Abstract
Three series of 4,6-dimethoxy-, 4,6-dipiperidino- and 4,6-dimorpholino-1,3,5-triazin-2-yl) amino acid derivatives were synthesized and characterized. A preliminary study for their monoamine oxidase inhibitory activity showed that compounds 7, 18, and 25 had MAO-A inhibition activity comparable to that of the standard clorgyline, with apparently more selective inhibitory activity toward MAO-A than MAO-B and no significant acute toxicity.
Keywords: 1,3,5-triazine derivatives; amino acids; morpholine; piperidine; monoamine oxidase
1. Introduction
Human monoamine oxidases A and B (MAO-A and B) are the most widely studied flavin-dependent amine oxidases. They are located in the mitochondrial outer membranes of neuronal, glial, and other cells particularly abundant in the liver and brain [1,2]. These FAD-dependent enzymes catalyze the oxidative deamination of several endogenous and exogenous monoamines and are responsible for the regulation and metabolism of major monoamine neurotransmitters, such as serotonin (5-OH tryptamine), noradrenaline, and dopamine [1,2,3]. The two mammalian isoforms of these enzymes are characterized by their distinct sensitivity to inhibitors and specificity to substrates. Thus, MAO-A is selectively inhibited by clorgyline and preferentially metabolizes serotonin: whereas MAO-B is inhibited by l-deprenyl and preferentially metabolizes benzylamine and phenylethylamine as substrates [4]. Among selective MAO inhibitors, those against MAO-A are used as anti-depressant and anti-anxiety drugs and have been claimed to protect neuronal cells against apoptosis [5,6]. In contrast, MAO-B inhibitors have been found to be beneficial in the treatment of Parkinson’s disease and Alzheimer’s disease. Early MAO-inhibitors introduced into clinical practice for the treatment of depression were abandoned due to adverse side effects, such as the “cheese effect”, which is characterized by hypertensive crises [4], and because the mechanism of interaction of several new drugs with MAOs has not been yet fully characterized. For these reasons, research has been aimed at the synthesis of new potential agents with clinical applications.
Recently, we have demonstrated a series of 3-benzyl-2-substituted quinoxalines as selective MAO-A inhibitors bearing substituted amino or hydrazino functionalities at position 2 [7] and novel structural variants of [1,2,4]triazolo[4,3-a]quinoxaline derivatives [8]. In addition, substituted pyridazine-1-yl acetic acid derivatives [9], and α-ketoamino acid ester derivatives [10] were established as selective monoamine oxidase-A inhibitors.
1,3,5-triazine derivatives are an important class of small molecules with anti-cancer [11,12,13,14,15,16] and anti-viral activity, among others [17]. These compounds are known to be VLA-4 integrin antagonists, anti-inflammatory agents [18], sorbitol dehydrogenase inhibitors [19], estrogen receptor modulators [20], potential anti-trypanosomal drugs [21], antimalarial agents [22,23,24,25,26,27,28,29], hypolipidemic agents, [30] and antimicrobial agents [31,32,33].
Here, we prepared three small libraries of molecules based on amino acid-substituted 1,3,5-triazine and evaluated their capacity to inhibit MOAs.
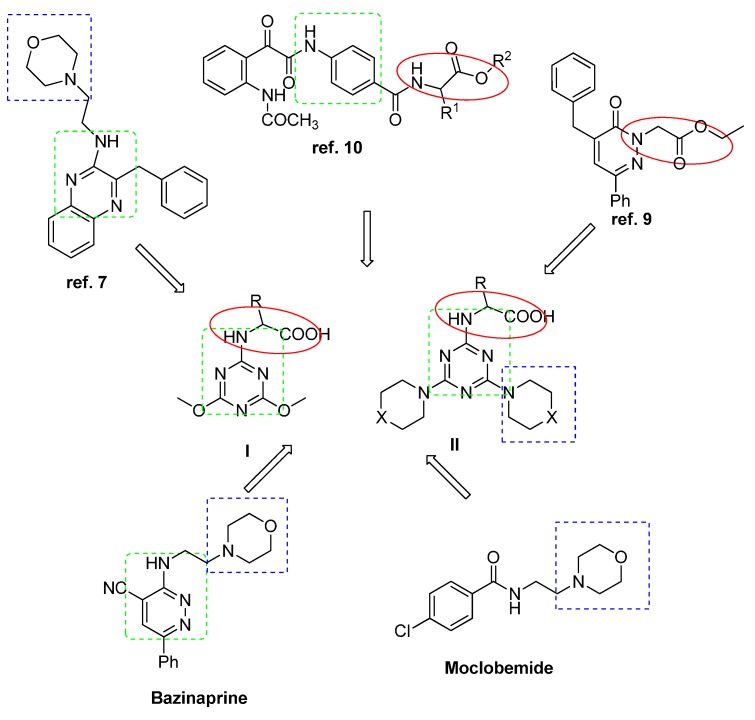
The aim of the present study was to tailor MAO-A inhibitors by designing a hybrid from different possible active sites of previously known MAO-A inhibitors, based on the following considerations: (i) the presence of electron-rich aromatic moieties (e.g., moclobemide [34],bazinaprine [35], quinoxaline derivatives [7,8]); (ii) the presence of morpholine moiety (e.g., moclobemide [34], bazinaprine [35]); and (iii) the presence of amino acid moiety [9,10]). The target compounds were designed to study the effect of molecular variation on MAO inhibitory activity, Figure 1.
Figure 1.
Planned modification and newly designed MAO inhibitors. I = (4,6-dimethoxy-1,3,5-triazin-2-yl) amino acid derivatives; X = O; II is (4,6-dimorpholino-1,3,5-triazin-2-yl) amino acid derivatives or X = CH2; II is (4,6-dipiperidino-1,3,5-triazin-2-yl) amino acid derivatives.
2. Results and Discussion
2.1. Chemistry
We replaced two chlorine atoms with cyanuric chloride and two methoxy, two piperidino or two morpholino groups, while the third chlorine was replaced with free α-amino acid. Accordingly, 4,6-dimethoxy-, 4,6-dipiperidino- and 4,6-dimorpholino-1,3,5-triazine-based amino acid derivatives were prepared by subsequent displacement of chlorine atoms.
The small library of (4,6-dimethoxy-1,3,5-triazin-2-yl) amino acid derivatives 3–9 were prepared by reaction of 2-chloro-4,6-dimethoxy triazine 1 and α-amino acids in the presence of triethyl amine as acid scavenger in a 1,4-dioxane/water (1:1) solvent mixture at room temperature (Scheme 1). The reaction was started by addition of N,N,N-triethyl amine (Et3N) to a solution of 2-chloro-4,6-dimethoxy triazine 1 in 1,4-dioxane and stirring until a white suspension of 4,6-dimethoxy-1,3,5-triazin-2-yl triethyl ammonium chloride salt 2 was formed. An aqueous solution of α-amino acid and Et3N was then added to this white suspension and stirred to give the desired products after neutralization with 5% citric acid or 1 N HCl. The structures of compounds 3–9 were confirmed by spectroscopic methods (IR, 1H- and 13C-NMR) and by elemental analysis.
Scheme 1.
Synthesis of (4,6-dimethoxy-1,3,5-triazin-2-yl) amino acid derivatives 3–9.
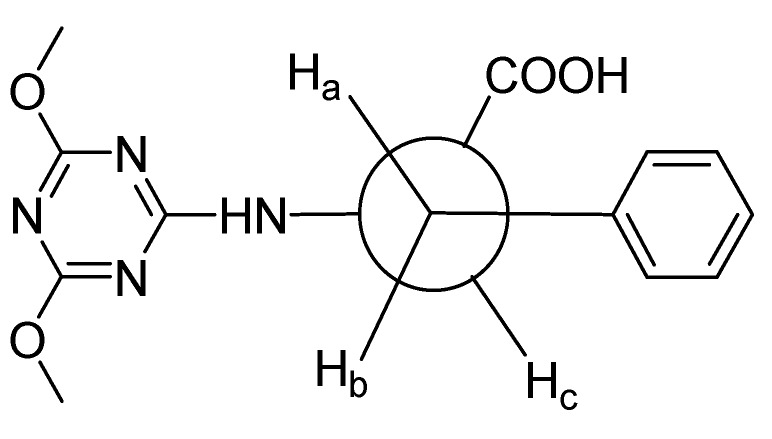
The 1H-NMR spectrum of 7 in DMSO-d6 (Supplementary Data Figure S9) showed two doublet of doublet (dd) peaks at δ 3.00 ppm and 3.14 ppm, corresponding to the two diastereotopic methylene protons Ha and Hb, respectively, as shown in the staggered conformation using Newman projection (Figure 2).
Figure 2.
Newman projection formula for 2-(4,6-dimethoxy-1,3,5-triazin-2-ylamino)-3-phenylpropanoic acid 7.
Ha showed a doublet of doublet peak caused by coupling with the germinal proton Hb with 2J = 13.9 Hz, and then with the vicinal proton Hc with 3J = 10.2 Hz (Anti-interaction, dihedral angle = 180°). Similarly, Hb showed a doublet of doublet peak as a result of coupling with the germinal proton Ha with 2J = 13.9 Hz, and then with the vicinal proton Hc with 3J = 3.7 Hz (Gauche interaction, dihedral angle = 60°). Two singlet peaks at δ 3.77 ppm and 3.80 ppm corresponding to the two methoxy groups were observed. A multiplet peak appeared at δ 4.54–4.59 ppm corresponding to the α-proton. The peaks corresponding to the aromatic protons appeared as a multiplet at δ 7.18–7.31 ppm. A doublet peak at δ 8.20 ppm with J = 8.0 Hz, which is D2O-exchangeable, was also observed, corresponding to the NH proton (Supplementary Data Figure S9).
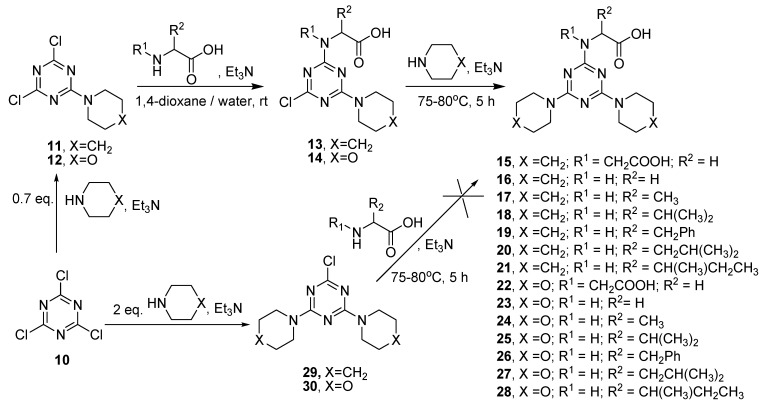
In addition, N-(4,6-dipiperidino/dimorpholino-1,3,5-triazin-2-yl) amino acid derivatives 15–28 were prepared through the following sequential reaction: cyanuric chloride 10 was reacted first with piperidine/morpholine in the presence of sodium carbonate (acid scavenger) to afford the corresponding products 2,4-dichloro-6-(piperidin-1-yl)-1,3,5-triazine 11 and 2,4-dichloro-6-morpholino-1,3,5-triazine 12 [28], respectively. Compounds 11 or 12 were allowed to react with free α-amino acids at room temperature. The formed products 13 and 14, respectively, were then allowed to react directly without isolation with piperidine/morpholine in the presence of Et3N, to give the corresponding products, N-(4,6-dipiperidino-1,3,5-triazin-2-yl) amino acid derivatives 15–21 and N-(4,6-dimorpholino-1,3,5-triazin-2-yl) amino acid derivatives 22–28 (Scheme 2).
Scheme 2.
Synthesis of N-(4,6-dipiperidino/dimorpholino-1,3,5-triazin-2-yl) amino acid derivatives 15–28.
Compounds 29 or 30 were prepared by reaction of cyanuric chloride with equiv. of piperidine/morpholine in the presence of Et3N (Scheme 2) [36]. In contrast, the preparation of N-(4,6-dipiperidino/dimorpholino-1,3,5-triazin-2-yl) amino acid derivatives through the reaction of free α-amino acids with 29/30 was not successful (Scheme 2). The difficulty to displace the third chlorine by the rather weak nucleophilicity of the amino group of α-amino acids can be attributed to the presence of two electron-donating piperidine/morpholine groups, which decreases the positivity of the third chlorine-bearing carbon and prevents the departure of the chlorine atom. The structures of compounds 15–28 were confirmed by spectroscopic methods (IR, 1H- and 13C-NMR) and by elemental analysis (Supplementary Data Figures S15–S35).
2.2. Preliminary Biology
The newly synthesized compounds 3–9 and 15–28 were tested to determine selectivity for MAO-A and MAO-B in the presence of the specific substrate serotonin or benzylamine, respectively. Compounds 7, 18, and 25 showed MAO-A inhibition activity comparable to that of the standard clorgyline with apparently more selective inhibitory activity toward MAO-A than MAO-B, and without no significant acute toxicity. More formal studies to confirm these preliminary results will be carried out and published elsewhere.
3. Experimental Section
3.1. Chemistry
Solvents and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Unless otherwise stated, the normal workup from organic solvent involved drying over Na2SO4 and rotary evaporation. TLC was performed using aluminum-backed Merck Silica Gel 60 F-254 plates and suitable solvent systems. Spots were visualized by a Spectroline UV Lamp (254 or 365 nm) or I2 vapor. Melting points were obtained in open capillary tubes using a MEL-Temp II melting point apparatus and uncorrected. Infrared spectra (IR) were recorded on a Perkin-Elmer (Waltham, MA, USA) 1600 series Fourier transform instrument as KBr pellets. The absorption bands (νmax) are given in wave numbers (cm−1). Nuclear magnetic resonance (NMR) spectra (1H-NMR and 13C-NMR) were recorded on a JEOL (Tokyo, Japan) 400 MHz and JEOL 500 MHz spectrometer at room temperature. Chemical shifts are reported in parts per million (ppm) and are referenced relative to residual solvent (e.g., CHCl3 at δH 7.26 ppm for CDCl3, DMSO at δH 2.50 ppm for DMSO-d6). Spin multiplicities are represented by the following signals: singlet (s), broad singlet (br s), doublet (d), broad doublet (br d), doublet of doublets (dd), triplet (t), doublet of triplets (dt), quartet (q), sextet (sex) and multiplet (m). Elemental analyses were performed on a Perkin-Elmer 2400 elemental analyzer, with the values found being within ±0.3% of the theoretical values.
3.1.1. General Procedure for the Synthesis of (4,6-Dimethoxy-1,3,5-triazin-2-yl) Amino Acid Derivatives 3–9
A solution of 2-chloro-4,6-dimethoxy triazine 1 (0.88 g, 5 mmol) and triethyl amine (1.04 mL, 7.5 mmol) in dioxane was stirred at room temperature until a white suspension of 4,6-dimethoxy-1,3,5-trazin-2-yl triethyl ammonium chloride was formed. A solution of α-amino acid (5 mmol) and triethyl amine (1.04 mL, 7.5 mmol) in 6 mL dioxane:water (1:1) was added to the white suspension, forming a clear mixture. The mixture was stirred overnight and then neutralized with 1N HCl to yield a white solid, which was then filtered and dried.
N-(4,6-Dimethoxy-1,3,5-triazin-2-yl)iminodiacetic acid (3). The product was obtained as a white powder, 6.81 g (83.4%) yield, mp 180–181 °C; IR (KBr): 3500–2589 (br, OH, acid), 1709 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 3.83 (s, 6H, 2 × OCH3), 4.29 (s, 4H, 2 × α-CH2), 12.77 (br s, 2H, 2 × COOH); 13C-NMR (100 MHz, DMSO-d6): 50.20, 54.87, 167.77, 171.20, 172.22. Elemental Analysis Calcd. for C9H12N4O6: C, 39.71; H, 4.44; N, 20.58. Found: C, 39.76; H, 4.38; N, 20.51.
2-(4,6-Dimethoxy-1,3,5-triazin-2-ylamino)acetic acid (4). The product was obtained as a white solid, 0.64 g (60.0%) yield; mp: 164–166 °C; IR (KBr): 3574–2522 (br, OH, acid), 3255 (NH, amine), 1725 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 3.83 (s, 3H, O-CH3), 3.80 (s, 3H, O–CH3), 3.91 (d, 2H, J = 5.9 Hz, CH2), 8.13 (t, 1H, J = 5.9 Hz, N–H); 13C-NMR (100 MHz, DMSO-d6): 40.67, 54.63, 54.75, 168.56, 171.8, 172.25, 172.36. Elemental Analysis Calcd. for C7H10N4O4: C, 39.25; H, 4.71; N, 26.16. Found: C, 39.49; H, 4.45; N, 25.94.
2-(4,6-Dimethoxy-1,3,5-triazin-2-ylamino)propanoic acid (5). The product was obtained as a white solid, 0.77 g (66.6%) yield; mp: 98–102 °C; IR (KBr): 3557–2567 (br, OH, acid), 3372 (NH, amine), 1721 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 1.35 (d, 3H, J = 7.3 Hz, CH3), 3.80 (s, 3H, O–CH3), 3.83 (s, 3H, O–CH3), 4.34 (quint, 1H, J = 7.3 Hz, α-CH), 8.20 (d, 1H, J = 7.3 Hz, N–H), 11.18 (br s, 1H, COOH); 13C-NMR (100 MHz, DMSO-d6): 17.30, 49.86, 54.63, 54.75, 167.86, 172.26, 174.85. Elemental Analysis Calcd. for C8H12N4O4: C, 42.10; H, 5.30; N, 24.55. Found: C, 42.17; H, 5.22; N, 24.71.
2-(4,6-Dimethoxy-1,3,5-triazin-2-ylamino)-3-methylbutanoic acid (6). The product was obtained as a white solid, 0.78 g (61.4%) yield; mp: 146–188 °C; IR (KBr): 3570–2539 (br, OH, acid), 3259 (NH, amine), 1720 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 0.94 (d, 3H, J = 6.6 Hz, CH3), 0.95 (d, 3H, J = 6.6 Hz, CH3), 2.13 (octet, 1H, J = 6.6 Hz, CH), 3.81 (s, 3H, O–CH3), 3.84 (s, 3H, O–CH3), 4.21 (t, 1H, J = 6.6 Hz, α-CH), 8.03 (d, 1H, J = 7.3 Hz, N–H); 13C-NMR (100 MHz, DMSO-d6): 19.11, 19.73, 29.94, 54.65, 54.76, 60.24, 168.48, 172.28, 173.68. Elemental Analysis Calcd. for C10H16N4O4: C, 46.87; H, 6.29; N, 21.86. Found: C, 47.02; H, 6.19; N, 21.79.
2-(4,6-Dimethoxy-1,3,5-triazin-2-ylamino)-3-phenylpropanoic acid (7). The product was obtained as a white solid, 0.98 g (64.1%) yield; mp: 153–155 °C; IR (KBr): 3431–2650 (br, OH, acid), 3256 (NH, amine), 1714 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 3.00 (dd, 1H, 2J = 13.9 Hz, 3J = 10.2 Hz, CH2–Ph), 3.14 (dd, 1H, 2J = 13.9 Hz, 3J = 3.7 Hz, CH2–Ph), 3.77 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 4.54–4.59 (m, 1H, α-CH), 7.18–7.31 (m, 5H, Ar–H), 8.20 (d, 1H, J = 8.0 Hz, N–H); 13C-NMR (100 MHz, DMSO-d6): 36.74, 54.66, 54.76, 56.11, 126.96, 128.77, 129.62, 138.55, 168.25, 172.21, 173.75. Elemental Analysis Calcd. for C14H16N4O4: C, 55.26; H, 5.30; N, 18.41. Found: C, 55.02; H, 5.51; N, 18.55.
2-(4,6-Dimethoxy-1,3,5-triazin-2-ylamino)-4-methylpentanoic acid (8). The product was obtained as a white solid, 0.91 g (67.4%) yield; mp: 102–103 °C; IR (KBr): 3443–2551 (br, OH, acid), 3282 (NH, amine) 1725 (CO, acid) cm−1;1H-NMR (400 MHz, DMSO-d6): δ 0.86 (d, 3H, J = 5.9 Hz, CH3), 0.90 (d, 3H, J = 6.6 Hz, CH3), 1.52 (m, 1H, CH), 1.68 (m, 2H, CH2), 3.80 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 4.35–4.38 (m, 1H, α-CH), 8.16 (d, 1H, J = 7.3 Hz, N–H); 13C-NMR (100 MHz, DMSO-d6): 21.67, 23.50, 24.97, 46.18, 52.6, 54.64, 54.75, 168.27, 172.25, 172.31, 174.76. Elemental Analysis Calcd. for C11H18N4O4: C, 48.88; H, 6.71; N, 20.73. Found: C, 49.08; H, 6.46; N, 20.97.
2-(4,6-Dimethoxy-1,3,5-triazin-2-ylamino)-3-methylpentanoic acid (9). The product was obtained as a white solid, 0.98 g (72.5%) yield; mp: 118–119 °C; IR (KBr): 3500–2536 (br, OH, acid), 3261 (NH, amine), 1715 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 0.85 (t, 3H, J = 7.4 Hz, CH3CH2), 0.91 (d, 3H, J = 6.6 Hz, CH3CH), 1.26–1.31 (m, 1H, CH2), 1.44–1.48 (m, 1H, CH2), 1.85–1.88 (m, 1H, CH), 3.81 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 4.27 (t, 1H, J = 7.4 Hz, α-CH), 8.04 (d, 1H, J = 7.4 Hz, N–H); 13C-NMR (100 MHz, DMSO-d6): 11.75, 16.14, 25.60, 36.31, 54.65, 54.75, 58.98, 168.35, 172.28, 173.63. Elemental Analysis Calcd. for C11H18N4O4: C, 48.88; H, 6.71; N, 20.73. Found: C, 48.69; H, 6.79; N, 20.83.
3.1.2. General Procedure for the Synthesis of N-(4,6-Dipiperidino-1,3,5-triazin-2-yl) Amino Acid Derivatives 15–21
A mixture of 2,4-dichloro-6-(piperidin-1-yl)-1,3,5-triazine 11 (0.47 g, 2 mmol) and triethyl amine (0.42 mL, 3 mmol) in 1,4-dioxane (5 mL) was stirred at room temperature until a white suspension was formed. A solution of α-amino acid (2.4 mmol) and triethyl amine (0.42 mL, 3 mmol) in water (2 mL) was added to the suspension to afford a clear mixture. The mixture was stirred overnight at room temperature. Subsequently, piperidine (0.3 mL, 3 mmol) and triethyl amine (0.56 mL, 4 mmol) were added to the reaction mixture and stirred at between 75 °C and 80 °C for 5 h. The reaction mixture was neutralized with 5% citric acid or 1 N HCl. The white precipitate was filtered and recrystallized from ethanol/water to obtain the desired products.
N-(4,6-Dipiperidino-1,3,5-triazin-2-yl)iminodiacetic acid (15). The product was obtained as a white solid, 0.44 g (79.1%) yield; mp: 128–130 °C; IR (KBr): 3593–2853 (br, OH, acid), 1729 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 1.44 (br s, 8H, 4 × a-CH2), 1.58 (m, 4H, 2 × b-CH2), 3.62 (br s, 8H, 4 × CH2N), 4.16 (s, 4H, 2 × α-CH2), 12.55 (br s, 2H, COOH); 13C-NMR (100 MHz, DMSO-d6): 24.97, 25.90, 40.06, 50.26, 164.81, 165.00, 172.48. Elemental Analysis Calcd. for C17H26N6O4: C, 53.96; H, 6.93; N, 22.21. Found: C, 53.77; H, 7.14; N, 22.43.
2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-ylamino)acetic acid (16). The product was obtained as a white solid, 0.51 g (79.0%) yield; mp: 211–214 °C; IR (KBr): 3628–2664 (br, OH, acid), 3269 (NH, amine) 1676 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 1.44 (br s, 8H, 4 × a-CH2), 1.58 (br s, 4H, 2 × b-CH2), 3.62 (br s, 8H, 4 × CH2N), 3.79 (d, 2H, J = 5.8 Hz, α-CH2), 6.85 (t, 1H, J = 5.8 Hz, NH), 12.37 (br s, 1H, COOH); 13C-NMR (100 MHz, DMSO-d6): 25.01, 25.93, 42.89, 43.95, 165.04, 166.45, 172.89. Elemental Analysis Calcd. for C15H24N6O2: C, 56.23; H, 7.55; N, 26.23. Found: C, 56.03; H, 7.73; N, 26.33.
2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-ylamino)propanoic acid (17). The product was obtained as a white solid, 0.52 g (78.1%) yield; mp: 126–128 °C; IR (KBr): 3571–2856 (br, OH, acid), 3454 (NH, amine), 1667 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 1.29 (d, 3H, J = 7.3 Hz, CH3), 1.43 (br s, 8H, 4 × a-CH2), 1.58 (br s, 4H, 2 × b-CH2), 3.62 (br s, 8H, 4 × CH2N), 4.22 (quint, 1H, J = 7.3 Hz, α-CH), 6.82 (d, 1H, J = 6.6 Hz, NH); 13C-NMR (100 MHz, DMSO-d6): 19.11, 25.01, 25.94, 43.96, 56.58, 164.00, 165.88, 175.97. Elemental Analysis Calcd. for C16H26N6O2: C, 57.46; H, 7.84; N, 25.13. Found: C, 57.59; H, 7.66; N, 25.31.
2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-ylamino)-3-methylbutanoic acid (18). The product was obtained as a white solid, 0.50 g (70.0%) yield; mp: 183–185 °C; IR (KBr): 3586–2853 (br, OH, acid), 3429 (NH, amine), 1723 (CO, acid) cm−1; 1H-NMR (500 MHz, DMSO-d6): δ 0.89 (s, 6H, 2 × CH3), 1.39 (br s, 8H, 4 × a-CH2), 1.53 (br s, 4H, 2 × b-CH2), 2.01 (s, 1H, CH), 3.54 (br s, 8H, 4 × CH2N), 4.07 (s, 1H, α-CH), 6.41 (s, 1H, NH, D2O exchangeable); Elemental Analysis Calcd. for C18H30N6O2: C, 59.64; H, 8.34; N, 23.19. Found: C, 59.59; H, 8.41; N, 23.07.
2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-ylamino)-3-phenylpropanoic acid (19). The product was obtained as a white solid, 0.67 g (82.0%) yield; mp: 132–134 °C; IR (KBr): 3571–2853 (br, OH, acid), 3316 (NH, amine), 1721 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 1.42 (br s, 8H, 4 × a-CH2), 1.57 (br s, 4H, 2 × b-CH2), 2.95–3.07 (m, 2H, CH2–Ph), 3.60 (br s, 8H, 4 × CH2N), 4.32–4.53 (m, 1H, α-CH), 6.77 (d, 1H, J = 7.3 Hz, NH), 7.17–7.31 (m, 5H, Ph–H), 12.5 (br s, 1H, COOH); 13C-NMR (100 MHz, DMSO-d6): 24.99, 25.93, 37.00, 43.96, 56.14, 126.80, 128.69, 129.68, 138.99, 164.77, 164.94, 166.14, 174.90. Elemental Analysis Calcd. for C22H30N6O2: C, 64.37; H, 7.37; N, 20.47. Found: C, 64.18; H, 7.43; N, 20.67.
2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-ylamino)-4-methylpentanoic acid (20). The product was obtained as a white solid, 0.54 g (71.8%) yield; mp:158–161 °C; IR (KBr): 3614–2855 (br, OH, acid), 3320 (NH, amine) 1721 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 0.86 (d, 3H, J = 6.6 Hz, CH3), 0.88 (d, 3H, J = 6.6 Hz, CH3), 1.38–1.49 (m, 1H, CH), 1.44 (br s, 8H, 4 × a-CH2), 1.57 (br s, 4H, 2 × b-CH2), 1.57–1.73 (m, 2H, CH2), 3.62–3.64 (m, 8H, 4 × CH2N), 4.27–4.29 (m, 1H, α-CH), 6.78 (d, 1H, J = 7.3 Hz, NH), 12.26 (br s, 1H, COOH); 13C-NMR (100 MHz, DMSO-d6): 21.87, 23.59, 24.90, 25.02, 25.89, 26.01, 43.94, 52.36, 164.99, 166.30, 175.88. Elemental Analysis Calcd. for C19H32N6O2: C, 60.61; H, 8.57; N, 22.32. Found: C, 60.45; H, 8.72; N, 22.16.
2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-ylamino)-3-methylpentanoic acid (21). The product was obtained as a white solid, 0.60 g (79.7%) yield; mp:176–178 °C; IR (KBr): 3614–2854 (br, OH, acid), 3315 (NH, amine) 1726 (CO, acid) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 0.85 (t, 3H, J = 6.6 Hz, CH3), 0.88 (d, 3H, J = 6.6 Hz, CH3), 1.22–1.52 (m, 2H, CH2), 1.44 (br s, 8H, 4 × a-CH2), 1.58 (br s, 4H, 2 × b-CH2), 1.77–1.82 (m, 1H, CH), 3.63 (br s, 8H, 4 × CH2N), 4.17 (t, 1H, J = 7.3 Hz, α-CH), 6.51 (d, 1H, J = 7.3 Hz, NH), 12.33 (br s, 1H, COOH); 13C-NMR (100 MHz, DMSO-d6): 11.73, 16.22, 25.01, 25.91, 26.02, 36.46, 43.95, 58.64, 164.84, 164.99, 166.30, 174.68. Elemental Analysis Calcd. for C19H32N6O2: C, 60.61; H, 8.57; N, 22.32. Found: C, 60.83; H, 8.36; N, 22.16.
3.1.3. General Procedure for the Synthesis of N-(4,6-Dimorpholino-1,3,5-triazin-2-yl) Amino Acid Derivatives 22–28
The mixture of 2,4-dichloro-6-morpholino-1,3,5-triazine 12 (0.47 g, 2 mmol) and triethyl amine (0.42 mL, 3 mmol) in 1,4-dioxane (5 mL) was stirred at room temperature until a white suspension was formed. A solution of α-amino acid (1.2 equiv.) and triethyl amine (0.42 mL, 3 mmol) in water (2 mL) was added to the suspension to afford a clear mixture. The mixture was stirred overnight at room temperature. Subsequently, morpholine (0.26 mL, 3 mmol) and triethyl amine (0.56 mL, 4 mmol) were added to the reaction mixture and stirred at between 75 °C and 80 °C for 5 h. The reaction mixture was neutralized with 5% citric acid or 1 N HCl. The white precipitate was filtered and recrystallized from ethanol/water to obtain the desired products.
N-(4,6-Dimorpholino-1,3,5-triazin-2-yl)iminodiacetic acid (22). The product was obtained as a white solid, 0.48 g (63.0%) yield; mp: 260 °C (decom.); IR (KBr): 3700–2660 (br, OH, acid), 1718 (CO, acid) cm−1; 1H-NMR (500 MHz, DMSO-d6): δ 3.50–3.53 (m, 8H, 4 × CH2N), 3.55–3.57 (m, 8H, 4 × CH2O), 3.94 (s, 4H, 2 × α-CH2), 11.63 (br s, 2H, 2 × COOH). Elemental Analysis Calcd for C15H22N6O6: C, 47.12; H, 5.80; N, 21.98. Found: C, 47.02; H, 5.94; N, 22.09.
2-(4,6-Dimorpholino-1,3,5-triazin-2-ylamino)acetic acid (23). The product was obtained as a white solid, 0.44 g (68.0%) yield; mp: 224–226 °C; IR (KBr): 3436 (br, OH, acid), 3296 (NH, amine), 1679 (CO, acid) cm−1; 1H-NMR (500 MHz, DMSO-d6): δ 3.54 (t, 8H, J = 4.6 Hz, 4 × CH2N), 3.58 (t, 8H, J = 4.6 Hz, 4 × CH2O), 3.78 (d, 2H, J = 6.1 Hz, α-CH2), 6.98 (t, 1H, J = 6.1 Hz, N–H), 11.92 (br s, 1H, COOH); 13C-NMR (125 MHz, DMSO-d6): 43.66, 44.82, 66.55, 165.05, 165.23, 166.29, 172.76. Elemental Analysis Calcd. for C13H20N6O4: C, 48.14; H, 6.22; N, 25.91. Found: C, 48.01; H, 6.34; N, 26.07.
2-(4,6-Dimorpholino-1,3,5-triazin-2-ylamino)propanoic acid (24). The product was obtained as a white solid, 0.42 g (61.8%) yield; mp: 194–196 °C; IR (KBr): 3642–2860 (br, OH, acid), 3428 (NH, amine), 1681 (CO, acid) cm−1; 1H-NMR (500 MHz, DMSO-d6): δ 1.26 (d, 3H, J = 7.7 Hz, CH3), 3.53–3.56 (m, 8H, 4 × CH2N), 3.57–3.3.61 (m, 8H, 4 × CH2O), 4.19 (quint, 1H, J = 6.9 Hz, α-CH), 6.99 (d, 1H, J = 6.9 Hz, NH), 12.07 (br s, 1H, COOH). Elemental Analysis Calcd. for C14H22N6O4: C, 49.70; H, 6.55; N, 24.84. Found: C, 49.59; H, 6.77; N, 24.91.
2-(4,6-Dimorpholino-1,3,5-triazin-2-ylamino)-3-methylbutanoic acid (25). The product was obtained as a white solid, 0.49 g (67.5%) yield; mp: 170–172 °C; IR (KBr): 3438 (br, OH, acid), 3305 (NH, amine), 1723 (CO, acid) cm−1; 1H-NMR (500 MHz, DMSO-d6): δ 0.88 (d, 3H, J = 7.7 Hz, CH3), 0.89 (d, 3H, J = 6.9 Hz, CH3), 1.97–2.04 (m, 1H, CH), 3.53 (s, 8H, 4 × CH2N), 3.60 (s, 8H, 4 × CH2O), 4.05 (t, 1H, J = 6.9 Hz, α-CH), 6.73 (d, 1H, J = 6.9 Hz, NH). Elemental Analysis Calcd. for C16H26N6O4: C, 52.45; H, 7.15; N, 22.94. Found: C, 52.29; H, 6.99; N, 23.05.
2-(4,6-Dimorpholino-1,3,5-triazin-2-ylamino)-3-phenylpropanoic acid (26). The product was obtained as a white solid, 0.69 g (83.1%) yield; mp: 126–128 °C; IR (KBr): 3642–2855 (br, OH, acid), 3427 (NH, amine), 1729 (CO, acid) cm−1; 1H-NMR (500 MHz, DMSO-d6) isomer A (83.0%): δ 2.91–2.99 (m, 1H, CH2–Ph), 3.01 (dd, 1H, J = 13.8 Hz, J = 5.4 Hz, CH2–Ph), 3.53 (s, 8H, 4 × CH2N), 3.57 (t, 8H, J = 5.4 Hz, 4 × CH2O), 4.37–4.41 (m, 1H, α-CH), 6.92 (d, 1H, J = 7.7 Hz, NH), 7.10–7.24 (m, 5H, Ph–H); isomer B (17.0%): δ 2.76–2.90 (m, 2H, CH2–Ph), 3.53 (s, 8H, 4 × CH2N), 3.57 (t, 8H, J = 5.4 Hz, 4 × CH2O), 4.52–4.54 (m, 1H, α-CH), 6.82 (d, 1H, J = 8.4 Hz, NH), 7.10–7.24 (m, 5H, Ph–H).Elemental Analysis Calcd. for C20H26N6O4: C, 57.96; H, 6.32; N, 20.28. Found: C, 57.76; H, 6.51; N, 20.33.
2-(4,6-Dimorpholino-1,3,5-triazin-2-ylamino)-4-methylpentanoic acid (27). The product was obtained as a white solid, 0.48 g (62.7%) yield; mp: 120–122 °C; IR (KBr): 3720–2500 (br, OH, acid), 3426 (NH, amine), 1728 (CO, acid) cm−1; 1H-NMR (500 MHz, DMSO-d6): δ 0.82 (d, 3H, J = 6.1 Hz, CH3), 0.85 (d, 3H, J = 6.1 Hz, CH3), 1.38–1.44 (m, 1H, CH), 1.57–1.67 (m, 2H, CH2), 3.53–3.55 (m, 8H, 4 × CH2N), 3.56–3.60 (m, 8H, 4 × CH2O), 4.21–4.25 (m, 1H, α-CH), 6.94 (d, 1H, J = 6.9 Hz, NH), 12.13 (br.s, 1H, COOH). Elemental Analysis Calcd. for C17H28N6O4: C, 53.67; H, 7.42; N, 22.09. Found: C, 53.54; H, 7.34; N, 22.19.
2-(4,6-Dimorpholino-1,3,5-triazin-2-ylamino)-3-methylpentanoic acid (28). The product was obtained as a white solid, 0.65 g (85.6%) yield; mp: 96–98 °C; IR (KBr): 3609–2661 (br, OH, acid), 3485 (NH, amine), 1670 (CO, acid) cm−1; 1H-NMR (500 MHz, CDCl3): δ 0.87–0.92 (m, 3H, CH3CH2), 0.93 (d, 3H, J = 6.2 Hz, CH3CH), 1.15–1.29 (m, 1H, CH2), 1.42–1.61 (m, 1H, CH2), 1.96–2.01 (m, 1H, CH), 3.66–3.74 (m, 8H, 4 × CH2N), 3.75–3.84 (m, 8H, 4 × CH2O), 4.50–4.64 (m, 1H, α-CH), 7.56–7.78 (m, 1H, NH), 8.24 (br s, 1H, COOH). Elemental Analysis Calcd. for C17H28N6O4: C, 53.67; H, 7.42; N, 22.09. Found: C, 53.78; H, 7.33; N, 21.98.
4. Conclusions
The synthesis and a preliminary biochemical evaluation of the newly synthesized N-(2,4-disubstituted-1,3,5-triazin-2-yl) amino acid derivatives as MAO inhibitors were described. Compounds 7, 18 and 25 showed the highest activity within the test compounds comparable to that of the standard clorgyline. These preliminary tests have also shown remarkable selectivity within the test compounds as MAO-A inhibitors. Therefore, such compounds would represent a fruitful matrix for the development of a new class of MAO-A inhibitors that would deserve further investigation and derivatization.
Acknowledgments
The authors thank the Science and Technology Development Fund (STDF), for funding this work through Research Project TC/12/RSG/2012 (Proposal ID (4769). The authors thank the Deanship of Scientific Research at King Saud University for partially funding this work through research group no. RGP-234 (Saudi Arabia).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/09/15976/s1.
Author Contributions
The main part of the work was carried out by Hosam H. Khalil, with the direct supervision of Ayman El-Faham, Sherine N. Khattab, Adnan A. Bekhit, and Mohamed Mokbel Abd El-Rahman. Conceptually, the work was designed by Ayman El-Faham and Fernando Albericio. The first draft was written by Hosam H. Khalil with the input from all the other authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the all compounds are available from the authors.
References
- 1.Kalgutkar A.S., Dalvie D.K., Castagnoli N., Taylor T. Interactions of Nitrogen-Containing Xenobiotics with Monoamine Oxidase (MAO) Isozymes A and B: SAR Studies on MAO Substrates and Inhibitors. Chem. Res. Toxicol. 2001;14:1139–1162. doi: 10.1021/tx010073b. [DOI] [PubMed] [Google Scholar]
- 2.Legoabe L.J., Petzer A., Petzer J.P. Selected C7-substituted chromone derivatives as monoamine oxidase inhibitors. Bioorg. Chem. 2012;45:1–11. doi: 10.1016/j.bioorg.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Youdim M.B.H., Bakhle Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006;147:S287–S296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson M.C., Hasan F., McCrodden J.M. Monoamine oxidase inhibitors and the cheese effect. Neurochem. Res. 1993;18:1145–1149. doi: 10.1007/BF00978365. [DOI] [PubMed] [Google Scholar]
- 5.Tetrud J.W., Koller W.C. A novel formulation of selegiline for the treatment of Parkinson’s disease. Neurology. 2004;63:S2–S6. doi: 10.1212/WNL.63.7_suppl_2.S2. [DOI] [PubMed] [Google Scholar]
- 6.Riederer P., Danielczyk W., Grunblatt E. Monoamine-oxidase-B inhibition in Alzheimer’s disease. Neurotoxicology. 2004;25:271–277. doi: 10.1016/S0161-813X(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 7.Hassan S.Y., Khattab S.N., Bekhit A.A., Amer A. Synthesis of 3-benzyl-2-substituted quinoxalines as novel monoamine oxidase A inhibitors. Bioorg. Med. Chem. Lett. 2006;16:1753–1756. doi: 10.1016/j.bmcl.2005.11.088. [DOI] [PubMed] [Google Scholar]
- 8.Khattab S.N., Hassan S.Y., Bekhit A.A., El-Massry A., Langer V., Amer A. Synthesis of new series of quinoxaline based MAO-inhibitors and docking studies. Eur. J. Med. Chem. 2010;45:4479–4489. doi: 10.1016/j.ejmech.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Khattab S.N., Bekhit A.A., El-Faham A., El-Massry A., Amer A. Synthesis of Some Pyridazinyl acetic Acid Derivatives as a Novel Class of Monoamine Oxidase-A Inhibitors. Chem. Pharm. Bull. 2008;56:1717–1721. doi: 10.1248/cpb.56.1717. [DOI] [PubMed] [Google Scholar]
- 10.El-Faham A., Al Marhoon Z., Abdel-Megeed A., Khattab S.N., Bekhit A., Albericio F. α-Ketoamino acid ester derivatives as promising MAO inhibitors. Biol. Org. Med. Chem. Lett. 2015;25:70–74. doi: 10.1016/j.bmcl.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Foster B.J., Harding B.J., Leyland-Jones B., Hoth D. Hexamethylmelamine: A critical review of an active drug. Cancer Treat. Rev. 1986;38:197–217. doi: 10.1016/0305-7372(86)90006-X. [DOI] [PubMed] [Google Scholar]
- 12.Tranchand B., Catimel G., Lucas C., Sarkany M., Bastian G., Evene E., Guastalla J.P., Negrier S., Rebattu P., Dumortier A., et al. Phase-I clinicalandpharmacokinetic study of S9788, anew multidrug-resistance reversal agent given alone and in combination with Doxorubicin to patients with advanced solid tumers. Cancer Chemother. Pharmacol. 1998;41:281–291. doi: 10.1007/s002800050741. [DOI] [PubMed] [Google Scholar]
- 13.Ono M., Kawahara N., Goto D., Wakabayashi Y., Ushiro S., Yoshida S., Izumi H., Kuwano M., Sato Y. Inhibition of tumor growth and neovascularization by an anti-gastric ulcer agent, irsogladine. Cancer Res. 1996;56:1512–1516. [PubMed] [Google Scholar]
- 14.Maeda M., Iigo M., Tsuda H., Fujita H., Yonemura Y., Nakagawa K., Endo Y., Sasaki T. Antimetastatic and antitumor effects of 2,4-diamino-6-(pyridine-4-yl)-1,3,5-triazine(4PyDAT) on the high lung metastatic colon 26 tumor in mice. Anti-Cancer Drug Des. 2000;15:217–223. [PubMed] [Google Scholar]
- 15.Menicagli R., Samaritani S., Signore G., Vaglini F., Via L.D. In vitro cytotoxic activities of 2-alkyl-4,6-diheteroalkyl-1,3,5-triazines: New molecules in anticancer research. J. Med. Chem. 2004;47:4649–4652. doi: 10.1021/jm0495374. [DOI] [PubMed] [Google Scholar]
- 16.Baindur N., Chadha N., Brandt B.M., Asgari D., Patch R.J., Schalk-Hihi C., Carver T.E., Petrounia I.P., Baumann C.A., Ott H., et al. 2-Hydroxy-4,6-diamino-[1,3,5]triazines: A Novel Class of VEGF-R2 (KDR) Tyrosine Kinase Inhibitors. J. Med. Chem. 2005;48:1717–1720. doi: 10.1021/jm049372z. [DOI] [PubMed] [Google Scholar]
- 17.Pandey V.K., Tusi S., Tusi Z., Joshi M., Bajpai S. Synthesis and biological activity of substituted 2,4,6-s-triazines. Acta Pharm. 2004;54:1–12. [PubMed] [Google Scholar]
- 18.Porter J.R., Archibald S.C., Brown J.A., Childs K., Critchley D., Head J.C., Hutchinson B., Parton T.A.H., Robinson M.K., Shock A., et al. Discovery and evaluation of N-(triazin-1,3,5-yl) phenylalanine derivatives as VLA-4 integrin antagonists. Bioorg. Med. Chem. Lett. 2002;12:1591–1594. doi: 10.1016/S0960-894X(02)00237-8. [DOI] [PubMed] [Google Scholar]
- 19.Mylari B.L., Withbroe G.J., Beebe D.A., Brackett N.S., Conn E.L., Coutcher J.B., Oates P.J., Zembrowski W.J. Design and synthesis of a novel family of triazine-based inhibitors of sorbitol dehydrogenase with oral activity: 1-{4-[3R,5S-dimethyl-4-(4-methyl-[1,3,5]triazin-2-yl)-piperazin-1-yl]-[1,3,5]triazin-2-yl}-(R) ethanol. Bioorg. Med. Chem. 2003;11:4179–4188. doi: 10.1016/S0968-0896(03)00490-5. [DOI] [PubMed] [Google Scholar]
- 20.Henke B.R., Consler T.G., Go N., Hale R.L., Hohman D.R., Jones S.A., Lu A.T., Moore L.B., Moore J.T., Orband-Miller L.A., et al. A New Series of Estrogen Receptor Modulators That Display Selectivity for Estrogen Receptor β. J. Med. Chem. 2002;45:5492–5505. doi: 10.1021/jm020291h. [DOI] [PubMed] [Google Scholar]
- 21.Klenke B., Stewart M., Barrett M.P., Brun R., Gilbert I.H. Synthesis and Biological Evaluation of s-Triazine Substituted Polyamines as Potential New Anti-Trypanosomal Drugs. J. Med. Chem. 2001;44:3440–3452. doi: 10.1021/jm010854+. [DOI] [PubMed] [Google Scholar]
- 22.Jensen N.P., Ager A.L., Bliss R.A., Canfield C.J., Kotecka B.M., Rieckmann K.H., Terpinski J., Jacobus D.P. Phenoxypropoxybiguanides, prodrugs of DHFR-inhibiting diaminotriazine antimalarials. J. Med. Chem. 2001;44:3925–3931. doi: 10.1021/jm010089z. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal A., Srivastava K., Puri S.K., Chauhan P.M.S. Syntheses of 2,4,6-trisubstituted triazines as antimalarial agents. Bioorg. Med. Chem. Lett. 2005;15:531–533. doi: 10.1016/j.bmcl.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 24.Srinivas K., Srinivas U., Harakishore K., Jayathirha R.V., Bhanuprakash K., Murthy U.S.N. Synthesis and antibacterial activity of 2,4,6-tri substituted s-triazines. Bioorg. Med. Chem. Lett. 2005;15:1121–1123. doi: 10.1016/j.bmcl.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 25.McKay G.A., Reddy R., Arhin F., Belley A., Lehoux D., Moeck G., Sarmiento I., Parr T.R., Gros P., Pelletier J., et al. Triaminotriazine DNA helicase inhibitors with antibacterial activity. Bioorg. Med. Chem. Lett. 2006;16:1286–1290. doi: 10.1016/j.bmcl.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 26.Ghaib A., Menager S., Verite P., Lafont O. Synthesis of variously 9,9-dialkylated octahydropyrimido [3,4-a]-s-triazines with potential antifungal activity. IL Farmaco. 2002;57:109–116. doi: 10.1016/S0014-827X(01)01181-8. [DOI] [PubMed] [Google Scholar]
- 27.Lubbers T., Angehrn P., Gmunder H., Herzig S., Kulhanek J. Design, synthesis, and structure-activity relationship studies of ATP analogues as DNA gyrase inhibitors. Bioorg. Med. Chem. Lett. 2000;10:821–826. doi: 10.1016/S0960-894X(00)00109-8. [DOI] [PubMed] [Google Scholar]
- 28.Lebreton S., Newcombe N., Bradley M. Antibacterial single-bead screening. Tetrahedron. 2003;59:10213–10222. doi: 10.1016/j.tet.2003.10.070. [DOI] [Google Scholar]
- 29.Sunduru N., Sharma M., Srivastava K., Rajakumar S., Puri S.K., Saxena J.K., Chauhan P.M.S. Synthesis of oxalamide and triazine derivatives as a novel class of hybrid 4-aminoquinoline with potent antiplasmodial activity. Bioorg. Med. Chem. 2009;17:6451–6462. doi: 10.1016/j.bmc.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 30.D’Atri G., Gomarasca P., Resnati G., Tronconi G., Scolastico C., Sirtori C.R. Novel pyrimidine and 1,3,5-triazine hypolipemic agents. J. Med. Chem. 1984;27:1621–1629. doi: 10.1021/jm00378a016. [DOI] [PubMed] [Google Scholar]
- 31.Silen J.L., Lu A.T., Solas D.W., Gore M.A., Maclean D., Shah N.H., Coffin L.M., Bhinderwala N.S., Wang Y., Tsutsui K.L., et al. Screening for novel antimicrobials from encoded combinatorial libraries by using a two-dimensional agar format. Antimicrob. Agents Chemother. 1998;42:1447–1453. doi: 10.1128/aac.42.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou C., Min J., Liu Z., Young A., Deshazer H., Gao T., Chang Y., Kallenbach N.R. Synthesis and biological evaluation of novel 1,3,5-triazine derivatives as antimicrobial agents. Bioorg. Med. Chem. Lett. 2008;18:1308–1311. doi: 10.1016/j.bmcl.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Koc Z.E., Bingol H., Saf A.O., Torlak E., Coskun A. Synthesis of novel tripodal-benzimidazole from 2,4,6-tris(p-formylphenoxy)-1,3,5-triazine: Structural, electrochemical and antimicrobial studies. J. Hazard. Mater. 2010;183:251–255. doi: 10.1016/j.jhazmat.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Santo R.D., Costi R., Roux A., Artico M., Befani O., Meninno T., Agostinelli E., Palmegiani P., Turini P., Girilli R., et al. Design, Synthesis, and Biological Activities of Pyrrolylethanoneamine Derivatives, a Novel Class of Monoamine Oxidases Inhibitors. J. Med. Chem. 2005;48:4220–4223. doi: 10.1021/jm050172c. [DOI] [PubMed] [Google Scholar]
- 35.Tao G., Irie Y., Li D.J., Keung W.M. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorg. Med. Chem. 2005;13:4777–4788. doi: 10.1016/j.bmc.2005.04.081. [DOI] [PubMed] [Google Scholar]
- 36.Kurteva V., Afonso C. Solvent-free synthesis of melamines under microwave irradiation. Green Chem. 2004;6:183–187. doi: 10.1039/b313689b. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.