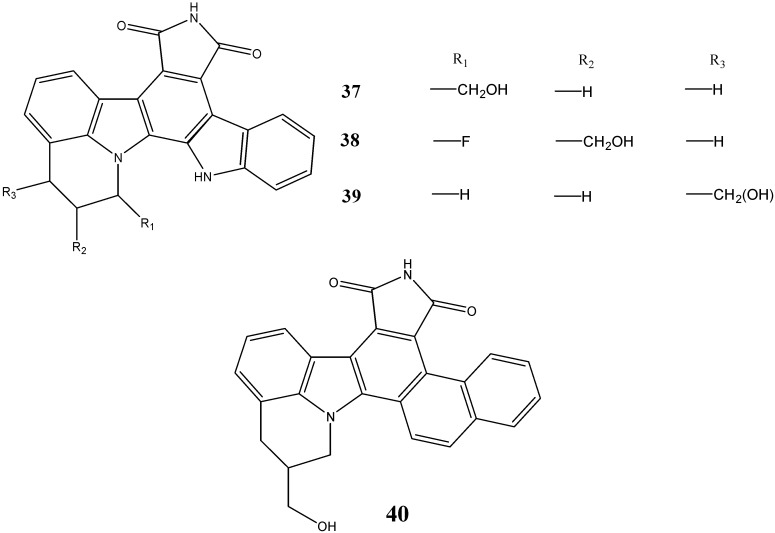
Zhu et al. [51] have documented the synthesis of N-annulated indolocarbazoles and further evaluated their cyclin dependent kinases (CDKs) inhibitory activity. The compounds 37, 38 and 39 were found to be potent inhibitors of cyclin D1/CDK4 and were effective antiproliferative agents against two human carcinoma cell lines, HCT116 (colon) and NCI-460 (lung). The replacement of the indole moiety with a naphthyl group (compound 40) also resulted in potent antiproliferative activity, with MIC values ranging between 0.37 and 0.96 µM (Figure 14).
Figure 14.
Structures of indolo- and naphtho-carbazoles 37–40.
N-substituted carbazoles, synthesized by Sharma et al. [48], have been screened for their antitumor activity. The compounds 5-[(9H-carbazol-9-yl)-methyl]-N-[(substituted phenyl)(piperazin-1-yl)methyl]-1,3,4-oxadiazol-2-amines 41–45 were found to be effective against human breast cancer cell lines (MCF-7). The LC50 values (µg/mL) of compounds 41-45 were 60.6, 60.2, 53.6, 80.0 and 35.6, respectively (Figure 15).
Figure 15.
Structures of piperazinyl-oxadiazole carbazoles (41–45).
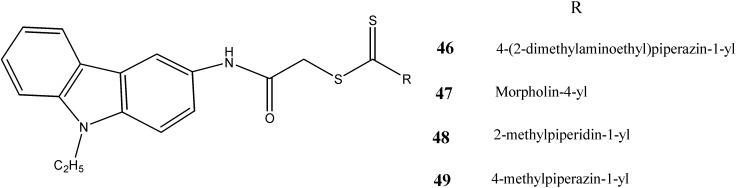
Ciftci et al. [52] have studied the apoptotic effects of N-ethyl-carbazole derivatives on A549 lung carcinoma and C6 glioma cell lines. The compounds 46 (IC50 = 5.9 µg/mL) and 47 (IC50 = 25.7 µg/mL) were found to be highly active against C6 and A549 cancer cell lines, respectively whereas compounds 48 and 49 showed moderate cytotoxic activity against both cell lines (Figure 16).
Figure 16.
Structures of N-ethyl carbazoles 46–49.
Howorko et al. [53] have reported the cytotoxic potential of compounds 50–53 against three human tumor cell lines, namely CCRF/CEM (T lymphoblast leukemia), A549, and MCF7 cell line. In another study, carried out by Li et al. [54], the compound 54 was found to be cytotoxic against cell line A549 (IC50 = 0.07 µM) and colon cancer HT29 cells (IC50 = 0.11 µM). The structures of these compounds are given in Figure 17.
Figure 17.
Structures of pyrido-carbazoles 50–54.
Roy et al. [55] have synthesized two indolocarbazoles and reported that compound 55 was found to be a novel check point kinase (ChK1) inhibitor.
Nakamura et al. [56] have studied the antitumor activity of a novel N-substituted carbazole, namely {[12,13-dihydro-5-[2-(dimethylamino)-ethyl]-4H-benzo[c]pyrimido[5,6,1-jk]carbazole-4,6,10-(5H,-11H)-trione hydrochloride]} (56). It inhibited topoisomerase II activity at a concentration of 2.5 µM which is a 10 times lower concentration than that of etoposide (standard).
Signal transducers and activators of transcription (STATs) are latent cytoplasmic transcription factors that upon activation, results into the transduction of signals from the cell membrane to the nucleus. The seven STATs namely STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6 have so far been identified in mammals. Among them, STAT3 is the most intimately linked to tumorigenesis. Saturnino et al. [57] have synthesized a series of N-alkylcarbazole derivatives and evaluated their activity on STAT3. Compounds 57–59 were revealed to inhibit the STAT3 activation by 50%, 90% and 95%, respectively, at a concentration of 50 µM (Figure 18).
Figure 18.
Structures of indolo-, pyrimido- and N-alkyl-carbazoles 55–59.
2.3. Neuroprotective Activity
Neuroprotection refers to the strategies and relative mechanisms able to defend the central nervous system (CNS) against neuronal injury due to both acute (e.g., stroke or trauma) and chronic neurodegenerative disorders [58,59]. Increased oxidative stress has been recognized as a common culprit of many neurological disorders including Alzheimer’s disease, Parkinson’s disease and stroke [60,61]. The β-Amyloid (Aβ) deposition is one of the hallmarks of Alzheimer’s disease. Therefore inhibition of Aβ peptide accumulation may be a preventive strategy for Alzheimer’s disease [62].
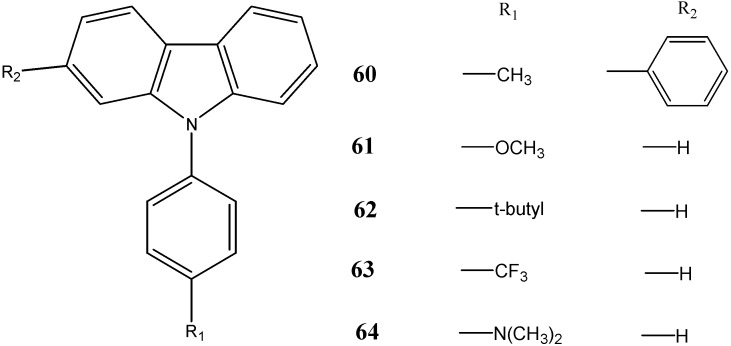
Zhu et al. [63] in 2013 have synthesized a series of N-substituted carbazoles and studied their neuroprotective effects on neuronal cells HT22 against cell injury induced by glutamate or homocysteic acid. The compound 2-phenyl-9-(p-tolyl)-9H-carbazole (60) displayed considerable neuroprotective ability at the concentration as low as 3 µM which might result from its antioxidative activity with GSH-independent mechanism. The compounds substituted with bulky groups such as methoxy-phenyl (compound 61), t-butyl-phenyl (62), trifluoro-phenyl (63), and N,N-dimethyl-phenyl (64) at the N-position of the carbazole also possessed significant neuroprotective activity at a concentration of 30 µM (Figure 19). It was observed that the presence of a substituent at the N-position of carbazole is essential for neuroprotective activity.
Figure 19.
Structures of phenyl-carbazoles 60–64.
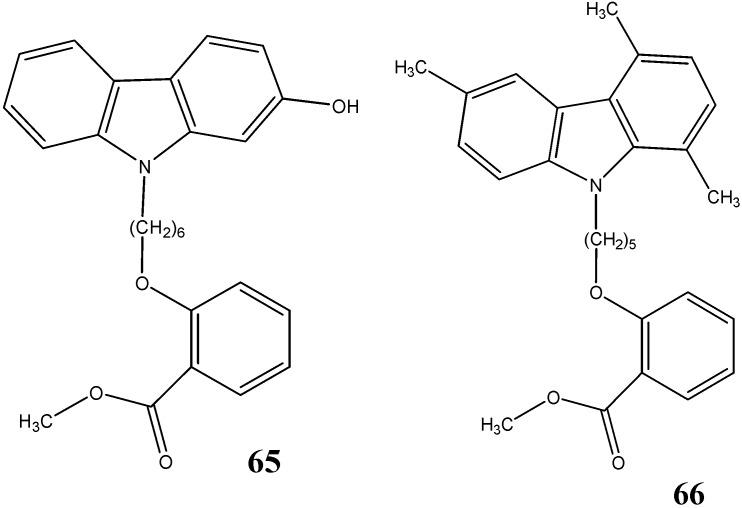
A series of N-alkyl-carbazole derivatives synthesized by Saturnino et al. [64], have been investigated for their ability to promote an increase of soluble amyloid-β (Aβ) peptides. Among the tested compounds, 65 was found to be responsible for a 30%–35% increase in the concentration of soluble Aβ peptides whereas compound 66 showed 65%–70% increase in concentration of soluble Aβ peptides at 10 µM (Figure 20). In both compounds, the distance between oxygen on the chain and the N-atom of carbazole scaffold play a decisive role in the interaction with Aβ peptides.
Figure 20.
Structures of N-alkyl-carbazoles 65 and 66.
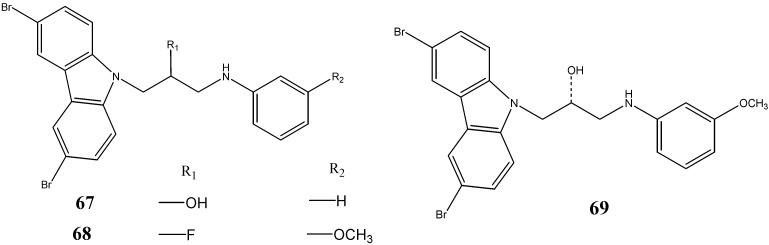
Among many genes reported to impact adult neurogenesis is the gene encoding NPAS3, a central nervous system-specific transcription factor that is associated with learning disability and mental illness [65,66]. NPAS3 deficit mice displayed the behavioral abnormalities [67] and a profound loss of adult hippocampal neurogenesis [68]. An aminopropyl-carbazole 67, designated as P7C3, exerts its pro-neurogenic activity by protecting newborn neurons from apoptosis and was capable of enhancing hippocampal neurogenesis in NPAS3 defecit mice. Pieper et al. [69] carried out a SAR study with thirty seven analogues of P7C3. The compound P7C3A20 (compound 68) was found to possess greater potency and efficacy than P7C3, whereas the (R)-enantiomer of P7C3-OMe (69) retained an activity equivalent to that of the parent compound (Figure 21).
Figure 21.
Structures of aminopropyl-carbazoles 67–69.
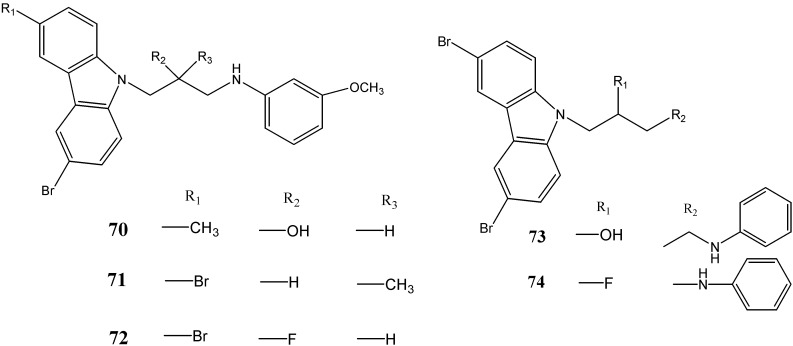
MacMillan et al. [70] have developed a series of P7C3 derivatives and, among them, compounds 70–74 were found to be more active than the parent compound (Figure 22).
Figure 22.
Structures of aminopropyl- and aminobutyl-carbazoles 70–74.
An aminopropyl-carbazole (P7C3) has been reported to block 1-methyl-4-phenyl-1,-2,-3,-6-tetrahydropyridine (MPTP)-mediated cell death of dopaminergic neurons in Parkinson’s disease. Cortes et al. [71] have screened P7C3 analogues (P7C3-S7, P7C3-S8, P7C3-S25, P7C3-S40, P7C3-S41, P7C3-S54, P7C3-S165 and P7C3-S184) for neuroprotective efficacy. The compounds P7C3-S7 (75), P7C3-S25 (76) and (S)-enantiomer of P7C3-S41 (77) were found to be active in MPTP protection assay. The compound P7C3-S184 (78) has been reported as a β-secretase inhibitor and prevented the formation of Aβ-peptide from amyloid precursor protein thereby proposed as a therapeutic approach for Alzheimer’s disease (Figure 23).
Figure 23.
Structures of P7C3 derivatives 75–78.
Yoon et al. [72] have developed and screened 25 aminopropyl-carbazole derivatives that can enhance neurogenesis of cultured neural stem cells. Among these analogues, compounds 79–81 demonstrated excellent proneurogenic and neuroprotective activity with no apparent toxicity (Figure 24).
Figure 24.
Structures of aminopropyl-carbazoles 79–81.
2.4. Anti-Epileptic and Antinociceptive Activities
Epilepsy is the most frequent neurologic infection characterized by excessive temporary neuronal discharge [73]. The overall prevalence of the disease is 1.0% of the population and up to 50 million people worldwide. Previous studies showed that a significant percent of individuals (20%–30%) using anti-epileptic drugs are resistant to the currently used therapeutic agent [74]. Therefore researchers are trying to find more active and less toxic compounds to control the seizures and produce a more comfortable life for the patients [75,76].
The N-substituted carbazoles 82–87 have been introduced by Rjamanickam et al. [77] and were screened for their antilepileptic and antinociceptive activities (Figure 25). Compound 86 showed highly significant anti-epileptic potential at a concentration of 20 mg/kg. This may be due to the substituent, 2-(2,-3-dimethyl-phenyl)-amino-benzoic acid. The compounds 82–87 have been reported to possess analgesic potential. The details of the antinociceptive activity of these compounds are given in Table 1.
Figure 25.
Structures of N-alkyl-carbazoles 82–87.
Table 1.
Antinociceptive evaluation of compounds (82–87).
| Treatment | Basel Reaction Time (s) before Treatment (Mean ± SEM) | Reaction Time (s) after Administration (Mean ± SEM) | |||
|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 120 min | ||
| Control | 4.1232 ± 0.2342 | 4.0228 ± 0.2322 | 4.1244 ± 0.2478 | 4.3244 ± 0.2286 | 4.3646 ± 0.2672 |
| Std a | 4.5120 ± 0.9012 | 8.800 ± 0.9031 ** | 10.22 ± 0.2642 ** | 11.48 ± 0.3476 ** | 13.22 ± 0.2974 ** |
| 82 | 4.9400 ± 0.6274 | 5.560 ± 0.6325 * | 6.48 ± 0.2224 * | 8.66 ± 0.2462 ** | 10.34 ± 0.2874 ** |
| 83 | 4.8819 ± 0.4654 | 5.042 ± 0.4761 * | 6.58 ± 0.2978 * | 8.44 ± 0.2536 ** | 10.48 ± 0.3576 ** |
| 84 | 4.7258 ± 0.4839 | 5.702 ± 0.4830 * | 7.46 ± 0.2564 ** | 9.26 ± 0.2978 ** | 11.22 ± 0.6428 ** |
| 85 | 4.5276 ± 0.5432 | 5.226 ± 0.5428 ** | 7.62 ± 0.2464 ** | 8.70 ± 0.3260 ** | 9.86 ± 0.2642 ** |
| 86 | 4.5276 ± 0.5324 | 5.406 ± 0.5428 * | 7.44 ± 0.4242 ** | 9.66 ± 0.2484 ** | 10.26 ± 0.2564 ** |
| 87 | 4.6863 ± 0.4912 | 5.863 ± 0.3726 * | 7.54 ± 0.4264 ** | 9.02 ± 0.2478 ** | 10.68 ± 0.2346 ** |
** p < 0.001 vs. control indicates highly significant; a Pentazocine standard; * p < 0.01 vs. control indicates significant.
3. Conclusions
Carbazole is a unique template associated with several biological activities. Due to the diverse and versatile biological properties of carbazole derivatives, particularly N-substituted carbazoles, they are of great interest to the research community. In particular, their antimicrobial, anticancer and antinociceptive activities makes these compounds attractive candidates, not only for microbe borne diseases, but also for the several other conditions like Alzheimer’s disease and epilepsy. This article has presented a comprehensive review of potent N-substituted carbazoles reported for their biological activities. More research must be carried out to explore their therapeutic potential for many other diseases like AIDS, hepatitis and diabetes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Saini M.S., Kumar A., Dwivedi J., Singh R. A review: Biological significances of heterocyclic compounds. Int. J. Pharm. Sci. Res. 2013;4:66–77. [Google Scholar]
- 2.Rathavi A., Shukla M., Thakor M.K. Synthesis and in vitro antibacterial and antifungal activity of trisubstituted S-triazines. J. Chem. Biol. Phys. Sci. Sec. A. 2014;4:85–92. [Google Scholar]
- 3.Dua R., Shrivastava S., Sonwane S.K., Srivastava S.K. Pharmacological significance of synthetic heterocycles scaffold: A review. Adv. Biol. Res. 2011;5:120–144. [Google Scholar]
- 4.Valverde M.G., Torroba T. Sulfur-nitrogen heterocycles. Molecules. 2005;10:318–320. doi: 10.3390/10020318. [DOI] [Google Scholar]
- 5.Nandy B.C., Gupta A.K., Mittal A., Vyas V. Carbazole: It’s biological activity. J. Biomed. Pharm. Res. 2014;3:42–48. [Google Scholar]
- 6.Knölker H.J., Reddy K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002;102:4303–4428. doi: 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]
- 7.Knölker H.J., Fröhner W., Reddy K.R. Iron-mediated synthesis of carbazomycin G and carbazomycin H, the first carbazole-1,4-quinol alkaloids from Streptoverticillium ehimense. Eur. J. Org. Chem. 2003;4:740–746. doi: 10.1002/ejoc.200390116. [DOI] [Google Scholar]
- 8.Hagiwara H., Choshi T., Fujimoto H., Sugino E., Hibino S. A novel total synthesis of antibiotic carbazole alkaloid carbazomycin G. Tetrahedron. 2000;56:5807–5811. doi: 10.1016/S0040-4020(00)00543-3. [DOI] [Google Scholar]
- 9.Cuong N.M., Wilhelm H., Porzel A., Arnold N., Wessjohann L. 1-O-Substituted derivatives of murrayafoline A and their antifungal. Nat. Prod. Res. 2008;22:1428–1434. doi: 10.1080/14786410802006033. [DOI] [PubMed] [Google Scholar]
- 10.Bouaziz Z., Issa S., Gentili J., Gratz A., Bollacke A., Kassack M., Jose J., Herfindal L., Gausdal G., Døskeland S.O., et al. Biologically active carbazole derivatives: Focus on oxazinocarbazoles and related compounds. J. Enzym. Inhib. Med. Chem. 2015;30:180–188. doi: 10.3109/14756366.2014.899594. [DOI] [PubMed] [Google Scholar]
- 11.Ha J.D., Kang S.K., Cheon H.G., Choi J.K. Synthesis of tetrahydrocarbazole derivatives as potent β3-adrenoceptor agonists. Bull. Korean Chem. Soc. 2004;25:1784–1790. [Google Scholar]
- 12.Asche C., Frank W., Albert A., Kucklaender U. Synthesis, antitumour activity and structure-activity relationships of 5H-benzo[b]carbazoles. Bioorg. Med. Chem. 2005;13:819–837. doi: 10.1016/j.bmc.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Hajbi Y., Neagoie C., Biannic B., Chilloux A., Vedrenne E., Bladeyrou B., Bailly C., Mérour J.Y., Rosca S., Routier S., et al. Synthesis and biological activities of new furo[3,4-b]carbazoles: Potential topoisomerase II inhibitors. Eur. J. Med. Chem. 2010;45:5428–5437. doi: 10.1016/j.ejmech.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Tylinska B., Howorko R.J., Mastalarz H., Klopotowska D., Fillip B., Wietrzyk J. Synthesis and structure-activity relationship analysis of new olivacine derivatives. Acta Pol. Pharm. Drug Res. 2010;67:495–502. [PubMed] [Google Scholar]
- 15.Giraud F., Akué-Gédu R., Nauton L., Candelon N., Debiton E., Théry V., Anizon F., Moreau P. Synthesis and biological activities of 4-substituted pyrrolo[2,3-a]carbazole Pim kinase inhibitors. Eur. J. Med. Chem. 2012;56:225–236. doi: 10.1016/j.ejmech.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Akué-Gédu R., Letribot B., Saugues E., Debiton E., Anizon F., Moreau P. Kinase inhibitory potencies and in vitro antiproliferative activities of N-10 substituted pyrrolo[2,3-a]carbazole derivatives. Bioorg. Med. Chem. Lett. 2012;22:3807–3809. doi: 10.1016/j.bmcl.2012.03.098. [DOI] [PubMed] [Google Scholar]
- 17.Lee H.J., Schaefer G., Heffron T.P., Shao L., Ye X., Sideris S., Malek S., Chan E., Merchant M., La H., et al. Noncovalent wild-typesparing inhibitors of EGFR T790M. Cancer Discov. 2013;3:168–181. doi: 10.1158/2159-8290.CD-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue M., Suzuki T., Nakada M. Asymmetric catalysis of Nozaki−Hiyama allylation and methallylation with a new tridentate bis(oxazolinyl)carbazole Ligand. J. Am. Chem. Soc. 2003;125:1140–1141. doi: 10.1021/ja021243p. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch G.H. Heterocyclic analogues of carbazole alkaloids. Curr. Org. Chem. 2001;5:507–518. doi: 10.2174/1385272013375409. [DOI] [Google Scholar]
- 20.Achab S., Diker K., Potier P. A short route to functionalized imidazo[4,5-c]carbazoles. Synthesis of the first example of the imidazo[4,5-c]β-carboline ring system. Tetrahedron Lett. 2001;42:8825–8828. doi: 10.1016/S0040-4039(01)01915-3. [DOI] [Google Scholar]
- 21.Chabane H., Lamazzi C., Thiery V., Guillaumet G., Besson T. Synthesis of novel 2-cyanothiazolocarbazoles analogues of ellipticine. Tetrahedron Lett. 2002;43:2483–2486. doi: 10.1016/S0040-4039(02)00353-2. [DOI] [Google Scholar]
- 22.Oliveria M.M., Salvador M.A., Coelho P.J., Carvalho L.M. New benzopyranocarbazoles: Synthesis and photochromic behavior. Tetrahedron. 2005;61:1681–1691. doi: 10.1016/j.tet.2004.12.055. [DOI] [Google Scholar]
- 23.Kawaguchi K., Nakano K., Nozaki K. Synthesis, structures, and properties of unsymmetrical heteroacenes containing both pyrrole and furan rings. Org. Lett. 2008;10:1199–1202. doi: 10.1021/ol703110g. [DOI] [PubMed] [Google Scholar]
- 24.Martin A.E., Prasad K.J.R. Synthesis and characterization of carbazole derivatives and their antimicrobial studies. Acta Pharm. 2006;56:79–86. [PubMed] [Google Scholar]
- 25.Guillonneau C., Pierre A., Charton Y., Guilbard N., Berthier L.K., Leonce S., Michael A., Bisagni E., Atassi G. Synthesis of 9-O-substituted derivatives of 9-hydroxy-5,6-dimethyl-6H- pyrido[4,3-b]carbazole-1-carboxylic acid (2-(Dimethylamino)ethyl)amide and their 10- and 11-methyl analogues with improved antitumor activity. J. Med. Chem. 1999;42:2191–2203. doi: 10.1021/jm981093m. [DOI] [PubMed] [Google Scholar]
- 26.Bandgar B.P., Adsul L.K., Chavan H.V., Jalde S.S., Shringare S.N., Shaikh R., Meshram R.J., Gacche R.N., Masand V. Synthesis, biological evaluation, and docking studies of 3-(substituted)-aryl-5-(9-methyl-3-carbazole)-1H-2-pyrazolines as potent anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2012;22:5839–5844. doi: 10.1016/j.bmcl.2012.07.080. [DOI] [PubMed] [Google Scholar]
- 27.Biamonte M.A., Wanner J., Roch K.G.L. Recent advances in malaria drug discovery. Bioorg. Med. Chem. Lett. 2013;23:2829–2843. doi: 10.1016/j.bmcl.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caruso A., Chiret A.S.V., Lancelot J.C., Sinicropi M.S., Garofalo A., Rault S. Efficient and Simple Synthesis of 6-Aryl-1,4-dimethyl-9H-carbazoles. Molecules. 2008;13:1312–1320. doi: 10.3390/molecules13061312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarty M., Ghosh N., Harigaya Y. A clay-mediated, regioselective synthesis of 2-(aryl/alkyl)amino-thiazolo[4,5-c]carbazoles. Tetrahedron Lett. 2004;45:4955–4957. doi: 10.1016/j.tetlet.2004.04.129. [DOI] [Google Scholar]
- 30.Issa S., Walchshofer N., Kassab I., Termoss H., Chamat S., Geahchan A., Bouaziz Z. Synthesis and antiproliferative activity of oxazinocarbazole and N,N-bis(carbazolylmethyl)amine derivatives. Eur. J. Med. Chem. 2010;45:2567–2577. doi: 10.1016/j.ejmech.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 31.Danish A.I., Prasad K.J.R. A one-pot synthesis of 1,2,4,5-tetraazaspiro [5.5]-6,7,8, 9-tetrahydrocarbazol-3-thiones and their antibacterial activities. Indian J. Heterocycl. Chem. 2006;14:19–22. [Google Scholar]
- 32.Indumati T., Fronczek F.R., Prasad K.J.R. Synthesis of 2-amino-8-chloro-4-phenyl-5,11-dihydro-6H-pyrido[2,3-a]carbazole-3-carbonitrile: Structural and biological evaluation. J. Mol. Struct. 2012;1016:134–139. doi: 10.1016/j.molstruc.2012.01.032. [DOI] [Google Scholar]
- 33.Kantevari S., Yempala T., Surineni G., Sridhar B., Sriram D. Synthesis and antitubercular evaluation of novel dibenzo[b,d]furan and 9-methyl-9H-carbazole derived hexahydro-2H-pyrano[3,2-c]quinolines via Povarov reaction. Eur. J. Med. Chem. 2011;46:4827–4833. doi: 10.1016/j.ejmech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Uttley A.H.C., Collins C.H., Naidoo J., George R.C. Vancomycin-resistant enterococci. Lancet. 1988;1:57–58. doi: 10.1016/S0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 35.Fox R., Neal K.R., Leen C.L.S., Ellis M.E., Mandal B.K. Fluconazole resistant candida in AIDS. J. Infect. 1991;22:201–204. doi: 10.1016/0163-4453(91)91767-R. [DOI] [PubMed] [Google Scholar]
- 36.Pfaller M.A., Bale M., Buschelman B. Selection of candidate quality control isolates and tentative quality control ranges for in vitro susceptibility testing of yeast isolates by National Committee for Clinical Laboratory Standards proposed standard methods. J. Clin. Microbiol. 1994;32:1650–1653. doi: 10.1128/jcm.32.7.1650-1653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu W., Wang S.F. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Med. Chem. 2010;45:4692–4696. doi: 10.1016/j.ejmech.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 38.Yaqub G., Hannan A., Akbar E., Usman M., Hamid A., Sadiq Z., Iqbal M. Synthesis, antibacterial, and antifungal activities of novel pyridazino carbazoles. J. Chem. 2013;2013:818739. doi: 10.1155/2013/818739. [DOI] [Google Scholar]
- 39.Zhang F.F., Gan L.L., Zhou G.H. Synthesis, anti-bacterial and antifungal activites of some carbazole derivatives. Bioorg. Med. Chem. 2010;20:1881–1884. doi: 10.1016/j.bmcl.2010.01.159. [DOI] [PubMed] [Google Scholar]
- 40.Gu W., Qiao C., Wang S.F., Hao Y., Miao T.T. Synthesis and biological evaluation of novel N-substituted 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2014;24:328–331. doi: 10.1016/j.bmcl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Kaissy W.W.N.A., Tuama S.H.F., Majidi S.M.H.A. Synthesis, characterization and evaluation of antimicrobial activity of some new acetylenic amine and 2- oxoazetidine of carbazole. Am. J. Sci. Ind. Res. 2013;4:389–398. [Google Scholar]
- 42.Reddy S.V.L., Naresh K., Raju C.N. New sulfonamide and carbamate derivatives of 4-(oxiran-2-ylmethoxy)-9Hcarbazole: Synthesis, characterization, antimicrobial and antioxidant activities. Der Pharm. Lett. 2013;5:221–231. [Google Scholar]
- 43.Kumar N., Sharma G.K., Pathak D. Microwave assisted and parallel synthesis of novel substituted carbazole derivatives of biological interest. Int. J. Pharm. Chem. Sci. 2013;2:273–282. [Google Scholar]
- 44.Kaushik K., Kumar N., Pathak D. Synthesis of some newer carbazole derivatives and evaluation for their pharmacological activity. Der Pharm. Sin. 2012;3:470–478. [Google Scholar]
- 45.Segall A.I., Vitale M.F., Perez V.L., Pizzorno M.T. HPLC analysis of 5H-benzo[a]carbazole with antifungal activity. J. Pharm. Biomed. Anal. 2003;31:1021–1026. doi: 10.1016/S0731-7085(02)00704-5. [DOI] [PubMed] [Google Scholar]
- 46.Thevissen K., Marchand A., Chaltin P., Meert E.M.K., Cammue B.P.A. Antifungal carbazoles. Curr. Med. Chem. 2009;16:2205–2211. doi: 10.2174/092986709788612701. [DOI] [PubMed] [Google Scholar]
- 47.Salih N., Salimon J., Yousif E. Synthesis and antimicrobial activities of 9H-carbazole derivatives. Arabian J. Chem. 2011;2011 doi: 10.1016/j.arabjc.2011.08.013. [DOI] [Google Scholar]
- 48.Sharma D., Kumar N., Pathak D. Synthesis, characterization and biological evaluation of some newer carbazole derivatives. J. Serb. Chem. Soc. 2014;79:125–132. doi: 10.2298/JSC130123069S. [DOI] [Google Scholar]
- 49.Gautam N., Mantha A.K., Mittal S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014;2014:154106. doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giraud F., Bourhis M., Nauton L., Théry V., Herfindal L., Døskeland S.O., Anizon F., Moreau P. New N-1,N-10-bridged pyrrolo[2,3-a]carbazole-3-carbaldehydes: Synthesis and biological activities. Bioorg. Chem. 2014;57:108–115. doi: 10.1016/j.bioorg.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Zhu G., Conner S.E., Zhou X., Chan H.K., Shih C., Engler T.A., Awar R.S.A., Brooks H.B., Watkins S.A., Spencer C.D., et al. Synthesis of 1,7-annulated indoles and their applications in the studies of cyclin dependent kinase inhibitors. Bioorg. Med. Chem. Lett. 2004;14:3057–3061. doi: 10.1016/j.bmcl.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 52.Ciftci G.A., Temel H.E., Yildirim S.U., Kaplancikli Z.A., Altintop M.D., Genc L. Apoptotic effects of some carbazole derivatives on lung carcinoma and glioma cell lines. Med. Chem. Res. 2013;22:3751–3759. doi: 10.1007/s00044-012-0325-2. [DOI] [Google Scholar]
- 53.Howorko R.J., Tylinska B., Biadun B., Gebarowski T., Gasiorowski K. New pyridocarbazole derivatives. Synthesis and their in Vitro anticancer activity. Act. Pol. Pharm. Drug Res. 2013;70:823–832. [PubMed] [Google Scholar]
- 54.Li B., Yue Z.Z., Feng J.M., He Q., Miao Z.H., Yang C.H. Design and Synthesis of Pyrido[3,2-α]carbazole derivatives and their analogues as potent antitumor agents. Eur. J. Med. Chem. 2013;66:531–539. doi: 10.1016/j.ejmech.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 55.Roy S., Eastman A., Gribble G.W. Synthesis of bisindolylmaleimides related to GF109203x and their efficient conversion to the bioactive indolocarbazoles. Org. Biomol. Chem. 2006;4:3228–3234. doi: 10.1039/b607504e. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura K., Sugumi H., Yamaguchi A., Uenaka T., Kotake Y., Okada T., Kamata J., Niijima J., Nagasu T., Koyanagi N., et al. Antitumor activity of ER-37328, a novel carbazole topoisomerase II inhibitor. Mol. Cancer Ther. 2002;1:169–175. [PubMed] [Google Scholar]
- 57.Saturnino C., Palladino C., Napoli M., Sinicropi M.S., Botta A., Sala M., Carcereri de Prati A., Novellino E., Suzuki H. Synthesis and biological evaluation of new N-alkylcarbazole derivatives as STAT3 inhibitors: Preliminary study. Eur. J. Med. Chem. 2013;60:112–119. doi: 10.1016/j.ejmech.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Kumar G.P., Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012;6:81–90. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. 2006;20:1023–1035. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- 60.Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 62.Doré V., Villemagne V.L., Bourgeat P., Fripp J., Acosta O., Chetélat G., Zhou L., Martins R., Ellis K.A., Masters C.L., et al. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70:903–911. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- 63.Zhu D., Chen M., Li M., Luo B., Zhao Y., Huang P., Xue F., Rapposelli S., Pi R., Wen S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013;68:81–88. doi: 10.1016/j.ejmech.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 64.Saturnino C., Iacopetta D., Sinicropi M.S., Rosano C., Caruso A., Caporale A., Marra N., Marengo B., Pronzato M.A., Parisi O.I., et al. N-alkyl carbazole derivatives as new tools for Alzheimer’s disease: Preliminary studies. Molecules. 2014;19:9307–1917. doi: 10.3390/molecules19079307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamnasaran D., Muir W., Ferguson-Smith M., Cox D. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J. Med. Genet. 2003;40:325–332. doi: 10.1136/jmg.40.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickard B.S., Pieper A.A., Porteous D.J., Blackwood D.H., Muir W.J. The NPAS3 gene—Emerging evidence for a role in psychiatric illness. Ann. Med. 2006;38:439–448. doi: 10.1080/07853890600946500. [DOI] [PubMed] [Google Scholar]
- 67.Erbel-Sieler C., Dudley C., Zhou Y., Wu X., Estill S.J., Han T., Diaz-Arrastia R., Brunskill E.W., Potter S.S., McKnight S.L. Behavioral and regulatory abnormalities in mice deficient in NPAS1 and NPAS3 transcription factors. Proc. Natl. Acad. Sci. USA. 2004;101:13648–13653. doi: 10.1073/pnas.0405310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pieper A.A., Wu X., Han T.W., Estill S.J., Dang W., Wu L.C., Reece-Fincanon S., Dudley C.A., Richardson J.A., Brat D.J., et al. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc. Natl. Acad. Sci. USA. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pieper A.A., Xie S., Capota E., Estill S.J., Zhong J., Long J.M., Becker G.L., Huntington P., Goldman S.E., Shen C.H., et al. Discovery of a Pro-neurogenic, Neuroprotective Chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacMillan K.S., Naidoo J., Liang J., Melito L., Williams N.S., Morlock L., Huntington P.J., Estill S.J., Longgood J., Becker G.L., et al. Development of proneurogenic, neuroprotective small molecules. J. Am. Chem. Soc. 2011;133:1428–1437. doi: 10.1021/ja108211m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cortés H.D.J., Xu P., Drawbridge J., Estill S.J., Huntington P., Tran S., Britt J., Tesla R., Morlock L., Naidoo J., et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. PNAS. 2012;109:17010–17015. doi: 10.1073/pnas.1213956109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon H.J., Kong S.Y., Park M.H., Cho Y., Kim S.E., Shin J.Y., Jung S.H., Lee J., Farhanullah, Kim H.J., et al. Aminopropyl carbazole analogues as potent enhancers of neurogenesis. Bioorg. Med. Chem. 2013;21:7165–7174. doi: 10.1016/j.bmc.2013.08.066. [DOI] [PubMed] [Google Scholar]
- 73.McCagh J., Fisk J.E., Baker G.A. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. 2009;86:1–14. doi: 10.1016/j.eplepsyres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Shindikar A.V., Khan F., Viswanathan C.L. Design, synthesis and in vivo anticonvulsant screening in mice of novel phenylacetamides. Eur. J. Med. Chem. 2006;41:786–792. doi: 10.1016/j.ejmech.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Brodie M.J. Do we need any more new antiepileptic drugs? Epilepsy Res. 2001;45:3–6. doi: 10.1016/S0920-1211(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 76.Bialer M., Johannessen S.I., Kupferberg H.J., Levy R.Y., Loiseau P., Perucca E. Progress report on new antiepileptic drugs: A summary of the sixth Eilat conference (EILAT VI) Epilepsy Res. 2002;51:31–71. doi: 10.1016/S0920-1211(02)00106-7. [DOI] [PubMed] [Google Scholar]
- 77.Rajamanickam V., Rajasekarena A., Palanivelu M., Anandarajagopal K., Elahi A.L.A., Umaranib N. Anti-nociceptive and anti-epileptic evaluation of N-Mannich bases of some substituted carbazoles. Int. J. Chem. Sci. 2008;6:1669–1675. [Google Scholar]