Abstract
Previous studies showed that hybrid grapes often have qualitatively and quantitatively higher polyphenolic contents than the common V. vinifera grape varieties. In general, these compounds are studied for grape chemotaxonomy and for nutraceutical purposes due to their relevant antioxidant activity. Non-anthocyanic flavonoid composition of five red hybrid grape varieties produced by crossing of V. vinifera, V. aestivalis, V. cinerea, V. berlandieri, V. labrusca, V. lincecumii, and V. rupestris were studied by liquid chromatography/high-resolution mass spectrometry. Thirty-one compounds were identified, including methylnaringenin, a tetrahydroxy-dimethoxyflavanone-hexoside, two flavonols (quercetin and a pentahydroxyflavone isomer), 20 glycoside flavonols (four quercetin, two myricetin, two kaempferol, three isorhamnetin, one laricitrin, two syringetin, one kaempferide and two dihydroflavonol derivatives; myricetin-glucoside-glucuronide; myricetin-diglucoside; syringetin-dihexoside), three flavan-3-ols (−)-epicatechin, (+)-catechin, (−)-epicatechin gallate) and four proantocyanidins (procyanidin B1, procyanidin B2, procyanidin B3 or B4/B5, procyanidin T2 or T3/T4/C1). Seibel 19881, Seyve Villard 12-347 and Seyve Villard 29-399 were particularly rich in polyphenols. These findings emphasize that these grapes are especially interesting for the production of antioxidant extracts for nutraceutical and pharmaceutical uses.
Keywords: antioxidants, hybrid grapes, polyphenols, high-resolution mass spectrometry
1. Introduction
The health benefits of grape and wine consumption are mainly associated with their polyphenolic content (the so-called French paradox [1]). Polyphenols are antioxidant compounds that act as reducing agents by donating hydrogen, acting as chelators, quenching singlet oxygen and by trapping free radicals [2]. Moreover, their activity in reducing coronary heart diseases, atherosclerosis [3,4], various types of cancer, and dermal disorders, was demonstrated [5,6].
Grape polyphenols can be divided in anthocyanic and non-anthocyanic compounds. The latter mainly include flavan-3-ols and their oligomers (proanthocyanidins and tannins), glycoside flavonols, hydroxycinnamates, hydroxybenzoates, and stilbenes derivatives [7].
Main flavan-3-ols in grape are catechin, epicatechin, gallocatechin, epigallocatechin, and epicatechin-3-O-gallate [7,8,9]. These compounds are located in the berry skin and seeds and are potent antioxidants characterized by important biological, pharmacological and medicinal properties: e.g., they are proven to protect human low density lipoprotein (LDL) against oxidation more efficiently than α-tocopherol on a molar basis, and act as cardioprotective agents [6,10,11,12].
Flavonols are located in the skin where are involved in the shielding from UV rays [13]. Principal are myricetin, quercetin and kaempferol mainly present as glucoside and glucuronide derivatives [14]. As well as glucoside, laricitrin, isorhamnetin and syringetin were found in grape in galactoside and glucuronide forms [15,16,17]. Flavonols are antioxidant and bioactive compounds present in dietary plants used for human nutrition [18]. For example, in vitro studies showed quercetin inhibits human platelet aggregation and acts as potential anticancer by inhibiting carcinogens and cancer cell growth in many experimental and human tumors [19,20].
Tannins are divided in condensed and hydrolysable. Condensed tannins (proanthocyanidins) are flavan-3-ol polymers mainly present in the solid parts of grape (skin and seeds) and, in minor amount, in the pulp [21]. In-vivo studies showed that grape seed procyanidin extract has an antioxidant activity as important as vitamin E, by preventing oxidative damage of tissues by reduction of the lipid oxidation and/or inhibition of free radicals production [22].
Traditional analytical methods for analysis of low molecular weight (MW) flavonoids are performed by reverse-phase high performance liquid chromatography (HPLC) coupled with UV-Vis spectrophotometry or mass spectrometry (MS) [14,23]. In general, liquid chromatography/mass spectrometry (LC/MS) and multiple mass spectrometry (MS/MS and MSn) are effective for the structural characterization [24]. LC coupled to high-resolution mass spectrometry (HRMS) has proven to be very effective in grape metabolomics, in particular for characterization of stilbene derivatives, flavonols and anthocyanins [25]. Recently, a database for grape and wine metabolomics (GrapeMetabolomics), which currently contains around 1100 putative compounds identified by HRMS, was constructed [26].
In previous studies, some hybrid grape varieties were highlighted as particularly interesting for their qualitative and quantitative anthocyanin and flavonol profiles [27,28]. In the present work, the study of more promising varieties was extended to the other non-anthocyanic flavonoids to complete their evaluation as sources of bioactive and antioxidant compounds potentially useful for industrial purposes. Polyphenols of their seed extracts were recently investigated [29], as a consequence the study was focalized on the skin/pulp extract. Characterization of compounds by ultra-high performance-liquid chromatography/quadrupole-time of flight mass spectrometry (UHPLC/QTOF) analysis, was performed.
2. Results and Discussion
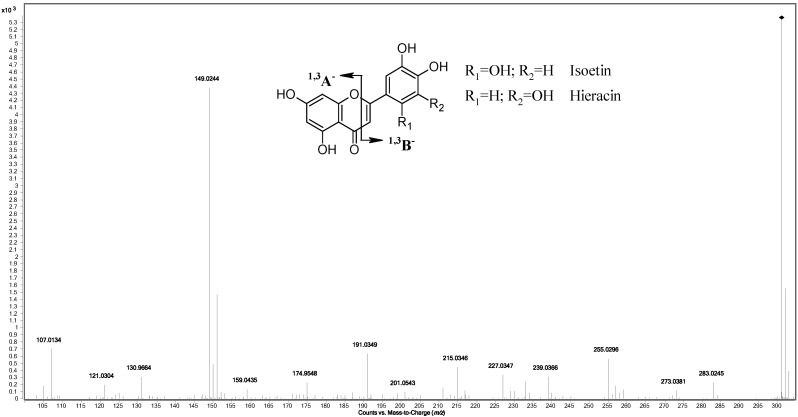
In high-resolution MS metabolomics, the identification of the molecular formula (MF) of compounds relies on the measurement of the mono-isotopic mass and isotopic pattern (relative abundances and m/z distances) followed by the raw data processing performed by using specific algorithms [30,31]. In this study, the first “targeted” identification of compounds was performed by comparing the theoretical isotopic pattern of the metabolites present in GrapeMetabolomics with the experimental ones calculated by the algorithms using the signals measured. Then, “untargeted” data processing of the remaining raw data was performed and provided the identification of possible other compounds not included in the database. Identification of compounds was based on the [M − H]− ion isotopic pattern and confirmed by MS/MS analysis. In the five grape samples, thirty-one non-anthocyanic flavonoids reported in Table 1 were identified including nineteen flavonols, two flavanonols, three flavan-3-ols, two flavanones, one flavone and four proanthocyanidins. Among them, methylnaringenin, pentahydroxy flavone quercetin isomer, syringetin-dihexoside, and tetrahydroxy-dimethoxyflavanone-hexoside were identified by the “untargeted” method. Almost all the compounds were identified with low mass error (maximum 2.7 ppm) and identification scores >90%. Just three of them—dihydrokaempferol-3-O-rhamnoside, kaempferide-p-coumaroylhexoside, and isorhamnetin-glucuronide—had a lower identification score (73%, 80% and 79%, respectively) probably due to their low signal intensity. For glucoside and glucuronide flavonols different characteristic fragmentations, were observed: glucoside compounds showed high signal of radical aglycone ion [Y0 − H]−• instead the glucuronide derivatives had higher signal of aglycone ion Y0−. A putative quercetin isomer was also identified: main MS/MS fragments at m/z 149.0244, which corresponds to the formula C8H5O3, and at m/z 151.0035 of ion C7H3O4, were observed (Table 1). Potentially, they form by 1,3B− fragmentation of flavones and flavonols [32] and the putative structure proposed for the compound is shown in Figure 1.
Table 1.
[M − H]− ion of non-anthocyanic flavonoids identified in the five hybrid grape samples studied. ppm: mass error; Id score: overall identification score calculated on the isotopic pattern signals of the compound. MS/MS, main identification fragments (underlined, the more abundant).
| Flavonoids Identified | Rt | Formula | [M − H]− Ion | Error | Id Score | MS/MS | |
|---|---|---|---|---|---|---|---|
| (min) | Experimental Theoretical | (ppm) | (%) | m/z | |||
| (−)-epicatechin | 13.63 | C15H14O6 | 289.0723 | 289.0718 | 1.7 | 97.8 | 205.0502 151.0399 |
| (+)-catechin | 12.61 | C15H14O6 | 289.0715 | 289.0718 | −1.0 | 99.3 | 205.0500 151.0396 |
| (−)-epicatechin-3-O-gallate | 15.11 | C22H18O10 | 441.0831 | 441.0827 | 0.9 | 98.3 | 289.0719 169.0142 |
| dihydrokaempferol-3-O-rhamnoside | 15.98 | C21H22O10 | 433.1148 | 433.1140 | 1.8 | 73.6 | 287.0563 269.0457 |
| dihydroquercetin-3-O-hexoside | 14.34 | C21H22O12 | 465.1048 | 465.1038 | 2.1 | 90.0 | 303.0512 151.0040 |
| isorhamnetin-3-O-glucoside | 15.72 | C22H22O12 | 477.1045 | 477.1038 | 1.5 | 98.5 | 314.0431 271.0242 |
| isorhamnetin-glucuronide | 15.90 | C22H20O13 | 491.0842 | 491.0831 | 2.2 | 79.7 | 315.0514 271.0243 |
| isorhamnetin-p-coumaroylglucoside | 17.67 | C31H28O14 | 623.1412 | 623.1406 | 0.9 | 93.8 | 477.1021 315.0511 |
| kaempferide-p-coumaroylhexoside | 17.80 | C31H28O13 | 607.1459 | 607.1457 | 0.3 | 80.4 | 461.1088 299.0557 |
| kaempferol-3-O-galactoside | 15.41 | C21H20O11 | 447.0942 | 447.0933 | 2.0 | 93.2 | 284.0337 255.0305 |
| kaempferol-3-O-glucoside | 15.61 | C21H20O11 | 447.0943 | 447.0933 | 2.2 | 94.3 | 284.0333 255.0299 |
| laricitrin-3-O-glucoside | 15.01 | C22H22O13 | 493.0994 | 493.0988 | 1.2 | 98.0 | 330.0377 315.0143 |
| methylnaringenin | 22.37 | C16H14O5 | 285.0773 | 285.0768 | 1.7 | 98.3 | 270.0539 164.0116 |
| myricetin-3-O-glucoside | 14.27 | C21H20O13 | 479.0845 | 479.0831 | 2.9 | 95.3 | 316.0233 271.0248 |
| myricetin-3-O-glucuronide | 14.20 | C21H18O14 | 493.0622 | 493.0624 | −0.4 | 98.9 | 317.0303 271.0242 |
| myricetin-dihexoside | 14.11 | C27H30O18 | 641.1362 | 641.1359 | 0.5 | 99.5 | 479.0838 316.0231 |
| myricetin-glucoside-glucuronide | 14.10 | C27H28O19 | 655.1153 | 655.1152 | 0.1 | 95.0 | 479.0815 317.0299 |
| pentahydroxy flavone (isom. quercetin) | 16.66 | C15H10O7 | 301.0356 | 301.0354 | 0.7 | 99.7 | 149.0244 151.0035 |
| procyanidin B1 | 12.02 | C30H26O12 | 577.1348 | 577.1351 | −0.5 | 99.2 | 407.0772 289.0718 |
| procyanidin B3/B4/B5 | 12.35 | C30H26O12 | 577.1345 | 577.1351 | −1.0 | 94.6 | 407.0779 289.0724 |
| procyanidin B2 | 13.25 | C30H26O12 | 577.1353 | 577.1351 | 0.3 | 95.1 | 407.0771 289.0716 |
| procyanidin T2/T3/T4/C1 | 12.84 | C45H38O18 | 865.1987 | 865.1985 | 0.2 | 91.4 | 577.1349 289.0716 |
| quercetin | 18.01 | C15H10O7 | 301.0356 | 301.0354 | 0.7 | 99.5 | 273.0406 151.0040 |
| quercetin-3-O-galactoside | 14.91 | C21H20O12 | 463.0892 | 463.0882 | 2.2 | 97.1 | 300.0284 151.0038 |
| quercetin-3-O-glucoside | 15.02 | C21H20O12 | 463.0889 | 463.0882 | 1.5 | 98.4 | 300.0284 151.0036 |
| quercetin-3-O-glucuronide | 14.96 | C21H18O13 | 477.0688 | 477.0675 | 2.7 | 96.5 | 301.0360 151.0036 |
| rutin (querc-3-O-rutinoside) | 14.63 | C27H30O16 | 609.1467 | 609.1461 | 1.0 | 97.4 | 463.0877 300.0279 |
| syringetin-3-O-galactoside | 15.53 | C23H24O13 | 507.1147 | 507.1144 | 0.6 | 94.7 | 345.0623 330.0390 |
| syringetin-3-O-glucoside | 15.68 | C23H24O13 | 507.1148 | 507.1144 | 0.8 | 98.1 | 344.0536 329.0298 |
| syringetin-dihexoside | 13.94 | C29H34O18 | 669.1672 | 669.1672 | 0.0 | 99.7 | 507.1144 345.0621 |
| tetrahydroxy-dimethoxyflavanone-hexoside | 14.19 | C23H26O13 | 509.1308 | 509.1301 | 1.4 | 98.6 | 346.0694 329.0674 |
Figure 1.
MS/MS spectrum of putative quercetin isomer flavone-type identified.
By performing analysis of samples collected in two consecutive vintages (harvests 2011 and 2012) it was possible to estimate the semi-quantitative content of the compounds reported in Table 2. Among the compounds identified two new flavonoids—isorhamnetin-p-coumaroylglucoside and kaempferide-p-coumaroylhexoside—were recently found in grape [33]. They were more abundant in the samples Siebel 19881 and Siebel 8357 (Table 2).
Table 2.
Semi-quantitative contents of non-anthocyanic flavonoids identified in the samples. Mean data of four samples (harvests 2011 and 2012), are reported.
| Flavonoids | Unknown Red | Seibel 19881 | Seyve Villard 12-347 | Seyve Villard 29-399 | Seibel 8357 |
|---|---|---|---|---|---|
| μg/kg Grape | |||||
| Flavan-3-ols | |||||
| (−)-epicatechin a | 4555 ± 1631 | 6117 ± 241 | 7467 ± 3491 | 11640 ± 2133 | 7126 ± 2447 |
| (+)-catechin b | 5578 ± 1173 | 13186 ± 802 | 11450 ± 3299 | 13257 ± 813 | 1032 ± 531 |
| (−)-epicatechin-3-O-gallate c | 1140 ± 146 | 1028 ± 293 | 673 ± 154 | 2111 ± 270 | 674 ± 74 |
| Total flavan-3-ols | 11273 | 20331 | 19590 | 27009 | 8832 |
| Flavanonols | |||||
| dihydrokaempferol-3-O-rhamnoside d | 15 ± 1 | 1082 ± 155 | 25 ± 4 | 374 ± 88 | 19 ± 2 |
| dihydroquercetin-3-O-hexoside e | 40 ± 7 | 2602 ± 680 | n.d. | 224 ± 94 | n.d. |
| Flavonols | |||||
| isorhamnetin-3-O-glucoside f | 46 ± 15 | 1090 ± 119 | 591 ± 192 | 258 ± 26 | 254 ± 56 |
| isorhamnetin-glucuronide f | n.d. | 6 ± 1 | n.d. | n.d. | n.d. |
| isorhamnetin-p-coumaroylglucoside f | n.d. | 46 ± 4 | 7 ± 2 | n.d. | 22 ± 5 |
| kaempferide-p-coumaroylhexoside d | n.d. | 39 ± 8 | n.d. | n.d. | 34 ± 1 |
| kaempferol-3-O-galactoside d | 9 ± 0 | 579 ± 10 | 933 ± 51 | 45 ± 9 | 36 ± 15 |
| kaempferol-3-O-glucoside d | 29 ± 5 | 2124 ± 75 | 2456 ± 47 | 161 ± 40 | 162 ± 78 |
| laricitrin-3-O-glucoside e | 560 ± 5 | 1681 ± 165 | 410 ± 60 | 177 ± 50 | 3240 ± 1240 |
| myricetin-3-O-glucoside h | 26702 ± 511 | 41569 ± 2324 | 24859 ± 4236 | 12458 ± 2148 | 35751 ± 1204 |
| myricetin-3-O-glucuronide h | 269 ± 32 | 202 ± 12 | 68 ± 1 | 50 ± 2 | 154 ± 23 |
| myricetin-diglucoside h | 538 ± 137 | 376 ± 74 | 612 ± 103 | 334 ± 70 | 682 ± 5 |
| myricetin-glucoside-glucuronide h | 46 ± 8 | 23 ± 2 | n.d. | n.d. | n.d. |
| pentahydroxy flavone (isom. quercetin) f | 98 ± 22 | 444 ± 125 | 100 ± 23 | 75 ± 46 | 522 ± 50 |
| quercetin f | 34 ± 8 | 316 ± 29 | 182 ± 49 | 52 ± 11 | 119 ± 45 |
| quercetin-3-O-galactoside e | 159 ± 2 | 5124 ± 331 | 4803 ± 452 | 705 ± 338 | 1027 ± 637 |
| quercetin-3-O-glucoside e | 2347 ± 380 | 12559 ± 69 | 16381 ± 548 | 6943 ± 1928 | 6124 ± 1758 |
| quercetin-3-O-glucuronide e | 618 ± 21 | 11411 ± 33 | 2501 ± 610 | 1142 ± 395 | 2220 ± 896 |
| rutin (querc-3-O-rutinoside) h | n.d | 13468 ± 1381 | 9887 ± 1496 | 2747 ± 1337 | 6091 ± 3070 |
| syringetin-3-O-galactoside f | 13 ± 0 | 57 ± 15 | 21 ± 2 | 22 ± 8 | 25 ± 4 |
| syringetin-3-O-glucoside f | 252 ± 70 | 1823 ± 139 | 228 ± 29 | 350 ± 49 | 1327 ± 388 |
| syringetin-dihexoside f | 8 ± 2 | 176 ± 49 | 37 ± 6 | 39 ± 18 | 18 ± 7 |
| Total flavonols * | 31632 | 92669 | 63976 | 25484 | 57285 |
| Procyanidins | |||||
| procyanidin B1 i | 3638 ± 903 | 2698 ± 515 | 7418 ± 1253 | 9698 ± 2364 | 1528 ± 636 |
| procyanidin B3/B4/B5 i | 1117 ± 127 | 1332 ± 81 | 3929 ± 1363 | 3913 ± 110 | 2684 ± 886 |
| procyanidin B2 l | 3836 ± 445 | 1603 ± 212 | 5029 ± 2393 | 6135 ± 4 | 634 ± 129 |
| procyanidin T2/T3/T4/C1 i | 339 ± 17 | 389 ± 32 | 617 ± 246 | 849 ± 17 | 591 ± 166 |
| Total procyanidins | 8930 | 6022 | 16992 | 20596 | 5437 |
| Flavanones | |||||
| methylnaringenin g | 114 ± 7 | 203 ± 7 | 88 ± 13 | 114 ± 11 | 136 ± 2 |
| tetrahydroxy-dimethoxyflavanone-hexoside f | 949 ± 294 | 3600 ± 630 | 910 ± 546 | 530 ± 398 | 7427 ± 449 |
| Total flavonoids | 53052 | 126953 | 101681 | 74406 | 79660 |
Superindexes indicate the standard used to calculate the concentration: a concentration calculated with analytical response factor of (−)-epicatechin; b (+)-catechin; c (−)-epicatechin gallate; d kaempferol-3-O-glucoside; e quercetin-3-O-glucoside; f isorhamnetin-3-O-glucoside; g 4ʹ,5,7-trihydroxy flavanone; h myricetin glucoside; i procyanidin B1; l procyanidin B2. *, pentahydroxy flavone (isom. quercetin) was not counted.
Currently, the grape varieties studied are not used for production and just five plants of each variety are present in the CREA-VIT vine collection. As a consequence, a quantitative study really representative of the compounds produced in the grape was not possible. Anyway, a semi-quantitative analysis of the compounds was performed in order to have main information on their presence in the grape. For some compounds, such as catechin, rutin, some quercetin derivatives, high variability was observed (±50%, Table 2), mainly due to the differences between two vintages. In general, flavonols were the most abundant non-anthocyanic flavonoids identified with a total content between 25 and 93 mg/kg calculated as sum of the single compounds in Table 2 (34%–73% of the total non-anthocyanic flavonoids identified). Total flavan-3-ol content was between 9 and 27 mg/kg (11%–36% of the total non-anthocyanic flavonoids) and proanthocyanidins between 5 and 20 mg/kg (5%–27% of the non-anthocyanic flavonoids). In a previous study, high contents of flavonols were found also in other hybrid red grape varieties, such as Seibel 9280 and Seibel 6339, and the white grape Seyve-Villard 12.303 [34]. In all samples, the flavonol profile was dominated by myricetin (myricetin-3-O-glucoside between 12.4 and 41.5 mg/kg) and the highest flavanol was procyanidin B1 (between 1.5 and 9.7 mg/kg). Similarly, high contents of procyanidin B1 and myricetin-3-O-glucoside were found in the V. labrusca grape variety Bordô and in skin of the table grape varieties BRS Clara and BRS Morena [35,36]. Myricetin-3-O-glucoside was the more abundant flavonol also in the hybrid grape cultivar BRS Violeta, but in this case the main flavanol was (+)-catechin in both skin and flesh [37].
Among the samples studied, Seibel 19881 had the highest total content of flavonols (93 mg/kg) and of flavanonols (3.7 mg/kg). In particular, this variety had considerable amounts of quercetin (43 mg/kg), myricetin (42 mg/kg), syringetin (2.0 mg/kg), and isorhamnetin (1.1 mg/kg). Moreover, this sample was characterized by relevant content of isorhamnetin-p-coumaroylglucoside (46 μg/kg), a compound recently identified in Raboso Piave V. vinifera grape together with other two p-coumaroylglucoside flavonoids (dihydrokaempferide-3-O-p-coumaroylhexoside and kaempferide-p-coumaroylhexoside) [33], by the presence of isorhamnetin-glucuronide, and significant contents of dihydrokaempferol-3-O-rhamnoside, dihydroquercetin-3-O-hexoside, and myricetin-3-O-glucoside-glucuronide. Both Seibel grape samples had high contents of pentahydroxy flavone and tetrahydroxy-dimethoxyflavanone-hexoside. Seyve Villard 29-399 was characterized by the highest contents of total flavan-3-ols and proantocyanidins, in particular (−)-epicatechin (11.6 mg/kg), (+)-catechin (13.2 mg/kg), epicatechin-3-O-gallate (2.1 mg/kg), procyanidin B1 and procyanidin B2 (9.7 mg/kg and 6.1 mg/kg, respectively) (Table 2). Also the other Seyve Villard sample (Seyve Villard 12-347) was characterized by high procyanidin B1 and procyanidin B2 (7.4 mg/kg and 5.0 mg/kg, respectively), (−)-epicatechin and (+)-catechin (7.5 mg/kg and 11.4 mg/kg, respectively), and by the highest contents of quercetin-3-O-glucoside (16.4 mg/kg) and kaempferol-3-O-glucoside (2.4 mg/kg).
3. Experimental Section
3.1. Chemicals, Samples and Sample Preparation
Standards of myricetin-3-O-glucoside, quercetin, quercetin-3-O-glucoside, isorhamnetin-3-O-glucoside, kaempferol-3-O-glucoside, rutin, procyanidin B1, procyanidin B2, (+)-catechin, (−)-epicatechin, (−)-epicatechin-3-O-gallate, (−)-epigallocatechin gallate, (−)-epigallocatechin were purchased from Extrasynthese (Genay, France); 4ʹ,5,7-trihydroxyflavanone was purchased from Sigma-Aldrich (Milan, Italy). Grape samples studied were: An “unknown hybrid red grape variety”, Seibel 8357 (Seibel 6150 × Seibel 5455: Seibel 6150 = {[(V. lincecumii × V. rupestris) × V. vinifera] × (Aramon × Ganzin)} × [(V. lincecumii × V. rupestris) × Aramon]; Seibel 5455 = {[(Aramon × Ganzin) × Bouboulenc (V. vinifera)] × [(V. lincecumii × V. rupestris) × (V. aestivalis × V. cinerea)] × V. berlandieri}; VIVC number 2768, http://www.vivc.de/), Seibel 19881 (unknown; VIVC number 11461), Seyve Villard 29-399 (unknown; VIVC number 11663) and Seyve Villard 12-347 (Seibel 6468 × Seibel 6905: Seibel 6468 = {[(Aramon × Ganzin) × Bouboulenc] × Sicilien (V. vinifera)]} × {[(V. lincecumii × V. rupestris) × (V. aestivalis × V. cinerea)] × V. berlandieri} × [(V. vinifera × V. labrusca) × V. rupestris] × V. vinifera; Seibel 6905 = Seibel 451 (unknown; VIVC number 10942) × {[(V. lincecumii × V. rupestris) × V. vinifera] × (Aramon × Ganzin)} × {[(V. lincecumii × V. rupestris) × V. vinifera] × (Aramon × Ganzin)} × {[(V. rupestris) × V. rupestris)] × Blanc Royal}; VIVC number 11587). Grapes were harvested in 2011 and 2012 at ripeness from the five plants of each variety present in the CREA-VIT grapevine germplasm repository (Susegana, Veneto, Italy). Mean sugar content of samples at the harvests were: Unknown hybrid red grape variety °Brix = 18.2 ± 0.8; Seibel 8357 °Brix= 19.4 ± 1.0; Seibel 19881 °Brix = 21.3 ± 1.1; Seyve Villard 29-399 °Brix = 17.7 ± 0.2; Seyve Villard 12-347 °Brix = 18.9 ± 1.0. For each sample, about 100 berries were picked randomly and immediately frozen at −20 °C. For sample preparation, twenty berries were weighed and homogenized using liquid nitrogen and the resulting powder was immediately extracted with pure methanol in ratio 2:1 v/w under stirring for 20 min in the dark. After addition to the extract of 200 μg/L of a 4ʹ,5,7-trihydroxyflavanone 500 mg/L solution as internal standard, the vial sample was wrapped with an aluminum foil to limit possible photo-oxidation and centrifuged at 10 °C for 20 min. The solution was filtered with an Acrodisc GHP 0.22 μm filter (Waters, Milford, MA, USA) and collected in a vial for LC/MS analysis. To perform quantitative analysis a standard solution was analyzed and RFIS/RFanalyte ratio of each compound was calculated. RFIS/RFanalyte coefficients were: Procyanidin B1 = 29.2, procyanidin B2 = 58.4; quercetin = 2.4; quercetin-3-O-glucoside = 8.2; rutin = 12.7; isorhamnetin-3-O-glucoside = 2.4; kaempferol-3-O-glucoside = 2.5; myricetin-3-O-glucoside = 14.1; (+)-catechin = 5.8; (−)-epicatechin = 6.6; (−)-epicatechin gallate = 16.7; (−)-epigallocatechin gallate = 64.3; (−)-epigallocatechin = 11.1.
3.2. LC/QTOF Mass Spectrometry
The analytical system used was Agilent UHPLC 1290 Infinity coupled to Agilent 1290 Infinity Autosampler (G4226A) and Agilent 6540 accurate-mass Q-TOF mass spectrometer (nominal resolution 40.000) with Jet Stream Ionization source (Agilent Technologies, Santa Clara, CA, USA). For each sample, two negative ionization mode analyses were performed with full scan acquisition. After each sample a blank was run. The data acquisition software was Agilent MassHunter version B.04.00 (B4033.2). Chromatography was performed using a Zorbax reverse-phase column (RRHD SB-C18 3 mm × 150 mm, 1.8 μm) (Agilent Technologies). The mobile phase was composed of (A) 0.1% v/v aqueous formic acid; and (B) 0.1% v/v formic acid in acetonitrile. Gradient elution program: 5% B isocratic for 8 min, from 5% to 45% B in 10 min, from 45% to 65% B in 5 min, from 65% to 90% in 4 min, and 90% B isocratic for 10 min. Flow rate was 0.4 mL/min; sample injection was 10 μL; column temperature was 35 °C.
QTOF conditions: sheath gas nitrogen 10 L/min at 400 °C, drying gas 8 L/min at 350 °C, nebulizer pressure 60 psi, nozzle voltage 1 kV, and capillary voltage 3.5 kV. Signals in the m/z 100–1700 range were recorded. Negative mass calibration was performed with standard mix G1969-85000 (Supelco Inc., Bellefonte, PA, USA) and had residual error for the expected masses between ±0.2 ppm. Lock masses were: TFA anion at m/z 112.9856 and HP-0921(+formate) at m/z 966.0007 in negative-ion mode.
MS/MS conditions: collision energies between 20 and 60 eV were used to fragment ions in the m/z 100–1700 range. Acquisition rate was 2 spectra/s.
Data processing was performed with Agilent Mass Hunter Qualitative Analysis software version B.05.00 (5.0.519.0) (Agilent Technologies). Confidence of the compound identification was based on accurate mass and isotope pattern and was expressed by an “overall identification score” computed as a weighted average of the compound isotopic pattern signals, such as exact masses, relative abundances, and m/z distances (spacing). Weight parameters were Wmass = 100, Wabundance = 60, and Wspacing = 50; mass expected data variation 2.0 mDa + 5.6 ppm, mass isotope abundance 7.5%, and mass isotope grouping peak spacing tolerance 0.0025 m/z + 7.0 ppm.
4. Conclusions
Among the samples studied, Seibel 19881 and the two Seyve Villard varieties are characterized by peculiar richness of non-anthocyanic flavonoids. In particular, Seyve Villard samples have high content of flavan-3-ols, procyanidin B1 and procyanidin B2. It is worth to highlight that these compounds have protective activity towards LDL oxidation greater than standard antioxidants like trolox and ascorbic acid [38]. Seibel 19881 is rich in antioxidant compounds, such as flavonols and flavanonols, and has relevant content of high isorhamnetin derivatives.
A previous study showed that these Seyve Villard grape varieties have low anthocyanin content [27]. On the other hand, these findings emphasize that they can be interesting for extraction of non-anthocyanic antioxidant compounds. Seibel 19881 confirmed its richness of non-anthocyanic polyphenols by showing that it is particularly interesting for production of extracts for nutraceutical and pharmaceutical uses. Despite in the previous study grape Seibel 8357 showed the highest anthocyanins content, this variety is not particularly rich in non-anthocyanic flavonoids, even if is characterized by relevant syringetin content. This step of the research was to investigate the presence in grape of non-anthocyanic flavonoids potentially interesting for nutraceutical purposes. If an industrial interest should arise, separated analysis of skins and pulps will have to be performed.
Acknowledgments
This study was developed within the VITENERGY1 Project, with funding from the Italian Ministry of Agricultural, Food and Forestry Policies, duration 2010–2013. The authors also thanks RGV-FAO Project, funded by Italian Ministry of Agricultural, Food and Forestry Policies, for the contribution to maintaining the CREA-VIT germplasm collection.
Author Contributions
M.G. and R.F. designed the research; M.D.R., A.P., A.D.V. and R.F. performed the research and analyzed the data; M.D.R., A.P. and R.F. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Labinskyy N., Csiszar A., Veress G., Stef G., Pacher P., Oroszi G., Wu J., Ungvari Z. Vascular dysfunction in aging: Potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr. Med. Chem. 2006;13:989–996. doi: 10.2174/092986706776360987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacopini P., Baldi M., Storchi P., Sebastiani L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008;21:589–598. doi: 10.1016/j.jfca.2008.03.011. [DOI] [Google Scholar]
- 3.Goldberg D.M., Hahn S.E., Parkes J.G. Beyond alcohol: Beverage consumption and cardiovascular mortality. Clin. Chim. Acta. 1995;237:155–187. doi: 10.1016/0009-8981(95)06069-P. [DOI] [PubMed] [Google Scholar]
- 4.Renaud S., Lorgeril D. Wine, alcohol, platelets, and the French paradox for coronary heart. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 5.German J.B., Frankel E.N., Waterhouse A.L., Hansen R.J., Walzem R.L. Wine phenolics and targets of chronic disease. In: Watkins T.R., editor. Wine, Nutritional and Therapeutic Benefits. American Chemical Society; Washington, DC, USA: 1997. pp. 196–214. [Google Scholar]
- 6.Yilmaz Y., Toledo R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin and gallic acid. J. Agric. Food Chem. 2004;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- 7.Riberéau-Gayon P., Glories Y., Maujean A., Dubourdieu D. Handbook of Enology: The Chemistry of Wine. Stabilization and Treatments. 2nd ed. Volume 2. John Wiley & Sons Ltd.; Chichester, UK: 2006. Phenolic compounds; pp. 141–203. [Google Scholar]
- 8.Shahidi F., Naczk M. Phenolics in Food and Nutraceuticals. CRC PRESS, Taylor and Francis Group; Boca Raton, FL, USA: 2004. Phenolic compounds of beverages; p. 271. [Google Scholar]
- 9.Delcambre A., Saucier C. Identification of new flavan-3-ol monoglycosides by UHPLC-ESI-Q-TOF in grapes and wine. J. Mass Spectrom. 2012;47:727–736. doi: 10.1002/jms.3007. [DOI] [PubMed] [Google Scholar]
- 10.Frankel E.N., Kanner J., German J.B., Parks E., Kinsella J.E. Inhibition of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457. doi: 10.1016/0140-6736(93)90206-V. [DOI] [PubMed] [Google Scholar]
- 11.Frankel E.N., Waterhouse A.L., Teissedre P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agric. Food Chem. 1995;43:890–894. doi: 10.1021/jf00052a008. [DOI] [Google Scholar]
- 12.Meyer A.S., Heinonen M., Frankel E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin and ellagic acid on human LDL oxidation. Food Chem. 1998;61:71–75. doi: 10.1016/S0308-8146(97)00100-3. [DOI] [Google Scholar]
- 13.Castillo-Muñoz N., Gómez-Alonso S., García-Romero E., Hermosín-Gutiérrez I. Flavonol profiles of Vitis vinifera white grape cultivars. J. Food Compos. Anal. 2010;23:699–705. doi: 10.1016/j.jfca.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Di Stefano R., Flamini R. High performance liquid chromatography analysis of grape and wine polyphenols. In: Flamini R., editor. Hyphenated Techniques in Grape and Wine Chemistry. John Wiley & Sons; Hoboken, NJ, USA: 2008. pp. 33–80. [Google Scholar]
- 15.Castillo-Muñoz N., Gómez-Alonso S., García-Romero E., Hermosín-Gutiérrez I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007;55:992–1002. doi: 10.1021/jf062800k. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Muñoz N., Gómez-Alonso S., García-Romero E., Gómez M.V., Velders A.H., Hermosín-Gutiérrez I. Flavonol 3-O-glycosides series of Vitis vinifera Cv. Petit Verdot red wine grapes. J. Agric. Food Chem. 2009;57:209–219. doi: 10.1021/jf802863g. [DOI] [PubMed] [Google Scholar]
- 17.Mattivi F., Guzzon R., Vrhovsek U., Stefanini M., Velasco R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006;54:7692–7702. doi: 10.1021/jf061538c. [DOI] [PubMed] [Google Scholar]
- 18.Boulton R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001;52:67–87. [Google Scholar]
- 19.Sakkiadi A.V., Stavrakakis M.N., Haroutounian S.A. Direct HPLC assay of five biologically interesting phenolic antioxidants in varietal Greek red wines. Lebensm. Wiss. Technol. 2001;34:410–413. doi: 10.1006/fstl.2001.0792. [DOI] [Google Scholar]
- 20.Schwarz M., Picazo-Bacete J.J., Winterhalter P., Hermosín-Gutiérrez I. Effect of copigments and grape cultivar on the color of red wines fermented after the addition of copigments. J. Agric. Food Chem. 2005;53:8372–8381. doi: 10.1021/jf051005o. [DOI] [PubMed] [Google Scholar]
- 21.Mané C., Souquet J.M., Ollé D., Véran F., Mazerolles G., Cheynier V., Fulcrand H. Optimisation of simultaneous flavanol, phenolic acid, and anthocyanin extraction from grapes using an experimental design: Application to the characterization of Champagne grape varieties. J. Agric. Food Chem. 2007;55:7224–7233. doi: 10.1021/jf071301w. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz Y., Toledo R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006;19:41–48. doi: 10.1016/j.jfca.2004.10.009. [DOI] [Google Scholar]
- 23.Flamini R., De Rosso M. Polyphenols analysis by liquid mass spectrometry. In: Flamini R., editor. Hyphenated Techniques in Grape and Wine Chemistry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2008. pp. 81–128. [Google Scholar]
- 24.Flamini R., Traldi P. Grape and wine polyphenols. In: Flamini R., Traldi P., editors. Mass Spectrometry in Grape and Wine Chemistry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2010. pp. 163–225. [Google Scholar]
- 25.Flamini R., de Rosso M., Bavaresco L. Study of grape polyphenols by liquid chromatography-high-resolution mass spectrometry (UHPLC/QTOF) and suspect screening analysis. J. Anal. Methods Chem. 2015;2015:1–10. doi: 10.1155/2015/350259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flamini R., de Rosso M., de Marchi F., Dalla Vedova A., Panighel A., Gardiman M., Maoz I., Bavaresco L. An innovative approach to grape metabolomics: Stilbene profiling by suspect screening analysis. Metabolomics. 2013;9:1243–1253. doi: 10.1007/s11306-013-0530-0. [DOI] [Google Scholar]
- 27.De Rosso M., Tonidandel L., Larcher R., Nicolini G., Dalla Vedova A., de Marchi F., Gardiman M., Giust M., Flamini R. Study of anthocyanic profiles of twenty-one hybrid grape varieties by liquid chromatography and precursor-ion mass spectrometry. Anal. Chim. Acta. 2012;732:120–129. doi: 10.1016/j.aca.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 28.De Rosso M., Tonidandel L., Larcher R., Nicolini G., Dalla Vedova A., de Marchi F., Gardiman M., Giust M., Flamini R. Identification of new flavonols in hybrid grapes by combined liquid chromatography-mass spectrometry approaches. Food Chem. 2014;163:244–251. doi: 10.1016/j.foodchem.2014.04.110. [DOI] [PubMed] [Google Scholar]
- 29.De Marchi F., Seraglia R., Molin L., Traldi P., de Rosso M., Panighel A., Dalla Vedova A., Gardiman M., Giust M., Carraro R., et al. Characterization of seed proanthocyanidins of thirty-two red and white hybrid grape varieties. Vitis. 2015;54:121–128. [Google Scholar]
- 30.Kueger S., Steinhauser D., Willmitzer L., Giavalisco P. High resolution plant metabolomics: From mass spectral features to metabolites and from whole-cell analysis to subcellular metabolite distributions. Plant J. 2012;70:39–50. doi: 10.1111/j.1365-313X.2012.04902.x. [DOI] [PubMed] [Google Scholar]
- 31.Sana T.R., Roark J.C., Li X., Waddell K., Fischer S.M. Molecular formula and METLIN personal metabolite database matching applied to the identification of compounds generated by LC/TOF-MS. J. Biomol. Technol. 2008;9:258–266. [PMC free article] [PubMed] [Google Scholar]
- 32.Fabre N., Rustan I., de Hoffmann E., Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001;12:707–715. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 33.Panighel A., de Rosso M., Dalla Vedova A., Flamini R. Putative identification of new p-coumaroyl glycoside flavonoids in grape by ultra-high performance liquid chromatography/high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2015;29:357–366. doi: 10.1002/rcm.7115. [DOI] [PubMed] [Google Scholar]
- 34.Liang Z., Yang Y., Cheng L., Zhong G.Y. Characterization of polyphenolic metabolites in grape hybrids. Vitis. 2013;52:51–59. doi: 10.1021/jf2046637. [DOI] [PubMed] [Google Scholar]
- 35.Lago-Vanzela E.S., Da-Silva R., Gómes E., García-Romero E., Hermosín-Gutiérrez I. Phenolic composition of the edible parts (flesh and skin) of Bordô Grape (V. labrusca) using HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2011;59:13136–13146. doi: 10.1021/jf203679n. [DOI] [PubMed] [Google Scholar]
- 36.Lago-Vanzela E.S., Da-Silva R., Gómes E., García-Romero E., Hermosín-Gutiérrez I. Phenolic composition of the Brazilian Seedless Table Grape Varieties BRS Clara and BRS Morena. J. Agric. Food Chem. 2011;59:8314–8323. doi: 10.1021/jf201753k. [DOI] [PubMed] [Google Scholar]
- 37.Rebello L.P.G., Lago-Vanzela E.S., Barcia M.T., Ramos A.M., Stringheta P.C., Da-Silva R., Castillo-Muñoz N., Gómez-Alonso S., Hermosín-Gutiérrez I. Phenolic composition of the berry parts of hybrid grape cultivar BRS Violeta (BRS Rubea × IAC 1398-21) using HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013;54:356–366. doi: 10.1016/j.foodres.2013.07.024. [DOI] [Google Scholar]
- 38.Leite da Silva Porto P.A., Nave Laranjinha J.A., Pererira de Freitas V.A. Antioxidant protection of low density lipoprotein by procyanidins: Structure/activity relationships. Biochem. Pharmacol. 2003;66:947–954. doi: 10.1016/S0006-2952(03)00458-1. [DOI] [PubMed] [Google Scholar]